Huanglongbing as a Persistent Threat to Citriculture in Latin America
Simple Summary
Abstract
1. Introduction
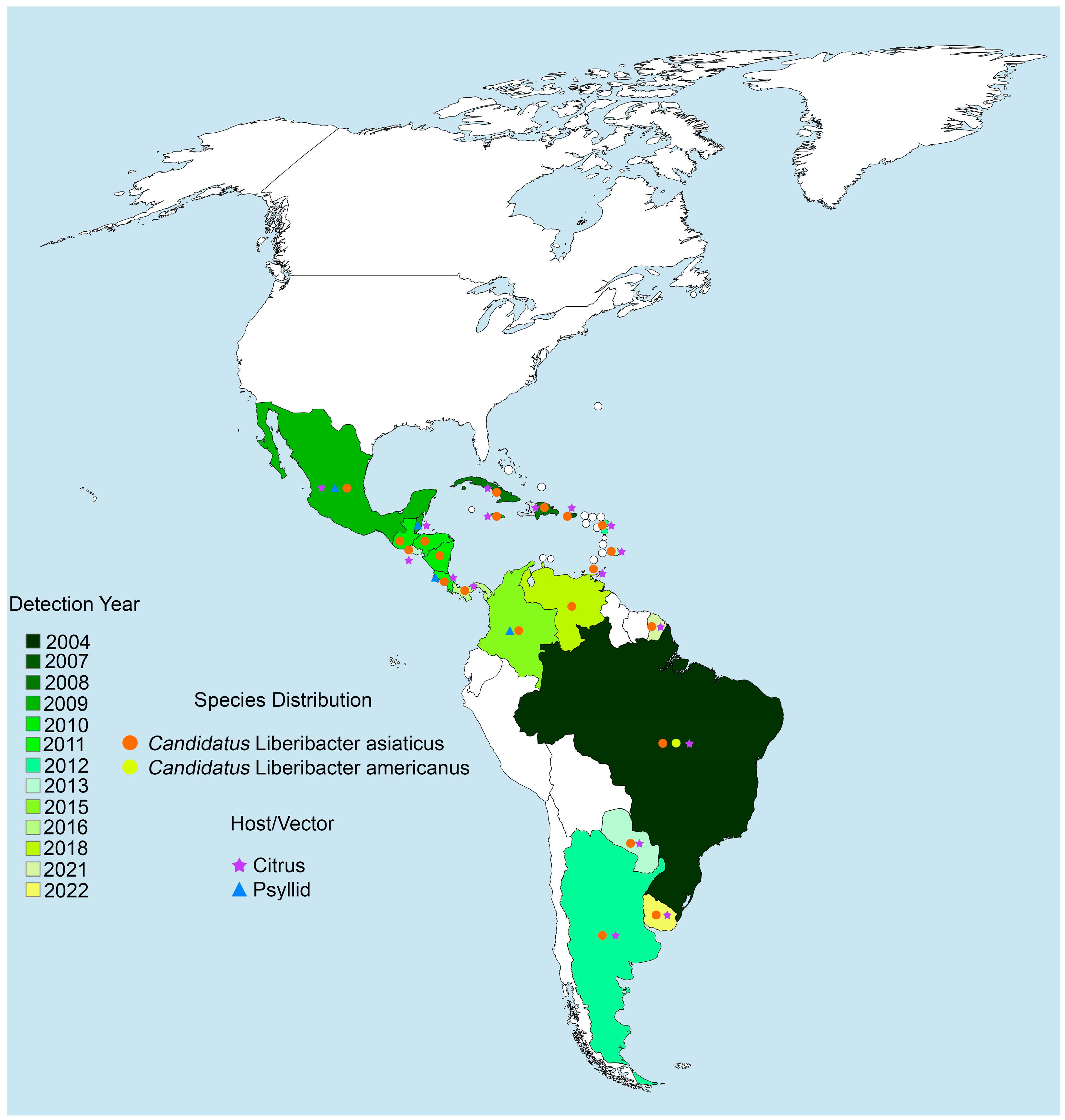
2. Epidemiology
3. Genetic Diversity
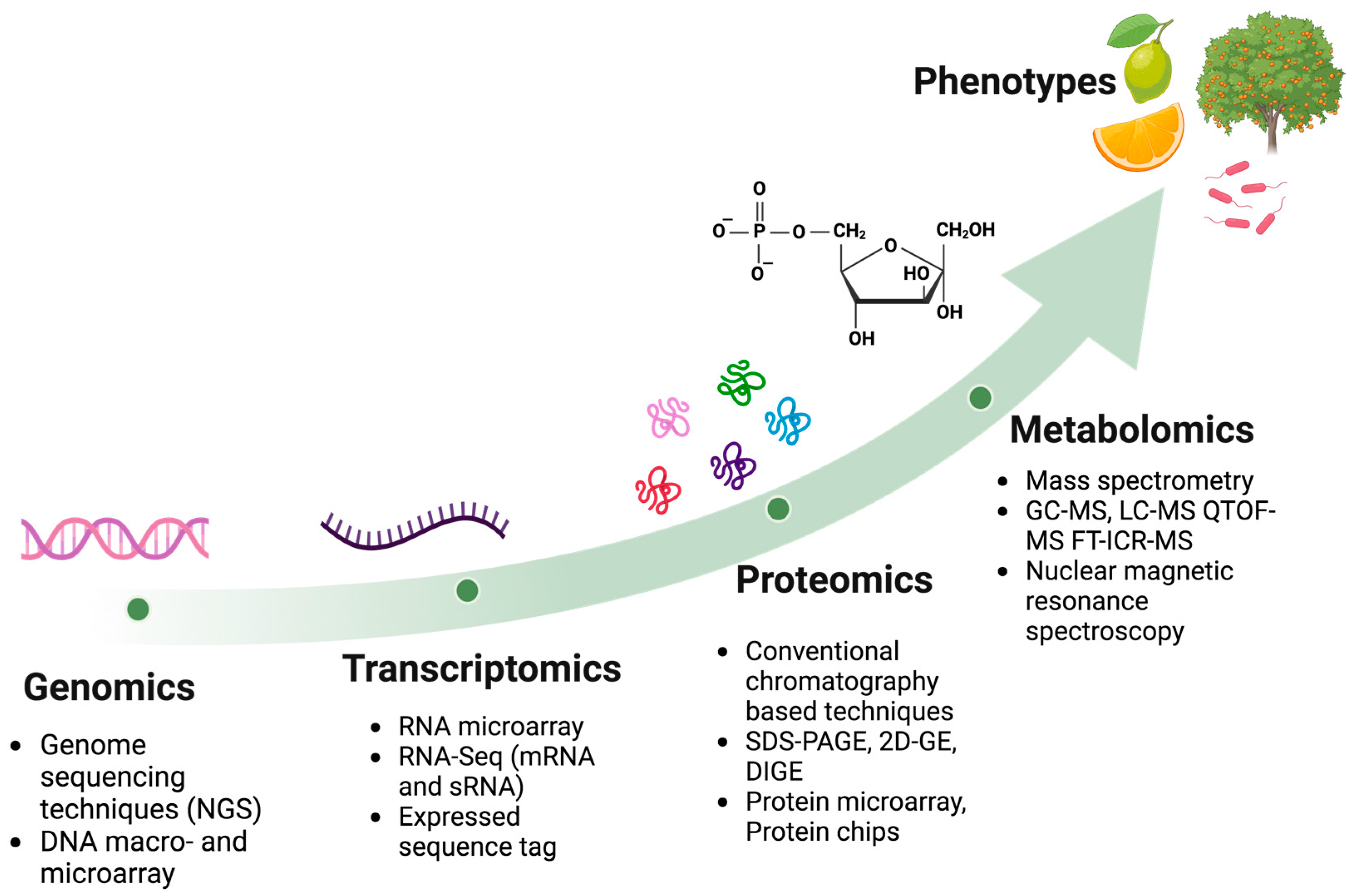
4. Omics Studies to Decipher Citrus–Candidatus Liberibacter Interaction
4.1. Genomics
4.1.1. Citrus Genomics
4.1.2. Candidatus Liberibacter Genomics
4.2. Transcriptomics
4.3. Proteomics
4.4. Metabolomics
5. Diagnostic Methods
6. Disease Management Methods
6.1. Government Policies
6.2. Chemical Management
6.3. Sustainable Management
6.4. Cultural Management
6.5. Emerging Biotechnology Approaches
7. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Food and Agriculture Organization of the United Nations. FAOSTAT Statistical Database. 2023. Available online: https://www.fao.org/faostat/en/#data/QCL/visualize (accessed on 5 March 2025).
- Bové, J.M. Huanglongbing: A Destructive, Newly-Emerging, Century-Old Disease of Citrus. J. Plant Pathol. 2006, 88, 7–37. [Google Scholar]
- USDA FAS. World Agricultural Production. Available online: https://fas.usda.gov/data/production (accessed on 5 March 2025).
- Etxeberria, E.; Gonzalez, P.; Achor, D.; Albrigo, G. Anatomical Distribution of Abnormally High Levels of Starch in HLB Affected Valencia Orange Trees. Physiol. Mol. Plant Pathol. 2009, 74, 76–83. [Google Scholar] [CrossRef]
- Gottwald, T.R. Current Epidemiological Understanding of Citrus Huanglongbing. Annu. Rev. Phytopathol. 2010, 48, 119–139. [Google Scholar] [PubMed]
- Lin, K.H. Yellow Shoot of Citrus. Symptomatology. Investigations in the Cause of Huanglongbing. Natural Transmission and spread. General conclusions. Acta Phytopathol. Sin. 1956, 2, 1–42. [Google Scholar]
- EPPO Global Database. 2023. Available online: https://gd.eppo.int/ (accessed on 15 October 2023).
- de Andrade, E.C.; Girardi, E.A.; Stuchi, E.S.; Moreira, A.S.; Freitas-Astua, J.; Fancelli, M.; Laranjeira, F.F. Citrus Huanglongbing (HLB) and the Brazilian Efforts to Overcome the Disease. Outlooks Pest Manag. 2021, 32, 189–194. [Google Scholar] [CrossRef]
- Servicio de Información Agroalimentaria y Pesquera (SIAP SAGARPA). 2023. Available online: https://www.gob.mx/siap/ (accessed on 4 November 2023).
