Community Composition of Epibiont Hydroids of the Naturalized Alien Macroalga Acanthophora spicifera in Pichilingue, Mexico
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Sample Collection
2.2. Sample Processing
2.3. Data Analysis
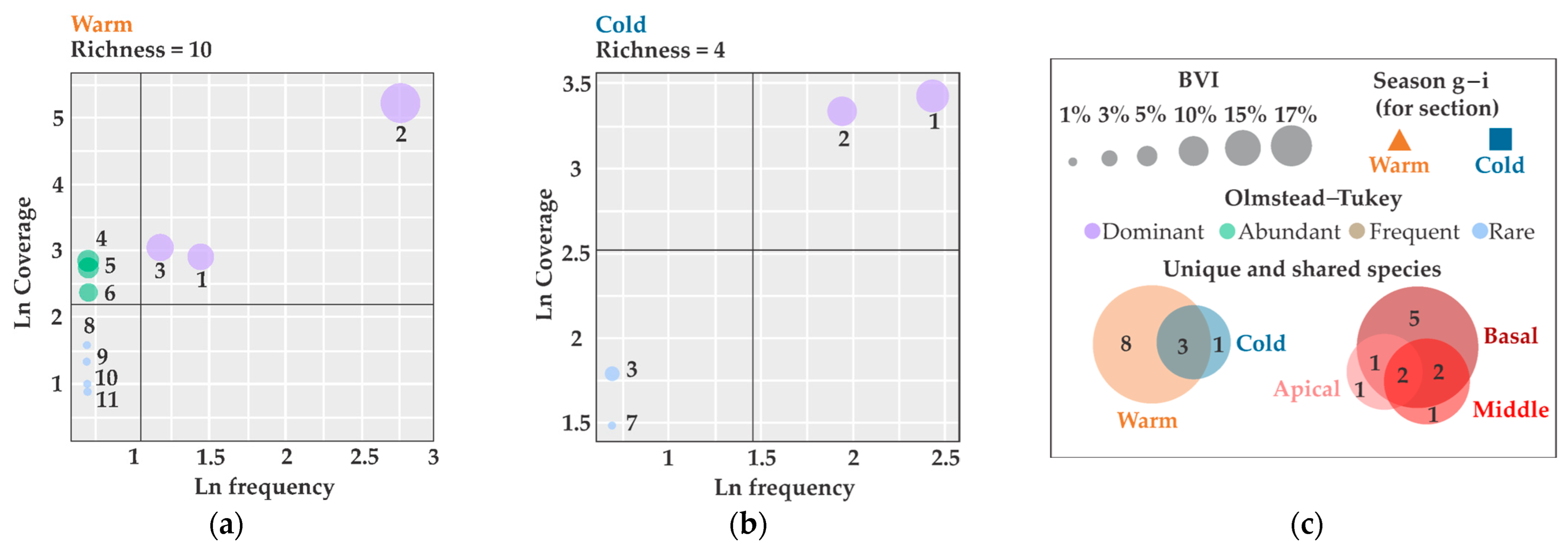
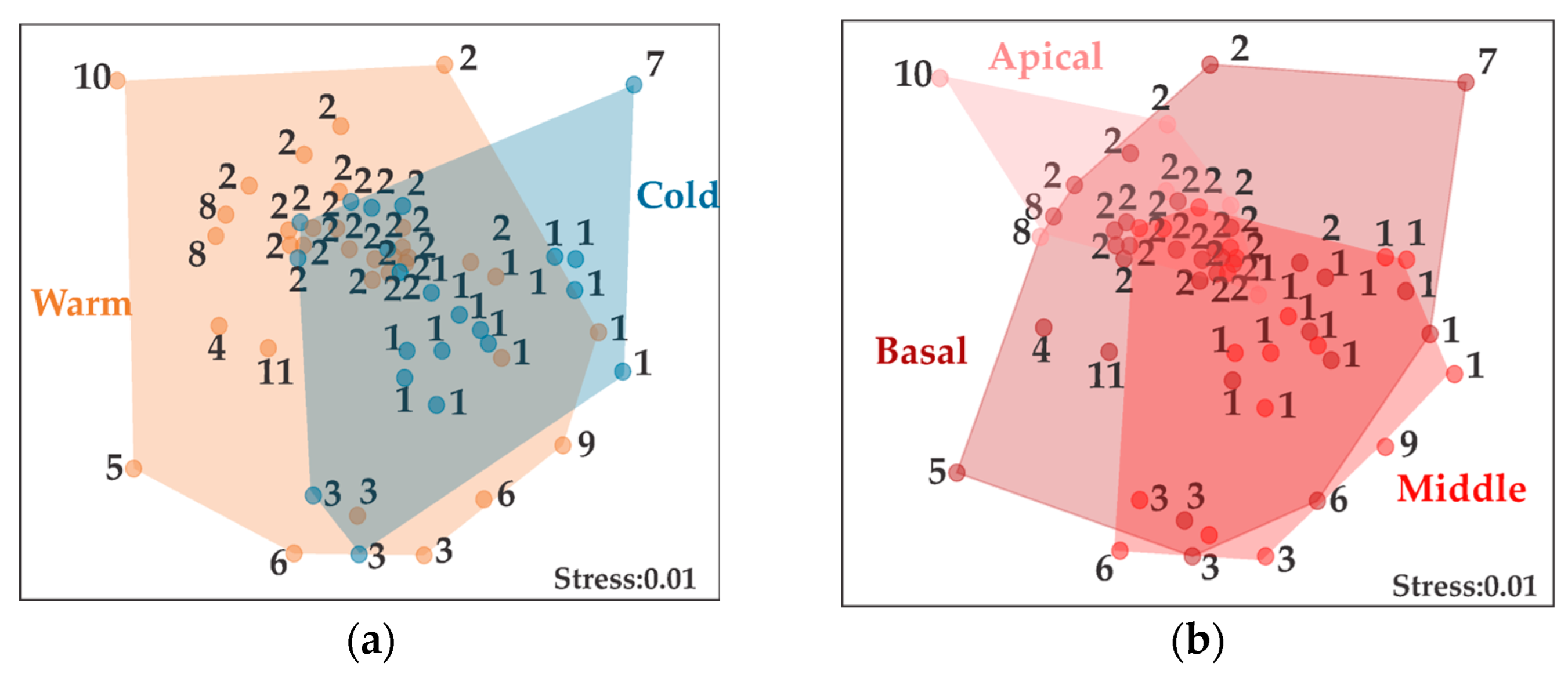
3. Results
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Hanley, M.E.; Firth, L.B.; Foggo, A. Victim of Changes? Marine macroalgae in a changing world. Ann. Bot. 2024, 133, 1–16. [Google Scholar] [CrossRef] [PubMed]
- Gan, S.X.; Tay, Y.C.; Huang, D. Effects of macroalgal morphology on marine epifaunal diversity. J. Mar. Biol. Assoc. UK 2019, 99, 1697–1707. [Google Scholar] [CrossRef]
- Schmidt, A.L.; Scheibling, R.E. A Comparison of epifauna and epiphytes on native Kelps (Laminaria species) and an invasive alga (Codium fragile ssp. tomentosoides) in Nova Scotia, Canada. Bot. Mar. 2006, 49, 315–330. [Google Scholar] [CrossRef]
- Sarma, A.L.N. The phytal fauna of Ulva fasciata off Visakhapatnam coast. Proc. Indian Acad. Sci. 1974, 80, 147–161. [Google Scholar] [CrossRef]
- Withers, R.G.; Farnham, W.F.; Lewey, S.; Jephson, N.A.; Haythorn, J.M.; Gray, P.W.G. The epibionts of Sargassum muticum in British waters. Mar. Biol. 1975, 31, 79–86. [Google Scholar] [CrossRef]
- Kitching, J.A. The flora and fauna associated with Himanthalia elongata (L.) S. F. Gray in relation to water current and wave action in the Lough Hyne marine nature reserve. Estuar. Coast. Shelf Sci. 1987, 25, 663–676. [Google Scholar] [CrossRef]
- Migotto, A.E. Benthic shallow-water hydroids (Cnidaria, Hydrozoa) of the coast of São Sebastião, Brazil, including a checklist of Brazilian hydroids. Zool. Verh. 1996, 306, 1–125. [Google Scholar]
- Wikström, S.A.; Kautsky, L. Invasion of a habitat-forming seaweed: Effects on associated biota. Biol. Invasions 2004, 6, 141–150. [Google Scholar] [CrossRef]
