Thermal Tolerance of Crassostrea (Magallana) ariakensis to Nuclear Plant Warm Water Discharges
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Experimental Materials
2.2. Experimental Methods
2.2.1. Acclimation
Thermal Tolerance Experiment
Long-Term Experiment on Suitable Growth
2.2.2. Dynamic Thermal Tolerance Experiment
2.2.3. Static Thermal Tolerance Experiment
2.2.4. Long-Term Experiment on Suitable Growth and Sampling
2.2.5. Growth and Nutritional Quality of C. ariakensis
2.2.6. Amylase Activity in the Digestive Gland of C. ariakensis
2.3. Statistical Analysis
3. Results
3.1. Thermal Tolerance Experiment
3.1.1. Discomfort Temperature and CTM
3.1.2. Incipient Lethal Temperature (ILT50)
3.2. Long-Term Experiment on Suitable Growth
3.2.1. Effects of Temperature Rise on the Growth of C. ariakensis
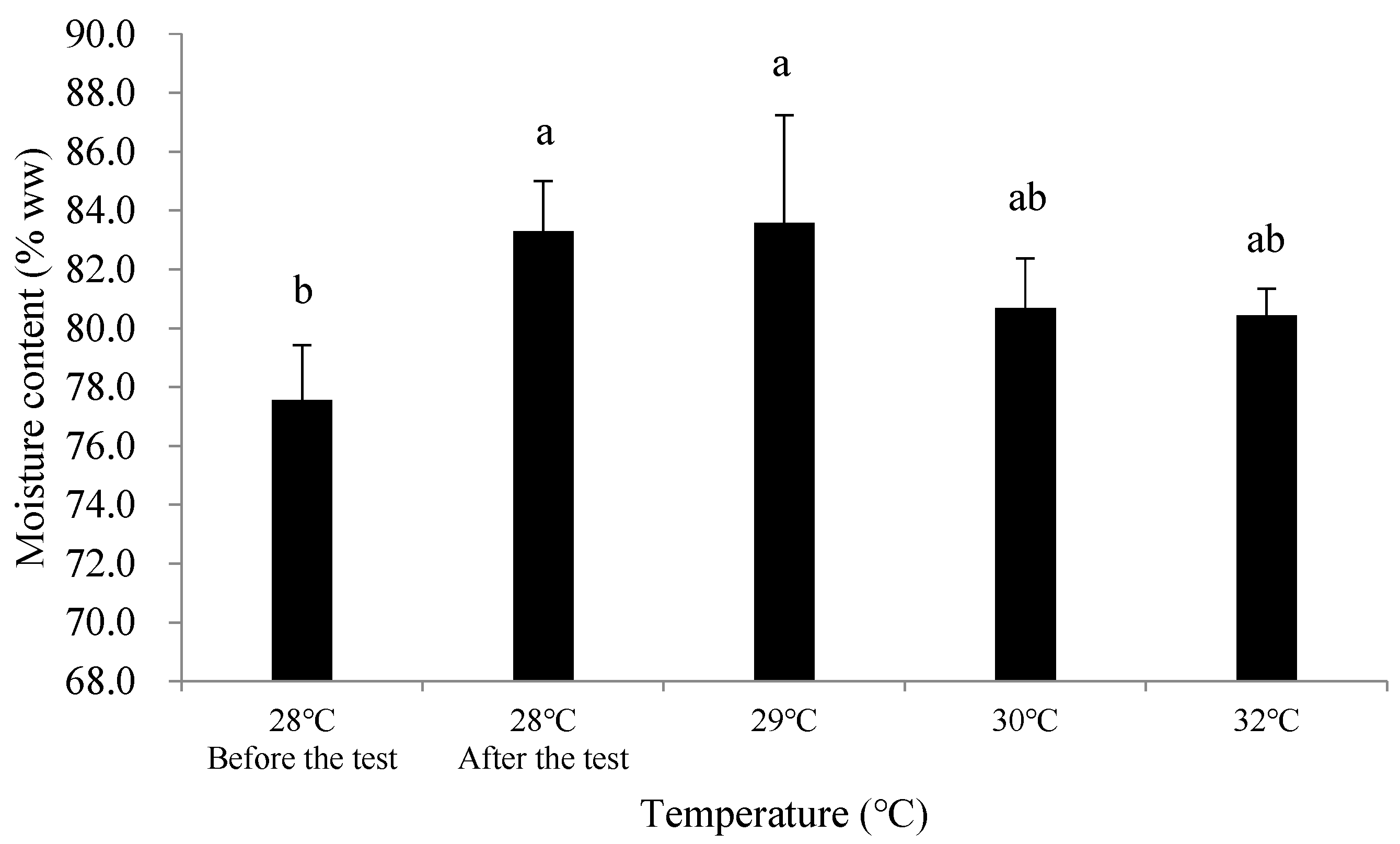
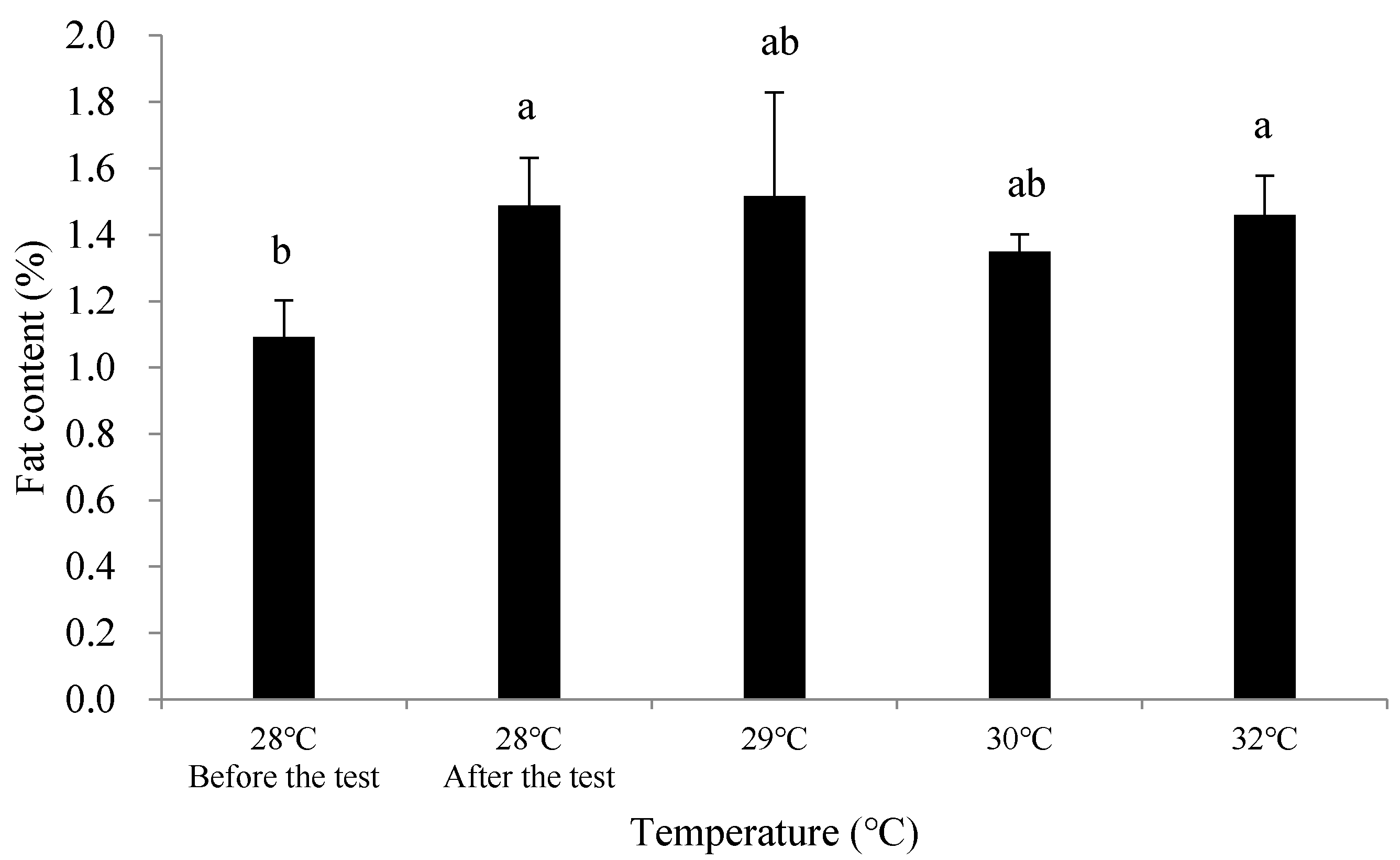
3.2.2. Effects of Temperature Rise on the Nutritional Quality of C. ariakensis
3.2.3. Effects of Temperature Rise on Amylase Activity in the Digestive Gland of C. ariakensis
