The Genetic and Epigenetic Arms of Human Ageing and Longevity
Simple Summary
Abstract
1. Introduction
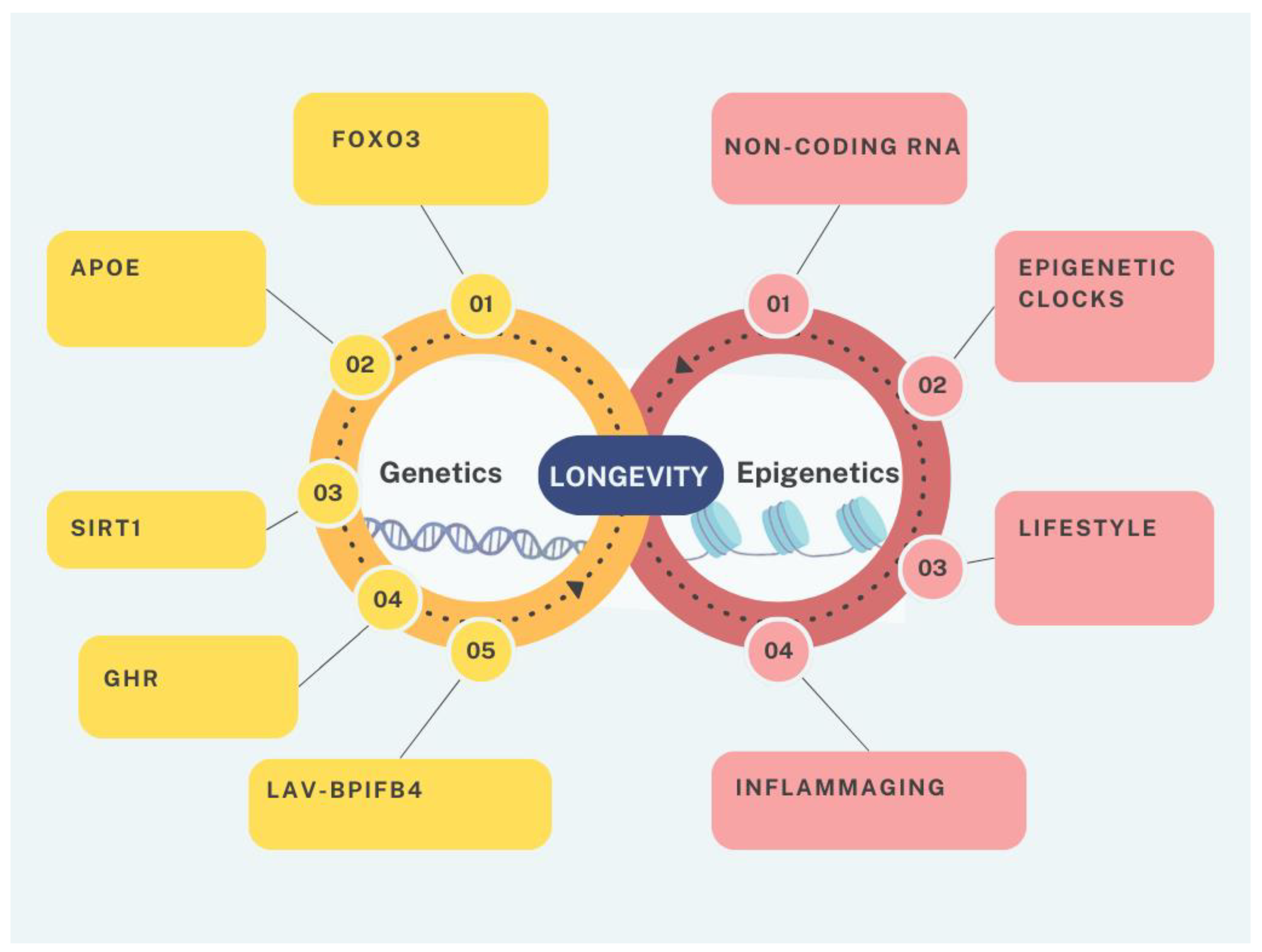
2. The Genetics of Longevity
3. Epigenetics and Ageing
4. Genetic Variants Influencing DNA Epigenetic Modifications
5. Modulation of Ageing by Non-Coding RNAs
6. Inflammageing at the Interface Between Genetics and Epigenetics, a New Prognostic Factor for Human Longevity
7. The Lifestyle Factors for Successful Epigenetic Changes
8. The Power of the Epigenetic Clocks as Prognostic and Diagnostic Biomarker of Ageing and Longevity
9. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Caruso, C.; Puca, A.A. Special Issue “Centenarians-A Model to Study the Molecular Basis of Lifespan and Healthspan”. Int. J. Mol. Sci. 2021, 22, 2044. [Google Scholar] [CrossRef] [PubMed]
- Herskind, A.M.; McGue, M.; Holm, N.V.; Sorensen, T.I.; Harvald, B.; Vaupel, J.W. The heritability of human longevity: A population-based study of 2872 Danish twin pairs born 1870–1900. Hum. Genet. 1996, 97, 319–323. [Google Scholar] [CrossRef] [PubMed]
- Quach, A.; Levine, M.E.; Tanaka, T.; Lu, A.T.; Chen, B.H.; Ferrucci, L.; Ritz, B.; Bandinelli, S.; Neuhouser, M.L.; Beasley, J.M.; et al. Epigenetic clock analysis of diet, exercise, education, and lifestyle factors. Aging 2017, 9, 419–446. [Google Scholar] [CrossRef] [PubMed]
- Perls, T.T.; Wilmoth, J.; Levenson, R.; Drinkwater, M.; Cohen, M.; Bogan, H.; Joyce, E.; Brewster, S.; Kunkel, L.; Puca, A. Life-long sustained mortality advantage of siblings of centenarians. Proc. Natl. Acad. Sci. USA 2002, 99, 8442–8447. [Google Scholar] [CrossRef]
- De Benedictis, G.; Franceschi, C. The unusual genetics of human longevity. Sci. Aging Knowl. Environ. 2006, 2006, pe20. [Google Scholar] [CrossRef]
- Brooks-Wilson, A.R. Genetics of healthy aging and longevity. Hum. Genet. 2013, 132, 1323–1338. [Google Scholar] [CrossRef]
- Smulders, L.; Deelen, J. Genetics of human longevity: From variants to genes to pathways. J. Intern. Med. 2024, 295, 416–435. [Google Scholar] [CrossRef]
- Deelen, J.; Uh, H.W.; Monajemi, R.; van Heemst, D.; Thijssen, P.E.; Bohringer, S.; van den Akker, E.B.; de Craen, A.J.; Rivadeneira, F.; Uitterlinden, A.G.; et al. Gene set analysis of GWAS data for human longevity highlights the relevance of the insulin/IGF-1 signaling and telomere maintenance pathways. Age 2013, 35, 235–249. [Google Scholar] [CrossRef]
- Passtoors, W.M.; Beekman, M.; Deelen, J.; van der Breggen, R.; Maier, A.B.; Guigas, B.; Derhovanessian, E.; van Heemst, D.; de Craen, A.J.; Gunn, D.A.; et al. Gene expression analysis of mTOR pathway: Association with human longevity. Aging Cell 2013, 12, 24–31. [Google Scholar] [CrossRef]
- Zhang, H.L.; Wu, J.; Zhu, J. The immune-modulatory role of apolipoprotein E with emphasis on multiple sclerosis and experimental autoimmune encephalomyelitis. Clin. Dev. Immunol. 2010, 2010, 186813. [Google Scholar] [CrossRef]
- Ciaglia, E.; Montella, F.; Maciag, A.; Scala, P.; Ferrario, A.; Banco, C.; Carrizzo, A.; Spinelli, C.C.; Cattaneo, M.; De Candia, P.; et al. Longevity-Associated Variant of BPIFB4 Mitigates Monocyte-Mediated Acquired Immune Response. J. Gerontol. A Biol. Sci. Med. Sci. 2019, 74, S38–S44. [Google Scholar] [CrossRef] [PubMed]
- Cattaneo, M.; Baragetti, A.; Malovini, A.; Ciaglia, E.; Lopardo, V.; Olmastroni, E.; Casula, M.; Ciacci, C.; Catapano, A.L.; Puca, A.A. Longevity-associated BPIFB4 gene counteracts the inflammatory signaling. Immun. Ageing 2024, 21, 19. [Google Scholar] [CrossRef] [PubMed]
