Probiotic Fermentation of Astragalus membranaceus and Raphani Semen Ameliorates Cyclophosphamide-Induced Immunosuppression Through Intestinal Short-Chain Fatty Acid-Dependent or -Independent Regulation of B Cell Function
Simple Summary
Abstract
1. Introduction
Germ
2. Materials and Methods
2.1. Probiotic Fermentation of A. membranaceus and Raphani Semen
2.2. Component Analysis of the Fermented A. membranaceus and Raphani Semen
2.3. Animal, Induction of Immunosuppression and Experimental Design
2.4. Histopathological Analysis of the Colon
2.5. Quantitative Real-Time Polymerase Chain Reaction (qPCR) Analysis
2.6. Flow Cytometry-Based Detection of Lymphocyte Subsets of Mice
2.7. Enzyme-Linked Immunosorbent Assay (ELISA)
2.8. Fecal DNA Extraction and Analysis of 16S rRNA Gene Sequence
2.9. Detection of Short-Chain Fatty Acids (SCFA) from Fecal Samples
2.10. Statistical Analysis
3. Results
3.1. Change in the Composition of A. membranaceus and Raphani Semen After Probiotic Fermentation
3.2. PROAS Improves the Intestinal Barrier Function in Immunosuppressed Mice
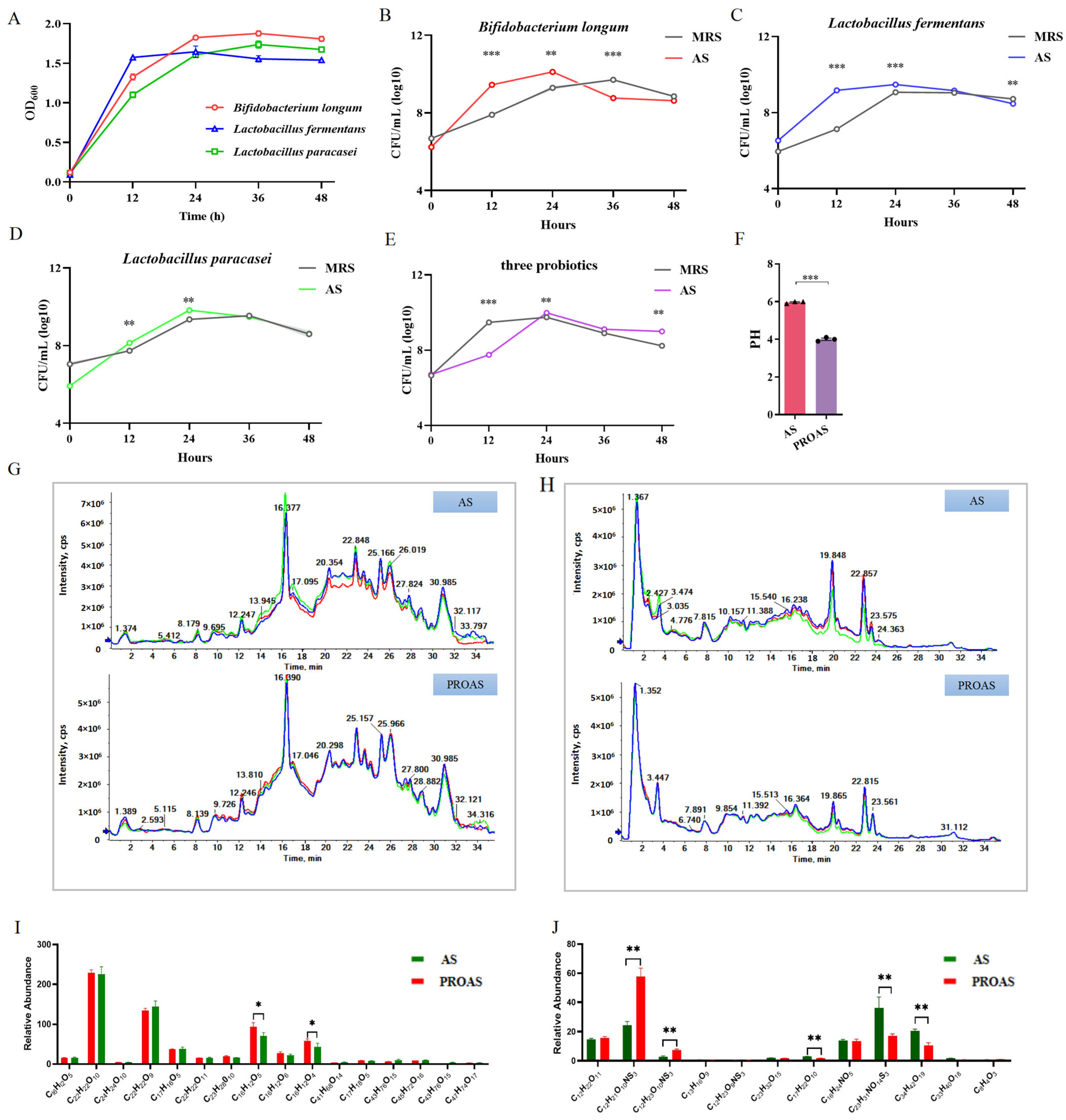
3.3. PROAS Improves Immune Function in Immunosuppressed Mice
3.4. PROAS Modulates the Gut Microbiome Composition in Immunosuppressed Mice

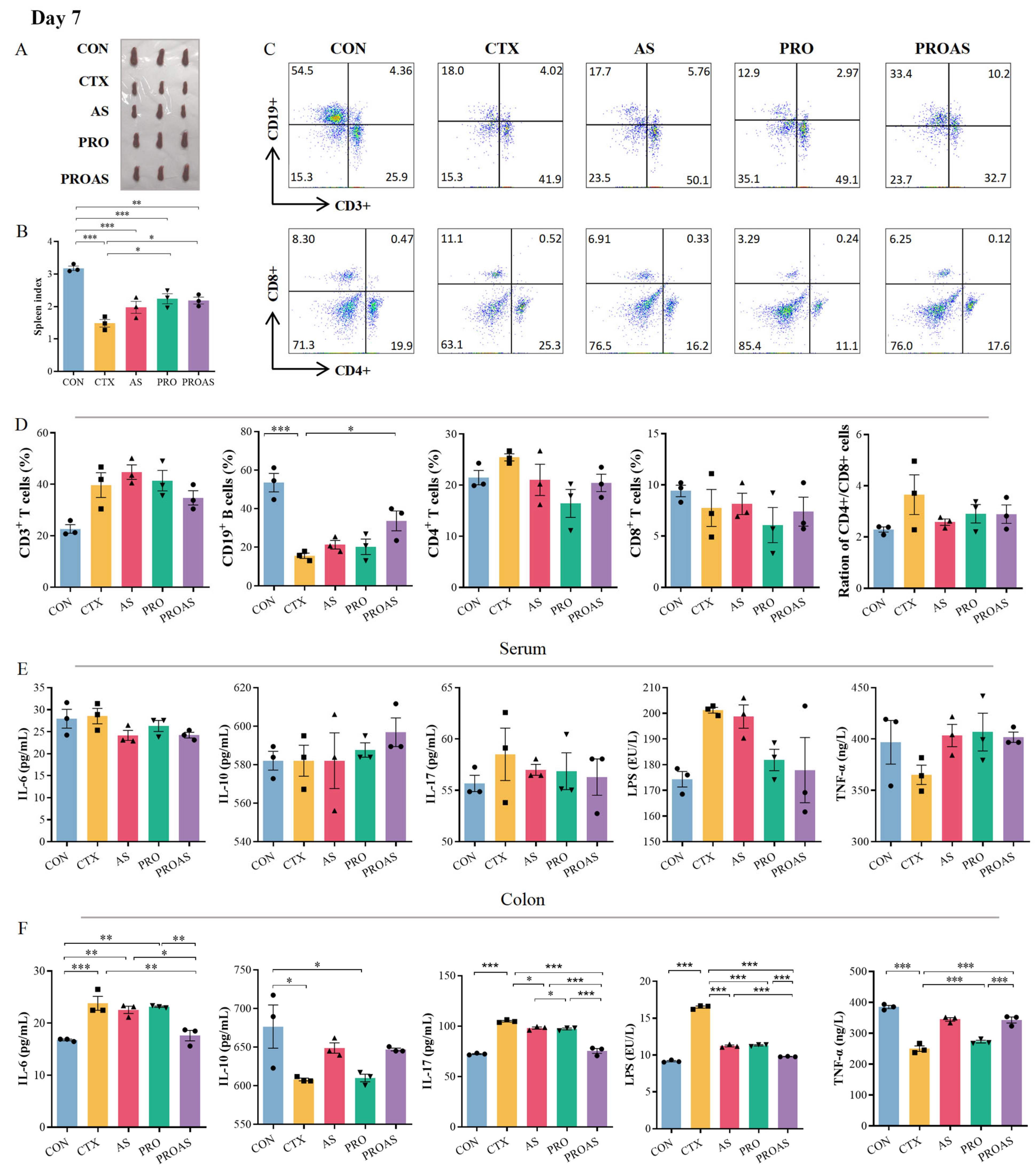
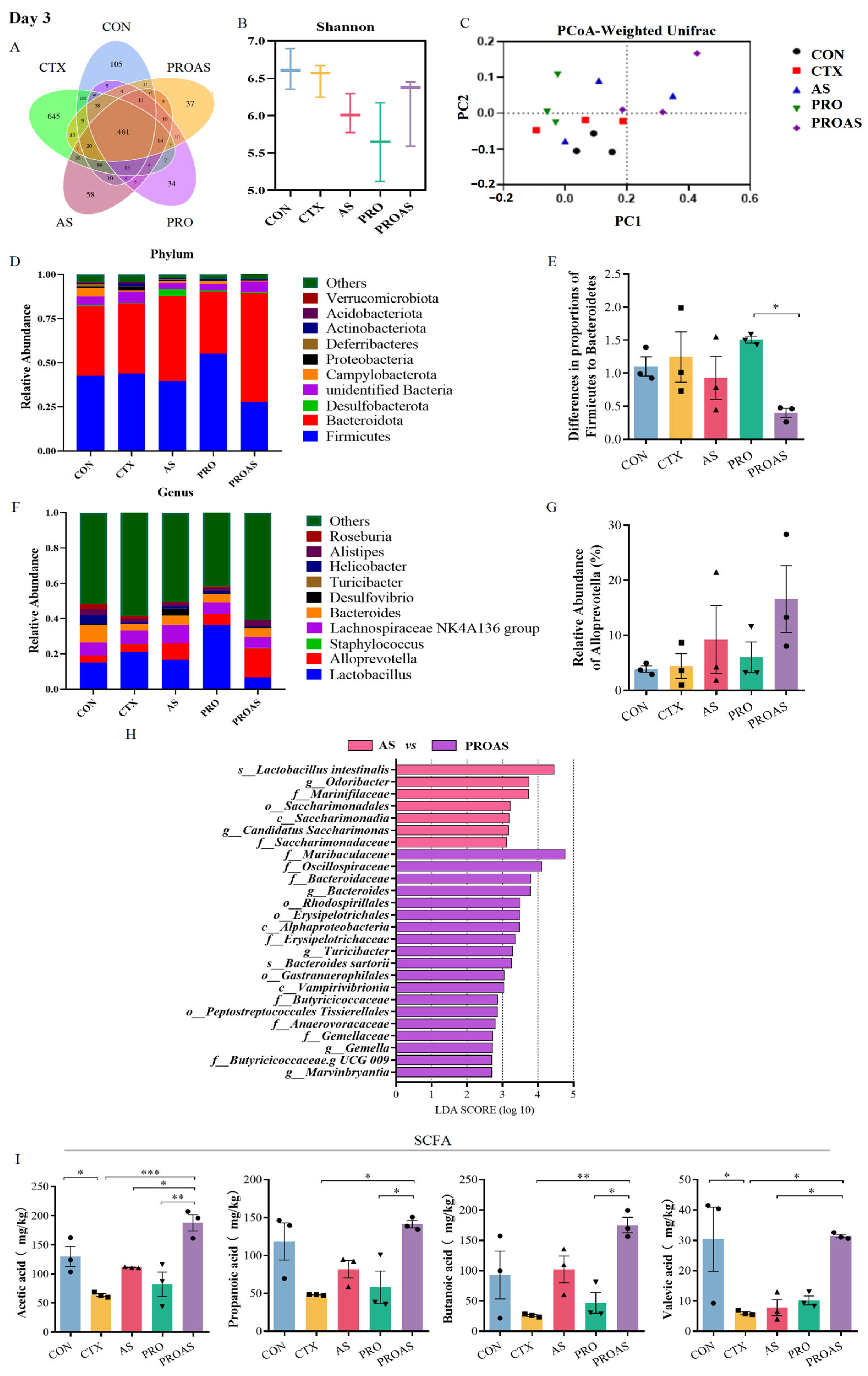

3.5. Effects of PROAS on Intestinal SCFA Production in Immunosuppressed Mice
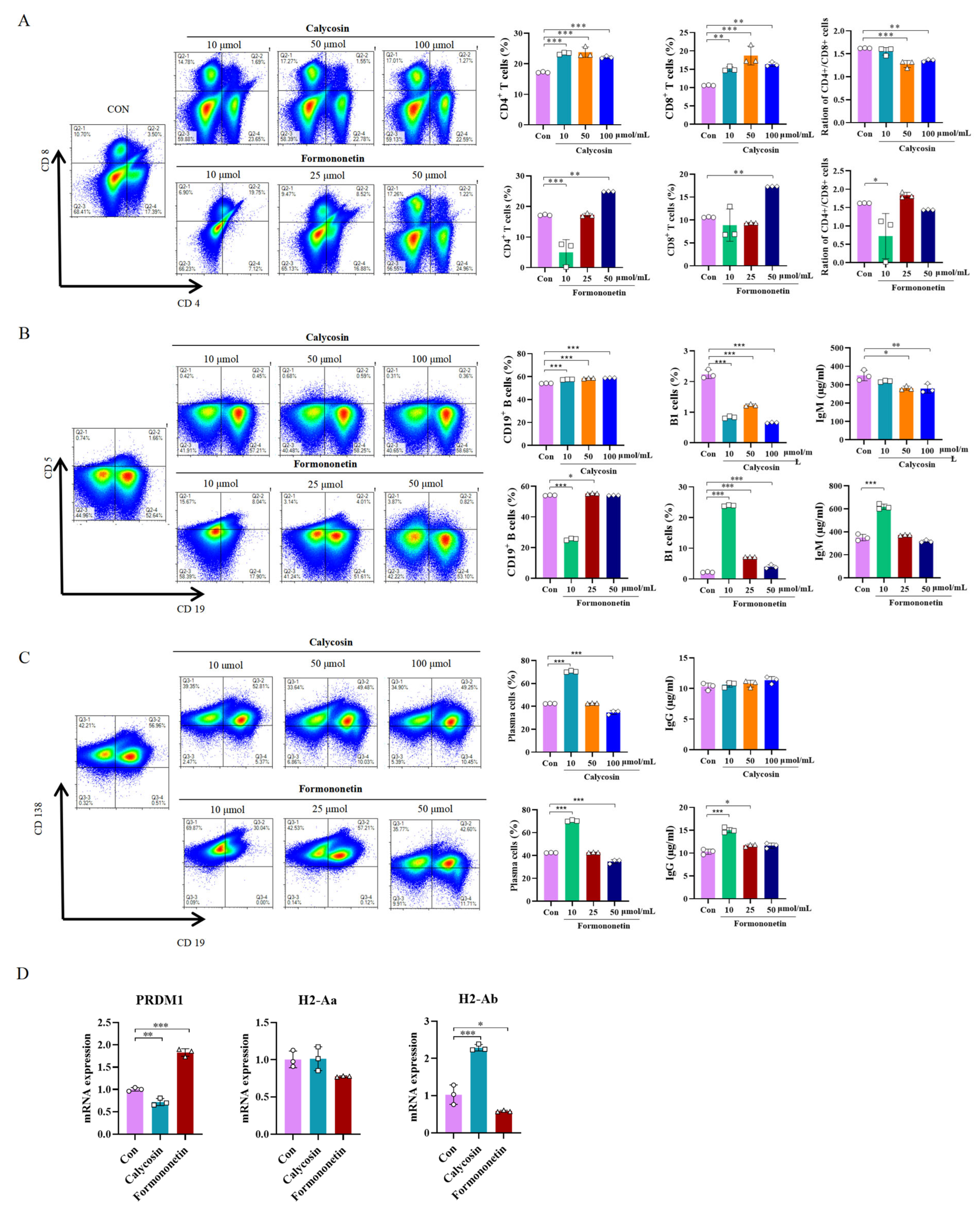
3.6. Effects of Calycosin and Formononetin from Astragalus on Mice Spleen Cells
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Chaplin, D.D. Overview of the immune response. J. Allergy Clin. Immunol. 2010, 125, S3–S23. [Google Scholar] [CrossRef]
- Lambring, C.B.; Siraj, S.; Patel, K.; Sankpal, U.T.; Mathew, S.; Basha, R. Impact of the Microbiome on the Immune System. Crit. Rev. Immunol. 2019, 39, 313–328. [Google Scholar] [CrossRef]
- Nagarajan, A.; Scoggin, K.; Gupta, J.; Threadgill, D.W.; Andrews-Polymenis, H.L. Using the collaborative cross to identify the role of host genetics in defining the murine gut microbiome. Microbiome 2023, 11, 149. [Google Scholar] [CrossRef] [PubMed]
- Belkaid, Y.; Hand, T.W. Role of the microbiota in immunity and inflammation. Cell 2014, 157, 121–141. [Google Scholar] [CrossRef]
- Wang, X.Q.; Zhang, A.H.; Miao, J.H.; Sun, H.; Yan, G.L.; Wu, F.F.; Wang, X.J. Gut microbiota as important modulator of metabolism in health and disease. RSC Adv. 2018, 8, 42380–42389. [Google Scholar] [CrossRef] [PubMed]