4. Discussion
4.1. Effects of Nuclear Plant Thermal Discharges on Water Temperature and C. ariakensis
4.2. Effects of Temperature Rise on the Heat Tolerance of C. ariakensis
4.3. Effects of Temperature Rise on the Nutritional Quality of C. ariakensis
4.4. Effects of Temperature Rise on the Amylase Activity in the Digestive Gland of C. ariakensis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Yue, Q.; He, J.; Zhi, S.; Dong, H. Fuel cycles optimization of nuclear power industry in China. Ann. Nucl. Energy 2018, 111, 635–643. [Google Scholar] [CrossRef]
- Chen, Y.; Martin, G.; Chabert, C.; Eschbach, R.; He, H.; Ye, G.-A. Prospects in China for nuclear development up to 2050. Prog. Nucl. Energy 2018, 103, 81–90. [Google Scholar] [CrossRef]
- Lin, J.; Zou, X.; Huang, F.; Yao, Y. Quantitative estimation of sea surface temperature increases resulting from the thermal discharge of coastal power plants in China. Mar. Pollut. Bull. 2021, 164, 112020. [Google Scholar] [CrossRef] [PubMed]
- Arieli, R.N.; Almogi-Labin, A.; Abramovich, S.; Herut, B. The effect of thermal pollution on benthic foraminiferal assemblages in the Mediterranean shoreface adjacent to Hadera power plant (Israel). Mar. Pollut. Bull. 2011, 62, 1002–1012. [Google Scholar] [CrossRef]
- Saravanan, P.; Priya, A.M.; Sundarakrishnan, B.; Venugopalan, V.P.; Rao, T.S.; Jayachandran, S. Effects of thermal discharge from a nuclear power plant on culturable bacteria at a tropical coastal location in India. J. Therm. Biol. 2008, 33, 385–394. [Google Scholar] [CrossRef]
- Teixeira, T.P.; Neves, L.M.; Araujo, F.G. Effects of a nuclear power plant thermal discharge on habitat complexity and fish community structure in Ilha Grande Bay, Brazil. Mar. Environ. Res. 2009, 68, 188–195. [Google Scholar] [CrossRef]
- Ding, X.; Tian, W.; Chen, Q.; Wei, G. Policies on water resources assessment of coastal nuclear power plants in China. Energy Policy 2019, 128, 170–178. [Google Scholar] [CrossRef]
- Fisheries Bureau of the Ministry of Agriculture and Rural Affairs of the People’s Republic of China. China Fisheries Yearbook; China Agriculture Press: Beijing, China, 2021; p. 158.