- Ciaglia, E.; Montella, F.; Carrizzo, A.; Lopardo, V.; Esposito, R.M.; Basile, C.; Damato, A.; De Lucia, M.; Maciag, A.; Spinetti, G.; et al. The longevity-associated BPIFB4 gene guarantees vascular homeostasis and immune protection through platelets. Geroscience 2024, 46, 6347–6359. [Google Scholar] [CrossRef] [PubMed]
- Ciaglia, E.; Montella, F.; Lopardo, V.; Scala, P.; Ferrario, A.; Cattaneo, M.; Carrizzo, A.; Malovini, A.; Madeddu, P.; Vecchione, C.; et al. Circulating BPIFB4 Levels Associate With and Influence the Abundance of Reparative Monocytes and Macrophages in Long Living Individuals. Front. Immunol. 2020, 11, 1034. [Google Scholar] [CrossRef]
- Di Pardo, A.; Ciaglia, E.; Cattaneo, M.; Maciag, A.; Montella, F.; Lopardo, V.; Ferrario, A.; Villa, F.; Madonna, M.; Amico, E.; et al. The longevity-associated variant of BPIFB4 improves a CXCR4-mediated striatum-microglia crosstalk preventing disease progression in a mouse model of Huntington’s disease. Cell Death Dis. 2020, 11, 546. [Google Scholar] [CrossRef]
- Ciaglia, E.; Lopardo, V.; Montella, F.; Carrizzo, A.; Di Pietro, P.; Malavolta, M.; Giacconi, R.; Orlando, F.; Cattaneo, M.; Madeddu, P.; et al. Transfer of the longevity-associated variant of BPIFB4 gene rejuvenates immune system and vasculature by a reduction of CD38(+) macrophages and NAD(+) decline. Cell Death Dis. 2022, 13, 86. [Google Scholar] [CrossRef]
- Santos, B.F.; Grenho, I.; Martel, P.J.; Ferreira, B.I.; Link, W. FOXO family isoforms. Cell Death Dis. 2023, 14, 702. [Google Scholar] [CrossRef]
- Flachsbart, F.; Dose, J.; Gentschew, L.; Geismann, C.; Caliebe, A.; Knecht, C.; Nygaard, M.; Badarinarayan, N.; ElSharawy, A.; May, S.; et al. Identification and characterization of two functional variants in the human longevity gene FOXO3. Nat. Commun. 2017, 8, 2063. [Google Scholar] [CrossRef]
- Rahman, S.; Islam, R. Mammalian Sirt1: Insights on its biological functions. Cell Commun. Signal 2011, 9, 11. [Google Scholar] [CrossRef]
- Kilic, U.; Gok, O.; Erenberk, U.; Dundaroz, M.R.; Torun, E.; Kucukardali, Y.; Elibol-Can, B.; Uysal, O.; Dundar, T. A remarkable age-related increase in SIRT1 protein expression against oxidative stress in elderly: SIRT1 gene variants and longevity in human. PLoS ONE 2015, 10, e0117954. [Google Scholar] [CrossRef]
- Brunet, A.; Sweeney, L.B.; Sturgill, J.F.; Chua, K.F.; Greer, P.L.; Lin, Y.; Tran, H.; Ross, S.E.; Mostoslavsky, R.; Cohen, H.Y.; et al. Stress-dependent regulation of FOXO transcription factors by the SIRT1 deacetylase. Science 2004, 303, 2011–2015. [Google Scholar] [CrossRef] [PubMed]
- Schachter, F.; Faure-Delanef, L.; Guenot, F.; Rouger, H.; Froguel, P.; Lesueur-Ginot, L.; Cohen, D. Genetic associations with human longevity at the APOE and ACE loci. Nat. Genet. 1994, 6, 29–32. [Google Scholar] [CrossRef] [PubMed]
- Huang, Y.; Mahley, R.W. Apolipoprotein E: Structure and function in lipid metabolism, neurobiology, and Alzheimer’s diseases. Neurobiol. Dis. 2014, 72 Pt A, 3–12. [Google Scholar] [CrossRef]
- Ben-Avraham, D.; Govindaraju, D.R.; Budagov, T.; Fradin, D.; Durda, P.; Liu, B.; Ott, S.; Gutman, D.; Sharvit, L.; Kaplan, R.; et al. The GH receptor exon 3 deletion is a marker of male-specific exceptional longevity associated with increased GH sensitivity and taller stature. Sci. Adv. 2017, 3, e1602025. [Google Scholar] [CrossRef] [PubMed]
- Donlon, T.A.; Chen, R.; Masaki, K.H.; Willcox, D.C.; Allsopp, R.C.; Willcox, B.J.; Morris, B.J. Association of growth hormone receptor gene variant with longevity in men is due to amelioration of increased mortality risk from hypertension. Aging 2021, 13, 14745–14767. [Google Scholar] [CrossRef]