- Albrecht, U.; Fiehn, O.; Bowman, K.D. Metabolic Variations in Different Citrus Rootstock Cultivars Associated with Different Responses to Huanglongbing. Plant Physiol. Biochem. 2012, 107, 33–44. [Google Scholar] [CrossRef]
- Bassanezi, R.B.; Lopes, S.A.; de Miranda, M.P.; Wulff, N.A.; Volpe, H.X.L.; Ayres, A.J. Overview of Citrus Huanglongbing Spread and Management Strategies in Brazil. Trop. Plant Pathol. 2020, 45, 251–264. [Google Scholar] [CrossRef]
- Lv, Y.; Zhong, Y.; Jiang, B.; Yan, H.; Ren, S.; Cheng, C. microRNA miR171b Positively Regulates Resistance to Huanglongbing of Citrus. Int. J. Mol. Sci. 2023, 24, 5737. [Google Scholar] [CrossRef]
- Li, J.; Gmitter, F.G., Jr.; Zhang, B.; Wang, Y. Uncovering Interactions between Plant Metabolism and Plant-Associated Bacteria in Huanglongbing-Affected Citrus Cultivars Using Multiomics Analysis and Machine Learning. J. Agric. Food Chem. 2023, 71, 16391–16401. [Google Scholar] [CrossRef]
- Li, J.; Wang, Y.Z.; Gmitter Jr, F.G.; Wang, Y. Identifying the Earliest Citrus Responses to Candidatus Liberibacter asiaticus Infection: A Temporal Metabolomics Study. Front. Plant Sci. 2024, 15, 1455344. [Google Scholar] [CrossRef]
- Teixeira, D.C.; Danet, J.L.; Eveillard, S.; Martins, E.C.; Jesus, W.C., Jr.; Yamamoto, P.T.; Lopes, S.A.; Bassanezi, R.B.; Ayres, A.J.; Saillard, C. Citrus Huanglongbing in São Paulo State, Brazil: PCR Detection of the ‘Candidatus’ Liberibacter Species Associated with the Disease. Mol. Cell. Probes 2005, 19, 173–179. [Google Scholar] [CrossRef] [PubMed]
- Phahladira, M.N.B.; Viljoen, R.; Pietersen, G. Widespread Occurrence of “Candidatus Liberibacter africanus subspecies capensis” in Calodendrum capense in South Africa. Eur. J. Plant Pathol. 2012, 134, 39–47. [Google Scholar] [CrossRef][Green Version]
- Roberts, R.; Pietersen, G. A Novel Subspecies of ‘Candidatus Liberibacter africanus’ Found on Native Teclea gerrardii (Family: Rutaceae) from South Africa. Antonie Van Leeuwenhoek 2017, 110, 437–444. [Google Scholar] [CrossRef] [PubMed]
- Roberts, R.; Steenkamp, E.T.; Pietersen, G. Three Novel Lineages of ‘Candidatus Liberibacter africanus’ Associated with Native Rutaceous Hosts of Trioza erytreae in South Africa. Int. J. Syst. Evol. Microbiol. 2015, 65, 723–731. [Google Scholar] [CrossRef]
- Saponari, M.; De Bac, G.; Breithaupt, J.; Loconsele, G.; Yokomi, R.K.; Catalano, L. First Report of “Candidatus Liberibacter asiaticus” Associated with Huanglongbing in Sweet Orange in Ethiopia. Plant Dis. 2010, 94, 482. [Google Scholar] [CrossRef]
- Ajene, I.J.; Khamis, F.M.; van Asch, B.; Pietersen, G.; Seid, N.; Rwomushana, I.; Ombura, F.L.O.; Momanyi, G.; Finyange, P.; Rasowo, B.A.; et al. Distribution of Candidatus Liberibacter Species in Eastern Africa, and the First Report of Candidatus Liberibacter asiaticus in Kenya. Sci. Rep. 2020, 10, 3919. [Google Scholar] [CrossRef]
- Teixeira, D.C.; Wulff, N.A.; Martins, E.C.; Kitajima, E.W.; Bassanezi, R.; Ayres, A.J.; Eveillard, S.; Saillard, C.; Bové, J.M. A Phytoplasma Closely Related to the Pigeon Pea Witches’-Broom Phytoplasma (16Sr IX) Is Associated with Citrus Huanglongbing Symptoms in the State of São Paulo, Brazil. Phytopathology 2008, 98, 977–984. [Google Scholar] [CrossRef]
- Chen, J.; Pu, X.; Deng, X.; Liu, H.; Civerolo, E. A Phytoplasma Related to ‘Candidatus Phytoplasma asteris’ Detected in Citrus Showing Huanglongbing (Yellow Shoot Disease) Symptoms in Guangdong, P.R. China. Phytopathology 2009, 99, 236–242. [Google Scholar] [CrossRef] [PubMed]
- Arratia-Castro, A.A.; Santos-Cervantes, M.E.; Fernández-Herrera, E.; Chávez-Medina, J.A.; Flores-Zamora, G.L.; Camacho-Beltrán, E.; Méndez-Lozano, J.; Leyva-López, N.E. Occurrence of ‘Candidatus Phytoplasma asteris’ in Citrus Showing Huanglongbing Symptoms in Mexico. Crop Protection 2014, 62, 144–151. [Google Scholar] [CrossRef]
- Lou, B.; Bai, X.; Bai, Y.; Deng, C.; RoyChowdhury, M.; Chen, C.; Song, Y. Detection and Molecular Characterization of a 16SrII-A* Phytoplasma in Grapefruit (Citrus paradisi) with Huanglongbing-like Symptoms in China. J. Phytopathol. 2014, 162, 387–395. [Google Scholar] [CrossRef]
- Wulff, N.A.; Fassini, C.G.; Marques, V.V.; Martins, E.C.; Coletti, D.A.B.; Teixeira, D.C.; Sanches, M.M.; Bové, J.M. Molecular Characterization and Detection of 16SrIII Group Phytoplasma Associated with Huanglongbing Symptoms. Phytopathology 2019, 109, 366–374. [Google Scholar] [PubMed]
- Quiroga, N.; Gamboa, C.; Medina, G.; Contaldo, N.; Torres, F.; Bertaccini, A.; Zamorano, A.; Fiore, N. Survey for ‘Candidatus Liberibacter’ and ‘Candidatus Phytoplasma’ in Citrus in Chile. Pathogens 2021, 11, 48. [Google Scholar] [CrossRef]
- Duan, D.P.; Gottwald, T.; Zhou, L.J.; Gabriel, D.W. First Report of Dodder Transmisión of ‘Candidatus Liberibacter asiaticus’ to Tomato (Lycopersicon esculentum). Plant Dis. 2008, 92, 831. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Lopes, S.A.; Massari, C.A.; Barbosa, J.C.; Ayres, A.J. Huanglongbing in the State of São Paulo–Brazil: Current Situation, Regulation, Management and Economic Impact. In Abstracts International Workshop on Citrus Quarantine Pests Villahermosa, Tabasco, México. 2009; Available online: https://www.calcitrusquality.org/wp-content/uploads/2009/05/huanglongbing-in-the-state-of-sp-word-2007.pdf (accessed on 17 March 2025).
- SENASA. Servicio Nacional de Sanidad y Calidad Agroalimentaria. “Resolución SENASA N° 0930/2009”. 2009. Available online: https://digesto.senasa.gob.ar/items/sho (accessed on 17 March 2025).
- Ammar, E.D.; Shatters, R.G.; Hall, D.G. Localization of Candidatus Liberibacter asiaticus, Associated with Citrus Huanglongbing Disease, in Its Psyllid Vector using Fluorescence in situ Hybridization. J. Phytopathol. 2011, 159, 726–734. [Google Scholar]
- Xu, C.F.; Xia, Y.H.; Li, K.B.; Ke, C. Study on the Law of Transmission of Citrus Huanglongbing by Psyllid, Diaphorina citri and the Distribution of Pathogen in the Adult. J. Agric. Sci. 1988, 3, 57–61. [Google Scholar]
- Inoue, H.; Ohnishi, J.; Ito, T.; Tomimura, K.; Miyata, S.; Iwanami, T.; Ashihara, W. Enhanced Proliferation and Efficient Transmission of Candidatus Liberibacter asiaticus by Adult Diaphorina citri After Acquisition Feeding in the Nymphal Stage. Ann. Appl. Biol. 2009, 155, 29–36. [Google Scholar]
- Martini, X.; Coy, M.; Kuhns, E.; Stelinski, L.L. Temporal Decline in Pathogen-Mediated Release of Methyl Salicylate Associated with Decreasing Vector Preference for Infected Over Uninfected Plants. Front. Ecol Evol. 2018, 6, 185. [Google Scholar]
- Capoor, S.; Rao, D.; Viswanath, S. Greening Disease of Citrus in the Deccan Trap Country and Its Relationship with the Vector, Diaphorina citri Kuwayama. Int. Organ. Citrus Virol. Conf. Proc. 1974, 6, 43–49. [Google Scholar]
- Pelz-Stelinski, K.S.; Brlansky, R.H.; Ebert, T.A.; Rogers, M.E. Transmission Parameters for Candidatus Liberibacter asiaticus by Asian Citrus Psyllid (Hemiptera: Psyllidae). J. Econ. Entomol. 2010, 103, 1531–1541. [Google Scholar] [CrossRef]
- Hall, D.G.; Hentz, M.G. Seasonal Flight Activity by the Asian Citrus Psyllid in East Central Florida. Entomol. Exp. Appl. 2011, 139, 75–85. [Google Scholar]
- Ammar, E.D.; Hall, D.G.; Shatters, R.G. Stylet Morphometrics and Citrus Leaf Vein Structure in Relation to Feeding Behavior of the Asian Citrus Psyllid Diaphorina citri, Vector of Citrus Huanglongbing Bacterium. PLoS ONE 2013, 8, e59914. [Google Scholar] [CrossRef] [PubMed]
- Folimonova, S.Y.; Achor, D.S. Early Events of Citrus Greening (Huanglongbing) Disease Development at the Ultrastructural Level. Phytopathology 2010, 100, 949–958. [Google Scholar] [PubMed]
- Canale, M.C.; Tomaseto, A.F.; Haddad, M.L.; Coletta-Filho, H.D.; Lopes, J.R.S. Latency and Persistence of ‘Candidatus Liberibacter asiaticus’ in Its Psyllid Vector, Diaphorina citri (Hemiptera: Liviidae). Phytopathology 2017, 107, 264–272. [Google Scholar] [CrossRef] [PubMed]
- Coletta-Filho, H.D.; Targon, M.L.P.N.; Takita, M.A.; De Negri, J.D.; Pompeu, J., Jr.; Machado, M.A. First Report of the Causal Agent of Huanglongbing (“Candidatus Liberibacter asiaticus”) in Brazil. Plant Dis. 2004, 88, 1382. [Google Scholar] [CrossRef] [PubMed]
- Trujillo-Arriaga, J. Situación Actual, Regulación y Manejo del HLB en México. 2 Taller Internacional Sobre el Huanglongbing y el Psílido Asiático de los Cítricos 2010; Servicio Nacional de Sanidad, Inocuidad y Calidad Agroalimentaria y Organización Norteamericana de Protección de Plantas: Mérida, Yucatán, Mexico, 2010.