- Oliveira, O.M.P.; Marques, A.C. Epiphytic hydroids (Hydrozoa: Anthoathecata and Leptothecata) of the world. Check List 2007, 3, 21–38. [Google Scholar] [CrossRef]
- Oliveira, O.M.P.; Marques, A.C. Global and local patterns in the use of macrophytes as substrata by hydroids (Hydrozoa: Anthoathecata and Leptothecata). Mar. Biol. Res. 2011, 7, 786–795. [Google Scholar] [CrossRef]
- Avila, C.; Angulo-Preckler, C.; Martín-Martín, R.P.; Figuerola, B.; Griffiths, H.J.; Waller, C.L. Invasive marine species discovered on non–native kelp rafts in the warmest Antarctic island. Sci. Rep. 2020, 10, 1639. [Google Scholar] [CrossRef] [PubMed]
- Méndez-Trejo, C.; Riosmena-Rodriguez, R.; López-Vivas, J.; Sentíes, A. Evaluación de la invasión de Acanthophora spicifera (Rhodophyta) sobre la epifauna en Bahía de La Paz, B.C.S. In Especies Invasoras Acuáticas: Casos de Estudio en Ecosistemas de México; Low, A., Quijón, P., Peters, E., Eds.; SEMARNAT: Mexico City, Mexico, 2014; pp. 433–456. ISBN 978-1-304-90189-7. [Google Scholar]
- Mendoza-Becerril, M.A.; Pedroche, F.F.; Estrada-González, M.C.; Serviere-Zaragoza, E. Records of the non-native alga Acanthophora spicifera (Rhodophyta) and their colonial epibionts in La Paz Bay, Gulf of California. Biodivers. Data J. 2023, 11, e114262. [Google Scholar] [CrossRef] [PubMed]
- Mendoza-Becerril, M.A.; Murillo-Torres, P.; Serviere-Zaragoza, E.; León-Cisneros, K.; Mazariegos-Villarreal, A.; López-Vivas, J.M.; Agüero, J. First records of hydroid epibionts on the introduced macroalga Gracilaria parvispora in the Mexican Pacific. Biodivers. Data J. 2024, 12, e130248. [Google Scholar] [CrossRef] [PubMed]
- Carral-Murrieta, C.O.; Serviere-Zaragoza, E.; Rivero, F.R.C.; Marques, A.C.; Mendoza-Becerril, M.A. Sargassum species as hydrozoans substrates: Key patterns of association or just availability? Aquat. Bot. 2024, 191, 103738. [Google Scholar] [CrossRef]