- Villa, F.; Carrizzo, A.; Spinelli, C.C.; Ferrario, A.; Malovini, A.; Maciag, A.; Damato, A.; Auricchio, A.; Spinetti, G.; Sangalli, E.; et al. Genetic Analysis Reveals a Longevity-Associated Protein Modulating Endothelial Function and Angiogenesis. Circ. Res. 2015, 117, 333–345. [Google Scholar] [CrossRef]
- Villa, F.; Malovini, A.; Carrizzo, A.; Spinelli, C.C.; Ferrario, A.; Maciag, A.; Madonna, M.; Bellazzi, R.; Milanesi, L.; Vecchione, C.; et al. Serum BPIFB4 levels classify health status in long-living individuals. Immun. Ageing 2015, 12, 27. [Google Scholar] [CrossRef]
- Dang, Z.; Avolio, E.; Thomas, A.C.; Faulkner, A.; Beltrami, A.P.; Cervellin, C.; Carrizzo, A.; Maciag, A.; Gu, Y.; Ciaglia, E.; et al. Transfer of a human gene variant associated with exceptional longevity improves cardiac function in obese type 2 diabetic mice through induction of the SDF-1/CXCR4 signalling pathway. Eur. J. Heart Fail. 2020, 22, 1568–1581. [Google Scholar] [CrossRef]
- Puca, A.A.; Carrizzo, A.; Spinelli, C.; Damato, A.; Ambrosio, M.; Villa, F.; Ferrario, A.; Maciag, A.; Fornai, F.; Lenzi, P.; et al. Single systemic transfer of a human gene associated with exceptional longevity halts the progression of atherosclerosis and inflammation in ApoE knockout mice through a CXCR4-mediated mechanism. Eur. Heart J. 2020, 41, 2487–2497. [Google Scholar] [CrossRef]
- Cattaneo, M.; Aleksova, A.; Malovini, A.; Avolio, E.; Thomas, A.; Alvino, V.V.; Kilcooley, M.; Pieronne-Deperrois, M.; Ouvrard-Pascaud, A.; Maciag, A.; et al. BPIFB4 and its longevity-associated haplotype protect from cardiac ischemia in humans and mice. Cell Death Dis. 2023, 14, 523. [Google Scholar] [CrossRef]
- Cattaneo, M.; Beltrami, A.P.; Thomas, A.C.; Spinetti, G.; Alvino, V.V.; Avolio, E.; Veneziano, C.; Rolle, I.G.; Sponga, S.; Sangalli, E.; et al. The longevity-associated BPIFB4 gene supports cardiac function and vascularization in ageing cardiomyopathy. Cardiovasc. Res. 2023, 119, 1583–1595. [Google Scholar] [CrossRef] [PubMed]
- Cattaneo, M.; Maciag, A.; Milella, M.S.; Ciaglia, E.; Bruno, A.; Puca, A.A. Longevity-Associated Variant of BPIFB4 Confers Neuroprotection in the STHdh Cell Model of Huntington Disease. Int. J. Mol. Sci. 2022, 23, 15313. [Google Scholar] [CrossRef] [PubMed]
- Puca, A.A.; Lopardo, V.; Montella, F.; Di Pietro, P.; Cesselli, D.; Rolle, I.G.; Bulfoni, M.; Di Sarno, V.; Iaconetta, G.; Campiglia, P.; et al. The Longevity-Associated Variant of BPIFB4 Reduces Senescence in Glioma Cells and in Patients’ Lymphocytes Favoring Chemotherapy Efficacy. Cells 2022, 11, 294. [Google Scholar] [CrossRef] [PubMed]
- Vecchione, C.; Villa, F.; Carrizzo, A.; Spinelli, C.C.; Damato, A.; Ambrosio, M.; Ferrario, A.; Madonna, M.; Uccellatore, A.; Lupini, S.; et al. A rare genetic variant of BPIFB4 predisposes to high blood pressure via impairment of nitric oxide signaling. Sci. Rep. 2017, 7, 9706. [Google Scholar] [CrossRef]
- Pes, G.M.; Tolu, F.; Poulain, M.; Errigo, A.; Masala, S.; Pietrobelli, A.; Battistini, N.C.; Maioli, M. Lifestyle and nutrition related to male longevity in Sardinia: An ecological study. Nutr. Metab. Cardiovasc. Dis. 2013, 23, 212–219. [Google Scholar] [CrossRef]
- Yang, J.H.; Hayano, M.; Griffin, P.T.; Amorim, J.A.; Bonkowski, M.S.; Apostolides, J.K.; Salfati, E.L.; Blanchette, M.; Munding, E.M.; Bhakta, M.; et al. Loss of epigenetic information as a cause of mammalian aging. Cell 2023, 186, 305–326.e327. [Google Scholar] [CrossRef]
