Spatio-Temporal Distribution of Four Trophically Dependent Fishery Species in the Northern China Seas Under Climate Change
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
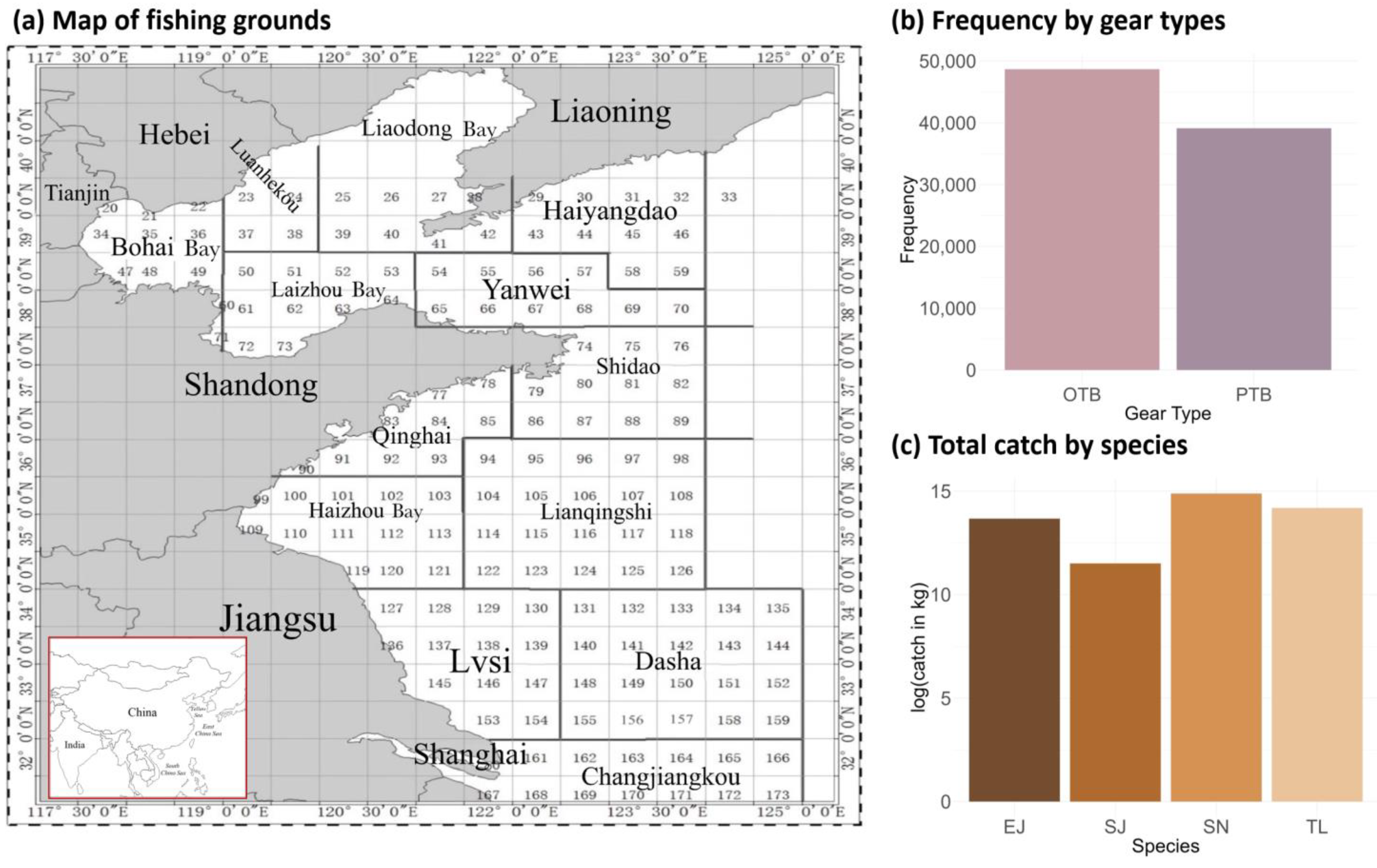
2.1. Data Source
2.2. Joint Species Distribution Modeling
2.3. Distributional Prediction and Spatial Overlap
3. Results
3.1. Model Performance
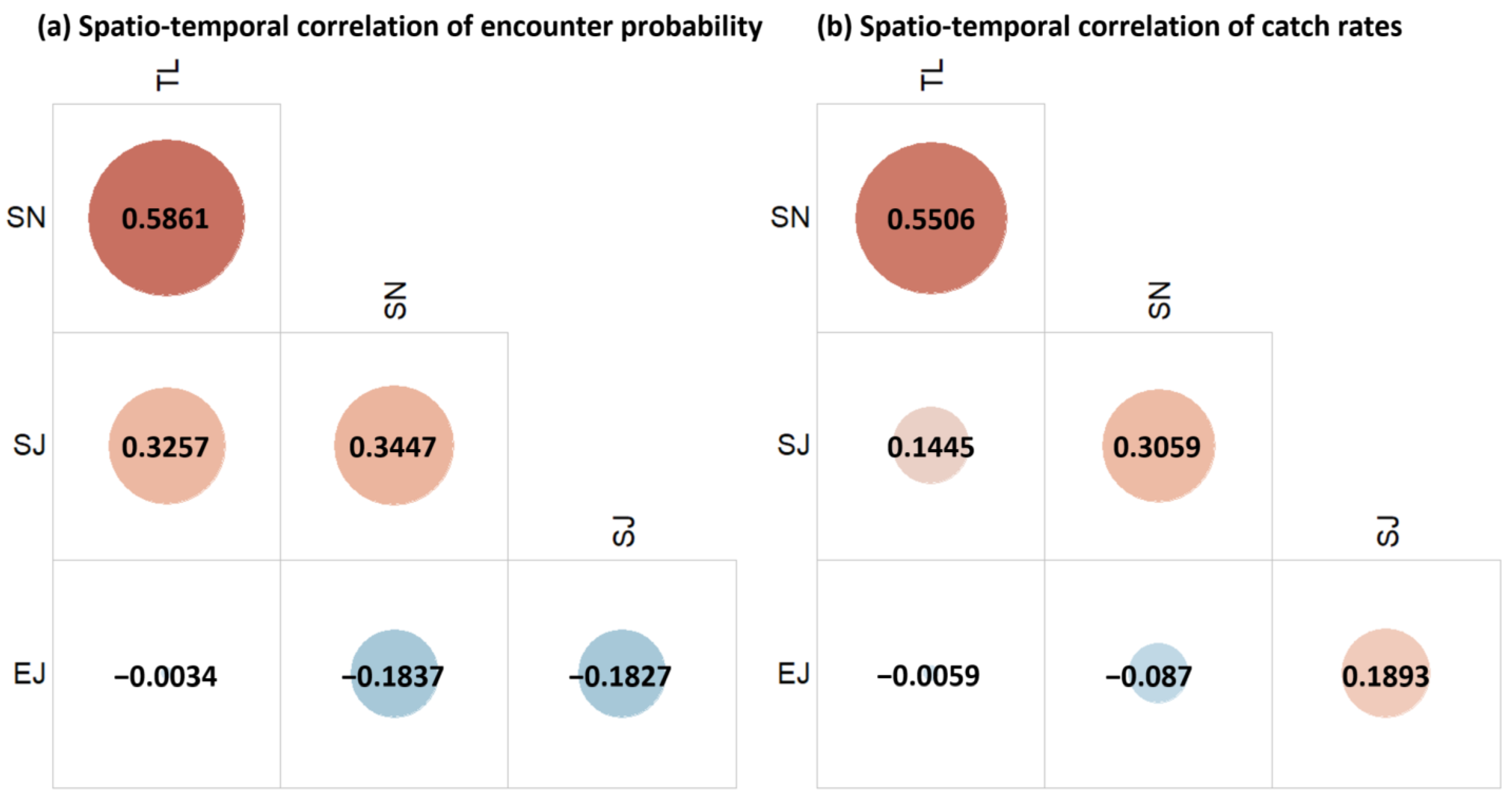
3.2. Environmental Effects and Species Correlations
3.3. Spatial Distribution and Overlaps
4. Discussion
4.1. The Effects of Environmental Factors
4.2. Interspecific Relationship and Spatial Overlap
4.3. Climate Change and Distributional Shift
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Cheung, W.W.L.; Frölicher, T.L. Marine heatwaves exacerbate climate change impacts for fisheries in the northeast Pacific. Sci. Rep. 2020, 10, 6678. [Google Scholar] [CrossRef] [PubMed]
- Jones, T.; Parrish, J.K.; Peterson, W.T.; Bjorkstedt, E.P.; Bond, N.A.; Ballance, L.T.; Bowes, V.; Hipfner, J.M.; Burgess, H.K.; Dolliver, J.E.; et al. Massive mortality of a planktivorous seabird in response to a marine heatwave. Geophys. Res. Lett. 2018, 45, 3193–3202. [Google Scholar] [CrossRef]
- Marin, M.; Feng, M.; Phillips, H.E.; Bindoff, N.L. A global, multiproduct analysis of coastal marine heatwaves: Distribution, characteristics, and long-term trends. J. Geophys. Res. Oceans 2021, 126, e2020JC016708. [Google Scholar] [CrossRef]
- Gulf of Maine Research Institute; Pershing, A.; Mills, K.; Dayton, A.; Franklin, B.; Kennedy, B. Evidence for adaptation from the 2016 marine heatwave in the northwest atlantic ocean. Oceanography 2018, 31, 152–161. [Google Scholar] [CrossRef]
- Wernberg, T.; Smale, D.A.; Tuya, F.; Thomsen, M.S.; Langlois, T.J.; de Bettignies, T.; Bennett, S.; Rousseaux, C.S. An extreme climatic event alters marine ecosystem structure in a global biodiversity hotspot. Nat. Clim. Chang. 2013, 3, 78–82. [Google Scholar] [CrossRef]
- Yang, T.; Shan, X.J.; Jin, X.S.; Chen, Y.L.; Teng, G.L.; Wei, X.J. Long-term changes in keystone species in fish community in spring in Laizhou Bay. Prog. Fish. Sci. 2018, 39, 1–11. [Google Scholar]
- Gallagher, C.A.; Chimienti, M.; Grimm, V.; Nabe-Nielsen, J. Energy-mediated responses to changing prey size and distribution in marine top predator movements and population dynamics. J. Anim. Ecol. 2021, 91, 241–254. [Google Scholar] [CrossRef]
- Gunther, K.; Baker, M.R.; Aydin, K. Using predator diets to infer forage fish distribution and assess responses to climate variability in the Eastern Bering Sea. Mar. Ecol. Prog. Ser. 2023, SPF2, 71–99. [Google Scholar] [CrossRef]