- SENASICA. Servicio Nacional de Sanidad, Inocuidad y Calidad Agroalimentaria. 2022. Available online: https://www.gob.mx/senasica (accessed on 5 October 2023).
- Mora-Aguilera, G.; Robles-García, P.; Lopez-Arroyo, J.I.; Flores-Sánchez, J.; Acevedo-Sánches, G.; Domínguez-Monge, S.; Gutierrez-Espinosa, A.; Loeza-Kuk, E. Situación Actual y Perspectivas del Manejo del HLB de los Cítricos. Rev. Mex. Fitopatol. 2014, 32, 108–119. [Google Scholar]
- SADER. Secretaría de Agricultura y Desarrollo Rural. Planeación Agrícola Citrícola Nacional; 2019. Available online: www.gob.mx/cms/uploads/attachment/file/257073/Potencial-C_tricos (accessed on 10 October 2023).
- SIAP-SAGARPA. Servicio de Información Agroalimentaria y Pesquera-Secretaría de Agricultura, Ganadería, Desarrollo Rural, Pesca y Alimentación. 2014. Available online: https://www.gob.mx/siap/acciones-y-programas/produccion-agricola-33119 (accessed on 15 October 2023).
- SENASICA. Servicio Nacional de Sanidad, Inocuidad y Calidad Agroalimentaria. 2015. Available online: https://www.gob.mx/senasica (accessed on 5 October 2023).
- López-Collado, J. Huanglongbing HLB en México; Colegio de Posgraduados, Campus Veracruz: Veracruz, Mexico, 2013. [Google Scholar]
- Rodríguez-Aguilar, O.; López-Collado, J.; Soto-Estrada, A.; Vargas-Mendoza, M.C.; García-Avila, C.J. Future Spatial Distribution of Diaphorina citri in Mexico under Climate Change Models. Ecol. Complex. 2023, 53, 101041. [Google Scholar]
- Pérez-Valencia, L.I.; Michel, A.P.; Moya-Raygoza, G.; Rodriguez, A. Genetic Variation and Structure of Diaphorina citri (Hemiptera: Liviidae) in Populations from México. Ann. Entomol. Soc. Am. 2019, 112, 379–387. [Google Scholar] [CrossRef]
- Alquézar, B.; Carmona, L.; Bennici, S.; Miranda, M.P.; Bassanezi, R.B.; Peña, L. Cultural Management of Huanglongbing: Current Status and Ongoing Research. Phytopathology 2022, 112, 11–25. [Google Scholar] [CrossRef]
- Fundo de Defesa da Citricultura. Alerta Fitossanitário. Available online: https://www.fundecitrus.com.br/alerta-fitossanitario (accessed on 5 October 2023).
- Wulff, N.A.; Daniel, B.; Sassi, R.S.; Moreira, A.S.; Bassanezi, R.B.; Sala, I.; Coletti, D.A.B.; Rodrigues, J.C. Incidence of Diaphorina citri Carrying Candidatus Liberibacter asiaticus in Brazil’s Citrus Belt. Insects 2020, 11, 672. [Google Scholar] [CrossRef]
- Suaste-Dzul, A.; Gallou, A.; Félix-Portillo, M.; Moreno-Carrillo, G.; Sánchez-González, J.; Palomares-Pérez, M.; Arredondo-Bernal, H. Seasonal Incidence of ‘Candidatus Liberibacter asiaticus’ (Rhizobiales: Rhizobiaceae) in Diaphorina citri (Hemiptera: Liviidae) in Colima, Mexico. Trop. Plant Pathol. 2017, 42, 410–415. [Google Scholar] [CrossRef]
- Hall, D.G. Incidence of “Candidatus Liberibacter asiaticus” in a Florida Population of Asian Citrus Psyllid. J. Appl. Entomol. 2018, 142, 97–103. [Google Scholar] [CrossRef]
- Lopes, S.A.; Luiz, F.Q.B.F.; Martins, E.C.; Fassini, C.G.; Sousa, M.C.; Barbosa, J.C.; Beattie, G.A.C. ‘Candidatus Liberibacter asiaticus’ Titers in Citrus and Acquisition Rates by Diaphorina citri are Decreased by Higher Temperature. Plant Dis. 2013, 97, 1563–1570. [Google Scholar] [CrossRef] [PubMed]
- Lopes, S.A.; Luiz, F.Q.B.F.; Oliveira, H.T.; Cifuentes-Arenas, J.C.; Raiol-Junior, L.L. Seasonal Variation of ‘Candidatus Liberibacter asiaticus’ Titers in New Shoots of Citrus in Distinct Environments. Plant Dis. 2017, 101, 583–590. [Google Scholar] [CrossRef] [PubMed]
- Agostini, J.P.; Preussler, C.; Outi, Y. Huanglongbing (HLB) Ex Greening. In Plagas Cuarentenarias de Frutales de la República Argentina Avances en los Resultados; INTA: Buenos Aires, Argentina, 2015; Volume 6, pp. 183–198. [Google Scholar]
- Badaracco, A.; Redes, F.J.; Preussler, C.A.; Agostini, J.P. Citrus Huanglongbing in Argentina: Detection and Phylogenetic Studies of ‘Candidatus Liberibacter asiaticus’. Australas. Plant Pathol. 2017, 46, 171–175. [Google Scholar] [CrossRef]
- Badaracco, A.; Redes, F.J.; Bustamente, K.M.; Bloch, N.; Schapovaloff, M.E.; Agostini, J.P. First Detection of Positive ‘Candidatus Liberibacter asiaticus’ Diaphorina citri in Argentina. Australas. Plant Pathol. 2022, 51, 9–12. [Google Scholar] [CrossRef]
- de Paula, L.B.; Lin, H.; Stuchi, E.S.; Francisco, C.S.; Safady, N.G.; Coletta-Filho, H.D. Genetic Diversity of ‘Candidatus Liberibacter asiaticus’ in Brazil Analyzed in Different Geographic Regions and Citrus Varieties. Eur. J. Plant Pathol. 2019, 154, 863–872. [Google Scholar] [CrossRef]
- Singh, Y.H.; Sharma, S.K.; Sinha, B.; Baranwal, V.K.; Singh, N.B.; Chanu, N.T.; Roy, S.S.; Ansari, M.A.; Ningombam, A.; Devi, P.S.; et al. Genetic Variability Based on Tandem Repeat Numbers in a Genomic Locus of ‘Candidatus Liberibacter asiaticus’ Prevalent in North East India. Plant Pathol. J. 2019, 35, 644–653. [Google Scholar] [CrossRef]
- Jagoueix, S.; Bove, J.M.; Garnier, M. Comparison of the 16S/23S Ribosomal Intergenic Regions of “Candidatus Liberobacter asiaticum” and “Candidatus Liberobacter africanum”, the Two Species Associated with Citrus Huanglongbing (Greening) Disease. Int. J. Syst. Evol. Microbiol. 1997, 47, 224–227. [Google Scholar] [CrossRef]
- Hocquellet, A.; Toorawa, P.; Bove, J.M.; Garnier, M. Detection and Identification of the Two Candidatus Liberobacter Species Associated with Citrus Huanglongbing by PCR Amplification of Ribosomal Protein Genes of theβ Operon. Mol. Cell. Probes 1999, 13, 373–379. [Google Scholar] [CrossRef]
- Bastianel, C.; Garnier-Semancik, M.; Renaudin, J.; Bové, J.M.; Eveillard, S. Diversity of “Candidatus Liberibacter asiaticus”, Based on the omp Gene Sequence. Appl. Environ. Microbiol. 2005, 71, 6473–6478. [Google Scholar]
- Deng, X.; Chen, J.; Feng, Z.; Shan, Z.; Guo, H.; Zhu, J.; Civerolo, E.Á. Identification and Characterization of the Huanglongbing Bacterium in Pummelo from Multiple Locations in Guangdong, P.R. China. Plant Dis. 2008, 92, 513–518. [Google Scholar]
- Duan, Y.; Zhou, L.; Hall, D.G.; Li, W.; Doddapaneni, H.; Lin, H.; Liu, L.; Vahling, C.M.; Gabriel, D.W.; Williams, K.P.; et al. Complete Genome Sequence of Citrus Huanglongbing Bacterium, ‘Candidatus Liberibacter asiaticus’ Obtained Through Metagenomics. Mol. Plant Microbe Interact. 2009, 22, 1011–1020. [Google Scholar] [PubMed]
- Zheng, Z.; Deng, X.; Chen, J. Whole-Genome Sequence of “Candidatus Liberibacter asiaticus” from Guangdong, China. Genome Announc. 2014, 2, e00273-14. [Google Scholar]
- Chen, J.; Deng, X.; Sun, X.; Jones, D.; Irey, M.; Civerolo, E. Guangdong and Florida Populations of ‘Candidatus Liberibacter asiaticus’ Distinguished by a Genomic Locus with Short Tandem Repeats. Phytopathology 2010, 100, 567–572. [Google Scholar] [PubMed]
- Islam, M.S.; Glynn, J.M.; Bai, Y.; Duan, Y.P.; Coletta-Filho, H.D.; Kuruba, G.; Civerolo, E.L.; Lin, H. Multilocus Microsatellite Analysis of ‘Candidatus Liberibacter asiaticus’ Associated with Citrus Huanglongbing Worldwide. BMC Microbiol. 2012, 12, 39. [Google Scholar]
- Matos, L.A.; Hilf, M.E.; Chen, J.; Folimonova, S.Y. Validation of ‘Variable Number of Tandem Repeat’-Based Approach for Examination of ‘Candidatus Liberibacter asiaticus’ Diversity and Its Applications for the Analysis of the Pathogen Populations in the Areas of Recent Introduction. PLoS ONE 2012, 8, e78994. [Google Scholar]
