Gait Asymmetry and Post-Traumatic Osteoarthritis Following Anterior Cruciate Ligament Rupture: A Preliminary Study
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
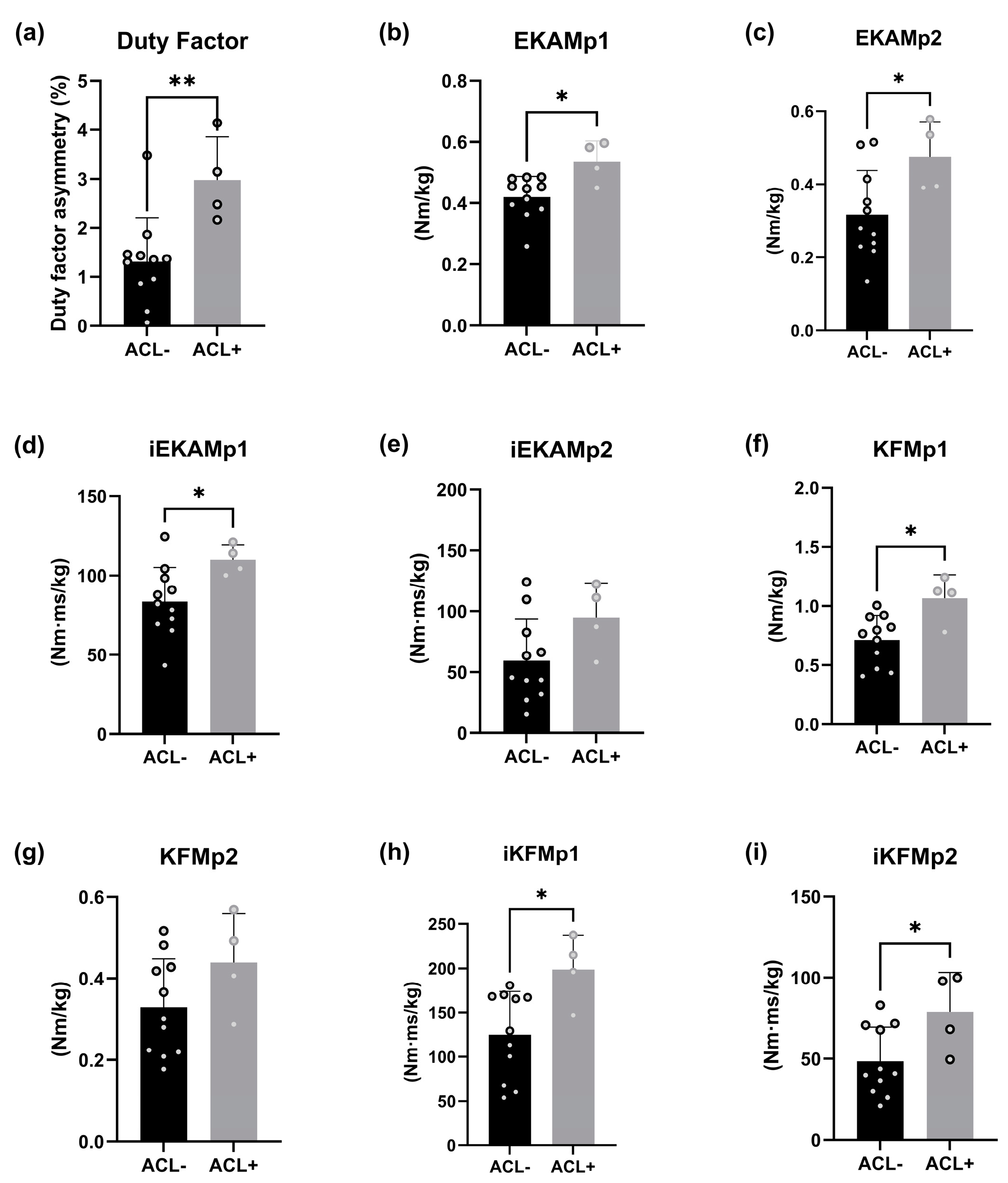
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Thomas, A.C.; Hubbard-Turner, T.; Wikstrom, E.A.; Palmieri-Smith, R.M. Epidemiology of Posttraumatic Osteoarthritis. J. Athl. Train. 2017, 52, 491–496. [Google Scholar] [CrossRef] [PubMed]
- Sharma, L. Osteoarthritis of the Knee. N. Engl. J. Med. 2021, 384, 51–59. [Google Scholar] [CrossRef]
- Hunter, D.J. Osteoarthritis. Best Pract. Res. Clin. Rheumatol. 2011, 25, 801–814. [Google Scholar] [CrossRef]
- Chang, J.C.; Sebastian, A.; Murugesh, D.K.; Hatsell, S.; Economides, A.N.; Christiansen, B.A.; Loots, G.G. Global molecular changes in a tibial compression induced ACL rupture model of post-traumatic osteoarthritis. J. Orthop. Res. 2017, 35, 474–485. [Google Scholar] [CrossRef] [PubMed]
- Mercurio, M.; Cerciello, S.; Corona, K.; Guerra, G.; Simonetta, R.; Familiari, F.; Galasso, O.; Gasparini, G. Factors Associated With a Successful Return to Performance After Anterior Cruciate Ligament Reconstruction: A Multiparametric Evaluation in Soccer Players. Orthop. J. Sports Med. 2024, 12, 23259671241275663. [Google Scholar] [CrossRef] [PubMed]
- Webster, K.E.; Hewett, T.E. Anterior Cruciate Ligament Injury and Knee Osteoarthritis: An Umbrella Systematic Review and Meta-analysis. Clin. J. Sport. Med. 2022, 32, 145–152. [Google Scholar] [CrossRef]
- Menendez, M.I.; Hettlich, B.; Wei, L.; Knopp, M.V. Feasibility of Na(18)F PET/CT and MRI for Noninvasive In Vivo Quantification of Knee Pathophysiological Bone Metabolism in a Canine Model of Post-traumatic Osteoarthritis. Mol. Imaging 2017, 16, 1536012117714575. [Google Scholar] [CrossRef] [PubMed]
- Riordan, E.A.; Little, C.; Hunter, D. Pathogenesis of post-traumatic OA with a view to intervention. Best Pract. Res. Clin. Rheumatol. 2014, 28, 17–30. [Google Scholar] [CrossRef]
- Lau, B.H.; Lafave, M.R.; Mohtadi, N.G.; Butterwick, D.J. Utilization and cost of a new model of care for managing acute knee injuries: The Calgary Acute Knee Injury Clinic. BMC Health Serv. Res. 2012, 12, 445. [Google Scholar] [CrossRef]
- Whittaker, J.L.; Culvenor, A.G.; Juhl, C.B.; Berg, B.; Bricca, A.; Filbay, S.R.; Holm, P.; Macri, E.; Urhausen, A.P.; Ardern, C.L.; et al. OPTIKNEE 2022: Consensus recommendations to optimise knee health after traumatic knee injury to prevent osteoarthritis. Br. J. Sports Med. 2022, 56, 1393–1405. [Google Scholar] [CrossRef]