- Speakman, C.N.; Hoskins, A.J.; Hindell, M.A.; Costa, D.P.; Hartog, J.R.; Hobday, A.J.; Arnould, J.P.Y. Influence of environmental variation on spatial distribution and habitat-use in a benthic foraging marine predator. R. Soc. Open Sci. 2021, 8. [Google Scholar] [CrossRef]
- Molinos, J.G.; Halpern, B.S.; Schoeman, D.S.; Brown, C.J.; Kiessling, W.; Moore, P.J.; Pandolfi, J.M.; Poloczanska, E.S.; Richardson, A.J.; Burrows, M.T. Climate velocity and the future global redistribution of marine biodiversity. Nat. Clim. Chang. 2015, 6, 83–88. [Google Scholar] [CrossRef]
- Olmos, M.; Ianelli, J.; Ciannelli, L.; Spies, I.; McGilliard, C.R.; Thorson, J.T. Estimating climate-driven phenology shifts and survey availability using fishery-dependent data. Prog. Oceanogr. 2023, 215. [Google Scholar] [CrossRef]
- Araujo, F.G.; Teixeira, T.P.; Guedes, A.P.P.; de Azevedo, M.C.C.; Pessanha, A.L.M. Shifts in the abundance and distribution of shallow water fish fauna on the southeastern Brazilian coast: A response to climate change. Hydrobiologia 2018, 814, 205–218. [Google Scholar] [CrossRef]
- Last, N.B.; Rhoades, E.; Miranker, A.D. Islet amyloid polypeptide demonstrates a persistent capacity to disrupt membrane integrity. Proc. Natl. Acad. Sci. USA 2011, 108, 9460–9465. [Google Scholar] [CrossRef]
- Milazzo, M.; Mirto, S.; Domenici, P.; Gristina, M. Climate change exacerbates interspecific interactions in sympatric coastal fishes. J. Anim. Ecol. 2012, 82, 468–477. [Google Scholar] [CrossRef] [PubMed]
- Kordas, R.L.; Harley, C.D.; O’Connor, M.I. Community ecology in a warming world: The influence of temperature on interspecific interactions in Marine Systems. J. Exp. Mar. Biol. Ecol. 2011, 400, 218–226. [Google Scholar] [CrossRef]
- Bond, N.; Thomson, J.; Reich, P.; Stein, J. Using species distribution models to infer potential climate change-induced range shifts of freshwater fish in south-eastern Australia. Mar. Freshw. Res. 2011, 62, 1043–1061. [Google Scholar] [CrossRef]
- Laman, E.A.; Rooper, C.N.; Turner, K.; Rooney, S.; Cooper, D.W.; Zimmermann, M. Using species distribution models to describe essential fish habitat in Alaska. Can. J. Fish. Aquat. Sci. 2018, 75, 1230–1255. [Google Scholar] [CrossRef]
- Leathwick, J.; Whitehead, D.; McLeod, M. Predicting changes in the composition of New Zealand’s indigenous forests in response to global warming: A modelling approach. Environ. Softw. 1996, 11, 81–90. [Google Scholar] [CrossRef]