- Ahumada-Rodríguez, J.S.; Cervantes-Santos, J.A.; Rivera-Villanueva, B.; Leyva-López, N.E.; Sandoval-Castro, E.; Méndez-Lozano, J.; Velázquez-Monreal, J.J.; Santos-Cervantes, M.E. Diferenciación de la Población Mexicana de Candidatus Libebacter asiaticus por Doble-Locus Genómico con Repeticiones Cortas en Tándem. Rev. Mex. Fitopatol. 2022, 40, 150–161. [Google Scholar]
- Deng, X.; Lopes, S.; Wang, X.; Sun, X.; Jones, D.; Irey, M.; Civerolo, E.; Chen, J. Characterization of ‘Candidatus Liberibacter asiaticus’ Populations by Double-Locus Analyses. Curr. Microbiol. 2014, 69, 554–560. [Google Scholar]
- Huang, J.; Alanís-Martínez, I.; Kumagai, L.; Dai, Z.; Zheng, Z.; Perez de Leon, A.A.; Chen, J.; Deng, X. Machine Learning and Analysis of Genomic Giversity of “Candidatus Liberibacter asiaticus” Strains from 20 Citrus Production States in Mexico. Front. Plant Sci. 2022, 13, 1052680. [Google Scholar]
- da Silva, P.A.; Fassini, C.G.; Sampaio, L.S.; Dequigiovanni, G.; Zucchi, M.I.; Wulff, N.A. Genetic Diversity of ‘Candidatus Liberibacter asiaticus’ Revealed by Short Tandem Repeats and Prophage Typing Indicates Population Homogeneity in Brazil. Phytopathology 2019, 109, 960–971. [Google Scholar] [CrossRef] [PubMed]
- Barbieri, H.B.; Fernandes, L.S.; de M. Pontes, J.G.; Pereira, A.K.; Fill, T.P. An Overview of the Most Threating Diseases that Affect Worldwide Citriculture: Main Features, Diagnose, and Current Control Strategies. Front. Nat. Prod. 2023, 2, 1045364. [Google Scholar] [CrossRef]
- Xu, Q.; Roose, M.L. Citrus Genomes: From Sequence Variations to Epigenetic Modifications. In The Citrus Genome. Compendium of Plant Genomes; Gentile, A., La Malfa, S., Deng, Z., Eds.; Springer: Cham, Switzerland, 2020. [Google Scholar]
- Cirillo, P. The Integration of Omics Approaches into Plant Disease Resistance. J. Plant Bio. Technol. 2023, 6, 148. [Google Scholar]
- Wu, G.A.; Terol, J.; Ibanez, V.; López-garcía, A.; Pérez-román, E.; Borredá, C.; Domingo, C.; Tadeo, F.R.; Carbonell-caballero, J.; Alonso, R.; et al. Genomics of the Origin and Evolution of Citrus. Nat. Publ. Gr. 2018, 554, 311–316. [Google Scholar] [CrossRef]
- Wang, X.; Xu, Y.; Zhang, S.; Cao, L.; Huang, Y.; Cheng, J.; Wu, G.; Tian, S.; Chen, C.; Liu, Y.; et al. Genomic Analyses of Primitive, Wild and Cultivated Citrus Provide Insights into Asexual Reproduction. Nat. Genet. 2017, 49, 765–772. [Google Scholar] [CrossRef] [PubMed]
- Folimonova, S.Y.; Robertson, C.J.; Garnsey, S.M.; Gowda, S.; Dawson, W.O. Examination of the Responses of Different Genotypes of Citrus to Huanglongbing (Citrus Greening) Under Different Conditions. Phytopathology 2009, 99, 1346–1354. [Google Scholar] [CrossRef]
- Ramadugu, C.; Keremane, M.L.; Halbert, S.E.; Duan, Y.P.; Roose, M.L.; Stover, E.; Lee, R.F. Long-Term Field Evaluation Reveals Huanglongbing Resistance in Citrus Relatives. Plant Dis. 2016, 100, 1858–1869. [Google Scholar] [CrossRef] [PubMed]
- Abutineh, M.; Pizzo, N.; Nifakos, N.; Jin, X.L.; Harlin, J.M.; Zhang, X.H. Genomic Analysis for Citrus Disease Detection. OBM Genet. 2021, 5, 124. [Google Scholar] [CrossRef]
- Huang, M.; Roose, M.L.; Yu, Q.; Du, D.; Yu, Y.; Zhang, Y.; Deng, Z.; Stover, E.; Gmitter, F.G., Jr. Construction of High-Density Genetic Maps and Detection of QTLs Associated with Huanglongbing Tolerance in Citrus. Front. Plant Sci. 2018, 9, 1694. [Google Scholar] [CrossRef]
- Gao, Y.; Xu, J.; Li, Z.; Zhang, Y.; Riera, N.; Xiong, Z.; Ouyang, Z.; Liu, X.; Lu, Z.; Seymour, Z.; et al. Citrus Genomic Resources Unravel Putative Genetic Determinants of Huanglongbing Pathogenicity. iScience 2023, 26, 106024. [Google Scholar] [CrossRef]
- Ying, X.; Wan, M.; Hu, L.; Zhang, J.; Li, H.; Lv, D. Identification of the Virulence Factors of Candidatus Liberibacter asiaticus via Heterologous Expression in Nicotiana benthamiana using Tobacco Mosaic Virus. Int. J. Mol. Sci. 2019, 20, 5575. [Google Scholar] [CrossRef]
- Hu, B.; Rao, M.J.; Deng, X.; Pandey, S.S.; Hendrich, C.; Ding, F.; Wang, N.; Xu, Q. Molecular Signatures between Citrus and Candidatus Liberibacter asiaticus ed. Christoph Dehio. PLoS Pathog. 2021, 17, e1010071. [Google Scholar] [CrossRef]
- Thapa, S.P.; De Francesco, A.; Trinh, J.; Gurung, F.B.; Pang, Z.; Vidalakis, G.; Wang, N.; Ancona, V.; Ma, W.; Coaker, G. Genome-wide Analyses of Liberibacter Species Provides Insights into Evolution, Phylogenetic Relationships, and Virulence Factors. Mol. Plant Pathol. 2020, 21, 716–731. [Google Scholar] [CrossRef] [PubMed]
- Leonard, M.T.; Fagen, J.R.; Davis-Richardson, A.G.; Davis, M.J.; Triplett, E.W. Complete Genome Sequence of Liberibacter crescens BT-1. Stand. Genomic Sci. 2012, 7, 271–283. [Google Scholar] [CrossRef] [PubMed]
- Jain, M.; Munoz-Bodnar, A.; Gabriel, D.W. Concomitant Loss of the Glyoxalase System and Glycolysis Makes the Uncultured Pathogen “Candidatus Liberibacter asiaticus” an Energy Scavenger. Appl. Environ. Microbiol. 2017, 83, e01670-17. [Google Scholar] [CrossRef] [PubMed]
- Merfa, M.V.; Pérez-López, E.; Naranjo, E.; Jain, M.; Gabriel, D.W.; De La Fuente, L. Progress and Obstacles in Culturing ‘Candidatus Liberibacter asiaticus’, the Bacterium Associated with Huanglongbing. Phytopathology 2019, 109, 1092–1101. [Google Scholar] [CrossRef]
- Lv, L.; Shao, X.; Chen, H.; Ho, C.T.; Sang, S. Genistein Inhibits Advanced Glycation End Product Formation by Trapping Methylglyoxal. Chem. Res. Toxicol. 2011, 24, 579–586. [Google Scholar] [CrossRef]
- Jain, M.; Munoz-Bodnar, A.; Gabriel, D.W. ‘Candidatus Liberibacter asiaticus’ Peroxiredoxin (LasBCP) Suppresses Oxylipin-Mediated Defense Signaling in Citrus. J. Plant Physiol. 2019, 31, 1312–1322. [Google Scholar] [CrossRef]
- Wang, N.; Trivedi, P. Citrus Huanglongbing: A Newly Relevant Disease Presents Unprecedented Challenges. Phytopathology 2013, 103, 652–665. [Google Scholar] [CrossRef]
- Zuñiga, C.; Peacock, B.; Liang, B.; McCollum, G.; Irigoyen, S.C.; Tec-Campos, D.; Marotz, C.; Weng, N.C.; Zepeda, A.; Vidalakis, G.; et al. Linking Metabolic Phenotypes to Pathogenic Traits Among “Candidatus Liberibacter asiaticus” and its Hosts. npj Syst. Biol. Appl. 2020, 6, 24. [Google Scholar] [CrossRef]
- Albrecht, U.; Bowman, K.D. Transcriptional Response of Susceptible and Tolerant Citrus to Infection with Candidatus Liberibacter asiaticus. Plant Sci. 2012, 185-186, 118–130. [Google Scholar] [CrossRef]
- Du, P.; Zhang, C.; Zou, X.; Zhu, Z.; Yan, H.; Wuriyanghan, H.; Li, W. “Candidatus Liberibacter asiaticus” Secretes Nonclassically Secreted Proteins That Suppress Host Hypersensitive Cell Death and Induce Expression of Plant Pathogenesis-Related Proteins. Appl. Environ. Microbiol. 2021, 87, AEM.00019-21. [Google Scholar]
- Tyagi, P.; Singh, D.; Mathur, S.; Singh, A.; Ranjan, R. Upcoming Progress of Transcriptomics Studies on Plants: An Overview. Front. Plant Sci. 2022, 13, 1030890. [Google Scholar]
- Albrecht, U.; Bowman, K.D. Gene Expression in Citrus sinensis (L.) Osbeck Following Infection with the Bacterial Pathogen Candidatus Liberibacter asiaticus Causing Huanglongbing in Florida. Plant Sci. 2008, 175, 291–306. [Google Scholar]