- Honda, K.; Littman, D.R. The microbiota in adaptive immune homeostasis and disease. Nature 2016, 535, 75–84. [Google Scholar] [CrossRef] [PubMed]
- Stalla, F.M.; Astegiano, M.; Ribaldone, D.G.; Saracco, G.M.; Pellicano, R. The small intestine: Barrier, permeability and microbiota. Minerva Gastroenterol. 2022, 68, 98–110. [Google Scholar] [CrossRef]
- Lu, Y.; Yuan, X.; Wang, M.; He, Z.; Li, H.; Wang, J.; Li, Q. Gut microbiota influence immunotherapy responses: Mechanisms and therapeutic strategies. J. Hematol. Oncol. 2022, 15, 47. [Google Scholar] [CrossRef]
- Rivera, C.A.; Lennon-Dumenil, A.M. Gut immune cells and intestinal niche imprinting. Semin. Cell Dev. Biol. 2023, 150–151, 50–57. [Google Scholar] [CrossRef]
- Cui, Y.; Zhang, L.; Liu, Y.; Liu, W.; Shi, W.; Bao, Y. Compound small peptide of Chinese medicine alleviates cyclophosphamide induced immunosuppression in mice by Th17/Treg and jejunum intestinal flora. Front. Microbiol. 2023, 14, 1039287. [Google Scholar] [CrossRef]
- Zhou, R.; He, D.; Xie, J.; Zhou, Q.; Zeng, H.; Li, H.; Huang, L. The Synergistic Effects of Polysaccharides and Ginsenosides from American Ginseng (Panax quinquefolius L.) Ameliorating Cyclophosphamide-Induced Intestinal Immune Disorders and Gut Barrier Dysfunctions Based on Microbiome-Metabolomics Analysis. Front. Immunol. 2021, 12, 665901. [Google Scholar] [CrossRef] [PubMed]
- Auyeung, K.K.; Han, Q.B.; Ko, J.K. Astragalus membranaceus: A Review of its Protection Against Inflammation and Gastrointestinal Cancers. Am. J. Chin. Med. 2016, 44, 1–22. [Google Scholar] [CrossRef] [PubMed]
- Li, C.X.; Liu, Y.; Zhang, Y.Z.; Li, J.C.; Lai, J. Astragalus polysaccharide: A review of its immunomodulatory effect. Arch. Pharm. Res. 2022, 45, 367–389. [Google Scholar] [CrossRef]
- Gao, L.; Li, H.; Li, B.; Shao, H.; Yu, X.; Miao, Z.; Zhang, L.; Zhu, L.; Sheng, H. Traditional uses, phytochemistry, transformation of ingredients and pharmacology of the dried seeds of Raphanus sativus L. (Raphani Semen), A comprehensive review. J. Ethnopharmacol. 2022, 294, 115387. [Google Scholar] [CrossRef] [PubMed]
- Li, M.; Gao, J.; Tang, Y.; Liu, M.; Wu, S.; Qu, K.; Long, X.; Li, H.; Liu, M.; Liu, Y.; et al. Traditional Herbal Medicine-Derived Sulforaphene LFS-01 Reverses Colitis in Mice by Selectively Altering the Gut Microbiota and Promoting Intestinal Gamma-Delta T Cells. Front. Pharmacol 2017, 8, 959. [Google Scholar] [CrossRef]