- Massimino, D.; Johnston, A.; Gillings, S.; Jiguet, F.; Pearce-Higgins, J.W. Projected reductions in climatic suitability for vulnerable British birds. Clim. Chang. 2017, 145, 117–130. [Google Scholar] [CrossRef]
- Pearson, R.G.; Dawson, T.P. Predicting the impacts of climate change on the distribution of species: Are bioclimate envelope models useful? Glob. Ecol. Biogeogr. 2003, 12, 361–371. [Google Scholar] [CrossRef]
- Dahms, C.; Killen, S.S. Temperature change effects on marine fish range shifts: A meta-analysis of ecological and methodological predictors. Glob. Change Biol. 2023, 29, 4459–4479. [Google Scholar] [CrossRef]
- Rodrigues, L.d.S.; Pennino, M.G.; Conesa, D.; Kikuchi, E.; Kinas, P.G.; Barbosa, F.G.; Cardoso, L.G. Modelling the distribution of marine fishery resources: Where are we? Fish Fish. 2022, 24, 159–175. [Google Scholar] [CrossRef]
- Spence, A.R.; Tingley, M.W. The challenge of novel abiotic conditions for species undergoing climate-induced range shifts. Ecography 2020, 43, 1571–1590. [Google Scholar] [CrossRef]
- Roberts, S.M.; Halpin, P.N.; Clark, J.S. Jointly modeling marine species to inform the effects of environmental change on an ecological community in the Northwest Atlantic. Sci. Rep. 2022, 12, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Clark, J.S.; Nemergut, D.; Seyednasrollah, B.; Turner, P.J.; Zhang, S. Generalized joint attribute modeling for biodiversity analysis: Median-zero, multivariate, Multifarious Data. Ecol. Monogr. 2017, 87, 34–56. [Google Scholar] [CrossRef]
- Pollock, L.J.; Tingley, R.; Morris, W.K.; Golding, N.; O’Hara, R.B.; Parris, K.M.; Vesk, P.A.; McCarthy, M.A. Understanding co-occurrence by modelling species simultaneously with a joint species distribution model (JSDM). Methods Ecol. Evol. 2014, 5, 397–406. [Google Scholar] [CrossRef]
- Wagner, T.; Hansen, G.J.; Schliep, E.M.; Bethke, B.J.; Honsey, A.E.; Jacobson, P.C.; Kline, B.C.; White, S.L. Improved understanding and prediction of freshwater fish communities through the use of joint species distribution models. Can. J. Fish. Aquat. Sci. 2020, 77, 1540–1551. [Google Scholar] [CrossRef]
- Dambrine, C.; Woillez, M.; Huret, M.; de Pontual, H. Characterising essential fish habitat using spatio-temporal analysis of fishery data: A case study of the European seabass spawning areas. Fish. Oceanogr. 2021, 30, 413–428. [Google Scholar] [CrossRef]