- Hull, S.L. Seasonal changes in diversity and abundance of ostracods on four species of intertidal algae with differing structural complexity. Mar. Ecol. Prog. Ser. 1997, 161, 71–82. [Google Scholar] [CrossRef]
- Cunha, A.F.; Jacobucci, G.B. Seasonal variation of epiphytic hydroids (Cnidaria: Hydrozoa) associated to a subtropical Sargassum cymosum (Phaeophyta: Fucales) Bed. Zool. Curitiba 2010, 27, 945–955. [Google Scholar] [CrossRef]
- Mendoza-Becerril, M.A.; Serviere-Zaragoza, E.; Mazariegos-Villarreal, A.; Rivera-Perez, C.; Calder, D.R.; Vázquez-Delfín, E.F.; Freile-Pelegrín, Y.; Agüero, J.; Robledo, D. Epibiont hydroids on beachcast Sargassum in the Mexican Caribbean. PeerJ 2020, 8, e9795. [Google Scholar] [CrossRef]
- Doty, M.S. Acanthophora, a possible invader of the marine flora of Hawaii. Pac. Sci. 1961, 15, 547–552. [Google Scholar]
- Ávila, E.; Méndez-Trejo, M.D.C.; Riosmena-Rodríguez, R.; López-Vivas, J.M.; Sentíes, A. Epibiotic traits of the invasive red seaweed Acanthophora spicifera in La Paz Bay, South Baja California (Eastern Pacific). Mar. Ecol. 2012, 33, 470–480. [Google Scholar] [CrossRef]
- Santamaría-del-Angel, E.; Alvarez-Borrego, S.; Müller-Karger, F.E. The 1982–1984 El Niño in the Gulf of California as seen in coastal zone color scanner imagery. J. Geophys. Res. Oceans 1994, 99, 7423–7431. [Google Scholar] [CrossRef]
- Flores-Ramírez, S.; Urban-R, J.; Villarreal-Chávez, G.; Valle-Jiménez, R. Spatial and temporal changes in the cetacean community structure at Bahía de La Paz, BCS, México (1988–1991). Cienc. Mar. 1996, 22, 151–173. [Google Scholar] [CrossRef]
- Guevara-Guillén, C.; Shirasago, B.; Perez-Lezama, E. The influence of large-scale phenomena on La Paz Bay hydrographic variability. Open J. Mar. Sci. 2015, 5, 146–157. [Google Scholar] [CrossRef][Green Version]