- Zuo, J.; Park, C.; Kung, J.Y.C.; Bou-Chacra, N.A.; Doschak, M.; Löbenberg, R. Traditional Chinese Medicine “Pill”, an Ancient Dosage Form with Surprising Modern Pharmaceutical Characteristics. Pharm. Res. 2021, 38, 199–211. [Google Scholar] [CrossRef]
- Kaur, A.P.; Bhardwaj, S.; Dhanjal, D.S.; Nepovimova, E.; Cruz-Martins, N.; Kuca, K.; Chopra, C.; Singh, R.; Kumar, H.; Sen, F.; et al. Plant Prebiotics and Their Role in the Amelioration of Diseases. Biomolecules 2021, 11, 440. [Google Scholar] [CrossRef]
- Kong, X.; Duan, W.; Li, D.; Tang, X.; Duan, Z. Effects of Polysaccharides from Auricularia auricula on the Immuno-Stimulatory Activity and Gut Microbiota in Immunosuppressed Mice Induced by Cyclophosphamide. Front. Immunol. 2020, 11, 595700. [Google Scholar] [CrossRef]
- Li, L.; Wang, L.; Fan, W.; Jiang, Y.; Zhang, C.; Li, J.; Peng, W.; Wu, C. The Application of Fermentation Technology in Traditional Chinese Medicine: A Review. Am. J. Chin. Med. 2020, 48, 899–921. [Google Scholar] [CrossRef]
- Li, Y.; Lei, Z.; Guo, Y.; Liu, Y.; Guo, X.; Wang, X.; Che, J.; Yuan, J.; Wang, C.; Li, M. Fermentation of Ganoderma lucidum and Raphani Semen with a probiotic mixture attenuates cyclophosphamide-induced immunosuppression through microbiota-dependent or -independent regulation of intestinal mucosal barrier and immune responses. Phytomedicine 2023, 121, 155082. [Google Scholar] [CrossRef]
- Choi, Y.J.; Yang, H.S.; Jo, J.H.; Lee, S.C.; Park, T.Y.; Choi, B.S.; Seo, K.S.; Huh, C.K. Anti-Amnesic Effect of Fermented Ganoderma lucidum Water Extracts by Lactic Acid Bacteria on Scopolamine-Induced Memory Impairment in Rats. Prev. Nutr. Food Sci. 2015, 20, 126–132. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Liu, H.; Qi, H.; Tang, W.; Zhang, C.; Liu, Z.; Liu, Y.; Wei, X.; Kong, Z.; Jia, S.; et al. Probiotic fermentation of Ganoderma lucidum fruiting body extracts promoted its immunostimulatory activity in mice with dexamethasone-induced immunosuppression. Biomed. Pharmacother. 2021, 141, 111909. [Google Scholar] [CrossRef] [PubMed]
- Li, M.; Liang, P.; Li, Z.; Wang, Y.; Zhang, G.; Gao, H.; Wen, S.; Tang, L. Fecal microbiota transplantation and bacterial consortium transplantation have comparable effects on the re-establishment of mucosal barrier function in mice with intestinal dysbiosis. Front. Microbiol. 2015, 6, 692. [Google Scholar] [CrossRef]
