Simple Summary
Wild ruminants may harbor pathogens transmitted by hematophagous arthropods, as well as those transmitted via oral and/or inhalation routes. Among these microorganisms, several bacteria and protozoa may also infect humans, livestock and companion animals. In fact, wild ruminants often serve as reservoirs without showing clinical signs, whereas other mammals can develop mild or severe diseases. Wild ruminants are largely present in different areas of Central Italy, but although some studies have been carried out on the occurrence of different bacterial and protozoan pathogens in Italian deer populations, the current epidemiology of these microorganisms in deer is not fully clear because the distribution and prevalence of the pathogens are constantly changing.
Abstract
Wild ruminants often harbor pathogens transmissible to other animals and humans, but their epidemiological role is not always defined for all microorganisms. In this survey, spleens, kidneys, and hearts sampled from 162 fallow deer (Dama dama) were subjected to molecular analyses to detect bacterial (Anaplasma phagocytophilum, Borrelia burgdorferi s.l., Brucella spp., Chlamydia abortus, Coxiella burnetii, Francisella tularensis, Leptospira spp.) and protozoan (piroplasms, Neospora caninum, Toxoplasma gondii) pathogens. Five (3.08%) spleens were positive for A. phagocytophilum, and twelve (7.40%) spleens were positive for Theileria cervi. The remaining pathogens investigated were not detected, and no coinfections were found. The analyzed animals do not seem to have a relevant role in the spreading of these pathogens; however, monitoring is pivotal to understand the epidemiological scenarios and take appropriate preventive measures in areas frequently visited by people.
1. Introduction
Wild ruminants, such as red deer (Cervus elaphus), roe deer (Capreolus capreolus) and fallow deer (Dama dama), often harbor pathogens transmitted by hematophagous arthropods, as well as agents transmitted via oral and/or inhalation routes [1,2,3,4]. Among these pathogens, several bacteria and protozoa may also be the cause of infections and diseases in human patients and domestic animals, i.e., farm and companion animals. In fact, wild ruminants often act as reservoirs without showing clinical signs, whereas other mammals can develop mild or severe diseases [3].
Wild ruminants are largely present in different areas of Central Italy; in particular, a fallow deer population live in the Regional Park Migliarino-San Rossore-Massaciuccoli, located in the northwest of Tuscany, Central Italy. From an epidemiological perspective, this protected area is of particular interest due to its proximity to the city center and the high number of visitors it receives daily [5]. Additionally, the deer population within the park exhibits a notably high density that reaches 99.8 heads/100 ha during the spring season [6]. As widely recognized, an increased ungulate population density facilitates the transmission and spread of diseases [7,8,9].
Even though some studies have been carried out on the occurrence of different bacterial and protozoan pathogens, in particular those transmitted by hematophagous arthropods, in Italian deer populations [10,11,12,13,14,15,16,17], the current epidemiology of these microorganisms in deer is not fully elucidated because the distribution and prevalence of the pathogens are constantly changing.
Therefore, the aim of the present survey was to verify the presence of bacterial (Anaplasma phagocytophilum, Borrelia burgdorferi sensu lato (s.l.), Brucella spp., Chlamydia abortus, Coxiella burnetii, Francisella tularensis, Leptospira spp.) and protozoan (piroplasms, Neospora caninum, Toxoplasma gondii) pathogens, most of which are transmitted by hematophagous arthropods, in fallow deer from the Regional Park.
2. Materials and Methods
2.1. Study Area
The analyzed animals lived in the San Rossore Estate, a protected area of Regional Park Migliarino-San Rossore-Massaciuccoli that covers a surface area of approximately 4950 hectares, characterized by wooded areas, wetlands and agricultural landscapes, close to the Pisa city (43°41′ N; 10°19′ E; Figure 1). The wooded areas are dominated by deciduous and coniferous forest where the predominant species are Quercus robur, Quercus ilex, Populus alba, Fraxinus spp., Pinus pinaster, and Pinus pinea. Wetlands show the presence of Carex spp., Phragmites australis and Juncus spp., while agricultural landscape are dominated by meadows and pasture. Fallow deer population exhibited a spring density with an estimated average of 42.8 deer/km2 (±8.1 SD), and in the recent past reached a peak of 99.8 deer/ km2 [18]. Several animal species are homed in the park: wildlife species are wild boar (Sus scrofa), wolf (Canis lupus), red fox (Vulpes vulpes), badger (Meles meles), weasel (Mustela nivalis), pine marten (Martes martes) and stone marten (Martes foina); domestic horse and cattle are also present. Numerous wild bird species reside in the park, which also serves as a stopover habitat for migratory birds [19].
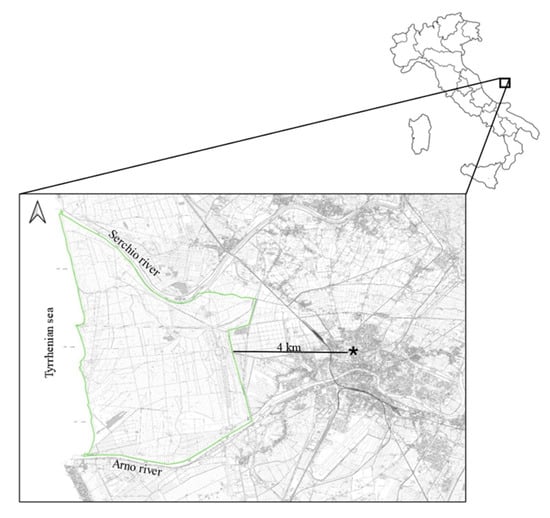
Figure 1.