- Monteforte-Sánchez, M. Integration of resident fisherfolk communities in marine protected areas by social micro-entrepreneurships of mariculture: A Case Study at La Paz Bay, South Baja California, Mexico. In Socio-Ecological Studies in Natural Protected Areas: Linking Community Development and Conservation in Mexico; Ortega-Rubio, A., Ed.; Springer International Publishing: Cham, Switzerland, 2020; pp. 537–566. ISBN 978-3-030-47264-1. [Google Scholar]
- de Jong, Y.; Hitipeuw, C.; Prud’homme van Reine, W.F. A taxonomic, phylogenetic and biogeographic study of the genus Acanthophora (Rhodomelaceae, Rhodophyta). Blumea Biodivers. Evol. Biogeogr. Plants 1999, 44, 217–249. [Google Scholar]
- Calder, D.R. Shallow-Water Hydroids of Bermuda: The Athecatae, 1st ed.; Royal Ontario Museum: Toronto, ON, Canada, 1988; ISBN 0-88854-339-5. [Google Scholar]
- Calder, D.R. Shallow-Water Hydroids of Bermuda. The Thecatae, Exclusive of Plumularioidea, 1st ed.; Royal Ontario Museum: Toronto, ON, Canada, 1991; ISBN 0-88854-354-9. [Google Scholar]
- Calder, D.R. Shallow-Water Hydroids of Bermuda: Superfamily Plumularioidea, 1st ed.; Royal Ontario Museum: Toronto, ON, Canada, 1997; ISBN 978-0-88854-339-4. [Google Scholar]
- Mendoza-Becerril, M.A.; Estrada-González, M.C.; Mazariegos-Villarreal, A.; Restrepo-Avendaño, L.; Villar-Beltrán, R.D.; Agüero, J.; Cunha, A.F. Taxonomy and diversity of Hydrozoa (Cnidaria, Medusozoa) of La Paz Bay, Gulf of California. Zootaxa 2020, 4808, 1–37. [Google Scholar] [CrossRef] [PubMed]
- Maronna, M.M.; Miranda, T.P.; Peña Cantero, Á.L.; Barbeitos, M.S.; Marques, A.C. Towards a phylogenetic classification of Leptothecata (Cnidaria, Hydrozoa). Sci. Rep. 2016, 6, 18075. [Google Scholar] [CrossRef] [PubMed]
- Ahyong, S.; Boyko, C.B.; Bernot, J.; Brandão, S.N.; Daly, M.; De Grave, S.; de Voogd, N.J.; Gofas, S.; Hernandez, F.; Hughes, L.; et al. World Register of Marine Species (WoRMS). Available online: https://www.marinespecies.org (accessed on 20 October 2024).