- O’Leary, C.A.; DeFilippo, L.B.; Thorson, J.T.; Kotwicki, S.; Hoff, G.R.; Kulik, V.V.; Ianelli, J.N.; Punt, A.E. Understanding transboundary stocks’ availability by combining multiple fisheries-independent surveys and oceanographic conditions in spatiotemporal models. ICES J. Mar. Sci. 2022, 79, 1063–1074. [Google Scholar] [CrossRef]
- Webster, R.A.; Soderlund, E.; Dykstra, C.L.; Stewart, I.J. Monitoring change in a dynamic environment: Spatiotemporal modelling of calibrated data from different types of fisheries surveys of Pacific halibut. Can. J. Fish. Aquat. Sci. 2020, 77, 1421–1432. [Google Scholar] [CrossRef]
- Cao, J.; Thorson, J.T.; Richards, R.A.; Chen, Y. Spatiotemporal Index Standardization improves the stock assessment of northern shrimp in the Gulf of Maine. Can. J. Fish. Aquat. Sci. 2017, 74, 1781–1793. [Google Scholar] [CrossRef]
- Grüss, A.; Walter, J.F.; Babcock, E.A.; Forrestal, F.C.; Thorson, J.T.; Lauretta, M.V.; Schirripa, M.J. Evaluation of the impacts of different treatments of spatio-temporal variation in catch-per-unit-effort standardization models. Fish. Res. 2019, 213, 75–93. [Google Scholar] [CrossRef]
- Zhou, S.; Campbell, R.A.; Hoyle, S.D. Catch per unit effort standardization using spatio-temporal models for Australia’s Eastern Tuna and billfish fishery. ICES J. Mar. Sci. 2019, 76, 1489–1504. [Google Scholar] [CrossRef]
- Bowlby, H.D.; Druon, J.-N.; Lopez, J.; Juan-Jordá, M.J.; Carreón-Zapiain, M.T.; Vandeperre, F.; Leone, A.; Finucci, B.; Sabarros, P.S.; Block, B.A.; et al. Global habitat predictions to inform spatiotemporal fisheries management: Initial steps within the framework. Mar. Policy 2024, 164. [Google Scholar] [CrossRef]
- Grüss, A.; Thorson, J.T. Developing spatio-temporal models using multiple data types for evaluating population trends and habitat usage. ICES J. Mar. Sci. 2019, 76, 1748–1761. [Google Scholar] [CrossRef]
- Hyman, A.C.; Chiu, G.S.; Fabrizio, M.C.; Lipcius, R.N. Spatiotemporal modeling of nursery habitat using bayesian inference: Environmental drivers of juvenile blue crab abundance. Front. Mar. Sci. 2022, 9, 834990. [Google Scholar] [CrossRef]
- Runnebaum, J.; Guan, L.; Cao, J.; O’brien, L.; Chen, Y. Habitat suitability modeling based on a spatiotemporal model: An example for cusk in the Gulf of Maine. Can. J. Fish. Aquat. Sci. 2018, 75, 1784–1797. [Google Scholar] [CrossRef]
- Grüss, A.; Thorson, J.T.; Babcock, E.A.; Tarnecki, J.H. Producing distribution maps for informing ecosystem-based fisheries management using a comprehensive survey database and spatio-temporal models. ICES J. Mar. Sci. 2017, 75, 158–177. [Google Scholar] [CrossRef]