Map of the study area. Light green line represents a border of the study area, while star represents the leaning tower.
2.2. Sampling
Sampling was conducted on fallow deer from July to December 2022, during maximum effort to implement the management plan. The animals were taken by gamekeepers in compliance with the management plan adopted and authorized by the park authority. Technical staff assisted the gamekeepers and conducted sampling following each fallow deer culling. Fallow deer did not show clinical signs and they did not undergo postmortem examination because they were destined for meat consumption. We supported the gamekeepers and carried out the sampling directly, between 1 and 3 h after the death of the deer.
In detail, spleen and kidney samples were collected from 162 fallow deer of which n = 25 bucks (male over 4 years old), n = 13 sores (male 2–4 y.o.), n = 11 prickets (male 1–2 y.o.), n = 29 fawns (male younger than 1 y.o.), n = 58 adult female (over 4 y.o.) and n = 29 fawns (female younger than 1 y.o.). Furthermore, from a subsample (78 deer of which n = 14 bucks, n = 11 sores, n = 11 prickets, n = 13 fawn males, n = 17 adult females, n = 12 fawn females), we also collected the hearts.
All samples were collected at the local butcher shop within the park using sterilized equipment to ensure aseptic conditions. Following killing, each fallow deer was immediately eviscerated in a refrigerated room. Using a sterilized scalpel, portions of the spleen, kidney, and heart were excised. Each sample weighed a minimum of 100 g and was collected in a sterilized plastic box. While awaiting transport to the analysis laboratory, the samples were stored in a refrigerator at a maximum temperature of 4 °C. Delivery to the laboratory occurred within 6 to 30 h after sampling.
2.3. Molecular Analyses
Samples were promptly submitted for DNA extraction. About 10 mg of tissue was collected from the inner spleen and myocardium; 10 mg of kidney cortex was taken after removing the renal capsule. All specimens were submitted to the DNA extraction with the DNeasy Tissue kit (Qiagen GmbH, Hilden, Germany) according to the manufacturer’s instructions; extraction controls to monitor cross-contamination of samples were included. DNA was quantified by measuring the optical density (OD) at 260 nm. Meanwhile, DNA purity was determined by calculating the ratio of absorbance at 260 and 280 nm; DNA extracts with an A260/A280 ratio of greater than 2.0 were considered of good quality and used in PCR assays. OD measurements were performed using a Nanodrop ND–1000 spectrophotometer (NanoDrop Technologies, Wilmington, DE, USA). DNAs were stored at 4 °C for 24–48 h, then used in PCR assays.
All spleens were tested for A. phagocytophilum, B. burgdorferi s.l., Brucella spp., C. abortus, C. burnetii, F. tularensis, N. caninum, T. gondii, and piroplasms. Heart samples were analyzed for T. gondii and N. caninum, and kidneys for Leptospira spp.
Different PCR protocols, summarized in Table 1, were employed to detect the investigated pathogens. Negative and positive controls were added in each PCR assay. Sterile distilled water was used instead of DNA in the negative control. DNA samples extracted from slides used for indirect immunofluorescent assay or bacterial cultures were included as positive controls (Table S1).

Table 1.
Target genes, primers and annealing temperature for the PCR assays carried out to detect the DNA of each pathogen.
For each protocol, PCR reactions were carried out in a 25 µL final volume, containing 12.5 µL EconoTaq PLUS 2× Master Mix (Lucigen Corporation, Middleton, WI, USA), 0.3 µM of each primer, 3 µL of extracted DNA and ultrapure water to reach the final volume. All PCR amplifications were performed in an automated thermal cycler SimpliAmp™ Thermal Cycler (Applied Biosystems, Waltham, MA, USA): 95 °C for 5 min of initial denaturation followed by 40 cycles at 95 °C for 1 min; annealing (temperatures reported in Table 1) for 1 min; and 72 °C for 2 min. A final step of 10 min at 72 °C completed the reaction.
For the detection of A. phagocytophilum DNA, a nested PCR protocol was used [20].
For the detection of piroplasms, the first PCR protocol was used [26]; positive samples were successively subjected to a second PCR assay, amplifying a longer fragment (about 1700 bp) of the 18S rRNA [27] in order to achieve correct species identification with sequencing analyses.
All PCR products were analyzed by electrophoresis on 1.5% agarose gel at 100 V for 45 min; gel was stained with ethidium bromide and observed. SharpMass™ 100 Plus Ladder (Euroclone, Milano, Italy) was added as a DNA marker.
Successively, amplicons of piroplasms obtained with the second PCR assay were sequenced by a commercial laboratory (BMR-Genomics, Padova, Italy) because most Babesia and Theileria species are amplified using the same set of primers for their similarity in the target gene. In order to confirm the positive results, A. phagocytophilum amplicons obtained with the second PCR step were submitted to sequencing analysis by the same commercial laboratory.
The resulting sequences were assembled and manually corrected through visual inspection of the electropherogram using BioEdit v.7.0.2. Subsequently, they were compared with reference sequences available in GenBank using the BLAST program 2.15.0 (http://www.ncbi.nlm.nih.gov/BLAST, accessed on 12 September 2024).
3. Results
We collected spleen, kidney, and heart samples, with the distribution reported in Table 2 and Table 3.

Table 2.