- Zhang, W.; Xu, J.H.; Yu, T.; Chen, Q.K. Effects of berberine and metformin on intestinal inflammation and gut microbiome composition in db/db mice. Biomed. Pharmacother. 2019, 118, 109131. [Google Scholar] [CrossRef]
- Musa, I.; Wang, Z.Z.; Yang, N.; Li, X.M. Formononetin inhibits IgE by huPlasma/PBMCs and mast cells/basophil activation via JAK/STAT/PI3-Akt pathways. Front. Immunol. 2024, 15, 1427563. [Google Scholar] [CrossRef] [PubMed]
- Malone, M.K.; Ujas, T.A.; Cotter, K.M.; Britsch, D.R.S.; Lutshumba, J.; Turchan-Cholewo, J.; Stowe, A.M. FACS to Identify Immune Subsets in Mouse Brain and Spleen. Methods Mol. Biol. 2023, 2616, 213–229. [Google Scholar]
- Zhao, M.; Xie, X.; Xu, B.; Chen, Y.; Cai, Y.; Chen, K.; Guan, X.; Ni, C.; Luo, X.; Zhou, L. Paeonol alleviates ulcerative colitis in mice by increasing short-chain fatty acids derived from Clostridium butyricum. Phytomedicine 2023, 120, 155056. [Google Scholar] [CrossRef]
- Xiao, Y.; Zhai, Q.; Zhang, H.; Chen, W.; Hill, C. Gut colonization mechanisms of Lactobacillus and Bifidobacterium: An argument for personalized designs. Annu. Rev. Food Sci. Technol. 2021, 12, 213–233. [Google Scholar] [CrossRef]
- Hiraku, A.; Nakata, S.; Murata, M.; Xu, C.; Mutoh, N.; Arai, S.; Odamaki, T.; Iwabuchi, N.; Tanaka, M.; Tsuno, T.; et al. Early probiotic supplementation of healthy term infants with Bifidobacterium longum subsp. infantis M-63 is safe andleads to the development of Bifidobacterium-predominant gut microbiota: A doubleblind, placebo-controlled trial. Nutrients 2023, 15, 1402. [Google Scholar] [CrossRef]
- Su, Y.; Cui, Z.Y.; Yang, X.Y.; Jiang, Y.; Zhang, W.; Zhang, Y.; Man, C. Lactobacillus paracasei JY062 and its exopolysaccharide enhance the intestinal barrier through macrophage polarization and Th17/Treg cell balance. Food Res. Int. 2024, 197 Pt 1, 115235. [Google Scholar] [CrossRef]
- Kaur, H.; Gupta, T.; Kapila, S.; Kapila, R. Lactobacillus fermentum (MTCC-5898) based fermented whey renders prophylactic action against colitis by strengthening the gut barrier function and maintaining immune homeostasis. Microb. Pathog. 2022, 173 Pt B, 105887. [Google Scholar] [CrossRef]
- Lin, W.S.; Hwang, S.E.; Koh, Y.C.; Ho, P.Y.; Pan, M.H. Modulatory Effects of Lactobacillus paracasei-Fermented Turmeric on Metabolic Dysregulation and Gut Microbiota in High-Fat Diet-Induced Obesity in Mice. J. Agric. Food Chem. 2024, 72, 17924–17937. [Google Scholar] [CrossRef] [PubMed]