- Thorson, J.T.; Pinsky, M.L.; Ward, E.J. Model-based inference for estimating shifts in species distribution, area occupied and centre of gravity. Methods Ecol. Evol. 2016, 7, 990–1002. [Google Scholar] [CrossRef]
- Thorson, J.T.; Rindorf, A.; Gao, J.; Hanselman, D.H.; Winker, H. Density-dependent changes in effective area occupied for sea-bottom-associated marine fishes. Proc. R. Soc. B Biol. Sci. 2016, 283, 20161853. [Google Scholar] [CrossRef]
- Cosandey-Godin, A.; Krainski, E.T.; Worm, B.; Flemming, J.M. Applying Bayesian spatiotemporal models to fisheries bycatch in the Canadian Arctic. Can. J. Fish. Aquat. Sci. 2015, 72, 186–197. [Google Scholar] [CrossRef]
- Stock, B.C.; Ward, E.J.; Thorson, J.T.; Jannot, J.E.; Semmens, B.X. The utility of spatial model-based estimators of unobserved bycatch. ICES J. Mar. Sci. 2018, 76, 255–267. [Google Scholar] [CrossRef]
- Chen, Y.; Shan, X.; Gorfine, H.; Dai, F.; Wu, Q.; Yang, T.; Shi, Y.; Jin, X. Ensemble projections of fish distribution in response to climate changes in the Yellow and Bohai Seas, China. Ecol. Indic. 2022, 146, 109759. [Google Scholar] [CrossRef]
- FishBase Team. FishBase. World Wide Web Electronic Publication. 2024. Available online: http://www.fishbase.se (accessed on 1 September 2024).
- Li, Y.S.; Xing, Y.N.; Pan, L.Z.; Zhang, Y.; Yu, W. Research progress on life history and model application of chub mackerel Scomber japonicus: A review. J. Dalian Ocean. Univ. 2021, 36, 694–705. [Google Scholar] [CrossRef]
- Liu, Z.H.; Han, D.Y.; Gao, C.X.; Ye, S. Feeding Habits of Hairtail Trichiurus japonicus in the Inshore Waters of Southern Zhejiang in Summer and Autumn. Fish. Sci. 2024, 43, 717–726. [Google Scholar] [CrossRef]
- Mu, X.X.; Zhang, C.; Zhang, C.L.; Xu, B.D.; Xue, Y.; Tian, Y.J.; Ren, Y.P. The fisheries biology of the spawning stock of Scomberomorus niphonius in the Bohai and Yellow Seas. J. Fish. Sci. China 2018, 25, 1308–1316. [Google Scholar] [CrossRef]
- Zhang, B. Feeding ecology of fishes in the Bohai Sea. Prog. Fish. Sci. 2018, 39, 11–22. [Google Scholar] [CrossRef]
- Li, X.W.; Zhao, J.M.; Liu, H.; Zhang, H.; Hou, X.Y. Status, problems and optimized management of spawning, feeding, overwintering grounds and migration route of marine fishery resources in Bohai Sea and Yellow Sea. Trans. Oceanol. Limnol. 2018, 5, 147–157. [Google Scholar] [CrossRef]
- Basher, Z.; Bowden, D.A.; Costello, M.J. Global Marine Environment Datasets (GMED). World Wide Web Electronic Publication. Version 2.0 (Rev.02.2018). 2018. Available online: http://gmed.auckland.ac.nz (accessed on 29 August 2024).