Distribution in relationship to the sex and age classes of fallow deer sampled for spleen and kidney.

Table 3.
Distribution in relationship to the sex and age classes of fallow deer sampled for heart.
All kidney samples were PCR-negative for Leptospira spp.
PCR for T. gondii and N. caninum carried out on all spleen and heart samples detected no positive reactions. All spleens were negative for F. tularensis, Brucella spp. C. abortus, C. burnetii and B. burgdorferi s.l.
Five (3.08%; 95% CI: 0.42–5.74%) spleens were positive for A. phagocytophilum, confirmed by the sequencing analyses of the amplicons obtained in the second PCR step. Twelve (7.40%; 95% CI: 3.37–11.43%) spleens were positive for piroplasm, and in all cases, the sequencing analyses of the amplicons obtained in both PCR assays identified Theileria cervi. No coinfections were detected.
4. Discussion
All tested fallow deer were negative for Brucella spp., in agreement with our recent study carried out in roe deer living in Central Italy [15]. These findings are strictly related to the Italian prophylaxis plans that have significantly reduced the circulation of brucellae; Tuscany is currently considered an officially free territory according to community legislation (EU legislation 1332 of 17 May 2024 for bovine brucellosis, EU legislation 2032 of 29 July 2024 for ovine-caprine brucellosis) [30]. Deer are mainly susceptible to B. abortus and B. melitensis infection, but they are not considered important in the epidemiology of these bacteria because they seem to act as dead-end reservoirs [31]. The limited cases of brucellosis reported in deer populations suggested this role [31,32,33], although serologically positive deer have been detected [34]. Brucella abortus and B. melitensis are pathogens responsible for severe disease in humans and animals, mainly ruminants, in which they cause reproductive disorders [32].
No fallow deer positive to F. tularensis were detected, in agreement with previous surveys carried out in deer [13] and other wild animals [35,36,37] in Central Italy. Francisella tularensis is the etiologic agent of the zoonosis called tularemia; it is a highly infectious bacterium transmitted by tick bites and via the oral/inhalation route [38]. Francisella tularensis has been isolated worldwide from more than 250 species, mainly lagomorphs and rodents, but also insectivores, carnivores, marsupials, ungulates, birds, amphibians, fishes, and invertebrates [38]. It is present in the Northern hemisphere, and it does not appear to be widespread in certain areas, such as Central Italy, where, however, the constant monitoring of wildlife is essential to better evaluate the risk of transmission to humans.
In this survey, all samples were C. burnetii-negative. This is the etiologic agent of the Q fever, a zoonotic infectious disease responsible for relevant reproductive disorders, mainly abortion, in ruminants and other mammal species [39]. Recently, C. burnetii DNA was found in 3/72 (4.16%) spleens sampled from roe deer in Central Italy [15], but the infection has also been documented in fallow, red, and roe deer, in different European areas [40]. The negative results of this survey may be attributed to the absence or minimal circulation of the pathogens in the selected area. This interpretation is supported by reports from technicians and park personnel, who did not observe cases of abortion in ungulates.
Similarly, the negative results for C. abortus are in agreement with our recent survey carried out in roe deer from Central Italy [15]. Chlamydia abortus is the causative agent of enzootic abortion in sheep, but it also often infects cattle; wild ruminants are known as susceptible hosts [23]. However, it is difficult to understand if these findings are related to the absence of the pathogen in the selected area or to scarce susceptibility of wild ruminants to C. abortus. In fact, information about chlamydiosis in deer is scant; only one case report described abortion related to C. abortus in a springbok antelope (Antidorcas marsupialis) in France [23], and further data are based on serological surveys. In particular, in Italy, the seroprevalence of 79% for C. psittaci was found in fallow deer [41]; the seroprevalences of 9.6% and 3.3% were observed in red deer for C. psittaci and C. suis, respectively, but no animals had antibodies to C. abortus and C. pecorum [42].
No samples had B. burgdorferi s.l. DNA, in agreement with a previous survey carried out in Poland, where all 74 analyzed red deer were PCR-negative for this pathogen [43]. Borrelia burgdorferi s.l., the causative agent of Lyme disease, is known as a tick-borne bacterium able to infect different animal species and cause disease in humans, dogs, horses and cattle [21]. The susceptibility of O. virginianus to Borrelia lonestari was demonstrated through an experimental infection; infected deer did not show overt clinical signs of disease at any time during the experiment, but they developed spirochetemia detectable by the direct examination of blood smears and/or by PCR [44].
Conversely, it has been supposed that deer are incompetent hosts for borrelia [45], and our results could be related to this aspect. In fact, borreliae are known to be sensitive to destruction by the complement system of host cervid species; the borreliacidal activity of white-tailed deer (Odocoileus virginianus) serum has been recently demonstrated [45]. However, few studies have been carried out to detect this pathogen in blood and tissues from deer. In Central Italy, 2/60 (3.33%) red deer and 1/72 (1.39%) roe deer were PCR-positive [13,16]. One roe deer (0.21%), among the 461 analyzed, was positive for B. burgdorferi s.l. in Netherland [2]. Lane et al. [46] found B. burgdorferi DNA in 5.12% and 20.31% of black-tailed (Odocoileus hemionus colombianus) in two different areas of Northern California, respectively, as did Trout-Fryxell et al. [47] in 21.2% of white-tailed deer blood samples in Arkansas (USA).