- Zaki, S.; Smith, M.M.; Little, C.B. Pathology-pain relationships in different osteoarthritis animal model phenotypes: It matters what you measure, when you measure, and how you got there. Osteoarthr. Cartil. 2021, 29, 1448–1461. [Google Scholar] [CrossRef]
- Watt, F.E.; Corp, N.; Kingsbury, S.R.; Frobell, R.; Englund, M.; Felson, D.T.; Levesque, M.; Majumdar, S.; Wilson, C.; Beard, D.J.; et al. Towards prevention of post-traumatic osteoarthritis: Report from an international expert working group on considerations for the design and conduct of interventional studies following acute knee injury. Osteoarthr. Cartil. 2019, 27, 23–33. [Google Scholar] [CrossRef]
- Whittaker, J.L.; Roos, E.M. A pragmatic approach to prevent post-traumatic osteoarthritis after sport or exercise-related joint injury. Best Pract. Res. Clin. Rheumatol. 2019, 33, 158–171. [Google Scholar] [CrossRef] [PubMed]
- Nations, U. World Population Prospects; United Nations Publications: New York, NY, USA, 2019. [Google Scholar]
- Eddo, O.O.; Lindsey, B.W.; Caswell, S.V.; Prebble, M.; Cortes, N. Unintended Changes in Contralateral Limb as a Result of Acute Gait Modification. J. Appl. Biomech. 2020, 36, 13–19. [Google Scholar] [CrossRef]
- Iijima, H.; Eguchi, R.; Aoyama, T.; Takahashi, M. Trunk movement asymmetry associated with pain, disability, and quadriceps strength asymmetry in individuals with knee osteoarthritis: A cross-sectional study. Osteoarthr. Cartil. 2019, 27, 248–256. [Google Scholar] [CrossRef]
- Metcalfe, A.J.; Andersson, M.L.; Goodfellow, R.; Thorstensson, C.A. Is knee osteoarthritis a symmetrical disease? Analysis of a 12 year prospective cohort study. BMC Musculoskelet. Disord. 2012, 13, 153. [Google Scholar] [CrossRef]
- Bonnaerens, S.; Fiers, P.; Galle, S.; Derie, R.; Aerts, P.; Frederick, E.; Kaneko, Y.; Derave, W.; De Clercq, D.; Segers, V. Relationship between duty factor and external forces in slow recreational runners. BMJ Open Sport Exerc. Med. 2021, 7, e000996. [Google Scholar] [CrossRef]
- Meder, K.G.; LoJacono, C.T.; Rhea, C.K. A systematic review of non-pharmacological interventions to improve gait asymmetries in neurological populations. Symmetry 2022, 14, 281. [Google Scholar] [CrossRef]
- Patoz, A.; Gindre, C.; Thouvenot, A.; Mourot, L.; Hébert-Losier, K.; Lussiana, T. Duty Factor Is a Viable Measure to Classify Spontaneous Running Forms. Sports 2019, 7, 233. [Google Scholar] [CrossRef] [PubMed]
- Patoz, A.; Lussiana, T.; Breine, B.; Gindre, C.; Malatesta, D. Accurate estimation of peak vertical ground reaction force using the duty factor in level treadmill running. Scand. J. Med. Sci. Sports 2023, 33, 169–177. [Google Scholar] [CrossRef] [PubMed]