- Thorson, J.T. Guidance for decisions using the vector autoregressive spatio-temporal (VAST) package in stock, ecosystem, habitat and climate assessments. Fish. Res. 2018, 210, 143–161. [Google Scholar] [CrossRef]
- Levins, R. Evolution in Changing Environments: Some Theoretical Explorations (No. 2); Princeton University Press: Princeton, NJ, USA, 1968. [Google Scholar]
- Schoener, T.W. Nonsynchronous spatial overlap of lizards in patchy habitats. Ecology 1970, 51, 408–418. [Google Scholar] [CrossRef]
- Pianka, E.R. The structure of lizard communities. Annu. Rev. Ecol. Syst. 1973, 4, 53–74. [Google Scholar] [CrossRef]
- Horn, H.S. Measurement of “overlap” in comparative ecological studies. Am. Nat. 1966, 100, 419–424. [Google Scholar] [CrossRef]
- Hu, C.; Zhang, H.; Zhang, Y.; Pan, G.; Xu, K.; Bi, Y.; Liang, J.; Wang, H.; Zhou, Y. Fish community structure and its relationship with environmental factors in the Nature Reserve of Trichiurus japonicus. J. Fish. China 2018, 42, 694–703. [Google Scholar]
- Rutterford, L.A.; Simpson, S.D.; Bogstad, B.; Devine, J.A.; Genner, M.J. Sea temperature is the primary driver of recent and predicted fish community structure across Northeast Atlantic shelf seas. Glob. Change Biol. 2023, 29, 2510–2521. [Google Scholar] [CrossRef] [PubMed]
- Sabatés, A.; Martín, P.; Lloret, J.; Raya, V. Sea warming and fish distribution: The case of the small pelagic fish, Sardinella aurita, in the western Mediterranean. Glob. Change Biol. 2006, 12, 2209–2219. [Google Scholar] [CrossRef]
- Townhill, B.L.; Couce, E.; Tinker, J.; Kay, S.; Pinnegar, J.K. Climate change projections of commercial fish distribution and suitable habitat around North Western Europe. Fish Fish. 2023, 24, 848–862. [Google Scholar] [CrossRef]
- Sun, P.; Chen, Q.; Fu, C.; Zhu, W.; Li, J.; Zhang, C.; Yu, H.; Sun, R.; Xu, Y.; Tian, Y. Daily growth of young-of-the-year largehead hairtail (Trichiurus japonicus) in relation to environmental variables in the East China Sea. J. Mar. Syst. 2020, 201, 103243. [Google Scholar] [CrossRef]
- Nguyen, K.Q.; Nguyena, V.Y. Changing of sea surface temperature affects catch of Spanish mackerel Scomberomorus commerson in the set-net fishery. Fish. Aquac. J. 2017, 8, 1–7. [Google Scholar] [CrossRef]
- Dickson, K.A.; Donley, J.M.; Sepulveda, C.; Bhoopat, L. Effects of temperature on sustained swimming performance and swimming kinematics of the chub mackerel Scomber japonicus. J. Exp. Biol. 2002, 205, 969–980. [Google Scholar] [CrossRef] [PubMed]
- Taga, M.; Kamimura, Y.; Yamashita, Y. Effects of water temperature and prey density on recent growth of chub mackerel Scomber japonicus larvae and juveniles along the Pacific coast of Boso–Kashimanada. Fish. Sci. 2019, 85, 931–942. [Google Scholar] [CrossRef]
- Biswas, B.K.; Svirezhev, Y.M.; Bala, B.K.; Wahab, M.A. Climate change impacts on fish catch in the World Fishing Grounds. Clim. Chang. 2008, 93, 117–136. [Google Scholar] [CrossRef]
- Jin, X.; Zhang, B.; Xue, Y. The response of the diets of four carnivorous fishes to variations in the Yellow Sea ecosystem. Deep Sea Res. Part II Top. Stud. Oceanogr. 2010, 57, 996–1000. [Google Scholar] [CrossRef]
- Zhang, W.; Ye, Z.; Tian, Y.; Yu, H.; Ma, S.; Ju, P.; Watanabe, Y. Spawning overlap of Japanese anchovy Engraulis japonicus and Japanese Spanish mackerel Scomberomorus niphonius in the coastal Yellow Sea: A prey–predator interaction. Fish. Oceanogr. 2022, 31, 456–469. [Google Scholar] [CrossRef]