- Yan, X.T.; Zhang, W.; Zhang, Y.; Zhang, Z.; Chen, D.; Wang, W.; Ma, W.; Qu, H.; Qian, J.Y.; Gu, R. In vitro anti-obesity effect of shenheling extract (SHLE) fermented with lactobacillus fermentum grx08. Foods 2022, 11, 1221. [Google Scholar] [CrossRef]
- Xu, C.; Ji, G.E. Bioconversion of flavones during fermentation in milk containing Scutellaria baicalensis extract by Lactobacillus brevis. J. Microbiol. Biotechnol. 2013, 23, 1422–1427. [Google Scholar] [CrossRef] [PubMed]
- Aliya, S.; Alhammadi, M.; Park, U.; Tiwari, J.N.; Lee, J.H.; Han, Y.K.; Huh, Y.S. The potential role of formononetin in cancer treatment: An updated review. Biomed. Pharmacother. 2023, 168, 115811. [Google Scholar] [CrossRef]
- Chen, L.; Zhou, Y.; Weng, Z.; Liu, S.; Li, T.; Wang, Y.; Yang, Y.; Liu, H.; Huang, W. Anti-cancer targets and molecular mechanisms of formononetin in treating osteosarcoma based on network pharmacology. Aging 2023, 15, 11489–11507. [Google Scholar] [CrossRef] [PubMed]
- Han, N.R.; Park, H.J.; Ko, S.G.; Moon, P.D. The Protective Effect of a Functional Food Consisting of Astragalus membranaceus, Trichosanthes kirilowii, and Angelica gigas or Its Active Component Formononetin against Inflammatory Skin Disorders through Suppression of TSLP via MDM2/HIF1α Signaling Pathways. Foods 2023, 12, 276. [Google Scholar] [CrossRef]
- Gu, J.; Sun, R.; Wang, Q.; Liu, F.; Tang, D.; Chang, X. Standardized Astragalus mongholicus Bunge-Curcuma aromatica Salisb. Extract Efficiently Suppresses Colon Cancer Progression Through Gut Microbiota Modification in CT26-Bearing Mice. Front. Pharmacol. 2021, 12, 714322. [Google Scholar] [CrossRef]
- Brummaier, T.; Pohanka, E.; Studnicka-Benke, A.; Pieringer, H. Using cyclophosphamide in inflammatory rheumatic diseases. Eur. J. Intern. Med. 2013, 24, 590–596. [Google Scholar] [CrossRef]
- Hennings, M.; Fremouw, T. The effect of doxorubicin or cyclophosphamide treatment on auditory brainstem response in mice. Exp. Brain. Res. 2022, 240, 2907–2921. [Google Scholar] [CrossRef]
- Chen, X.; Sun, W.; Xu, B.; Wu, E.; Cui, Y.; Hao, K.; Zhang, G.; Zhou, C.; Xu, Y.; Li, J.; et al. Polysaccharides from the Roots of Millettia speciosa Champ Modulate Gut Health and Ameliorate Cyclophosphamide-Induced Intestinal Injury and Immuno-suppression. Front. Immunol. 2021, 12, 766296. [Google Scholar]
- Zheng, S.; Zheng, H.; Zhang, R.; Piao, X.; Hu, J.; Zhu, Y.; Wang, Y. Immunomodulatory Effect of Ginsenoside Rb2 Against Cyclophosphamide-Induced Immunosuppression in Mice. Front. Pharmacol. 2022, 13, 927087. [Google Scholar] [CrossRef]