- Clarke, K.R.; Gorley, R.N. Getting Started with PRIMER V7; PRIMER-E, Ltd.: Plymouth, UK, 2015. [Google Scholar]
- Hsieh, T.C.; Ma, K.H.; Chao, A. iNEXT: Interpolation and Extrapolation for Species Diversity. 2020. Available online: http://chao.stat.nthu.edu.tw/wordpress/software_download/ (accessed on 20 October 2024).
- Olmstead, P.S.; Tukey, J.W. A Corner test for association. Ann. Math. Stat. 1947, 18, 495–513. [Google Scholar] [CrossRef]
- Sokal, R.R.; Rohlf, F.J. Biometry: The Principles and Practice of Statistics in Biological Research, 2nd ed.; W. H. Freeman and Company: New York, NY, USA, 1981; ISBN 978-0-7167-1254-1. [Google Scholar]
- Loya-Salinas, D.H.; Escofet, A. Contribution to the calculation of the biological value index (Sanders, 1960). Cienc. Mar. 1990, 16, 97–115. [Google Scholar] [CrossRef]
- R Development Core Team. R: A Language and Environment for Statistical Computing; Foundation for Statistical Computing: Vienna, Austria, 2024. [Google Scholar]
- Wickham, H. Ggplot2: Elegant Graphics for Data Analysis, 2nd ed.; Springer International Publishing: New York, NY, USA, 2016; ISBN 978-3-319-24275-0. [Google Scholar]
- Larsoon, J. Eulerr: Area-Proportional Euler and Venn Diagrams with Ellipse. 2024. Available online: https://cran.r-project.org/web/packages/eulerr/eulerr.pdf (accessed on 15 September 2024).
- Giraudoux, P. Pgirmess: Data Analysis in Ecology. 2013. Available online: https://www.researchgate.net/publication/309726221_Pgirmess_data_analysis_in_ecology (accessed on 15 September 2024).
- Kruskal, J.; Wish, M. Multidimensional Scaling; SAGE Publications, Inc.: Thousand Oaks, CA, USA, 2024. [Google Scholar]
- Clarke, K.R. Non-Parametric multivariate analyses of changes in community structure. Aust. J. Ecol. 1993, 18, 117–143. [Google Scholar] [CrossRef]
- Norton, T.A.; Benson, M.R. Ecological interactions between the brown seaweed Sargassum muticum and its associated fauna. Mar. Biol. 1983, 75, 169–177. [Google Scholar] [CrossRef]
- Gutow, L.; Beermann, J.; Buschbaum, C.; Rivadeneira, M.M.; Thiel, M. Castaways can’t be choosers—Homogenization of rafting assemblages on floating seaweeds. J. Sea Res. 2015, 95, 161–171. [Google Scholar] [CrossRef]
- Estrada-González, M.C.; Agüero, J.; Mendoza-Becerril, M.A. Medusozoans (Cnidaria) from the Mexican Pacific: A review based on historical literature and recent observations. J. Nat. Hist. 2023, 57, 784–853. [Google Scholar] [CrossRef]
- Mendoza-Becerril, M.A.; Estrada-González, M.C.; Agüero, J. Medusozoos de La Bahía de La Paz, 1st ed.; Petricor Cuadernos: La Paz, Mexico, 2022; ISBN 978-607-7634-31-7. [Google Scholar]
- Estrada-González, M.C.; Jiménez-López, M.E.; Huato-Soberanis, L.; Mendoza-Becerril, M.A. Socioenvironmental and spatial criteria as tools for the management and conservation of hydrozoans in protected and unprotected areas. Diversity 2023, 15, 182. [Google Scholar] [CrossRef]
- Martell, L.; Bracale, R.; Carrion, S.A.; Purcell, J.E.; Lezzi, M.; Gravili, C.; Piraino, S.; Boero, F. Successional Dynamics of Marine Fouling Hydroids (Cnidaria: Hydrozoa) at a Finfish aquaculture facility in the Mediterranean Sea. PLoS ONE 2018, 13, e0195352. [Google Scholar] [CrossRef]
- González-Martínez, A.I.; Barrios-Caballero, Y.; Born-Schmidt, G.; Koleff-Osorio, P. El sistema de información sobre especies invasoras (SNIB). Available online: https://www.snib.mx/ (accessed on 24 July 2024).