- Chang, A.H.; Moisio, K.C.; Chmiel, J.S.; Eckstein, F.; Guermazi, A.; Prasad, P.V.; Zhang, Y.; Almagor, O.; Belisle, L.; Hayes, K.; et al. External knee adduction and flexion moments during gait and medial tibiofemoral disease progression in knee osteoarthritis. Osteoarthr. Cartil. 2015, 23, 1099–1106. [Google Scholar] [CrossRef]
- Gerbrands, T.A.; Pisters, M.F.; Theeven, P.J.R.; Verschueren, S.; Vanwanseele, B. Lateral trunk lean and medializing the knee as gait strategies for knee osteoarthritis. Gait Posture 2017, 51, 247–253. [Google Scholar] [CrossRef]
- Teng, H.L.; MacLeod, T.D.; Link, T.M.; Majumdar, S.; Souza, R.B. Higher Knee Flexion Moment During the Second Half of the Stance Phase of Gait Is Associated With the Progression of Osteoarthritis of the Patellofemoral Joint on Magnetic Resonance Imaging. J. Orthop. Sports Phys. Ther. 2015, 45, 656–664. [Google Scholar] [CrossRef] [PubMed]
- Teng, H.L.; Powers, C.M. Sagittal plane trunk posture influences patellofemoral joint stress during running. J. Orthop. Sports Phys. Ther. 2014, 44, 785–792. [Google Scholar] [CrossRef]
- Boswell, M.A.; Uhlrich, S.D.; Kidziński, Ł.; Thomas, K.; Kolesar, J.A.; Gold, G.E.; Beaupre, G.S.; Delp, S.L. A neural network to predict the knee adduction moment in patients with osteoarthritis using anatomical landmarks obtainable from 2D video analysis. Osteoarthr. Cartil. 2021, 29, 346–356. [Google Scholar] [CrossRef]
- Chehab, E.F.; Favre, J.; Erhart-Hledik, J.C.; Andriacchi, T.P. Baseline knee adduction and flexion moments during walking are both associated with 5 year cartilage changes in patients with medial knee osteoarthritis. Osteoarthr. Cartil. 2014, 22, 1833–1839. [Google Scholar] [CrossRef] [PubMed]
- Hall, M.; Bennell, K.L.; Wrigley, T.V.; Metcalf, B.R.; Campbell, P.K.; Kasza, J.; Paterson, K.L.; Hunter, D.J.; Hinman, R.S. The knee adduction moment and knee osteoarthritis symptoms: Relationships according to radiographic disease severity. Osteoarthr. Cartil. 2017, 25, 34–41. [Google Scholar] [CrossRef]
- Creaby, M.W. It’s not all about the knee adduction moment: The role of the knee flexion moment in medial knee joint loading. Osteoarthr. Cartil. 2015, 23, 1038–1040. [Google Scholar] [CrossRef] [PubMed]
- Bennell, K.L.; Bowles, K.A.; Wang, Y.; Cicuttini, F.; Davies-Tuck, M.; Hinman, R.S. Higher dynamic medial knee load predicts greater cartilage loss over 12 months in medial knee osteoarthritis. Ann. Rheum. Dis. 2011, 70, 1770–1774. [Google Scholar] [CrossRef]