- Chen, S.; Chen, Z.; Wang, Y.; Hao, W.; Yuan, Q.; Zhou, H.; Gao, C.; Wang, Y.; Wu, X.; Wang, S. Targeted delivery of Chinese herb pair-based berberine/tannin acid self-assemblies for the treatment of ulcerative colitis. J. Adv. Res. 2022, 40, 263–276. [Google Scholar] [CrossRef]
- Chao, L.; Lin, J.; Zhou, J.; Du, H.; Chen, X.; Liu, M.; Qu, Q.; Lv, W.; Guo, S. Polyphenol Rich Forsythia suspensa Extract Alleviates DSS-Induced Ulcerative Colitis in Mice through the Nrf2-NLRP3 Pathway. Antioxidants 2022, 11, 475. [Google Scholar] [CrossRef] [PubMed]
- Wang, R.; Lin, F.; Ye, C.; Aihemaitijiang, S.; Halimulati, M.; Huang, X.; Jiang, Z.; Li, L.; Zhang, Z. Multi-omics analysis reveals therapeutic effects of Bacillus subtilis-fermented Astragalus membranaceus in hyperuricemia via modulation of gut microbiota. Food Chem. 2023, 399, 133993. [Google Scholar] [CrossRef]
- Hanus, M.; Parada-Venegas, D.; Landskron, G.; Wielandt, A.M.; Hurtado, C.; Alvarez, K.; Hermoso, M.A.; López-Köstner, F.; De la Fuente, M. Immune System, Microbiota, and Microbial Metabolites: The Unresolved Triad in Colorectal Cancer Microenvironment. Front. Immunol. 2021, 12, 612826. [Google Scholar] [CrossRef] [PubMed]
- Xie, Q.; Li, H.; Ma, R.; Ren, M.; Li, Y.; Li, J.; Chen, H.; Chen, Z.; Gong, D.; Wang, J. Effect of Coptis chinensis franch and Magnolia officinalis on intestinal flora and intestinal barrier in a TNBS-induced ulcerative colitis rats model. Phytomed. Int. J. Phytother. Phytopharm. 2022, 97, 153927. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Ma, Y.; Li, X.; Wang, Y.; Huo, Z.; Lin, Y.; Li, J.; Yang, H.; Zhang, Z.; Yang, P.; et al. Fermented Astragalus and its metabolites regulate inflammatory status and gut microbiota to repair intestinal barrier damage in dextran sulfate sodium-induced ulcerative colitis. Front. Nutr. 2022, 9, 1035912. [Google Scholar] [CrossRef]
- Parker, B.J.; Wearsch, P.A.; Veloo, A.C.M.; Rodriguez-Palacios, A. The genus Alistipes: Gut bacteria with emerging implications to inflammation, cancer, and mental health. Front. Immunol. 2020, 11, 906. [Google Scholar] [CrossRef]
- Zhou, X.; Pak, S.; Li, D.; Dong, L.; Chen, F.; Hu, X.; Ma, L. Bamboo Shoots Modulate Gut Microbiota, Eliminate Obesity in High-Fat-Diet-Fed Mice and Improve Lipid Metabolism. Foods 2023, 12, 1380. [Google Scholar] [CrossRef]
- Qiu, L.; Yan, C.; Yang, Y.; Liu, K.; Yin, Y.; Zhang, Y.; Lei, Y.; Jia, X.; Li, G. Morin alleviates DSS-induced ulcerative colitis in mice via inhibition of inflammation and modulation of intestinal microbiota. Int. Immunopharmacol. 2024, 140, 112846. [Google Scholar] [CrossRef] [PubMed]