- GBIIF Obelia dichotoma (Linnaeus, 1758). Available online: https://www.gbif.org/es/species/5185976 (accessed on 24 July 2024).
- Cunha, A.F.; Collins, A.G.; Marques, A.C. Phylogenetic relationships of Proboscoida Broch, 1910 (Cnidaria, Hydrozoa): Are traditional morphological diagnostic characters relevant for the delimitation of lineages at the species, genus, and family levels? Mol. Phylogenet. Evol. 2017, 106, 118–135. [Google Scholar] [CrossRef] [PubMed]
- Rackley, D.H. Hydroids of the pelagic Sargassum community of the Gulf Stream and Sargasso Sea; Virginia Institute of Marine Science: Gloucester Point, VA, USA, 1974. [Google Scholar]
- Faucci, A.; Boero, F. Structure of an epiphytic hydroid community on Cystoseira at two sites of different wave exposure. Sci. Mar. 2000, 64, 255–264. [Google Scholar] [CrossRef]
- Ronowicz, M.; Wlodarska-Kowalczuk, M.; Kuklinski, P. Factors influencing hydroids (Cnidaria: Hydrozoa) biodiversity and distribution in Arctic kelp forest. J. Mar. Biol. Assoc. UK 2008, 88, 1567–1575. [Google Scholar] [CrossRef]
- Borowitzka, M.A.; Lethbridge, R.C.; Charlton, L. Species richness, spatial distribution and colonisation pattern of algal and invertebrate epiphytes on the seagrass Amphibolis griffithii. Mar. Ecol. Prog. Ser. Oldendorf 1990, 64, 281–291. [Google Scholar] [CrossRef]
- Calder, D.R. Seasonal cycles of activity and inactivity in some hydroids from Virginia and South Carolina, U.S.A. Can. J. Zool. 1990, 68, 442–450. [Google Scholar] [CrossRef]
- Schnoller, V.C.G.; López-Vivas, J.M.L.; Riosmena-Rodríguez, R.; López Calderón, J.M. Evaluation of biomass and reproductive aspects of invasive algae Acanthophora spicifera in Punta Roca Caimancito, BCS Mexico. In Marine Benthos: Biology, Ecosystem Functions and Environmental Impact; Nova Science Publishers: New York, NY, USA, 2016; pp. 139–168. ISBN 978-1-63484-930-2. [Google Scholar]
- Fraschetti, S.; Terlizzi, A.; Bevilacqua, S.; Boero, F. The distribution of hydroids (Cnidaria, Hydrozoa) from micro- to macro-scale: Spatial patterns on habitat-forming algae. J. Exp. Mar. Biol. Ecol. 2006, 339, 148–158. [Google Scholar] [CrossRef]