- Milosic, F.; Hengstschlager, M.; Osmanagic-Myers, S. Premature aging in genetic diseases: What conclusions can be drawn for physiological aging. Front. Aging 2023, 4, 1327833. [Google Scholar] [CrossRef]
- Shamanna, R.A.; Croteau, D.L.; Lee, J.H.; Bohr, V.A. Recent Advances in Understanding Werner Syndrome. F1000Res 2017, 6, 1779. [Google Scholar] [CrossRef]
- Soto-Palma, C.; Niedernhofer, L.J.; Faulk, C.D.; Dong, X. Epigenetics, DNA damage, and aging. J. Clin. Invest. 2022, 132, e158446. [Google Scholar] [CrossRef]
- Pal, S.; Tyler, J.K. Epigenetics and aging. Sci. Adv. 2016, 2, e1600584. [Google Scholar] [CrossRef]
- Duan, R.; Fu, Q.; Sun, Y.; Li, Q. Epigenetic clock: A promising biomarker and practical tool in aging. Ageing Res. Rev. 2022, 81, 101743. [Google Scholar] [CrossRef] [PubMed]
- Jiraboonsri, S.; Hemvipat, P.; Kamolratanakul, S.; Bhummaphan, N.; Siritientong, T.; Kitkumthorn, N.; Mutirangura, A.; Meevassana, J. CpG methylation changes in Alu repetitive sequences in normal aging due to diastolic hypertension in human dermal fibroblasts from the facial area. Biomed. Rep. 2024, 20, 5. [Google Scholar] [CrossRef] [PubMed]
- Luo, Y.; Lu, X.; Xie, H. Dynamic Alu methylation during normal development, aging, and tumorigenesis. Biomed. Res. Int. 2014, 2014, 784706. [Google Scholar] [CrossRef] [PubMed]
- Hahn, A.; Pensold, D.; Bayer, C.; Tittelmeier, J.; Gonzalez-Bermudez, L.; Marx-Blumel, L.; Linde, J.; Gross, J.; Salinas-Riester, G.; Lingner, T.; et al. DNA Methyltransferase 1 (DNMT1) Function Is Implicated in the Age-Related Loss of Cortical Interneurons. Front. Cell Dev. Biol. 2020, 8, 639. [Google Scholar] [CrossRef]
- Yung, R.; Ray, D.; Eisenbraun, J.K.; Deng, C.; Attwood, J.; Eisenbraun, M.D.; Johnson, K.; Miller, R.A.; Hanash, S.; Richardson, B. Unexpected effects of a heterozygous dnmt1 null mutation on age-dependent DNA hypomethylation and autoimmunity. J. Gerontol. A Biol. Sci. Med. Sci. 2001, 56, B268–B276. [Google Scholar] [CrossRef]
- Mrabti, C.; Yang, N.; Desdin-Mico, G.; Alonso-Calleja, A.; Vilchez-Acosta, A.; Pico, S.; Parras, A.; Piao, Y.; Schoenfeldt, L.; Luo, S.; et al. Loss of H3K9 trimethylation leads to premature aging. bioRxiv 2024. [Google Scholar] [CrossRef]
- De Cecco, M.; Criscione, S.W.; Peterson, A.L.; Neretti, N.; Sedivy, J.M.; Kreiling, J.A. Transposable elements become active and mobile in the genomes of aging mammalian somatic tissues. Aging 2013, 5, 867–883. [Google Scholar] [CrossRef]
- Terry, D.M.; Devine, S.E. Aberrantly High Levels of Somatic LINE-1 Expression and Retrotransposition in Human Neurological Disorders. Front. Genet. 2019, 10, 1244. [Google Scholar] [CrossRef]
- Zhang, Y.; Wilson, R.; Heiss, J.; Breitling, L.P.; Saum, K.U.; Schottker, B.; Holleczek, B.; Waldenberger, M.; Peters, A.; Brenner, H. DNA methylation signatures in peripheral blood strongly predict all-cause mortality. Nat. Commun. 2017, 8, 14617. [Google Scholar] [CrossRef]
- Cheng, Y.; Cai, B.; Li, H.; Zhang, X.; D’Souza, G.; Shrestha, S.; Edmonds, A.; Meyers, J.; Fischl, M.; Kassaye, S.; et al. HBI: A hierarchical Bayesian interaction model to estimate cell-type-specific methylation quantitative trait loci incorporating priors from cell-sorted bisulfite sequencing data. Genome Biol. 2024, 25, 273. [Google Scholar] [CrossRef]
- Gaunt, T.R.; Shihab, H.A.; Hemani, G.; Min, J.L.; Woodward, G.; Lyttleton, O.; Zheng, J.; Duggirala, A.; McArdle, W.L.; Ho, K.; et al. Systematic identification of genetic influences on methylation across the human life course. Genome Biol. 2016, 17, 61. [Google Scholar] [CrossRef] [PubMed]