- Qu, Z.; Liu, H.; Yang, J.; Zheng, L.; Huang, J.; Wang, Z.; Xie, C.; Zuo, W.; Xia, X.; Sun, L.; et al. Selective utilization of medicinal polysaccharides by human gut Bacteroides and Parabacteroides species. Nat. Commun. 2025, 16, 638. [Google Scholar] [CrossRef]
- Li, X.; Sun, B.; Qin, Y.; Yue, F.; Lü, X. Amelioration of Obesity-Related Disorders in High-Fat Diet-Fed C57BL/6 Mice Following Fecal Microbiota Transplantation from DL-Norvaline-Dosed Mice. Mol. Nutr. Food Res. 2025, e202400577. [Google Scholar] [CrossRef]
- Hao, Y.; Ji, Z.; Shen, Z.; Xue, Y.; Zhang, B.; Yu, D.; Liu, T.; Luo, D.; Xing, G.; Tang, J.; et al. Increase dietary fiber intake ameliorates cecal morphology and drives cecal species-specific of short-chain fatty acids in white pekin ducks. Front. Microbiol. 2022, 13, 853797. [Google Scholar] [CrossRef]
- Zhao, H.; Gao, X.; Liu, Z.; Zhang, L.; Fang, X.; Sun, J.; Zhang, Z.; Sun, Y. Sodium Alginate Prevents Non-Alcoholic Fatty Liver Disease by Modulating the Gut-Liver Axis in High-Fat Diet-Fed Rats. Nutrients 2022, 14, 4846. [Google Scholar] [CrossRef] [PubMed]
- Leth, M.L.; Pichler, M.J.; Abou Hachem, M. Butyrate-producing colonic clostridia: Picky glycan utilization specialists. Essays Biochem. 2023, 67, 415–428. [Google Scholar] [PubMed]
- Xu, M.; Xiao, H.; Zou, X.; Pan, L.; Song, Q.; Hou, L.; Zeng, Y.; Han, Y.; Zhou, Z. Mechanisms of levan in ameliorating hyperuricemia: Insight into levan on serum metabolites, gut microbiota, and function in hyperuricemia rats. Carbohydr. Polym. 2025, 347, 122665. [Google Scholar] [CrossRef]
- Ding, M.; Bao, Y.; Liang, H.; Zhang, X.; Li, B.; Yang, R.; Zeng, N. Potential mechanisms of formononetin against inflammation and oxidative stress: A review. Front. Pharmacol. 2024, 15, 1368765. [Google Scholar] [CrossRef]
- Giacoppo, S.; Galuppo, M.; Iori, R.; De Nicola, G.R.; Bramanti, P.; Mazzon, E. The protective effects of bioactive (RS)-glucoraphanin on the permeability of the mice blood-brain barrier following experimental autoimmune encephalomyelitis. Eur. Rev. Med. Pharmacol. Sci. 2014, 18, 194–204. [Google Scholar]
- Holcomb, L.; Holman, J.M.; Hurd, M.; Lavoie, B.; Colucci, L.; Hunt, B.; Hunt, T.; Kinney, M.; Pathak, J.; Mawe, G.M.; et al. Early life exposure to broccoli sprouts confers stronger protection against enterocolitis development in an immunological mouse model of inflammatory bowel disease. Biorxiv. Prepr. Serv. Biol. 2023, 27, 525953. [Google Scholar]