- Gili, J.-M.; Hughes, R. The ecology of marine benthic hydroids. Oceanogr. Mar. Biol. Ann. Rev. 1995, 33, 351–426. [Google Scholar]
- Monteforte, M.; Cariño, M. Exploration and evaluation of natural stocks of pearl oysters Pinctada mazatlanica and Pteria sterna (Bivalvia: Pteriidae): La Paz Bay, South Baja California, Mexico. Ambio 1992, 21, 314–320. [Google Scholar]
- Torres-Hernández, M.-Y.; Trasviña-Castro, A.; Rosales-Villa, A.-R.; Souza, A.J. Dynamics of the surface circulation of La Paz Bay, Mexico. Cont. Shelf Res. 2022, 235, 104664. [Google Scholar] [CrossRef]
- Riedl, R. Biologie Der Meershöhlen, 1st ed.; Paul Parey: Hamburg, Germany, 1966; ISBN 978-3490063182. [Google Scholar]
- Gili, J.M.; Bouillon, J.; Pagès, F.; Palanques, A.; Puig, P.; Heussner, S. Origin and biogeography of the deep-water Mediterranean Hydromedusae including the description of two new species collected in submarine canyons of Northwestern Mediterranean. Sci. Mar. 1998, 62, 113–134. [Google Scholar]
- Koehl, M.A.R.; Daniel, T.L. Hydrodynamic interactions between macroalgae and their epibionts. Front. Mar. Sci. 2022, 9, 872960. [Google Scholar] [CrossRef]
- Kato, M.; Nakamura, K.; Hirai, E.; Kakinuma, Y. Interspecific relation in the colony formation among some hydrozoan species. Bull. Mar. Biol. Stn. Asamushi 1962, 11, 31–39. [Google Scholar]
- Sempere-Valverde, J.; Castro-Cadenas, M.D.; Guerra-García, J.M.; Espinosa, F.; García-Gómez, J.C.; Ros, M. Buoys are non-indigenous fouling hotspots in marinas regardless of their environmental status and pressure. Sci. Total Environ. 2024, 909, 168301. [Google Scholar] [CrossRef] [PubMed]
- Bavestrello, G.; Puce, S.; Cerrano, C.; Zocchi, E.; Boero, N. The problem of seasonality of benthic hydroids in temperate waters. Chem. Ecol. 2006, 22, S197–S205. [Google Scholar] [CrossRef]
- Puce, S.; Bavestrello, G.; Di Camillo, C.G.; Boero, F. Long-Term changes in hydroid (Cnidaria, Hydrozoa) assemblages: Effect of Mediterranean warming? Mar. Ecol. 2009, 30, 313–326. [Google Scholar] [CrossRef]