- Mozhui, K.; Kim, H.; Villani, F.; Haghani, A.; Sen, S.; Horvath, S. Pleiotropic influence of DNA methylation QTLs on physiological and ageing traits. Epigenetics 2023, 18, 2252631. [Google Scholar] [CrossRef] [PubMed]
- Hawe, J.S.; Wilson, R.; Schmid, K.T.; Zhou, L.; Lakshmanan, L.N.; Lehne, B.C.; Kuhnel, B.; Scott, W.R.; Wielscher, M.; Yew, Y.W.; et al. Genetic variation influencing DNA methylation provides insights into molecular mechanisms regulating genomic function. Nat. Genet. 2022, 54, 18–29. [Google Scholar] [CrossRef] [PubMed]
- Heyn, H.; Li, N.; Ferreira, H.J.; Moran, S.; Pisano, D.G.; Gomez, A.; Diez, J.; Sanchez-Mut, J.V.; Setien, F.; Carmona, F.J.; et al. Distinct DNA methylomes of newborns and centenarians. Proc. Natl. Acad. Sci. USA 2012, 109, 10522–10527. [Google Scholar] [CrossRef]
- Giuliani, M.E.; Barbi, V.; Bigossi, G.; Marcozzi, S.; Giacconi, R.; Cardelli, M.; Piacenza, F.; Orlando, F.; Ciaglia, E.; Cattaneo, M.; et al. Effects of Human LAV-BPIFB4 Gene Therapy on the Epigenetic Clock and Health of Aged Mice. Int. J. Mol. Sci. 2023, 24, 6464. [Google Scholar] [CrossRef]
- Swahari, V.; Nakamura, A.; Hollville, E.; Stroud, H.; Simon, J.M.; Ptacek, T.S.; Beck, M.V.; Flowers, C.; Guo, J.; Plestant, C.; et al. MicroRNA-29 is an essential regulator of brain maturation through regulation of CH methylation. Cell Rep. 2021, 35, 108946. [Google Scholar] [CrossRef]
- Rajado, A.T.; Silva, N.; Esteves, F.; Brito, D.; Binnie, A.; Araujo, I.M.; Nobrega, C.; Braganca, J.; Castelo-Branco, P.; Consortium, A.S. How can we modulate aging through nutrition and physical exercise? An epigenetic approach. Aging 2023, 15, 3191–3217. [Google Scholar] [CrossRef]
- Montella, F.; Lopardo, V.; Vecchione, C.; Puca, A.A.; Ciaglia, E. High TARC plasma levels confer protection to long living individuals by inducing M2 profile. Cytokine 2021, 137, 155305. [Google Scholar] [CrossRef]
- Singh, A.; Schurman, S.H.; Bektas, A.; Kaileh, M.; Roy, R.; Wilson, D.M., 3rd; Sen, R.; Ferrucci, L. Aging and Inflammation. Cold Spring Harb. Perspect. Med. 2024, 14, a041197. [Google Scholar] [CrossRef]
- Mourad, R.; Hsu, P.Y.; Juan, L.; Shen, C.; Koneru, P.; Lin, H.; Liu, Y.; Nephew, K.; Huang, T.H.; Li, L. Estrogen induces global reorganization of chromatin structure in human breast cancer cells. PLoS ONE 2014, 9, e113354. [Google Scholar] [CrossRef]
- Wijchers, P.J.; Festenstein, R.J. Epigenetic regulation of autosomal gene expression by sex chromosomes. Trends Genet. 2011, 27, 132–140. [Google Scholar] [CrossRef] [PubMed]
- Wilhelm, D.; Koopman, P. The makings of maleness: Towards an integrated view of male sexual development. Nat. Rev. Genet. 2006, 7, 620–631. [Google Scholar] [CrossRef] [PubMed]
- Kulkarni, O.P.; Lichtnekert, J.; Anders, H.J.; Mulay, S.R. The Immune System in Tissue Environments Regaining Homeostasis after Injury: Is “Inflammation” Always Inflammation? Mediat. Inflamm. 2016, 2016, 2856213. [Google Scholar] [CrossRef] [PubMed]
- Jazin, E.; Cahill, L. Sex differences in molecular neuroscience: From fruit flies to humans. Nat. Rev. Neurosci. 2010, 11, 9–17. [Google Scholar] [CrossRef]
- Wilson, C.A.; Davies, D.C. The control of sexual differentiation of the reproductive system and brain. Reproduction 2007, 133, 331–359. [Google Scholar] [CrossRef]
- Chen, G.; Yung, R. Meta-inflammaging at the crossroad of geroscience. Aging Med. 2019, 2, 157–161. [Google Scholar] [CrossRef]
- De Santa, F.; Totaro, M.G.; Prosperini, E.; Notarbartolo, S.; Testa, G.; Natoli, G. The histone H3 lysine-27 demethylase Jmjd3 links inflammation to inhibition of polycomb-mediated gene silencing. Cell 2007, 130, 1083–1094. [Google Scholar] [CrossRef]