- Shapiro-Shelef, M.; Calame, K. Regulation of plasma-cell development. Nat. Rev. Immunol. 2005, 5, 230–242. [Google Scholar] [CrossRef]
- Roche, P.A.; Furuta, K. The ins and outs of MHC class II-mediated antigen processing and presentation. Nat. Rev. Immunol. 2015, 15, 203–216. [Google Scholar] [CrossRef] [PubMed]
- Kim, M.; Qie, Y.; Park, J.; Kim, C.H. Gut microbial metabolites fuel host antibody responses. Cell Host Microbe 2016, 20, 202–214. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Miao, Q.; Pan, C.; Yin, J.; Wang, L.; Qu, L.; Yin, Y.; Wei, Y. Research advances in probiotic fermentation of Chinese herbal medicines. Imeta 2023, 2, e93. [Google Scholar] [CrossRef] [PubMed]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, Y.; Wei, X.; Rui, B.; Du, Y.; Lei, Z.; Guo, X.; Wang, C.; Yuan, D.; Wang, X.; Li, M.; et al. Probiotic Fermentation of Astragalus membranaceus and Raphani Semen Ameliorates Cyclophosphamide-Induced Immunosuppression Through Intestinal Short-Chain Fatty Acid-Dependent or -Independent Regulation of B Cell Function. Biology 2025, 14, 312. https://doi.org/10.3390/biology14030312
Chen Y, Wei X, Rui B, Du Y, Lei Z, Guo X, Wang C, Yuan D, Wang X, Li M, et al. Probiotic Fermentation of Astragalus membranaceus and Raphani Semen Ameliorates Cyclophosphamide-Induced Immunosuppression Through Intestinal Short-Chain Fatty Acid-Dependent or -Independent Regulation of B Cell Function. Biology. 2025; 14(3):312. https://doi.org/10.3390/biology14030312
Chicago/Turabian StyleChen, Yang, Xiaoqing Wei, Binqi Rui, Yutong Du, Zengjie Lei, Xiujie Guo, Chaoran Wang, Donglin Yuan, Xiuli Wang, Ming Li, and et al. 2025. "Probiotic Fermentation of Astragalus membranaceus and Raphani Semen Ameliorates Cyclophosphamide-Induced Immunosuppression Through Intestinal Short-Chain Fatty Acid-Dependent or -Independent Regulation of B Cell Function" Biology 14, no. 3: 312. https://doi.org/10.3390/biology14030312
APA StyleChen, Y., Wei, X., Rui, B., Du, Y., Lei, Z., Guo, X., Wang, C., Yuan, D., Wang, X., Li, M., Hou, B., & Liu, Y. (2025). Probiotic Fermentation of Astragalus membranaceus and Raphani Semen Ameliorates Cyclophosphamide-Induced Immunosuppression Through Intestinal Short-Chain Fatty Acid-Dependent or -Independent Regulation of B Cell Function. Biology, 14(3), 312. https://doi.org/10.3390/biology14030312