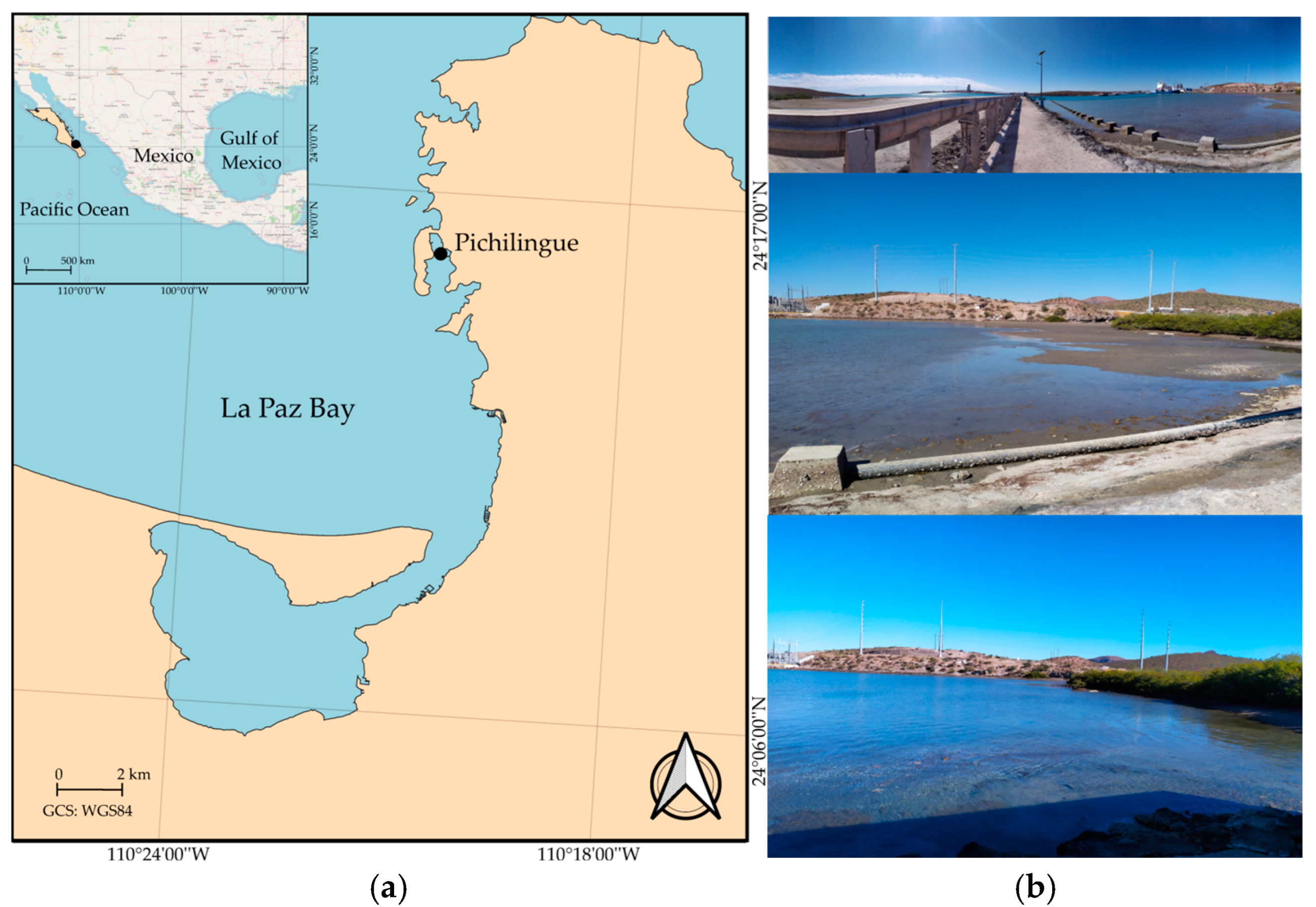





| Taxa | Warm Season (35 Ups, 28 °C) | Cold Season (35 Ups, 25 °C) | Section |
| Class Hydrozoa Owen, 1843 | |||
| Subclass Hydroidolina Collins, 2000 | |||
| Superorder “Anthoathecata” Cornelius, 1992 | |||
| Bimeria vestita Wright, 1859 | 1.5 | B | |
| Bougainvillia muscus (Allman, 1863) | 9.9 ± 5.3 | 5.0 | M, B |
| Capitata (Indet.) | 14.3 | B | |
| Corydendrium sp. (Indet.) | 3.4 | B | |
| Turritopsis sp. (Indet.) | 13.7 | B | |
| Superorder Leptothecata Cornelius, 1992 | |||
| Clytia cf. gracilis (M. Sars, 1851) | 1.6 | A | |
| Clytia linearis (Thornely, 1900) | 5.7 ± 3.1 | 2.9 ± 1.2 | A, M, B |
| Lafoeidae (Indet.) | 2.8 | M | |
| Obelia cf. dichotoma (Linnaeus, 1758) | 11.8 ± 12.8 | 4.6 ± 3.8 | A, M, B |
| Obelia oxydentata Stechow, 1914 | 3.9 | A, B | |
| Ventromma halecioides (Alder, 1859) | 8.1 | M, B |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Licona Angeles, J.; Castañeda Rivero, F.R.; Mendoza-Becerril, M.A. Community Composition of Epibiont Hydroids of the Naturalized Alien Macroalga Acanthophora spicifera in Pichilingue, Mexico. Biology 2025, 14, 44. https://doi.org/10.3390/biology14010044
Licona Angeles J, Castañeda Rivero FR, Mendoza-Becerril MA. Community Composition of Epibiont Hydroids of the Naturalized Alien Macroalga Acanthophora spicifera in Pichilingue, Mexico. Biology. 2025; 14(1):44. https://doi.org/10.3390/biology14010044
Chicago/Turabian StyleLicona Angeles, Jessica, Francisco Rubén Castañeda Rivero, and María A. Mendoza-Becerril. 2025. "Community Composition of Epibiont Hydroids of the Naturalized Alien Macroalga Acanthophora spicifera in Pichilingue, Mexico" Biology 14, no. 1: 44. https://doi.org/10.3390/biology14010044
APA StyleLicona Angeles, J., Castañeda Rivero, F. R., & Mendoza-Becerril, M. A. (2025). Community Composition of Epibiont Hydroids of the Naturalized Alien Macroalga Acanthophora spicifera in Pichilingue, Mexico. Biology, 14(1), 44. https://doi.org/10.3390/biology14010044