- Nardini, C.; Moreau, J.F.; Gensous, N.; Ravaioli, F.; Garagnani, P.; Bacalini, M.G. The epigenetics of inflammaging: The contribution of age-related heterochromatin loss and locus-specific remodelling and the modulation by environmental stimuli. Semin. Immunol. 2018, 40, 49–60. [Google Scholar] [CrossRef]
- Ramos-Lopez, O.; Milagro, F.I.; Riezu-Boj, J.I.; Martinez, J.A. Epigenetic signatures underlying inflammation: An interplay of nutrition, physical activity, metabolic diseases, and environmental factors for personalized nutrition. Inflamm. Res. 2021, 70, 29–49. [Google Scholar] [CrossRef]
- Kobayashi, S.; Isotani, A.; Mise, N.; Yamamoto, M.; Fujihara, Y.; Kaseda, K.; Nakanishi, T.; Ikawa, M.; Hamada, H.; Abe, K.; et al. Comparison of gene expression in male and female mouse blastocysts revealed imprinting of the X-linked gene, Rhox5/Pem, at preimplantation stages. Curr. Biol. 2006, 16, 166–172. [Google Scholar] [CrossRef]
- Skaletsky, H.; Kuroda-Kawaguchi, T.; Minx, P.J.; Cordum, H.S.; Hillier, L.; Brown, L.G.; Repping, S.; Pyntikova, T.; Ali, J.; Bieri, T.; et al. The male-specific region of the human Y chromosome is a mosaic of discrete sequence classes. Nature 2003, 423, 825–837. [Google Scholar] [CrossRef] [PubMed]
- Raefski, A.S.; O’Neill, M.J. Identification of a cluster of X-linked imprinted genes in mice. Nat. Genet. 2005, 37, 620–624. [Google Scholar] [CrossRef] [PubMed]
- Ligthart, S.; Marzi, C.; Aslibekyan, S.; Mendelson, M.M.; Conneely, K.N.; Tanaka, T.; Colicino, E.; Waite, L.L.; Joehanes, R.; Guan, W.; et al. DNA methylation signatures of chronic low-grade inflammation are associated with complex diseases. Genome Biol. 2016, 17, 255. [Google Scholar] [CrossRef] [PubMed]
- Fitzgerald, K.N.; Hodges, R.; Hanes, D.; Stack, E.; Cheishvili, D.; Szyf, M.; Henkel, J.; Twedt, M.W.; Giannopoulou, D.; Herdell, J.; et al. Potential reversal of epigenetic age using a diet and lifestyle intervention: A pilot randomized clinical trial. Aging 2021, 13, 9419–9432. [Google Scholar] [CrossRef]
- Willcox, D.C.; Scapagnini, G.; Willcox, B.J. Healthy aging diets other than the Mediterranean: A focus on the Okinawan diet. Mech. Ageing Dev. 2014, 136–137, 148–162. [Google Scholar] [CrossRef]
- Fontana, L.; Partridge, L.; Longo, V.D. Extending healthy life span--from yeast to humans. Science 2010, 328, 321–326. [Google Scholar] [CrossRef]
- Gensous, N.; Franceschi, C.; Santoro, A.; Milazzo, M.; Garagnani, P.; Bacalini, M.G. The Impact of Caloric Restriction on the Epigenetic Signatures of Aging. Int. J. Mol. Sci. 2019, 20, 2022. [Google Scholar] [CrossRef]
- Moreno, C.L.; Mobbs, C.V. Epigenetic mechanisms underlying lifespan and age-related effects of dietary restriction and the ketogenic diet. Mol. Cell Endocrinol. 2017, 455, 33–40. [Google Scholar] [CrossRef]
- Pandey, M.; Bansal, S.; Bar, S.; Yadav, A.K.; Sokol, N.S.; Tennessen, J.M.; Kapahi, P.; Chawla, G. miR-125-chinmo pathway regulates dietary restriction-dependent enhancement of lifespan in Drosophila. Elife 2021, 10, e62621. [Google Scholar] [CrossRef]
- Neufer, P.D.; Bamman, M.M.; Muoio, D.M.; Bouchard, C.; Cooper, D.M.; Goodpaster, B.H.; Booth, F.W.; Kohrt, W.M.; Gerszten, R.E.; Mattson, M.P.; et al. Understanding the Cellular and Molecular Mechanisms of Physical Activity-Induced Health Benefits. Cell Metab. 2015, 22, 4–11. [Google Scholar] [CrossRef]
- Ren, H.; Collins, V.; Clarke, S.J.; Han, J.S.; Lam, P.; Clay, F.; Williamson, L.M.; Andy Choo, K.H. Epigenetic changes in response to tai chi practice: A pilot investigation of DNA methylation marks. Evid. Based Complement. Altern. Med. 2012, 2012, 841810. [Google Scholar] [CrossRef] [PubMed]