The 3.08% prevalence found for A. phagocytophilum, while not high compared to previous surveys [48], confirms that deer are susceptible to the pathogen and indicates the presence of this bacterium in the geographic area where animals live. The small number of positive animals did not allow for statistically evaluating the differences between age/gender classes or months of sampling. This tick-borne bacterium can infect several animal species, most of which act as asymptomatic reservoirs; other species, such as dogs, cattle and horse, often develop disease characterized by mild or severe signs [49,50]. A past investigation in fallow deer living in the same natural park detected a prevalence of 72.4% of this bacterium [48]; in addition, A. phagocytophilum DNA was found in 40% of red deer [13] and 59.72% of roe deer [16] from other areas in Central Italy. Many surveys have been carried out in deer populations living in different European areas; prevalences ranging from 20% to up 90% were found [1,14,51,52,53,54]. Recently, it has been supposed that C. elaphus and C. capreolus may serve as reservoirs of zoonotic A. phagocytophilum strains [55].
All heart and spleen samples scored negative for N. caninum DNA. This finding partially agrees with data from the literature. Fallow deer, in fact, have been surveyed by serological methods [56,57,58,59,60] only, and seroprevalence resulted ranged from 0% [59,60,61] to 2.9% [57]. Although in a limited study in Mexico, 2 out of 19 tested fallow deer were serologically positive [56]. Molecular analyses show a lower sensitivity when compared to serology, so our results are not surprising. However, N. caninum could represent a threat for D. dama, being reported as responsible for a fatal case of meningoencephalitis in a 3-week-old fawn [62]. Even if not zoonotic, this parasite is a leading cause of bovine abortion and stillbirth, as well as capable of causing neuromuscular disease in dogs [56], and, being both animal species present in San Rossore Park, attention should be paid.
Similarly, DNA from T. gondii was not found in any specimens. This finding fully agrees with the results provided by Stollberg et al. [63] who found a 0% PCR prevalence rate in 80 fallow deer from Brandeburg (Germany), with a 6.8% seroprevalence. Similarly, a 1% seroprevalence was reported in D. dama from Czek Republic [58]. Furthermore, serological surveys yielded seroprevalences ranging from 0% [59] to 37.4% [64], showing a large variability of this value among different populations. Interestingly, a wild ungulates community from Spain showed the highest seroprevalence rates [64,65]. Other cervids have been checked for these protozoa in our region, with different results. A previous molecular survey on the blood samples of red deer from another area of Tuscany reported a prevalence of 22% and 28% for the DNA of T.gondii and N. caninum, respectively [66], while a further recent study on the spleen specimens of roe deer showed a prevalence of 1.38% for T. gondii and 0% for N. caninum [15].
Unfortunately, serum samples of the selected animals were not available in our study, precluding the evaluation of seroprevalence rates, so the occurrence of N. caninum and T. gondii, although low, cannot be ruled out.
Theileria cervi was detected in 7.40% of spleen specimens as the sole piroplasm species. Also, in this case, the small number of positive animals did not allow for statistically evaluating the differences between age/gender classes or months of sampling. Theileria cervi has been frequently reported in cervids from Canada and the USA [67], where it is primarily transmitted by Amblyomma americanum [68]. The piroplasm was identified in O. virginianus [69] and more recently in equids from Mexico [70], as well as in cervids from Argentina and Brazil [71,72]. The parasite is rarely responsible for clinical disease, except for animals living in areas with a high deer population, starved or coinfected with other agents [63,67]. To the best of our knowledge, T. cervi was not reported from Italy except for in a study carried out on Rhipicephalus sanguineus specimens from privately owned dogs in Italy [73], indicating the occurrence of this parasite in our country.
5. Conclusions
The fallow deer analyzed in the present investigation lived in a protected area where many wild animals, mammals and birds, are present. In addition, the park is largely frequented by people, including owners with their dogs. Most of the investigated pathogens were zoonotic, and can infect and cause diseases in dogs as well; therefore, being aware of the presence and diffusion of these agents in a given area allows for better understanding the risks of infections for people and dogs.
The tested animals were negative for most of the pathogens investigated, suggesting that they do not have a relevant role in the epidemiology of these agents. However, deer may be important for the epidemiology of the pathogens transmitted by hematophagous arthropods. The presence of deer can provide a source of blood meals for ticks in the absence of other hosts, potentially supporting larger tick populations within an environment [74].
Therefore, monitoring the spreading of pathogens in deer populations, with a particular emphasis on zoonotic ones, is pivotal to verify the epidemiological scenarios of the microorganisms and take appropriate preventive measures in areas like this park, frequently visited by people.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/biology14040342/s1, Table S1: Positive controls included in the PCR assays.