- Teng, H.L.; Calixto, N.E.; MacLeod, T.D.; Nardo, L.; Link, T.M.; Majumdar, S.; Souza, R.B. Associations between patellofemoral joint cartilage T1ρ and T2 and knee flexion moment and impulse during gait in individuals with and without patellofemoral joint osteoarthritis. Osteoarthr. Cartil. 2016, 24, 1554–1564. [Google Scholar] [CrossRef]
- Erhart-Hledik, J.; Chu, C.; Asay, J.; Favre, J.; Andriacchi, T. Longitudinal Changes in the Total Knee Joint Moment After Anterior Cruciate Ligament Reconstruction Correlate With Cartilage Thickness Changes. J. Orthop. Res. 2019, 37, 1546–1554. [Google Scholar] [CrossRef]
- Wellsandt, E.; Khandha, A.; Manal, K.; Axe, M.J.; Buchanan, T.S.; Snyder-Mackler, L. Predictors of knee joint loading after anterior cruciate ligament reconstruction. J. Orthop. Res. 2017, 35, 651–656. [Google Scholar] [CrossRef]
- Favero, M.; Ramonda, R.; Goldring, M.B.; Goldring, S.R.; Punzi, L. Early knee osteoarthritis. RMD Open 2015, 1 (Suppl. S1), e000062. [Google Scholar] [CrossRef]
- Nagano, H.; Tatsumi, I.; Sarashina, E.; Sparrow, W.A.; Begg, R.K. Modelling knee flexion effects on joint power absorption and adduction moment. Knee 2015, 22, 490–493. [Google Scholar] [CrossRef] [PubMed]
- Whittlesey, S.N.; Hamill, J.; Caldwell, G.E.; Robertson, D.G.E. Research Methods in Biomechanics; Human Kinetics: Champaign, IL, USA, 2014. [Google Scholar]
- Capin, J.J.; Zarzycki, R.; Arundale, A.; Cummer, K.; Snyder-Mackler, L. Report of the Primary Outcomes for Gait Mechanics in Men of the ACL-SPORTS Trial: Secondary Prevention With and Without Perturbation Training Does Not Restore Gait Symmetry in Men 1 or 2 Years After ACL Reconstruction. Clin. Orthop. Relat. Res. 2017, 475, 2513–2522. [Google Scholar] [CrossRef] [PubMed]
- Kato, N.; Fukusaki, C.; Leetawesup, K.; Kadokura, Y.; Ishii, N. Improvement in gait asymmetry during Nordic walking in patients with lower extremity osteoarthritis. J. Phys. Fit. Sports Med. 2020, 9, 65–73. [Google Scholar] [CrossRef]
- Butler, R.J.; Minick, K.I.; Ferber, R.; Underwood, F. Gait mechanics after ACL reconstruction: Implications for the early onset of knee osteoarthritis. Br. J. Sports Med. 2009, 43, 366–370. [Google Scholar] [CrossRef] [PubMed]
- Bates, N.A.; Schilaty, N.D.; Nagelli, C.V.; Krych, A.J.; Hewett, T.E. Multiplanar Loading of the Knee and Its Influence on Anterior Cruciate Ligament and Medial Collateral Ligament Strain During Simulated Landings and Noncontact Tears. Am. J. Sports Med. 2019, 47, 1844–1853. [Google Scholar] [CrossRef]
- Hewett, T.E.; Ford, K.R.; Xu, Y.Y.; Khoury, J.; Myer, G.D. Utilization of ACL Injury Biomechanical and Neuromuscular Risk Profile Analysis to Determine the Effectiveness of Neuromuscular Training. Am. J. Sports Med. 2016, 44, 3146–3151. [Google Scholar] [CrossRef] [PubMed]