- Murach, K.A.; Dimet-Wiley, A.L.; Wen, Y.; Brightwell, C.R.; Latham, C.M.; Dungan, C.M.; Fry, C.S.; Watowich, S.J. Late-life exercise mitigates skeletal muscle epigenetic aging. Aging Cell 2022, 21, e13527. [Google Scholar] [CrossRef] [PubMed]
- Dhar, P.; Moodithaya, S.S.; Patil, P. Epigenetic alterations-The silent indicator for early aging and age-associated health-risks. Aging Med. 2022, 5, 287–293. [Google Scholar] [CrossRef] [PubMed]
- Gensous, N.; Bacalini, M.G.; Pirazzini, C.; Marasco, E.; Giuliani, C.; Ravaioli, F.; Mengozzi, G.; Bertarelli, C.; Palmas, M.G.; Franceschi, C.; et al. The epigenetic landscape of age-related diseases: The geroscience perspective. Biogerontology 2017, 18, 549–559. [Google Scholar] [CrossRef]
- Wang, K.; Liu, H.; Hu, Q.; Wang, L.; Liu, J.; Zheng, Z.; Zhang, W.; Ren, J.; Zhu, F.; Liu, G.H. Epigenetic regulation of aging: Implications for interventions of aging and diseases. Signal Transduct. Target. Ther. 2022, 7, 374. [Google Scholar] [CrossRef]
- Horvath, S.; Raj, K. DNA methylation-based biomarkers and the epigenetic clock theory of ageing. Nat. Rev. Genet. 2018, 19, 371–384. [Google Scholar] [CrossRef]
- Freire-Aradas, A.; Giron-Santamaria, L.; Mosquera-Miguel, A.; Ambroa-Conde, A.; Phillips, C.; Casares de Cal, M.; Gomez-Tato, A.; Alvarez-Dios, J.; Pospiech, E.; Aliferi, A.; et al. A common epigenetic clock from childhood to old age. Forensic Sci. Int. Genet. 2022, 60, 102743. [Google Scholar] [CrossRef]
- Jiang, S.; Guo, Y. Epigenetic Clock: DNA Methylation in Aging. Stem Cells Int. 2020, 2020, 1047896. [Google Scholar] [CrossRef]
- Simpson, D.J.; Chandra, T. Epigenetic age prediction. Aging Cell 2021, 20, e13452. [Google Scholar] [CrossRef]
- Bocklandt, S.; Lin, W.; Sehl, M.E.; Sanchez, F.J.; Sinsheimer, J.S.; Horvath, S.; Vilain, E. Epigenetic predictor of age. PLoS ONE 2011, 6, e14821. [Google Scholar] [CrossRef]
- Tong, H.; Dwaraka, V.B.; Chen, Q.; Luo, Q.; Lasky-Su, J.A.; Smith, R.; Teschendorff, A.E. Quantifying the stochastic component of epigenetic aging. Nat. Aging 2024, 4, 886–901. [Google Scholar] [CrossRef] [PubMed]
- McCrory, C.; Fiorito, G.; Hernandez, B.; Polidoro, S.; O’Halloran, A.M.; Hever, A.; Ni Cheallaigh, C.; Lu, A.T.; Horvath, S.; Vineis, P.; et al. GrimAge Outperforms Other Epigenetic Clocks in the Prediction of Age-Related Clinical Phenotypes and All-Cause Mortality. J. Gerontol. A Biol. Sci. Med. Sci. 2021, 76, 741–749. [Google Scholar] [CrossRef] [PubMed]
- Margiotti, K.; Monaco, F.; Fabiani, M.; Mesoraca, A.; Giorlandino, C. Epigenetic Clocks: In Aging-Related and Complex Diseases. Cytogenet. Genome Res. 2023, 163, 247–256. [Google Scholar] [CrossRef] [PubMed]
- Belsky, D.W.; Caspi, A.; Arseneault, L.; Baccarelli, A.; Corcoran, D.L.; Gao, X.; Hannon, E.; Harrington, H.L.; Rasmussen, L.J.; Houts, R.; et al. Quantification of the pace of biological aging in humans through a blood test, the DunedinPoAm DNA methylation algorithm. Elife 2020, 9, e54870. [Google Scholar] [CrossRef]
- Zeidan, R.S.; McElroy, T.; Rathor, L.; Martenson, M.S.; Lin, Y.; Mankowski, R.T. Sex differences in frailty among older adults. Exp. Gerontol. 2023, 184, 112333. [Google Scholar] [CrossRef]
- Olshansky, S.J.; Willcox, B.J.; Demetrius, L.; Beltran-Sanchez, H. Implausibility of radical life extension in humans in the twenty-first century. Nat. Aging 2024, 4, 1635–1642. [Google Scholar] [CrossRef]
- Shen, X.; Wang, C.; Zhou, X.; Zhou, W.; Hornburg, D.; Wu, S.; Snyder, M.P. Nonlinear dynamics of multi-omics profiles during human aging. Nat. Aging 2024, 4, 1619–1634. [Google Scholar] [CrossRef]