Author Contributions
Conceptualization, V.V.E. and F.M.; methodology, V.V.E., P.B., C.T. and F.M.; formal analysis, V.V.E., C.T., F.B., G.C. and B.B.; investigation, C.T., G.C., B.B., P.B., M.D.F. and M.A.; writing—original draft preparation, V.V.E. and F.M.; writing—review and editing, V.V.E., P.B., M.D.F. and F.M.; supervision, V.V.E. and F.M.; funding acquisition, V.V.E. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the University of Pisa, grant number PRA_2020_88.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
All data are reported in the manuscript.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Silaghi, C.; Fröhlich, J.; Reindl, H.; Hamel, D.; Rehbein, S. Anaplasma phagocytophilum and Babesia Species of Sympatric Roe Deer (Capreolus capreolus), Fallow Deer (Dama dama), Sika Deer (Cervus nippon) and Red Deer (Cervus elaphus) in Germany. Pathogens 2020, 9, 968. [Google Scholar] [CrossRef]
- Wijburg, S.R.; Fonville, M.; de Bruin, A.; van Rijn, P.A.; Montizaan, M.G.E.; van den Broek, J.; Sprong, H.; Rijks, J.M. Prevalence and predictors of vector-borne pathogens in Dutch roe deer. Parasites Vectors 2022, 15, 76. [Google Scholar] [CrossRef] [PubMed]
- Middlebrook, E.A.; Romero, A.T.; Bett, B.; Nthiwa, D.; Oyola, S.O.; Fair, J.M.; Bartlow, A.W. Identification and distribution of pathogens coinfecting with Brucella spp., Coxiella burnetii and Rift Valley fever virus in humans, livestock and wildlife. Zoonoses Public Health 2022, 69, 175–194. [Google Scholar] [CrossRef] [PubMed]
- Dashti, A.; Köster, P.C.; Bailo, B.; de Las Matas, A.S.; Habela, M.Á.; Rivero-Juarez, A.; Vicente, J.; Serrano, E.; Arnal, M.C.; de Luco, D.F.; et al. Occurrence and limited zoonotic potential of Cryptosporidium spp., Giardia duodenalis, and Balantioides coli infections in free-ranging and farmed wild ungulates in Spain. Res. Vet. Sci. 2023, 159, 189–197. [Google Scholar] [CrossRef]
- Ciuti, S.; Apollonio, M. Ecological sexual segregation in fallow deer (Dama dama): A multisptial and multiptemporal approach. Behav. Ecol. Sociobiol. 2008, 62, 1747–1759. [Google Scholar]
- Brogi, R.; Bongi, P.; Del Frate, M.; Sieni, S.; Cavallera, A.; Apollonio, M. Intra-guild competition and ecosystem services of mammal scavengers in a new colonized wolf landscape. Behav. Ecol. Sociobiol. 2025, 79, 20. [Google Scholar] [CrossRef]
- Ostfeld, R.S.; Keesing, F. The function of biodiversity in the ecology of vector-borne zoonotic diseases. Can. J. Zool. 2000, 78, 2061–2078. [Google Scholar]
- LoGuidice, K.; Ostfeld, R.S.; Schmidt, K.A.; Keesing, F. The ecology of infectious disease: Effect of host diversity and community composition on Lyme disease risk. Proc. Natl. Acad. Sci. USA 2003, 100, 567–571. [Google Scholar]
- Böhm, M.; White, P.C.L.; Chambers, J.; Smith, L.; Hurchings, M.R. Wild deer as a source of infection for livestock and humans in the UK. Vet. J. 2007, 174, 270–276. [Google Scholar]
- Galuppi, R.; Aureli, S.; Bonoli, C.; Caffara, M.; Tampieri, M.P. Detection and molecular characterization of Theileria sp. in fallow deer (Dama dama) and ticks from an Italian natural preserve. Res. Vet. Sci. 2011, 91, 110–115. [Google Scholar] [CrossRef]
- Veronesi, F.; Galuppi, R.; Tampieri, M.P.; Bonoli, C.; Mammoli, R.; Piergili Fioretti, D. Prevalence of Anaplasma phagocytophilum in fallow deer (Dama dama) and feeding ticks from an Italy preserve. Res. Vet. Sci. 2011, 90, 40–43. [Google Scholar] [CrossRef] [PubMed]
- Menandro, M.L.; Martini, M.; Dotto, G.; Mondin, A.; Ziron, G.; Pasotto, D. Tick-borne zoonotic bacteria in fallow deer (Dama dama) in Euganean Hills Regional Park of Italy. Int. J. Infect. Dis. 2016, 53 (Suppl. S61), 19.086. [Google Scholar] [CrossRef][Green Version]
- Ebani, V.V.; Rocchigiani, G.; Bertelloni, F.; Nardoni, S.; Leoni, A.; Nicoloso, S.; Mancianti, F. Molecular survey on the presence of zoonotic arthropod-borne pathogens in wild red deer (Cervus elaphus). Comp. Immunol. Microbiol. Infect. Dis. 2016, 47, 77–80. [Google Scholar] [CrossRef]
- Cafiso, A.; Bazzocchi, C.; Cavagna, M.; Di Lorenzo, E.; Serra, V.; Rossi, R.; Comazzi, S. Molecular Survey of Babesia spp. and Anaplasma phagocytophilum in Roe Deer from a Wildlife Rescue Center in Italy. Animals 2021, 11, 3335. [Google Scholar] [CrossRef] [PubMed]