- Aritua, V.; Achor, D.; Gmitter, F.G.; Albrigo, G.; Wang, N. Transcriptional and Microscopic Analyses of Citrus Stem and Root Responses to Candidatus Liberibacter asiaticus Infection ed. Raffaele A. Calogero. PLoS ONE 2013, 8, e73742. [Google Scholar] [CrossRef] [PubMed]
- Xu, M.; Li, Y.; Zheng, Z.; Dai, Z.; Tao, Y.; Deng, X. Transcriptional Analyses of Mandarins Seriously Infected by “Candidatus liberibacter asiaticus”. PLoS ONE 2015, 10, e0133652. [Google Scholar]
- Fan, J.; Chen, C.; Yu, Q.; Khalaf, A.; Achor, D.S.; Brlansky, R.H.; Moore, G.A.; Li, Z.G.; Gmitter, F.G., Jr. Comparative Transcriptional and Anatomical Analyses of Tolerant Rough Lemon and Susceptible Sweet Orange in Response to ‘Candidatus Liberibacter asiaticus’ Infection. Mol. Plant Microbe Interact. 2021, 25, 1396–1407. [Google Scholar]
- Wang, Y.; Zhou, L.; Yu, X.; Stover, E.; Luo, F.; Duan, Y. Transcriptome Profiling of Huanglongbing (HLB) Tolerant and Susceptible Citrus Plants Reveals the Role of Basal Resistance in HLB Tolerance. Front. Plant Sci. 2016, 7, 933. [Google Scholar] [CrossRef]
- Hu, Y.; Zhong, X.; Liu, X.; Lou, B.; Zhou, C.; Wang, X. Comparative Transcriptome Analysis Unveils the Tolerance Mechanisms of Citrus hystrix in Response to “Candidatus Liberibacter Asiaticus” Infection. PLoS ONE 2017, 12, e0189229. [Google Scholar] [CrossRef]
- Curtolo, M.; de Souza Pacheco, I.; Boava, L.P.; Takita, M.A.; Granato, L.M.; Galdeano, D.M.; de Souza, A.A.; Cristofani-Yali, M.; Machado, M.A. Wide-ranging Transcriptomic Analysis of Poncirus trifoliata, Citrus sunki, Citrus sinensis and Contrasting Hybrids Reveals HLB Tolerance Mechanisms. Sci. Rep. 2020, 10, 20865. [Google Scholar]
- Arce-Leal, Á.P.; Bautista, R.; Rodríguez-Negrete, E.A.; Manzanilla-Ramírez, M.Á.; Velázquez-Monreal, J.J.; Méndez-Lozano, J.; Bejarano, E.R.; Castillo, A.G.; Claros, M.G.; Leyva-López, N.E. De novo Assembly and Functional Annotation of Citrus aurantifolia Transcriptome from Candidatus Liberibacter asiaticus Infected and non-Infected Trees. Data Brief 2020, 29, 105198. [Google Scholar] [CrossRef] [PubMed]
- Arce-Leal, Á.P.; Bautista, R.; Rodríguez-Negrete, E.A.; Manzanilla-Ramírez, M.A.; Velázquez-Monreal, J.J.; Santos-Cervantes, M.E.; Méndez-Lozano, J.; Beuzón, C.R.; Bejarano, E.R.; Castillo, A.G.; et al. Gene Expression Profile of Mexican Lime (Citrus aurantifolia) Trees in Response to Huanglongbing Disease Caused by Candidatus Liberibacter asiaticus. Microorganisms 2020, 8, 528. [Google Scholar] [CrossRef] [PubMed]
- Mallory, A.C.; Vaucheret, H. Functions of Micrornas and Related Small a RNAs in Plants. Nat. Genet. 2006, 38, S31–S36. [Google Scholar] [CrossRef]
- Navarro, L.; Dunoyer, P.; Jay, F.; Arnold, B.; Dharmasiri, N.; Estelle, M.; Voinnet, O.; Jones, J.D.G. A Plant miRNA Contributes to Antibacterial Resistance by Repressing Auxin Signaling. Science 2006, 312, 436–439. [Google Scholar] [CrossRef] [PubMed]
- Zhao, H.; Sun, R.; Albrecht, U.; Padmanabhan, C.; Wang, A.; Coffey, M.D.; Girke, T.; Wang, Z.; Close, T.J.; Roose, M.; et al. Small RNA Profiling Reveals Phosphorus Deficiency as a Contributing Factor in Symptom Expression for Citrus Huanglongbing Disease. Mol. Plant 2013, 6, 301–310. [Google Scholar] [CrossRef] [PubMed]
- Zhong, Y.; Cheng, C.; Moniruzzaman, M.; Jiang, B.; Jiang, N.; Zhong, G. Expression of miRNAs and their Target Genes in Roots of ‘Sanhu’ Tangerine (Citrus reticulata blanco cv. ‘Sanhu’) in Response to Candidatus Liberibacter asiaticus Infection. J. Plant Dis. Prot. 2021, 128, 407–420. [Google Scholar] [CrossRef]
- Bojórquez-Orozco, A.M.; Arce-Leal, Á.P.; Montes, R.A.C.; Santos-Cervantes, M.E.; Cruz-Mendívil, A.; Méndez-Lozano, J.; Castillo, A.G.; Rodríguez-Negrete, E.A.; Leyva-López, N.E. Differential Expression of miRNAs Involved in Response to Candidatus Liberibacter asiaticus Infection in Mexican Lime at Early and Late Stages of Huanglongbing Disease. Plants 2023, 12, 1039. [Google Scholar] [CrossRef]
- Elmore, J.M.; Griffin, B.D.; Walley, J.W. Advances in Functional Proteomics to Study Plant-Pathogen Interactions. Curr. Opin. Plant Biol. 2021, 63, 102061. [Google Scholar] [CrossRef]
- Martinelli, F.; Reagan, R.L.; Dolan, D.; Fileccia, V.; Dandekar, A.M. Proteomic Analysis Highlights the Role of Detoxification Pathways in Increased Tolerance to Huanglongbing Disease. BMC Plant Biol. 2016, 16, 167. [Google Scholar] [CrossRef]
- Nwugo, C.C.; Lin, H.; Duan, Y.; Civerolo, E.L. The Effect of “Candidatus Liberibacter asiaticus” Infection on the Proteomic Profiles and Nutritional Status of pre-Symptomatic and Symptomatic Grapefruit (Citrus paradisi) Plants. BMC Plant Biol. 2013, 13, 59. [Google Scholar] [CrossRef]
- Yao, L.; Yu, Q.; Huang, M.; Song, Z.; Grosser, J.; Chen, S.; Wang, Y.; Gmitter Jr, F.G. Comparative iTRAQ Proteomic Profiling of Sweet Orange Fruit on Sensitive and Tolerant Rootstocks Infected by ‘Candidatus Liberibacter asiaticus’. PLoS ONE 2020, 15, e0228876. [Google Scholar] [CrossRef]
- Guerra-Lupián, M.A.; Ruiz-Medrano, R.; Ramírez-Pool, J.A.; Ramírez-Ortega, F.A.; López-Buenfil, J.A.; Loeza-Kuk, E.; Morales-Galván, O.; Chavarin-Palacio, C.; Hinojosa-Moya, J.; Xoconostle-Cázares, B. Localized Expression of Antimicrobial Proteins Mitigates Huanglongbing Symptoms in Mexican Lime. J. Biotechnol. 2018, 285, 74–83. [Google Scholar] [PubMed]
- Rao, M.J.; Wu, S.; Duan, M.; Wang, L. Antioxidant Metabolites in Primitive, Wild, and Cultivated Citrus and Their Role in Stress Tolerance. Molecules 2021, 26, 5801. [Google Scholar] [CrossRef] [PubMed]
- Rao, M.J.; Ding, F.; Wang, N.; Deng, X.; Xu, Q. Metabolic Mechanisms of Host Species Against Citrus Huanglongbing (Greening Disease). Crit. Rev. Plant Sci. 2018, 37, 496–511. [Google Scholar]
- Dala Paula, B.M.; Raithore, S.; Manthey, J.A.; Baldwin, E.A.; Bai, J.; Zhao, W.; Glória, M.B.A.; Plotto, A. Active Taste Compounds in Juice from Oranges Symptomatic for Huanglongbing (HLB) Citrus Greening Disease. Lwt- Food Sci. Technol 2018, 91, 518–525. [Google Scholar]
- da Cruz, M.A.; Plotto, A.; Ferrarezi, R.S.; Leite Junior, R.P.; Bai, J. Effect of Huanglongbing on the Volatile Organic Compound Profile of Fruit Juice and Peel Oil in ‘Ray Ruby’ Grapefruit. Foods 2023, 12, 713. [Google Scholar] [CrossRef]
- Pontes, J.G.M.; Ohashi, W.Y.; Brasil, A.J.M.; Filgueiras, P.R.; Espíndola, A.P.D.M.; Silva, J.S.; Poppi, R.; Coletta-Filho, H.; Tasic, L. Metabolomics by NMR Spectroscopy in Plant Disease Diagnostic: Huanglongbing as a Case Study. ChemistrySelect 2016, 1, 1176–1178. [Google Scholar] [CrossRef]
- Wetterich, C.B.; Kumar, R.; Sankaran, S.; Belasque, J.; Ehsani, R.; Marcassa, L.G. A Comparative Study on Application of Computer Vision and Fluorescence Imaging Spectroscopy for Detection of Citrus Huanglongbing Disease in USA and Brazil. Opt. Infobase Conf. Pap. 2013, JW3A, JW3A.26. [Google Scholar]
- Bertolini, E.; Felipe, R.T.A.; Sauer, A.V.; Lopes, S.A.; Arilla, A.; Vidal, E.; Mourão Filho, F.A.A.; Nunes, W.M.C.; Bové, J.M.; López, M.M.; et al. Tissue-print and Squash Real-Time PCR for Direct Detection of ‘Candidatus Liberibacter’ species in Citrus Plants and Psyllid Vectors. Plant Pathol. 2014, 63, 1149–1158. [Google Scholar]
- Ranulfi, A.C.; Cardinali, M.C.B.; Kubota, T.M.K.; Freitas-Astúa, J.; Ferreira, E.J.; Bellete, B.S.; da Silva, M.F.G.F.; Villas Boas, P.R.; Magalhães, A.B.; Milori, D.M.B.P. Laser-Induced Fluorescence Spectroscopy Applied to Early Diagnosis of Citrus Huanglongbing. Biosyst. Eng. 2016, 144, 133–144. [Google Scholar]