| Gene | Pathway | Variant/SNPs | Clinical Effects of the Variant | Experimental Model/Methods | Ref. |
|---|---|---|---|---|---|
| FOXO3 | Inflammation, aerobic glycolysis, cardiometabolic and oxidative stress, apoptosis, DNA repair | rs2802292 | Lower incidence of ageing-associated diseases (cardiovascular, neurodegenerative, and cancer) | GWAS in different cohorts | [18] |
| SIRT1 | Energy metabolism, inflammation, DNA repair and senescence | rs7895833 | Interplay with FOXO3 prevents from oxidative stress conditions and apoptosis | GWAS in 1 cohort | [20] |
| APOE | Lipid metabolism | ε4 | Atherosclerosis | GWAS in different cohorts | [23] |
| ε2 | Lower incidence of ageing-associated diseases (cardiovascular and neurodegenerative) | GWAS in different cohorts | [23] | ||
| GHR | Interplay with IGF-1 for oxidative stress response | d3-GHR deletion | Enhanced oxidative stress response (less ROS production) | GWAS in different cohorts | [24] |
| BPIFB4 | Immune function, inflammation, cellular homeostasis, | rs2070325 (LAV-BPIFB4) | Reducing inflammation and neuroinflammation, shaping and rejuvenating immune system, reducing the senescence profile | GWAS in different cohorts; in vivo and in vitro models | [11,13,14,15,16,26,27,28,29,30,31,32,33] |
| rs2070325 (RV-BPIFB4) | Enhancer of frailty and cardiac dysfunctions | GWAS in different cohorts; in vivo and in vitro models | [34] |
| Epigenetic Change | Mechanism | Clinical Effects | Experimental Model/Methods | Ref. |
|---|---|---|---|---|
| DNA hypomethylation | Decreased Methylation of Alu | Osteoporosis, diabetes, Alzheimer’s disease | Analytical cross-sectional and in vitro studies | [42,43] |
| Reduction of DNTM1 | Reduced aging-associated transcriptional changes; interneuron survival and slight autoimmunity | In vivo C57BL/6 aged murine model | [40,41] | |
| Heterocromatin de-condensation | Loss of the H3K9me3 | Degeneration of multiple organs leading to premature aging | In vivo TKOCAGCre murine model | [42] |
| Activation of silent retrotransposons | Premature aging and neurodegeneration | In vivo and ex vivo C57BL/6 aged murine models | [43,44] | |
| Non-coding RNAs | Deletion of miR-29 (modulation of DNMT3A and TET enzymes) | Repression of genes associated with neuronal activity | In vivo murine models | [53] |
| nc886 (Reduction of p16INK4A, p21 and ROS) | Decreased senescence | In vitro fibroblast cell models | [54] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ciaglia, E.; Montella, F.; Lopardo, V.; Basile, C.; Esposito, R.M.; Maglio, C.; Longo, R.; Maciag, A.; Puca, A.A. The Genetic and Epigenetic Arms of Human Ageing and Longevity. Biology 2025, 14, 92. https://doi.org/10.3390/biology14010092
Ciaglia E, Montella F, Lopardo V, Basile C, Esposito RM, Maglio C, Longo R, Maciag A, Puca AA. The Genetic and Epigenetic Arms of Human Ageing and Longevity. Biology. 2025; 14(1):92. https://doi.org/10.3390/biology14010092
Chicago/Turabian StyleCiaglia, Elena, Francesco Montella, Valentina Lopardo, Cristina Basile, Roberta Maria Esposito, Clara Maglio, Roberta Longo, Anna Maciag, and Annibale Alessandro Puca. 2025. "The Genetic and Epigenetic Arms of Human Ageing and Longevity" Biology 14, no. 1: 92. https://doi.org/10.3390/biology14010092
APA StyleCiaglia, E., Montella, F., Lopardo, V., Basile, C., Esposito, R. M., Maglio, C., Longo, R., Maciag, A., & Puca, A. A. (2025). The Genetic and Epigenetic Arms of Human Ageing and Longevity. Biology, 14(1), 92. https://doi.org/10.3390/biology14010092