- Ebani, V.V.; Trebino, C.; Guardone, L.; Bertelloni, F.; Cagnoli, G.; Altomonte, I.; Vignola, P.; Bongi, P.; Mancianti, F. Retrospective Molecular Survey on Bacterial and Protozoan Abortive Agents in Roe Deer (Capreolus capreolus) from Central Italy. Animals 2022, 12, 3202. [Google Scholar] [CrossRef]
- Ebani, V.V.; Guardone, L.; Rocchigiani, G.; Bascherini, A.; Cagnoli, G.; Bertelloni, F.; Bongi, P.; Russo, C.; Riccioli, F.; Mancianti, F. Molecular survey on the presence of arthropod-borne bacteria and protozoans in roe deer (Capreolus capreolus) and ticks from Central Italy. Acta Trop. 2022, 233, 106586. [Google Scholar] [CrossRef]
- Floris, I.; Vannuccini, A.; Ligotti, C.; Musolino, N.; Romano, A.; Viani, A.; Bianchi, D.M.; Robetto, S.; Decastelli, L. Detection and Characterization of Zoonotic Pathogens in Game Meat Hunted in Northwestern Italy. Animals 2024, 14, 562. [Google Scholar] [CrossRef]
- Franceschi, S.; Bongi, P.; Del Frate, M.; Fattorini, L.; Apollonio, M. A sampling strategy for habitat selection, mapping, and abundance estimation of deer by pellet counts. J. Wildl. Manag. 2023, 87, e22345. [Google Scholar] [CrossRef]
- Gorreri, L.; Somoncini, Y. Nesting Birds in Migliarino—San Rossore—Massaciuccoli Regional Park; Cambi Editore: Siena, Italy, 2010; ISBN 888848213X. (In Italian) [Google Scholar]
- Massung, R.F.; Slater, K.; Owens, J.H.; Nicholson, W.L.; Mather, T.N.; Solberg, V.B.; Olson, J.G. Nested PCR assay for detection of granulocytic Ehrlichiae. J. Clin. Microbiol. 1998, 36, 1090–1095. [Google Scholar] [CrossRef]
- Chang, Y.F.; Novosol, V.; McDonough, S.P.; Chang, C.F.; Jacobson, R.H.; Divers, T.; Quimby, F.W.; Shin, S.; Lein, D.H. Experimental infection of ponies with Borrelia burgdorferi by exposure to Ixodid ticks. Vet. Pathol. 2000, 37, 68–76. [Google Scholar] [CrossRef]
- Romero, C.; Gamazo, C.; Pardo, M.; Lopez-Goni, I. Specific detection of Brucella DNA by PCR. J. Clin. Microbiol. 1995, 33, 615–617. [Google Scholar] [CrossRef] [PubMed]
- Berri, M.; Bernard, F.; Lecu, A.; Ollivet-Courtois, F.; Rodolakis, A. Molecular characterization and ovine live vaccine 1B evaluation toward a Chlamydophila abortus strain isolated from springbok antelope abortion. Vet. Microbiol. 2004, 103, 231–240. [Google Scholar] [CrossRef]
- Milutinovi’c, M.; Masuzawa, T.; Tomanovi’c, S.; Radulovi’c, Z.; Fukui, T.; Okamoto, Y. Borrelia burgdorferi sensu lato, Anaplasma phagocytophilum, Francisella tularensis and their co-infections in host-seeking Ixodes ricinus ticks collected in Serbia. Exp. Appl. Acarol. 2008, 45, 171–183. [Google Scholar] [CrossRef]
- Stoddard, R.A.; Gee, J.E.; Wilkins, P.P.; McCaustland, K.; Hoffmaster, A.R. Detection of pathogenic Leptospira spp. Through TaqMan polymerase chain reactionn targeting the LipL32 gene. Diagn. Microbiol. Infect. Dis. 2009, 64, 247–255. [Google Scholar] [CrossRef]
- Beck, R.; Vojta, L.; Mrljak, V.; Marinculi’c, A.; Beck, A.; Zivicnjak, T.; Cacciò, S.M. Diversity of Babesia and Theileria species in symptomatic and asymptomatic dogs in Croatia. Int. J. Parasitol. 2009, 39, 843–848. [Google Scholar] [CrossRef] [PubMed]
- Cacciò, S.M.; Antunovic, B.; Moretti, A.; Mangili, V.; Marinculic, A.; Baric, R.R.; Slemenda, S.B.; Pieniazek, N.J. Molecular characterisation of Babesia canis canis and Babesia canis vogeli from naturally infected European dogs. Vet. Parasitol. 2002, 106, 285–292. [Google Scholar] [CrossRef]
- Müller, N.; Zimmermann, V.; Hentrich, B.; Gottstein, B. Diagnosis of Neospora caninum and Toxoplasma gondii infection by PCR and DNA hybridization immunoassay. J. Clin. Microbiol. 1996, 34, 2850–2852. [Google Scholar] [CrossRef]
- Jones, C.D.; Okhravi, N.; Adamson, P.; Tasker, S.; Lightman, S. Comparison of PCR detection methods for B1, P30, and 18s rDNA genes of Toxoplasma gondii in aqueous humor. Investig. Ophthalmol. Vis. Sci. 2000, 41, 634–644. [Google Scholar]
- Bollettino Nazionale Epidemiologico Veterinario. Available online: https://www.izs.it/BENV_NEW/territori-indenni_en.html (accessed on 16 January 2025).