- Zhang, Q.; Qiu, M.; Wu, X.; Pan, J. Heat pretreatment induces thermotolerance in the Jinjiang oyster (Crassostrea ariakensis Gould). Ecol. Sci. 2005, 25, 35–37. (In Chinese) [Google Scholar] [CrossRef]
- Wang, R.; Yang, H.; Zhu, L.; Wu, C.; Chen, Y. Application of temperature rise envelop in thermal discharge from nuclear power plant. Adm. Tech. Environ. Monit. 2020, 32, 49–52. (In Chinese) [Google Scholar] [CrossRef]
- Cai, Z.; Chen, H.; Jin, Q.; Lian, J. Influence of thermal power effluent on three species of fishes in Daya Bay. J. Trop. Oceanogr. 1999, 18, 11–19. (In Chinese) [Google Scholar] [CrossRef]
- Rajaguru, S.; Ramachandran, S. Temperature tolerance of some estuarine fishes. J. Therm. Biol. 2001, 26, 41–45. [Google Scholar] [CrossRef] [PubMed]
- Chen, Q.; Zeng, J.; Gao, A.; Liao, Y.; Yang, G. Advances in study of temperature of thermal tolerance of fishes. J. Fish. China 2004, 28, 562–567. (In Chinese) [Google Scholar] [CrossRef]
- Holland, W.E.; Smith, M.H.; Gibbons, J.W.; Brown, D.H. Thermal tolerances of fish from a reservoir receiving heated effluent from a nuclear reactor. Physiol. Zool. 1974, 47, 110–118. [Google Scholar] [CrossRef]
- Lyytikäinen, T.; Koskela, J.; Rissanen, I. Thermal resistance and upper lethal temperatures of underyearling Lake Inari Arctic charr. J. Fish Biol. 1997, 51, 515–525. [Google Scholar] [CrossRef]
- Gabbott, P.A.; Bayne, B.L. Biochemical effects of temperature and nutritive stress on Mytilus edulis L. J. Mar. Biol. Assoc. U. K. 1973, 53, 269–286. [Google Scholar] [CrossRef]
- López-Bascón, M.A.; Luque de Castro, M.D. Chapter 11—Soxhlet Extraction. In Liquid-Phase Extraction; Poole, C.F., Ed.; Elsevier: Amsterdam, The Netherlands, 2020; pp. 327–354. [Google Scholar]
- Martinez, M.; Mangano, M.C.; Maricchiolo, G.; Genovese, L.; Mazzola, A.; Sara, G. Measuring the effects of temperature rise on Mediterranean shellfish aquaculture. Ecol. Indic. 2018, 88, 71–78. [Google Scholar] [CrossRef]
- Liu, C.; Guo, J.; Peng, Z.; Huang, J.; Liu, Z. Tolerance of juvenile shellfish Lutraria sieboldii to high-temperature and dry exposure. Fish. Sci. 2015, 34, 169–173. (In Chinese) [Google Scholar] [CrossRef]
- Wang, Q.; Li, S.; Fu, C.; Zhang, M.; Teng, W.; Liu, Z.; Liu, W.; Li, W.; Tan, K. Establishment of high temperature resistance families and use of laboratory assays to predict subsequent survival in juvenile stage of the Japanese scallop (Mizuhopecten yessoensis). J. Fish. China 2014, 38, 371–377. (In Chinese) [Google Scholar] [CrossRef]
- Liu, Z.; Wang, H.; Li, Z.; Zheng, Y. Upper incipient lethal temperature of Argopecten irradians concentricus Say. J. Fish. Sci. China 2007, 14, 778–785. (In Chinese) [Google Scholar] [CrossRef]
- Yu, J.; Yin, Z.; Zhang, Y.; Bi, J.; Yan, X.; Nie, H. Effects of high water temperature on physiology, survival, and resistance to high temperature air-exposure in the Manila clam Ruditapes philippinarum. Comp. Biochem. Physiol. Part C Toxicol. Pharmacol. 2022, 262, 109469. [Google Scholar] [CrossRef]
- Anestis, A.; Poertner, H.O.; Lazou, A.; Michaelidis, B. Metabolic and molecular stress responses of sublittoral bearded horse mussel Modiolus barbatus to warming sea water: Implications for vertical zonation. J. Exp. Biol. 2008, 211, 2889–2898. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Liu, Z.; Yao, R.; Luo, C.; Yan, J. Effect of temperature and salinity on the survival and growth of Meretrix lyrata juveniles. Acta Ecol. Sin. 2010, 30, 3406–3413. (In Chinese) [Google Scholar]