- Miyazaki, T.; Wada, M.; Kawahara, H.; Sato, M.; Baba, H.; Shimada, S. Dynamic load at baseline can predict radiographic disease progression in medial compartment knee osteoarthritis. Ann. Rheum. Dis. 2002, 61, 617–622. [Google Scholar] [CrossRef] [PubMed]
- Farrokhi, S.; Voycheck, C.A.; Tashman, S.; Fitzgerald, G.K. A biomechanical perspective on physical therapy management of knee osteoarthritis. J. Orthop. Sports Phys. Ther. 2013, 43, 600–619. [Google Scholar] [CrossRef] [PubMed]
- Ho, K.Y.; Blanchette, M.G.; Powers, C.M. The influence of heel height on patellofemoral joint kinetics during walking. Gait Posture 2012, 36, 271–275. [Google Scholar] [CrossRef]
- Garcia, S.A.; Brown, S.R.; Koje, M.; Krishnan, C.; Palmieri-Smith, R.M. Gait asymmetries are exacerbated at faster walking speeds in individuals with acute anterior cruciate ligament reconstruction. J. Orthop. Res. 2022, 40, 219–230. [Google Scholar] [CrossRef]
- Williams, J.; Neal, K.; Alfayyadh, A.; Capin, J.; Khandha, A.; Manal, K.; Potter, H.; Snyder-Mackler, L.; Buchanan, T. Early knee loading asymmetries may be at the root of eventual osteoarthritis development after anterior cruciate ligament reconstruction. Osteoarthr. Cartil. 2020, 28, S226–S227. [Google Scholar] [CrossRef]
- Teng, H.L.; Wu, D.; Su, F.; Pedoia, V.; Souza, R.B.; Ma, C.B.; Li, X. Gait Characteristics Associated With a Greater Increase in Medial Knee Cartilage T(1ρ) and T(2) Relaxation Times in Patients Undergoing Anterior Cruciate Ligament Reconstruction. Am. J. Sports Med. 2017, 45, 3262–3271. [Google Scholar] [CrossRef] [PubMed]
- Ebert, J.R.; Edwards, P.; Yi, L.; Joss, B.; Ackland, T.; Carey-Smith, R.; Buelow, J.-U.; Hewitt, B. Strength and functional symmetry is associated with post-operative rehabilitation in patients following anterior cruciate ligament reconstruction. Knee Surg. Sports Traumatol. Arthrosc. 2018, 26, 2353–2361. [Google Scholar] [CrossRef] [PubMed]
- Hadizadeh, M.; Amri, S.; Roohi, S.A.; Mohafez, H. Assessment of Gait Symmetry Improvements in National Athletes after Anterior Cruciate Ligament Reconstruction during Rehabilitation. Int. J. Sports Med. 2016, 37, 997–1002. [Google Scholar] [CrossRef]
- Brunner, J.; Schimmack, U. Estimating population mean power under conditions of heterogeneity and selection for significance. Meta-Psychology 2020, 4. [Google Scholar] [CrossRef]
- Wang, L.J.; Zeng, N.; Yan, Z.P.; Li, J.T.; Ni, G.X. Post-traumatic osteoarthritis following ACL injury. Arthritis Res. Ther. 2020, 22, 57. [Google Scholar] [CrossRef] [PubMed]