- Garin-Bastuji, B.; Hars, J.; Drapeau, A.; Cherfa, M.A.; Game, Y.; Le Horgne, J.M.; Rautureau, S.; Maucci, E.; Pasquier, J.J.; Jay, M.; et al. Reemergence of Brucella melitensis in wildlife, France. Emerg. Infect. Dis. 2014, 20, 157–1571. [Google Scholar] [CrossRef]
- Godfroid, J.; Cloeckaert, A.; Liautard, J.P.; Kohler, S.; Fretin, D.; Walravens, K.; Garín Bastuji, B.; Letesson, J.J. From the discovery of the Malta fever’s agent to the discovery of a marine mammal reservoir, brucellosis has continuously been a re-emerging zoonosis. Vet. Res. 2005, 36, 313–326. [Google Scholar] [CrossRef]
- Conner, M.M.; Ebinger, M.R.; Blanchong, J.A.; Cross, P.C. Infectious disease in cervids of North America. Ann. N. Y. Acad. Sci. 2008, 1134, 146–172. [Google Scholar] [CrossRef] [PubMed]
- Pires, H.; Cardoso, L.; Lopes, A.P.; Fontes, M.D.C.; Santos-Silva, S.; Matos, M.; Pintado, C.; Roque, N.; Fonseca, L.F.; Morgado, I.; et al. Hunting for Answers: Assessing Brucella spp. Seroprevalence and Risks in Red Deer and Wild Boar in Central Portugal. Pathogens 2024, 13, 242. [Google Scholar] [CrossRef] [PubMed]
- Pascucci, I.; Di Domenico, M.; Dall’Acqua, F.; Sozio, G.; Cammà, C. Detection of Lyme disease and Q fever agents in wild rodents in central Italy. Vector Borne Zoonotic Dis. 2015, 15, 404–411. [Google Scholar]
- Ebani, V.V.; Nardoni, S.; Giani, M.; Rocchigiani, G.; Archin, T.; Altomonte, I.; Poli, A.; Mancianti, F. Molecular survey on the occurrence of avian haemosporidia, Coxiella burnetii and Francisella tularensis in waterfowl from central Italy. Int. J. Parasitol. Parasites Wildl. 2019, 10, 87–92. [Google Scholar] [CrossRef]
- Ebani, V.V.; Rocchigiani, G.; Nardoni, S.; Bertelloni, F.; Vasta, V.; Papini, R.A.; Verin, R.; Poli, A.; Mancianti, F. Molecular detection of tick-borne pathogens in wild red foxes (Vulpes vulpes) from Central Italy. Acta Trop. 2017, 172, 197–200. [Google Scholar] [CrossRef] [PubMed]
- Ammam, I.; Brunet, C.D.; Boukenaoui-Ferrouk, N.; Peyroux, J.; Berthier, S.; Boutonnat, J.; Rahal, K.; Bitam, I.; Maurin, M. Francisella tularensis PCR detection in Cape hares (Lepus capensis) and wild rabbits (Oryctolagus cuniculus) in Algeria. Sci. Rep. 2022, 12, 21451. [Google Scholar] [CrossRef]
- Angelakis, E.; Raoult, D. Q Fever. Vet. Microbiol. 2010, 140, 297–309. [Google Scholar] [CrossRef]
- Celina, S.S.; Cerný, J. Coxiella burnetii in ticks, livestock, pets and wildlife: A mini-review. Front. Vet. Sci. 2022, 9, 1068129. [Google Scholar] [CrossRef]
- Giovannini, A.; Cancellotti, F.M.; Turilli, C.; Randi, E. Serological investigations for some bacterial and viral pathogens in fallow deer (Cervus dama) and wild boar (Sus. scrofa) of the San Rossore Preserve Tuscany, Italy. J. Wildl. Dis. 1988, 24, 127–132. [Google Scholar]
- Di Francesco, A.; Donati, M.; Nicoloso, S.; Orlandi, L.; Baldelli, R.; Salvatore, D.; Sarli, G.; Cevenini, R.; Morandi, F. Chlamydiosis: Seroepidemiologic survey in a red deer (Cervus elaphus) population in Italy. J. Wildl. Dis. 2012, 48, 488–491. [Google Scholar] [CrossRef]
- Wodeka, B. Significance of red deer (Cervus elaphus) in the ecology of Borrelia burgdorferi sensu lato. Wiad. Parazytol. 2007, 53, 231–237. [Google Scholar]
- Moyer, P.L.; Varela, A.S.; Luttrell, M.P.; Moore, V.A., 4th; Stallknecht, D.E.; Little, S.E. White-tailed deer (Odocoileus virginianus) develop spirochetemia following experimental infection with Borrelia lonestari. Vet. Microbiol. 2006, 115, 229–236. [Google Scholar] [CrossRef]
- Pearson, P.; Rich, C.; Feehan, M.J.R.; Ditchkoff, S.S.; Rich, S.M. White-Tailed Deer Serum Kills the Lyme Disease Spirochete, Borrelia burgdorferi. Vector Borne Zoonotic Dis. 2023, 23, 303–305. [Google Scholar] [CrossRef] [PubMed]
- Lane, R.S.; Mun, J.; Parker, J.M.; White, M. Columbian black-tailed deer (Odocoileus hemionus columbianus) as hosts for Borrelia spp. in northern California. J. Wildl. Dis. 2005, 41, 115–125. [Google Scholar] [CrossRef] [PubMed]
- Trout Fryxell, R.T.; Dayton Steelman, C.; Szalanski, A.L.; Kvamme, K.L.; Billingsley, P.M.; Williamson, P.C. Survey of Borreliae in ticks, canines, and white-tailed deer from Arkansas, USA. Parasites Vectors 2012, 5, 139. [Google Scholar] [CrossRef]