- Wang, J.; Gao, C.; Tian, S.; Han, D.; Ma, J.; Dai, L.; Ye, S. Shifts in composition and co-occurrence patterns of the fish community in the south inshore of Zhejiang, China. Glob. Ecol. Conserv. 2023, 44. [Google Scholar] [CrossRef]
- Bakhoum, S.A. Diet overlap of immigrant narrow-barred Spanish mackerel Scomberomorus commerson (lac., 1802) and the largehead hairtail ribbonfish Trichiurus Lepturus (L., 1758) in the Egyptian Mediterranean coast. Anim. Biodivers. Conserv. 2007, 30, 147–160. [Google Scholar] [CrossRef]
- Shi, Y.; Zhang, X.; Yang, S.; Dai, Y.; Cui, X.; Wu, Y.; Zhang, S.; Fan, W.; Han, H.; Zhang, H.; et al. Construction of CPUE standardization model and its simulation testing for chub mackerel (Scomber japonicus) in the Northwest Pacific Ocean. Ecol. Indic. 2023, 155. [Google Scholar] [CrossRef]
- Sun, Y.; Zhang, H.; Jiang, K.; Xiang, D.; Shi, Y.; Huang, S.; Li, Y.; Han, H. Simulating the changes of the habitats suitability of chub mackerel (Scomber japonicus) in the high seas of the North Pacific Ocean using ensemble models under medium to long-term future climate scenarios. Mar. Pollut. Bull. 2024, 207, 116873. [Google Scholar] [CrossRef] [PubMed]
- Xia, M.; Jia, H.; Wang, Y.; Zhang, H. Effects of Climate Change on the Distribution of Scomber japonicus and Konosirus punctatus in China’s Coastal and Adjacent Waters. Fishes 2024, 9, 395. [Google Scholar] [CrossRef]
- Liu, S.; Tian, Y.; Liu, Y.; Alabia, I.D.; Cheng, J.; Ito, S.-I. Development of a prey-predator species distribution model for a large piscivorous fish: A case study for Japanese Spanish mackerel Scomberomorus niphonius and Japanese anchovy Engraulis japonicus. Deep Sea Res. Part II Top. Stud. Oceanogr. 2022, 207. [Google Scholar] [CrossRef]
- Yang, T.; Liu, X.; Han, Z. Predicting the effects of climate change on the suitable habitat of japanese spanish mackerel (Scomberomorus niphonius) based on the species distribution model. Front. Mar. Sci. 2022, 9. [Google Scholar] [CrossRef]
- Mills, K.E.; Kemberling, A.; Kerr, L.A.; Lucey, S.M.; McBride, R.S.; Nye, J.A.; Pershing, A.J.; Barajas, M.; Lovas, C.S. Multispecies population-scale emergence of climate change signals in an ocean warming hotspot. ICES J. Mar. Sci. 2024, 81, 375–389. [Google Scholar] [CrossRef]
- Perry, A.L.; Low, P.J.; Ellis, J.R.; Reynolds, J.D. Climate change and distribution shifts in marine fishes. Science 2005, 308, 1912–1915. [Google Scholar] [CrossRef]
- Payne, M.R.; Barange, M.; Cheung, W.W.L.; MacKenzie, B.R.; Batchelder, H.P.; Cormon, X.; Eddy, T.D.; Fernandes, J.A.; Hollowed, A.B.; Jones, M.C.; et al. Uncertainties in projecting climate-change impacts in marine ecosystems. ICES J. Mar. Sci. 2016, 73, 1272–1282. [Google Scholar] [CrossRef]



| Indices | Description | Calculation | Range | Source |
|---|---|---|---|---|
| Levins’ Index | Measures the evenness of individual distribution within resource states; links the niche breadth to the species’ utilization of available resources | 0 to 1 | Levins (1968) [52] | |
| Schoener’s Index | Measures the degree to which species overlap in ecological space, usually in terms of overlap in food resource or habitat use | 0 to 1 | Schoener (1970) [53] | |