- Baag, S.; Mandal, S. Combined effects of ocean warming and acidification on marine fish and shellfish: A molecule to ecosystem perspective. Sci. Total Environ. 2022, 802, 149807. [Google Scholar] [CrossRef]
- Hernroth, B.; Sköld, H.N.; Wiklander, K.; Jutfelt, F.; Baden, S. Simulated climate change causes immune suppression and protein damage in the crustacean Nephrops norvegicus. Fish Shellfish Immunol. 2012, 33, 1095–1101. [Google Scholar] [CrossRef]
- Lam-Gordillo, O.; Douglas, E.J.; Hailes, S.F.; Cummings, V.; Lohrer, A.M. Effects of in situ experimental warming on metabolic expression in a soft sediment bivalve. Sci. Rep. 2025, 15, 1812. [Google Scholar] [CrossRef]
- Braga, A.C.; Pereira, V.; Marcal, R.; Marques, A.; Guilherme, S.; Costa, P.R.; Pacheco, M. DNA damage and oxidative stress responses of mussels Mytilus galloprovincialis to paralytic shellfish toxins under warming and acidification conditions—Elucidation on the organ-specificity. Aquat. Toxicol. 2020, 228, 105619. [Google Scholar] [CrossRef]
- Zeng, J. Ecological effect by thermal discharged water from subtropical coastal power plants. Ph.D. Thesis, Zhejiang University, Hangzhou, China, 2008. [Google Scholar]
- Matoo, O.B.; Lannig, G.; Bock, C.; Sokolova, I.M. Temperature but not ocean acidification affects energy metabolism and enzyme activities in the blue mussel, Mytilus edulis. Ecol. Evol. 2021, 11, 3366–3379. [Google Scholar] [CrossRef]
- Gao, F.; Zheng, R.; Zhang, K.; Ma, L.; Liu, K.; Huang, D.; Fang, C.; Lin, H.; Bo, J. Effects of thermal stress from nuclear power plants on the survival rate, behavioral changes, and biochemical and molecular responses of abalone. Aquac. Rep. 2024, 37, 102239. [Google Scholar] [CrossRef]
- Yu, D.; Shen, Z.; Zhang, Z.; Zhang, C.; Tang, Q.; Liu, H. Effect of temperature acclimation on the thermal tolerance of Rhynchocypris oxycephalus. Acta Hydrobiol. Sin. 2017, 41, 538–542. (In Chinese) [Google Scholar] [CrossRef]
- Liao, Y.; Chen, Q.; Zeng, J.; Gao, A.; Liu, J.; Sun, Q. Thermal tolerance of four commercial shellfishes. Mar. Sci. Bull. 2007, 26, 50–54. (In Chinese) [Google Scholar] [CrossRef]
- Fokina, N.N.; Lysenko, L.A.; Sukhovskaya, I.V.; Vdovichenko, E.A.; Borvinskaya, E.V.; Kantserova, N.P.; Krupnova, M.Y.; Ruokolainen, T.R.; Smirnov, L.P.; Vysotskaya, R.U.; et al. Biochemical response of blue mussels Mytilus edulis L. from the white sea to rapid changes in ambient temperature. J. Evol. Biochem. Physiol. 2015, 51, 378–387. [Google Scholar] [CrossRef]
- Valles-Regino, R.; Tate, R.; Kelaher, B.; Savins, D.; Dowell, A.; Benkendorff, K. Ocean warming and CO2− induced acidification impact the lipid content of a marine predatory gastropod. Mar. Drugs 2015, 13, 6019–6037. [Google Scholar] [CrossRef]
- Bowyer, J.N.; Qin, J.G.; Adams, L.R.; Thomson, M.J.S.; Stone, D.A.J. The response of digestive enzyme activities and gut histology in yellowtail kingfish (Seriola lalandi) to dietary fish oil substitution at different temperatures. Aquaculture 2012, 368, 19–28. [Google Scholar] [CrossRef]
- Khan, F.U.; Hu, M.; Kong, H.; Shang, Y.; Wang, T.; Wang, X.; Xu, R.; Lu, W.; Wang, Y. Ocean acidification, hypoxia and warming impair digestive parameters of marine mussels. Chemosphere 2020, 256, 127096. [Google Scholar] [CrossRef]