- Killiny, N.; Etxeberria, E.; Flores, A.P.; Blanco, P.G.; Reyes, T.F.; Cabrera, L.P. Laser-Induced Breakdown Spectroscopy (LIBS) as a Novel Technique for Detecting Bacterial Infection in Insects. Sci. Rep. 2019, 9, 2449. [Google Scholar] [CrossRef]
- Orjuela-Garzón, W.A.; Araque Echeverry, W.A.; Cabrera Pedraza, R. Identificación de Tecnologías y Métodos para la Detección Temprana del Huanglongbing (HLB) a través de Cienciometría en Artículos Científicos y Patentes. Cienc. Tecnol. Agropecuaria 2020, 21, e1208. [Google Scholar]
- Gómez-Flores, W.; Garza-Saldana, J.J.; Varela-Fuentes, S.E. A Huanglongbing Detection Method for Orange Trees Based on Deep Neural Networks and Transfer Learning. IEEE Access 2022, 10, 116686–116696. [Google Scholar]
- Stolowicz, F.; Larocca, L.; Werbajh, S.; Parma, Y.; Carrillo, C.; Ogas, L.; Agostini, J.P.; Redes, J.; Welin, B.; Castagnaro, A.; et al. A Colorimetric, Sensitive, Rapid, and Simple Diagnostic Kit for the HLB Putative Causal Agent Detection. Front. Agron. 2022, 4, 984360. [Google Scholar] [CrossRef]
- Morán, F.; Herrero-Cervera, M.; Carvajal-Rojas, S.; Marco-Noales, E. Real-Time On-Site Detection of the Three ‘Candidatus Liberibacter’ Species Associated with HLB Disease: A Rapid and Validated Method. Front. Plant Sci. 2023, 14, 1176513. [Google Scholar]
- Mathanker, S.K.; de Jensen, C.E.; Pagán-López, A.M.; Pérez-Alegría, L.R. UAV Color Images for Determination of Citrus Plant Parameters. J. Agric. Univ. Puerto Rico 2019, 103, 141–153. [Google Scholar] [CrossRef]
- Wetterich, C.B.; Kumar, R.; Sankaran, S.; Belasque Junior, J.; Ehsani, R.; Marcassa, L.G. A Comparative Study on Application of Computer Vision and Fluorescence Imaging Spectroscopy for Detection of Huanglongbing Citrus Disease in the USA and Brazil. J. Spectrosc. 2013, 2013, 841738. [Google Scholar]
- Mota, A.D.; Rossi, G.; De Castro, G.C.; Ortega, T.A.; De Castro, N.J.C. Portable Fluorescence Spectroscopy Platform for Huanglongbing (HLB) Citrus Disease in situ Detection. SPIE 2014, 9003, 90031U. [Google Scholar]
- Wetterich, C.B.; De Oliveira Neves, R.F.; Belasque, J.; Marcassa, L.G. Detection of Citrus Canker and Huanglongbing Using Fluorescence Imaging Spectroscopy and Support Vector Machine Technique. Appl. Opt. 2016, 55, 400–407. [Google Scholar] [CrossRef]
- Arredondo Valdés, R.; Delgado Ortiz, J.C.; Beltrán Beache, M.; Anguiano Cabello, J.; Cerna Chávez, E.; Rodríguez Pagaza, Y.; Ochoa Fuentes, Y.M. A Review of Techniques for Detecting Huanglongbing (Greening) in Citrus. Can. J. Microbiol. 2016, 62, 803–811. [Google Scholar] [CrossRef]
- Neves, R.F.O.; Wetterich, C.B.; Sousa, E.P.M.; Marcassa, L.G. Multiclass Classifier Based on Deep Learning for Detection of Citrus Disease Using Fluorescence Imaging Spectroscopy. Laser Phys. 2023, 33, 55602. [Google Scholar] [CrossRef]
- Cardinali, M.C.; Villas Boas, P.R.; Milori, D.M.; Ferreira, E.J.; França e Silva, M.; Machado, M.A.; Bellete, B.S.; da Silva, M.F. Infrared Spectroscopy: A Potential Tool in Huanglongbing and Citrus Variegated Chlorosis Diagnosis. Talanta 2012, 91, 1–6. [Google Scholar] [CrossRef]
- Moriya, É.A.; Imai, N.N.; Tommaselli, A.M.; Berveglieri, A.; Honkavaara, E.; Soares, M.A.; Marino, M. Detecting Citrus Huanglongbing in Brazilian Orchards Using Hyperspectral Aerial Images. Int. Arch. Photogramm. Remote Sens. Spatial Inf. Sci. 2019, XLII-2/W13, 1881–1886. [Google Scholar] [CrossRef]
- Pereira, F.M.; Milori, D.M.; Pereira-Filho, E.R.; Veníncio, A.L.; Russo, M.; Cardinali, M.C.; Martins, P.K.; Freitas-Astúa, J. Laser-Induced Fluorescence Imaging Method to Monitor Citrus Greening Disease. Comput. Electron. Agric. 2011, 79, 90–93. [Google Scholar] [CrossRef]
- Ponce, L.; Etxeberria, E.; Gonzalez, P.; Ponce, A.; Flores, T. Rapid Identification of Huanlongbing-Infected Citrus Plants Using Laser-Induced Breakdown Spectroscopy of Phloem Samples. Appl. Opt. 2018, 57, 8841–8844. [Google Scholar] [CrossRef]
- de Moraes Pontes, J.G.; Vendramini, P.H.; Fernandes, L.S.; de Souza, F.H.; Pilau, E.J.; Eberlin, M.N.; Magnani, R.F.; Wulff, N.A.; Fill, T.P. Mass Spectrometry Imaging as a Potential Technique for Diagnostic of Huanglongbing Disease Using Fast and Simple Sample Preparation. Sci. Rep. 2020, 10, 13457. [Google Scholar]
- Wang, H.; Ramnani, P.; Pham, T.; Villarreal, C.C.; Yu, X.; Liu, G.; Mulchandani, A. Asymptomatic Diagnosis of Huanglongbing Disease Using Metalloporphyrin Functionalized Single-Walled Carbon Nanotubes Sensor Arrays. Front. Chem. 2020, 8, 362. [Google Scholar] [CrossRef] [PubMed]
- de Chaves, M.Q.G.; Morán, F.; Barbé, S.; Bertolini, E.; de la Rosa, F.S.; Marco-Noales, E. A New and Accurate qPCR Protocol to Detect Plant Pathogenic Bacteria of the Genus ‘Candidatus Liberibacter’ in Plants and Insects. Sci. Rep. 2023, 13, 3338. [Google Scholar] [CrossRef] [PubMed]
- Wetterich, C.B.; De Oliveira Neves, R.F.; Belasque, J.; Ehsani, R.; Marcassa, L.G. Detection of Huanglongbing in Florida using Fluorescence Imaging Spectroscopy and Machine-Learning Methods. Appl. Opt. 2017, 56, 15–23. [Google Scholar] [CrossRef]
- Rigano, L.A.; Malamud, F.; Orce, I.G.; Filippone, M.P.; Marano, M.R.; do Amaral, A.M.; Castagnaro, A.P.; Vojnov, A.A. Rapid and Sensitive Detection of Candidatus Liberibacter asiaticus by Loop Mediated Isothermal Amplification Combined with a Lateral Flow Dipstick. BMC Microbiol. 2014, 14, 86. [Google Scholar] [CrossRef]
- CDASP (Coordenadoria de Defesa Agropecuária do Estado de São Paulo). Huanglongbing (HLB)-20 Anos: Huanglongbing (HLB)—20 Anos: Os Anos 2000; Informativo Defesa AgroSP nº 032; Secretaria de Agricultura e Abastecimento: Campinas, São Paulo, Brazil, 2024. Available online: https://www.defesa.agricultura.sp.gov.br/informativo/defesa-agrosp-no-032-marco2024/huanglongbing-hlb-20-anos-os-anos-2000/ (accessed on 18 March 2025).
- DOU (Diário Oficial Da União). Portaria Nº 317, de 21 de Maio de 2021. Institui o Programa Nacional de Prevenção e Controle à Doença Denominada Huanglongbing (HLB)—PNCHLB; Ministério da Agricultura, Pecuária e Abastecimento/Secretaria de Defesa Agropecuária, Governo do Brasil: Brasília, Brazil, 2021. Available online: https://www.in.gov.br/web/dou/-/portaria-n-317-de-21-de-maio-de-2021-321773783 (accessed on 20 March 2025).
- Graham, J.H.; Bassanezi, R.B.; Dawson, W.O.; Dantzler, R. Management of Huanglongbing of Citrus: Lessons from São Paulo and Florida. Annu. Rev. Phytopathol. 2024, 62, 243–262. [Google Scholar]
- NOM-EM-047-FITO-2009; NORMA Oficial Mexicana de Emergencia NOM-EM-047-FITO-2009, Por la que se Establecen las Acciones Fitosanitarias para Mitigar el Riesgo de Introducción y Dispersión del Huanglongbing (HLB) de los cítricos (Candidatus liberibacter spp.) en el Territorio Nacional. 2009. Available online: https://faolex.fao.org/docs/pdf/mex88549.pdf (accessed on 21 March 2025).
- Diario Oficial de La Federación (DOF). ACUERDO: Por el que se Dan a Conocer las Medidas Fitosanitarias que Deberán Aplicarse para el Control del Huanglongbing (Candidatus Liberibacter spp.) y su Vector; Diario Official de La Federación (DOF): Ciudad de México, Mexico, 2010.