- Kang, H. Sample size determination and power analysis using the G*Power software. J. Educ. Eval. Health Prof. 2021, 18, 17. [Google Scholar] [CrossRef]
- Krakowski, P.; Nogalski, A.; Jurkiewicz, A.; Karpiński, R.; Maciejewski, R.; Jonak, J. Comparison of Diagnostic Accuracy of Physical Examination and MRI in the Most Common Knee Injuries. Appl. Sci. 2019, 9, 4102. [Google Scholar] [CrossRef]
- Dare, D.; Rodeo, S. Mechanisms of post-traumatic osteoarthritis after ACL injury. Curr. Rheumatol. Rep. 2014, 16, 448. [Google Scholar] [CrossRef] [PubMed]
- Racine, J.; Aaron, R.K. Post-traumatic osteoarthritis after ACL injury. RI Med. J. 2014, 97, 25–28. [Google Scholar]
- Han, P.F.; Wei, L.; Duan, Z.Q.; Zhang, Z.L.; Chen, T.Y.; Lu, J.G.; Zhao, R.P.; Cao, X.M.; Li, P.C.; Lv, Z.; et al. Contribution of IL-1β, 6 and TNF-α to the form of post-traumatic osteoarthritis induced by “idealized” anterior cruciate ligament reconstruction in a porcine model. Int. Immunopharmacol. 2018, 65, 212–220. [Google Scholar] [CrossRef] [PubMed]
- Heard, B.J.; Barton, K.I.; Chung, M.; Achari, Y.; Shrive, N.G.; Frank, C.B.; Hart, D.A. Single intra-articular dexamethasone injection immediately post-surgery in a rabbit model mitigates early inflammatory responses and post-traumatic osteoarthritis-like alterations. J. Orthop. Res. 2015, 33, 1826–1834. [Google Scholar] [CrossRef] [PubMed]


| Demographic Variable | Group 1. ACL− | Group 2. ACL+ | p Value |
|---|---|---|---|
| Sex (male/female) (n) | 5/6 | 4/0 | |
| Age (y) * | 35 ± 6 | 32 ± 1 | 0.1158 |
| Mass (kg) * | 71.3 ± 12.5 | 86.6 ± 7.9 | 0.0998 |
| Height (cm) *† | 174 ± 11.3 | 185 ± 3.9 | <0.001 |
| Body mass index (kg/m2) * | 23 ± 2.3 | 25.2 ± 2.0 | 0.1442 |
| Years post-surgery | - | 6.0 ± 3.2 | - |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pringle, S.; D’Août, K. Gait Asymmetry and Post-Traumatic Osteoarthritis Following Anterior Cruciate Ligament Rupture: A Preliminary Study. Biology 2025, 14, 208. https://doi.org/10.3390/biology14020208
Pringle S, D’Août K. Gait Asymmetry and Post-Traumatic Osteoarthritis Following Anterior Cruciate Ligament Rupture: A Preliminary Study. Biology. 2025; 14(2):208. https://doi.org/10.3390/biology14020208
Chicago/Turabian StylePringle, Samuel, and Kristiaan D’Août. 2025. "Gait Asymmetry and Post-Traumatic Osteoarthritis Following Anterior Cruciate Ligament Rupture: A Preliminary Study" Biology 14, no. 2: 208. https://doi.org/10.3390/biology14020208
APA StylePringle, S., & D’Août, K. (2025). Gait Asymmetry and Post-Traumatic Osteoarthritis Following Anterior Cruciate Ligament Rupture: A Preliminary Study. Biology, 14(2), 208. https://doi.org/10.3390/biology14020208