- Areekijseree, M.; Engkagul, A.; Kovitvadhi, U.; Thongpan, A.; Mingmuang, M.; Pakkong, P.; Rungruangsak-Torrissen, K. Temperature and pH characteristics of amylase and proteinase of adult freshwater pearl mussel, Hyriopsis (Hyriopsis) bialatus Simpson 1900. Aquaculture 2004, 234, 575–587. [Google Scholar] [CrossRef]
- Zhang, S.; Zhu, S.; Saqib, M.N.; Yu, M.; Du, C.; Huang, D.; Li, Y. Extensive inhibition of starch digestion by exogenous proteins and inhibition mechanisms: A comprehensive review. Trends Food Sci. Technol. 2024, 143, 104303. [Google Scholar] [CrossRef]

| Acclimation Temperature (°C) | 16 | 26 | 30 |
|---|---|---|---|
| Discomfort temperature (°C) | 48.6 ± 1.2 | 57.8 ± 2.9 | 58.9 ± 3.0 |
| CTM (°C) | 51.6 ± 1.4 | 60.0 ± 1.6 | 61.2 ± 2.2 |
| Acclimation Temperature (°C) | Indicators | Exposure Temperature (°C) | ||||||
|---|---|---|---|---|---|---|---|---|
| 42 | 43 | 44 | 45 | 46 | 47 | 48 | ||
| 16 | Mortality rate (%) | 0 | 0 | 7.8 ± 1.9 | 38.9 ± 5.1 | 58.9 ± 8.8 | 82.2 ± 12.0 | 100.0 ± 0.0 |
| Time for 50% mortality (min) | - | - | - | - | 1045 ± 100.1 | 527 ± 52.9 | 125 ± 20.1 | |
| Acclimation Temperature (°C) | Indicators | Exposure Temperature (°C) | ||||||
| 50 | 51 | 52 | 53 | 54 | 55 | 56 | ||
| 26 | Mortality rate (%) | 0 | 5.6 ± 0.5 | 13.3 ± 1.0 | 30.0 ± 1.3 | 53.3 ± 1.7 | 74.4 ± 2.0 | 100.0 ± 0.0 |
| Time for 50% mortality (min) | - | - | - | - | 1231 ± 110.0 | 746 ± 88.1 | 357 ± 20.1 | |
| Acclimation Temperature (°C) | Indicators | Exposure Temperature (°C) | ||||||
| 52 | 53 | 54 | 55 | 56 | 57 | 58 | ||
| 30 | Mortality rate (%) | 0 | 0 | 4.4 ± 0.7 | 16.7 ± 0.7 | 52.2 ± 1.8 | 74.4 ± 2.6 | 100 ± 0.0 |
| Time for 50% mortality (min) | - | - | - | - | 1440 ± 156.5 | 932 ± 46.3 | 181 ± 10.6 | |
| Acclimation Temperature (°C) | 16 | 26 | 30 |
|---|---|---|---|
| ILT50 (°C) | 45.61 | 53.71 | 55.90 |
| 95% Confidence Interval (°C) | 45.43–45.82 | 53.50–53.94 | 55.70–56.13 |
| Indicators | Temperature (°C) | ||||
|---|---|---|---|---|---|
| 28 (Before the Test) | 28 (After the Test) | 29 | 30 | 31 | |
| Soft Tissue Wet Weight (g) | 4.1 ± 1.8 b | 6.9 ± 2.2 a | 6.7 ± 2.2 a | 6.9 ± 1.7 a | 7.0 ± 1.3 a |
| Condition Index (%) | 7.3 ± 3.6 c | 11.7 ± 1.2 a | 10.8 ± 1.8 a | 11.3 ± 1.9 a | 12.2 ± 1.2 a |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, L.; Liu, L.; Yan, C.; Wang, L.; Ye, Y.; Chen, L.; Zou, X.; Zhang, H.; Zeng, M.; Jiang, M. Thermal Tolerance of Crassostrea (Magallana) ariakensis to Nuclear Plant Warm Water Discharges. Biology 2025, 14, 311. https://doi.org/10.3390/biology14030311
Li L, Liu L, Yan C, Wang L, Ye Y, Chen L, Zou X, Zhang H, Zeng M, Jiang M. Thermal Tolerance of Crassostrea (Magallana) ariakensis to Nuclear Plant Warm Water Discharges. Biology. 2025; 14(3):311. https://doi.org/10.3390/biology14030311
Chicago/Turabian StyleLi, Lei, Longyu Liu, Cong Yan, Liang Wang, Yuanlv Ye, Lu Chen, Xiong Zou, Haijing Zhang, Mengni Zeng, and Mei Jiang. 2025. "Thermal Tolerance of Crassostrea (Magallana) ariakensis to Nuclear Plant Warm Water Discharges" Biology 14, no. 3: 311. https://doi.org/10.3390/biology14030311
APA StyleLi, L., Liu, L., Yan, C., Wang, L., Ye, Y., Chen, L., Zou, X., Zhang, H., Zeng, M., & Jiang, M. (2025). Thermal Tolerance of Crassostrea (Magallana) ariakensis to Nuclear Plant Warm Water Discharges. Biology, 14(3), 311. https://doi.org/10.3390/biology14030311