| Pianka’s Index | Assumes that individuals/species with similar diets are likely to compete over ecological niche | 0 to 1 | Pianka (1973) [54] | |
| Morisita–Horn Index | The simplified Morisita index to measure niche overlap, particularly applicable to the case where population density varies greatly | 0 to 1 | Horn (1966) [55] |
| Model | Effects Included | AIC | ΔAIC | Deviance | Percent Deviance Explained |
|---|---|---|---|---|---|
| M1 | Temporal + spatio-temporal + SST | 152,698.4 | 177.7 | 15,006.32 | 56.66% |
| M2 | Temporal + spatio-temporal + SST + Chl-A | 152,621.9 | 101.2 | 15,288.89 | 57.72% |
| M3 | Temporal + spatio-temporal + SST + Chl-A + SCV | 152,520.7 | - | 16,285.39 | 61.49% |
| Species Pairs | Indicators | Current | RCP2.6 | RCP8.5 | ||
|---|---|---|---|---|---|---|
| 2050 | 2100 | 2050 | 2100 | |||
| TL-SN | Levins | 0.8148 | 0.3699 | 0.0405 | 0.2077 | 0.3444 |
| Schoener | 0.7950 | 0.4805 | 0.2353 | 0.3856 | 0.4087 | |
| Pianka | 0.8667 | 0.3456 | 0.0665 | 0.2818 | 0.3038 | |
| Morisita–Horn | 0.8827 | 0.3844 | 0.0704 | 0.2929 | 0.4197 | |
| TL-SJ | Levins | 0.8888 | 0.6908 | 0.0239 | 0.0598 | 0.0401 |
| Schoener | 0.5070 | 0.2766 | 0.0998 | 0.1459 | 0.1090 | |
| Pianka | 0.3261 | 0.0743 | 0.0134 | 0.0120 | 0.0132 | |
| Morisita–Horn | 0.3922 | 0.2095 | 0.0302 | 0.0330 | 0.0199 | |
| TL-EJ | Levins | 0.7145 | 0.2490 | 0.0297 | 0.5320 | 0.1058 |
| Schoener | 0.5392 | 0.1907 | 0.1354 | 0.3102 | 0.1745 | |
| Pianka | 0.4275 | 0.0314 | 0.0154 | 0.0575 | 0.0370 | |
| Morisita–Horn | 0.5047 | 0.1054 | 0.0439 | 0.3704 | 0.0729 | |
| SN-SJ | Levins | 0.9835 | 0.2287 | 0.1261 | 0.1949 | 0.1094 |
| Schoener | 0.4520 | 0.1885 | 0.1101 | 0.1586 | 0.1156 | |
| Pianka | 0.2639 | 0.0359 | 0.0130 | 0.0068 | 0.0279 | |
| Morisita–Horn | 0.3801 | 0.0649 | 0.0519 | 0.0536 | 0.0382 | |
| SN-EJ | Levins | 0.7461 | 0.2007 | 0.4713 | 0.3155 | 0.0474 |
| Schoener | 0.5159 | 0.1948 | 0.3112 | 0.2165 | 0.1072 | |
| Pianka | 0.3296 | 0.0318 | 0.0969 | 0.0288 | 0.0009 | |
| Morisita–Horn | 0.4715 | 0.0798 | 0.2797 | 0.1152 | 0.0239 | |
| SJ-EJ | Levins | 0.1606 | 0.0331 | 0.0207 | 0.0010 | 0.0462 |
| Schoener | 0.3435 | 0.1102 | 0.0593 | 0.0167 | 0.1161 | |
| Pianka | 0.1865 | 0.0216 | 0.0048 | 0.0001 | 0.0021 | |
| Morisita–Horn | 0.2115 | 0.0397 | 0.0256 | 0.0015 | 0.0568 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ren, J.; Liu, Q.; Ma, Y.; Ji, Y.; Xu, B.; Xue, Y.; Zhang, C. Spatio-Temporal Distribution of Four Trophically Dependent Fishery Species in the Northern China Seas Under Climate Change. Biology 2025, 14, 168. https://doi.org/10.3390/biology14020168
Ren J, Liu Q, Ma Y, Ji Y, Xu B, Xue Y, Zhang C. Spatio-Temporal Distribution of Four Trophically Dependent Fishery Species in the Northern China Seas Under Climate Change. Biology. 2025; 14(2):168. https://doi.org/10.3390/biology14020168
Chicago/Turabian StyleRen, Jun, Qun Liu, Yihong Ma, Yupeng Ji, Binduo Xu, Ying Xue, and Chongliang Zhang. 2025. "Spatio-Temporal Distribution of Four Trophically Dependent Fishery Species in the Northern China Seas Under Climate Change" Biology 14, no. 2: 168. https://doi.org/10.3390/biology14020168
APA StyleRen, J., Liu, Q., Ma, Y., Ji, Y., Xu, B., Xue, Y., & Zhang, C. (2025). Spatio-Temporal Distribution of Four Trophically Dependent Fishery Species in the Northern China Seas Under Climate Change. Biology, 14(2), 168. https://doi.org/10.3390/biology14020168