- Villegas-Monter, A.; Mora-Aguilera, A. Avances de la Fruticultura en México. Rev. Bras. Frutic. 2011, 33, 179–186. [Google Scholar] [CrossRef]
- Hernández-Hernández, R.; Granados-Ramírez, G.R.; Mora-Aguilera, G.; Aguirre-Gómez, R.; León-García, I. Reconversión de cultivos como resultado de la presencia de Huanglongbing en Colima, México. Acta Universitaria 2019, 29, 1–13. [Google Scholar]
- Salcedo-Baca, D.H.; González-Hernández, E.; Rodríguez-Leyva, E.; Vera-Villagrán, C.; Múzquiz-Fragoso, A.; Hurtado-Arellano, A. Evaluación de la campaña contra el HLB en 2008, 2009 y 2010. IICA 2012, 126. [Google Scholar]
- Villar-Luna, H.; Santos-Cervantes, M.E.; Rodríguez-Negrete, E.A.; Méndez-Lozano, J.; Leyva-López, N.E. Economic and Social Impact of Huanglongbing on the Mexico Citrus Industry: A Review and Future Perspectives. Horticulturae 2024, 10, 481. [Google Scholar] [CrossRef]
- Vieira, J.G.A.; Santana, E.D.R.; Thiesen, L.V.; Matioli, T.F.; Yamamoto, P.T. Effect of Systemic Insecticides Applied via Drench on the Mortality of Diaphorina citri on Curry Leaf. Insects 2023, 14, 422. [Google Scholar] [CrossRef] [PubMed]
- Carmo-Sousa, M.; Garcia, R.B.; Wulff, N.A.; Fereres, A.; Miranda, M.P. Drench Application of Systemic Insecticides Disrupts Probing Behavior of Diaphorina citri (Hemiptera: Liviidae) and Inoculation of Candidatus Liberibacter asiaticus. Insects 2020, 11, 314. [Google Scholar] [CrossRef] [PubMed]
- Pérez-Zarate, L.A.; Osorio-Acosta, F.; Villanueva-Jiménez, J.A.; Ortega-Arenas, L.D.; Chiquito-Contreras, R.G. Factores que Inciden en el Control Químico de Diaphorina citri Kuwayama en Áreas Regionales de Control. Southwest. Entomol. 2016, 41, 1037–1050. [Google Scholar]
- Miranda, M.P.; da Silva Scapin, M.; Vizoni, M.C.; Zanardi, O.Z.; Eduardo, W.I.; Volpe, H.X.L. Spray Volumes and Frequencies of Insecticide Applications for Suppressing Diaphorina citri Populations in Orchards. Crop Prot. 2021, 140, 105406. [Google Scholar]
- Pardo, S.; Martínez, A.M.; Figueroa, J.I.; Chavarrieta, J.M.; Viñuela, E.; Rebollar-Alviter, Á.; Pineda, S. Insecticide Resistance of Adults and Nymphs of Asian Citrus Psyllid Populations from Apatzingán Valley, Mexico. Pest Manag. Sci. 2017, 74, 135–140. [Google Scholar]
- de Carli, L.F.; Miranda, M.P.; Volpe, H.X.L.; Zardini, O.Z.; Vizoni, M.C.; Martini, F.M.; Lopes, J.P.A. Leaf Age Affects the Efficacy of Insecticides to Control Asian citrus Psyllid, Diaphorina citri (Hemiptera: Liviidae). J. Appl. Entomol. 2018, 142, 689–695. [Google Scholar] [CrossRef]
- Assalin, M.R.; de Souza, D.R.C.; Rosa, M.A.; Duarte, R.R.M.; Castanha, R.F.; Vilela, E.S.D.; Tasic, L.; Durán, N. Thiamethoxam Used as Nanopesticide for the Effective Management of Diaphorina citri psyllid: An Environmental-Friendly Formulation. Int. J. Pest. Manag. 2022, 24, 1–9. [Google Scholar]
- Stephano-Hornedo, J.L.; Torres-Gutiérrez, O.; Toledano-Magaña, Y.; Gradilla-Martínez, I.; Pestryakov, A.; Sánchez-González, A.; Bogdanchikova, N. Argovit™ Silver Nanoparticles to Fight Huanglongbing Disease in Mexican limes (Citrus aurantifolia Swingle). RSC Adv. 2020, 10, 6146–6155. [Google Scholar] [CrossRef] [PubMed]
- Ghosh, D.; Kokane, S.; Savita, B.K.; Kumar, P.; Sharma, A.K.; Ozcan, A.; Kokane, A.; Santra, S. Huanglongbing Pandemic: Current Challenges and Emerging Management Strategies. Plants 2023, 12, 160. [Google Scholar] [CrossRef] [PubMed]
- Paiva, P.E.B.; Parra, J.R.P. Natural Parasitism of Diaphorina citri Kuwayama (Hemiptera, Psyllidae) Nymphs by Tamarixia radiata Waterston (Hymenoptera, Eulophidae) in São Paulo Orange Groves. Rev. Bras. Entomol. 2012, 56, 499–503. [Google Scholar] [CrossRef]
- Chavez, Y.; Chirinos, D.T.; González, G.; Lemos, N.; Fuentes, A.; Castro, R.; Kondo, T. Tamarixia radiata (Waterston) and Cheilomenes sexmaculata (Fabricius) as Biological Control Agents of Diaphorina citri Kuwayama in Ecuador. Chil. J. Agric. Res. 2017, 77, 180–184. [Google Scholar] [CrossRef]
- Cortez-Mondaca, E.; Lugo-Angulo, N.E.; Pérez-Márquez, J.; Apodaca-Sánchez, M.A. Primer Reporte de Enemigos Naturales y Parasitisme Sobre Diaphorina citri Kuwayama en Sinaloa, México. Southw. Entomol. 2011, 35, 113–116. [Google Scholar] [CrossRef]
- Arias-Ortega, P.L.; Restrepo-García, A.M.; Soto-Giraldo, A. Primer Registro de Diaphorencyrtus sp. (Hymenoptera: Encyrtidae) en Colombia. Bol. Cient. Mus. Hist. Nat. U. Caldas 2016, 20, 157–165. [Google Scholar] [CrossRef]
- Portalanza, D.E.; Sanchez, L.; Plúas, M.; Felix, I.; Costa, V.A.; Dias-Pini, N.D.S.; Gómez-Torres, M.L. First Records of Parasitoids Attacking the Asian Citrus Psyllid in Ecuador. Rev. Bras. Entomol. 2017, 61, 107–110. [Google Scholar] [CrossRef]
- Jorge, S.J.; Rueda-Ramírez, D.; de Moraes, G.J. Predation Capacity of Phytoseiid Mites (Mesostigmata: Phytoseiidae) from Brazil on Eggs of Diaphorina citri (Hemiptera: Liviidae). Phytoparasitica 2021, 49, 603–611. [Google Scholar] [CrossRef]
- Kalile, M.O.; Cardoso, A.C.; Pallini, A.; Fonseca, M.M.; Ferreira-Junior, T.A.; Janssen, A. A Predatory Mite that Suppresses Diaphorina citri Populations on Plants with Pollen and Oviposition Sites. Entomol. Exp. Appl. 2023, 171, 592–602. [Google Scholar]
- Pérez-González, O.; Rodríguez-Guerra, R.; López-Arroyo, J.I.; Sandoval-Coronado, C.F.; Maldonado-Blanco, M.G. Radial Growth, Sporulation, and Virulence of Mexican Isolates of Hirsutella citriformis against Diaphorina citri. Southwest. Entomol. 2015, 40, 111–120. [Google Scholar] [CrossRef]
- Pérez-González, O.; Gomez-Flores, R.; Tamez-Guerra, P. Insight into Biological Control Potential of Hirsutella citriformis Against Asian Citrus Psyllid as a Vector of Citrus Huanglongbing Disease in America. J. Fungi 2022, 8, 573. [Google Scholar] [CrossRef]
- Lezama-Gutiérrez, R.; Molina-Ochoa, J.; Chávez-Flores, O.; Angel-Sahagun, C.A.; Skoda, S.R.; Reyes-Martínez, G.; Foster, J.E. Use of the Entomopathogenic Fungi Metarhizium anisopliae, Cordyceps bassiana and Isaria fumosorosea to Control Diaphorina citri (Hemiptera: Psyllidae) in Persian Lime Under Field Conditions. Int. J. Trop. Insect Sci. 2012, 32, 39–44. [Google Scholar]
- Dorta, S.D.O.; Balbinotte, J.; Monnerat, R.; Lopes, J.R.S.; da Cunha, T.; Zanardi, O.Z.; de Freitas-Astúa, J. Selection of Bacillus thuringiensis Strains in Citrus and Their Pathogenicity to Diaphorina citri (Hemiptera: Liviidae) Nymphs. Insect Sci. 2020, 27, 519–530. [Google Scholar] [PubMed]
- Mendoza-García, E.E.; Ortega-Arenas, L.D.; Serrato-Cruz, M.Á.; Villanueva-Jiménez, J.A.; López-Arroyo, J.I.; Pérez-Pacheco, R. Chemical Composition, Toxicity, and Repellence of Plant Essential Oils Against Diaphorina citri (Hemiptera: Liviidae). Chil. J. Agric. Res. 2019, 79, 636–647. [Google Scholar]
- Orozco-Santos, M.; Robles-González, M.; Hernández-Fuentes, L.M.; Velázquez-Monreal, J.J.; de Jesús Bermudez-Guzmán, M.; Manzanilla-Ramírez, M.; Nieto-Ángel, D. Uso de Aceites y Extractos Vegetales para el Control de Diaphorina citri Kuwayama en Lima Mexicana en el Trópico Seco de México. Southwest. Entomol. 2016, 41, 1051–1066. [Google Scholar]
- Pistori, J.F.; Simionato, A.S.; Navarro, M.O.; Andreata, M.F.; Santos, I.M.; Meneguim, L.; Andrade, G. Low-Molecular-Weight Metabolites Produced by Pseudomonas aeruginosa as an Alternative to Control Huanglongbing in Citrus sinensis cv. Valencia. Trop. Plant Pathol. 2018, 43, 289–296. [Google Scholar]
- Canales, E.; Coll, Y.; Hernández, I.; Portieles, R.; Rodríguez García, M.; López, Y.; Borrás-Hidalgo, O. ‘Candidatus Liberibacter asiaticus’, Causal Agent of Citrus Huanglongbing, is Reduced by Treatment with Brassinosteroids. PLoS ONE 2016, 11, e0146223. [Google Scholar]
- da Silva, J.R.; de Alvarenga, F.V.; Boaretto, R.M.; Lopes, J.R.S.; Quaggio, J.A.; Coletta Filho, H.D.; Mattos, D. Following the Effects of Micronutrient Supply in HLB-infected Trees: Plant Responses and ‘Candidatus Liberibacter asiaticus’ Acquisition by the Asian Citrus Psyllid. Trop. Plant Pathol. 2020, 45, 597–610. [Google Scholar]
- Bassanezi, R.B.; Primiano, I.V.; Vescove, H.V. Effect of Enhanced Nutritional Programs and Exogenous Auxin Spraying on Huanglongbing Severity, Fruit Drop, Yield and Economic Profitability of Orange Orchards. Crop Prot. 2021, 145, 105609. [Google Scholar] [CrossRef]
- Ramirez-Godoy, A.; Puentes-Perez, G.; Restrepo-Diaz, H. An Evaluation of the Use of Calcium, Potassium and Silicon for the Management of Diaphorina citri Populations in Tahiti Lime Trees. Not. Bot. Horti Agrobot. Cluj Napoca 2018, 46, 546–552. [Google Scholar] [CrossRef]
- de Souza Ferraz, R.L.; de Andrade Barbosa, M.; Viégas, P.R.A.; da Silva Costa, P.; de Melo, A.S.; Magalhães, I.D.; Neto, J.D.; de Souza Medeiros, A. Nutritional Status of Orange Tree ‘Pera Rio’ variety after Huanglongbing Disease Infection, Leaf Spray Fertilization and Application of Resistance-Inducing Bioinductors. Aust. J. Crop Sci. 2017, 11, 1642–1650. [Google Scholar] [CrossRef]
- Hernández-Morales, L.M.; García-Pérez, E.; Cortés-Flores, J.I.; Villegas-Monter, Á.; Mora-Aguilera, J.A. Fertilización Integral en Árboles de Naranjo ‘MARRS’ en Producción con Síntomas de Virus de la Tristeza de los Cítricos (VTC) y Huanglongbing (HLB). Rev. Fitotec. Mex. 2021, 44, 59–66. [Google Scholar] [CrossRef]
- Román-Paoli, E.; Ortiz-López, J.; Zamora-Echevarría, J.; Román-Pérez, F.M. Fertilization Methods Affecting ‘Tahiti’ Lime (Citrus latifolia) Fruit Yield and Profitability. J. Agric. Univ. Puerto Rico 2021, 105, 163–177. [Google Scholar] [CrossRef]
- Moreira, A.S.; Stuchi, E.S.; Silva, P.R.B.; Bassanezi, R.B.; Girardi, E.A.; Laranjeira, F.F. Could Tree Density Play a Role in Managing Citrus Huanglongbing Epidemics? Trop. Plant Pathol. 2019, 44, 268–274. [Google Scholar] [CrossRef]
- Orozco-Santos, M.; Preciado, J.C.G.; Velázquez-Monreal, J.J.; Hernández-Fuentes, L.M.; Robles-González, M.M.; Manzanilla-Ramírez, M.Á.; Manzo-Sánchez, G. Uso de Acolchados Plásticos para Reducir Diaphorinia citri 1-Huanglongbing e Incrementar el Rendimiento de Lima Mexicana en el Trópico Seco de México. Southwest. Entomol. 2022, 47, 927–934. [Google Scholar] [CrossRef]
- Sun, L.; Nasrullah; Ke, F.; Nie, Z.; Wang, P.; Xu, J. Citrus Genetic Engineering for Disease Resistance: Past, Present, and Future. Int. J. Mol. Sci. 2019, 20, 5256. [Google Scholar] [CrossRef]
- Tavano, E.C.D.R.; Vieira, M.L.C.; Alves-Mourão, F.D.A.; Harakava, R.; Mendes, B.M.J. Genetic Transformation of Citrus sinensis ‘Hamlin’ with attacin a Driven by a Phloem Tissue-Specific Promoter for Resistance to Candidatus Liberibacter spp. Acta Hortic. 2015, 1065, 695–702. [Google Scholar] [CrossRef]
- Tavano, E.C.D.R.; Erpen, L.; Aluisi, B.; Harakava, R.; Lopes, J.R.S.; Vieira, M.L.C.; Piedade, S.; Mendes, B.J.; Mourão Filho, F.D.A.A. Sweet Orange Genetic Transformation with the attacin A Gene Under the Control of Phloem-Specific Promoters and Inoculation with Candidatus Liberibacter asiaticus. J. Hortic. Sci. Biotechnol. 2019, 94, 210–219. [Google Scholar] [CrossRef]
- Longhi, T.V.; de Carvalho, D.U.; Duin, I.M.; da Cruz, M.A.; Leite Junior, R.P. Transgenic Sweet Orange Expressing the Sarcotoxinia IA Gene Produces High-Quality Fruit and Shows Tolerance to ‘Candidatus Liberibacter asiaticus’. Int. J. Mol. Sci. 2022, 23, 9300. [Google Scholar] [CrossRef] [PubMed]
- de Oliveira Dorta, S.; Attílio, L.B.; Zanardi, O.Z.; Lopes, J.R.S.; Machado, M.A.; Freitas-Astúa, J. Genetic Transformation of ‘Hamlin’ and ‘Valencia’ Sweet Orange Plants Expressing the cry11A Gene of Bacillus thuringiensis as Another Tool to the Management of Diaphorina citri (Hemiptera: Liviidae). J. Biotechnol. 2023, 368, 60–70. [Google Scholar] [CrossRef] [PubMed]
- dos Santos Silva, J.; de Santana Cerqueira, L.R.; Hunter, W.B.; de Andrade, E.C. RNAi Feeding Bioassay: A Protocol for dsRNA Screening Against Asian Citrus Psyllid and Related Hemipteran Insects. In RNAi Strategies for Pest Management: Methods and Protocols in Molecular Biology; Vaschetto, L.M., Ed.; Humana: Totowa, NJ, USA, 2022; Volume 2360, pp. 85–90. [Google Scholar]
- de Andrade, E.C.; Hunter, W.B. RNAi Feeding Bioassay: Development of a Non-Transgenic Approach to Control Asian Citrus Psyllid and Other Hemipterans. Entomol. Exp. Appl. 2017, 162, 389–396. [Google Scholar] [CrossRef]
- Manzano-Galdeano, D.M.; Breton, M.C.; Lopes, J.R.S.; Falk, B.W.; Machado, M.A. Oral Delivery of Double-Stranded RNAs Induces Mortality in Nymphs and Adults of the Asian Citrus Psyllid, Diaphorina citri. PLoS ONE 2017, 12, e0171847. [Google Scholar]
- Pacheco, I.D.S.; Galdeano, D.M.; Maluta, N.K.P.; Lopes, J.R.S.; Machado, M.A. Gene Silencing of Diaphorina citri Candidate Effectors Promotes Changes in Feeding Behaviors. Sci. Rep. 2020, 10, 5992. [Google Scholar]
- Rueda-Silva, J.C.; González-Campos, L.I.; Durán-Armenta, L.F.; Karam-Coppola, A.; Antonio-Pérez, A.; Ordoñez-Rodríguez, J.; Saucedo_Tavitas, N.E.; Rico-Torres, V.; Cruz-Cruz, C.; García-Huante, Y.G.; et al. Novel Bacterial Plasmid Produces Small Interfering RNAs (siRNAs) that Induce Effective Gene Silencing in the Asian Citrus Psyllid Diaphorina citri. Electron. J. Biotechnol. 2023, 64, 59–68. [Google Scholar]


| Species | Subsp. | Abbr. | Characteristics | Distribution | Reference |
|---|---|---|---|---|---|
| Candidatus Liberibacter africanus | capenis | CLafC | Heat-sensitive (22–24 °C) | Africa | [2,16,17,18] |
| clausenae | CLafCl | ||||
| zanthoxyli | CLafZ | ||||
| vepridis | CLafV | ||||
| tecleae | CLafT | ||||
| Candidatus Liberibacter asiaticus | CLas | Heat-tolerant (≤35 °C) | Asia, Oceania, America, and Africa (Ethiopia and Kenya) | [2,15,19,20] | |
| Candidatus Liberibacter americanus | CLam | Heat-sensitive (≤32 °C) | Brazil | [15] |
| Candidatus Phytoplasma | Distribution | Reference |
|---|---|---|
| Candidatus Phytoplasma phoenicium | Brazil | [21] |
| Candidatus Phytoplasma asteris | China Mexico | [22,23] |
| Candidatus Phytoplasma aurantifolia | China | [24] |
| Candidatus Phytoplasma pruni | Brazil | [25] |
| Candidatus Phytoplasma ulmi | Chile | [26] |
| Candidatus Phytoplasma hispanicum | Chile | [26] |
| Species | Strain | GenBank Accession Number | Geographic Area | Size (Mb) | No. of Genes |
|---|---|---|---|---|---|
| CLas 1 | SGCA16 | VTLZ01 | California, USA | 1.21 | 1102 |
| SGCA5 | LMTO01 | 1.20 | 1112 | ||
| AHCA1 | CP029348.1 | 1.23 | 1110 | ||
| AHCA17 | VNFL01 | 1.21 | 1103 | ||
| A-SBCA19 | JADBIB01 | 1.19 | 1126 | ||
| HHCA | JMIL02 | 1.15 | 1212 | ||
| HHCA16 | VTLY01 | 1.21 | 1121 | ||
| DUR1TX1 | VTLT01 | Texas, USA | 1.21 | 1098 | |
| DUR2TX1 | VTLS01 | 1.21 | 1161 | ||
| GFR3TX3 | VTLR01 | 1.21 | 1109 | ||
| LBR19TX2 | VTMA01 | 1.20 | 1085 | ||
| LBR23TX5 | VTMB01 | 1.20 | 1087 | ||
| TX2351 | MTIM01 | 1.25 | 1191 | ||
| CRCFL16 | VTLW01 | Florida, USA | 1.21 | 1187 | |
| FL17 | JWHA01 | 1.23 | 1116 | ||
| MFL16 | VTLX01 | 1.20 | 1134 | ||
| JRPAMB1 | CP040636.1 | 1.24 | 1119 | ||
| Psy62 | CP001677.5 | 1.23 | 1114 | ||
| CoFLP | CP054558.1 | La Guajira, Colombia | 1.23 | 1114 | |
| 9PA | JABDRZ01 | Brazil | 1.23 | 1113 | |
| Mex8 | VTLU01 | Baja California, Mexico | 1.24 | 1141 | |
| BCSMX | JAOPHS01 | Baja California Sur, Mexico | 1.23 | 1116 | |
| YTMX | JAOPHR01 | Yucatan, Mexico | 1.23 | 1111 | |
| CLso 2 | RSTM | LLVZ01 | California, USA | 1.29 | 1210 |
| R1 | JNVH01 | 1.20 | 1143 | ||
| CLso-ZC1 | CP002371.1 | Texas, USA | 1.26 | 1169 | |
| HenneA | JQIG01 | 1.21 | 1146 | ||
| CLam 3 | PW_SP | AOFG01 | Sao Paulo, Brazil | 1.17 | 1018 |
| Sao Paulo | CP006604.1 | 1.19 | 1045 | ||
| Lcr 4 | BT-0 | CP010522.1 | Puerto Rico | 1.52 | 1389 |
| BT-1 | CP003789.1 | 1.50 | 1388 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cervantes-Santos, J.A.; Villar-Luna, H.; Bojórquez-Orozco, A.M.; Díaz-Navarro, J.E.; Arce-Leal, Á.P.; Santos-Cervantes, M.E.; Claros, M.G.; Méndez-Lozano, J.; Rodríguez-Negrete, E.A.; Leyva-López, N.E. Huanglongbing as a Persistent Threat to Citriculture in Latin America. Biology 2025, 14, 335. https://doi.org/10.3390/biology14040335
Cervantes-Santos JA, Villar-Luna H, Bojórquez-Orozco AM, Díaz-Navarro JE, Arce-Leal ÁP, Santos-Cervantes ME, Claros MG, Méndez-Lozano J, Rodríguez-Negrete EA, Leyva-López NE. Huanglongbing as a Persistent Threat to Citriculture in Latin America. Biology. 2025; 14(4):335. https://doi.org/10.3390/biology14040335
Chicago/Turabian StyleCervantes-Santos, Jael Arely, Hernán Villar-Luna, Ana Marlenne Bojórquez-Orozco, José Ernesto Díaz-Navarro, Ángela Paulina Arce-Leal, María Elena Santos-Cervantes, Manuel Gonzalo Claros, Jesús Méndez-Lozano, Edgar Antonio Rodríguez-Negrete, and Norma Elena Leyva-López. 2025. "Huanglongbing as a Persistent Threat to Citriculture in Latin America" Biology 14, no. 4: 335. https://doi.org/10.3390/biology14040335
APA StyleCervantes-Santos, J. A., Villar-Luna, H., Bojórquez-Orozco, A. M., Díaz-Navarro, J. E., Arce-Leal, Á. P., Santos-Cervantes, M. E., Claros, M. G., Méndez-Lozano, J., Rodríguez-Negrete, E. A., & Leyva-López, N. E. (2025). Huanglongbing as a Persistent Threat to Citriculture in Latin America. Biology, 14(4), 335. https://doi.org/10.3390/biology14040335