- Ebani, V.V.; Cerri, D.; Fratini, F.; Ampola, M.; Andreani, E. Anaplasma phagocytophilum infection in a fallow deer (Dama dama) population in a preserve of central Italy. New Microbiol. 2007, 30, 161–165. [Google Scholar] [PubMed]
- El Hamiani Khatat, S.; Daminet, S.; Duchateau, L.; Elhachimi, L.; Kachani, M.; Sahibi, H. Epidemiological and Clinicopathological Features of Anaplasma phagocytophilum Infection in Dogs: A Systematic Review. Front. Vet. Sci. 2021, 8, 686644. [Google Scholar] [CrossRef]
- Schäfer, I.; Silaghi, C.; Fischer, S.; Marsboom, C.; Hendrickx, G.; Gehlen, H.; Müller, E. Detection of Anaplasma phagocytophilum in horses from Germany by molecular and serological testing (2008–2021). Vet. Parasitol. 2022, 312, 109840. [Google Scholar] [CrossRef]
- Kauffmann, M.; Rehbein, S.; Hamel, D.; Lutz, W.; Heddergott, M.; Pfister, K.; Silaghi, C. Anaplasma phagocytophilum and Babesia spp. in roe deer (Capreolus capreolus), fallow deer (Dama dama) and mouflon (Ovis musimon) in Germany. Mol. Cell. Probes 2017, 31, 46–54. [Google Scholar] [CrossRef]
- Hornok, S.; Sug’ar, L.; Fern’andez de Mera, I.G.; de la Fuente, J.; Horv’ath, G.; Kov’acs, T.; Micsutka, A.; Gönczi, E.; Flaisz, B.; Tak’acs, N.; et al. Tick- and fly-borne bacteria in ungulates: The prevalence of Anaplasma phagocytophilum, haemoplasmas and rickettsiae in water buffalo and deer species in central Europe. Hungary. BMC Vet. Res. 2018, 14, 98. [Google Scholar] [CrossRef]
- Razanske, I.; Rosef, O.; Radzijevskaja, J.; Bratchikov, M.; Griciuviene, L.; Paulauskas, A. Prevalence and co-infection with tick-borne Anaplasma phagocytophilum and Babesia spp. in red deer (Cervus elaphus) and roe deer (Capreolus capreolus) in Southern Norway. Int. J. Parasitol. Parasites Wildl. 2019, 8, 127–134. [Google Scholar] [CrossRef] [PubMed]
- Grassi, L.; Franzo, G.; Martini, M.; Mondin, A.; Cassini, R.; Drigo, M.; Pasotto, D.; Vidorin, E.; Menandro, M.L. Ecotyping of Anaplasma phagocytophilum from wild ungulates and ticks shows circulation of zoonotic strains in Northeastern Italy. Animals 2021, 11, 310. [Google Scholar] [CrossRef] [PubMed]
- Myczka, A.W.; Steiner-Bogdaszewska, Ż.; Oloś, G.; Bajer, A.; Laskowski, Z. Diversity of Anaplasma phagocytophilum Strains from Roe Deer (Capreolus capreolus) and Red Deer (Cervus elaphus) in Poland. Animals 2024, 14, 637. [Google Scholar] [CrossRef]
- De La Torre, J.R.; Bautista-Piña, C.; Alfonso Ortega, S.J.; Cantu-Covarruvias, A.; Genoveva Alvarez-Ojeda, M.; Romero-Salas, D.; Henke, S.E.; Hilton, C.D.; Hewitt, D.G.; De Young, R.W.; et al. Neospora caninum in Axis Deer (Axis axis) and Fallow Deer (Dama dama) in Northern Mexico. J. Wildl. Dis. 2017, 53, 186–187. [Google Scholar] [CrossRef] [PubMed]
- Bień, J.; Moskwa, B.; Bogdaszewski, M.; Cabaj, W. Detection of specific antibodies anti-Neospora caninum in the fallow deer (Dama dama). Res. Vet. Sci. 2012, 92, 96–98. [Google Scholar] [CrossRef]
- Bartova, E.; Sedlak, K.; Pavlik, I.; Literak, I. Prevalence of Neospora caninum and Toxoplasma gondii antibodies in wild ruminants from the countryside or captivity in the Czech Republic. J. Parasitol. 2007, 93, 1216–1218. [Google Scholar] [CrossRef]
- De Craeye, S.; Speybroeck, N.; Ajzenberg, D.; Dardé, M.L.; Collinet, F.; Tavernier, P.; Van Gucht, S.; Dorny, P.; Dierick, K. Toxoplasma gondii and Neospora caninum in wildlife: Common parasites in Belgian foxes and Cervidae? Vet. Parasitol. 2011, 178, 64–69. [Google Scholar] [CrossRef]
- Huaman, J.L.; Pacioni, C.; Doyle, M.; Forsyth, D.M.; Helbig, K.J.; Carvalho, T.G. Evidence of Australian wild deer exposure to N. caninum infection and potential implications for the maintenance of N. caninum sylvatic cycle. BMC Vet. Res. 2023, 19, 153. [Google Scholar] [CrossRef]
- Almería, S.; Vidal, D.; Ferrer, D.; Pabón, M.; Fernández-de-Mera, M.I.; Ruiz-Fons, F.; Alzaga, V.; Marco, I.; Calvete, C.; Lavin, S.; et al. Seroprevalence of Neospora caninum in non-carnivorous wildlife from Spain. Vet. Parasitol. 2007, 143, 21–28. [Google Scholar] [CrossRef] [PubMed]
- Soldati, S.; Kiupel, M.; Wise, A.; Maes, R.; Botteron, C.; Robert, N. Meningoencephalomyelitis caused by Neospora caninum in a juvenile fallow deer (Dama dama). J. Vet. Med. A Physiol. Pathol. Clin. Med. 2004, 51, 280–283. [Google Scholar] [CrossRef]
- Stollberg, K.C.; Schares, G.; Mayer-Scholl, A.; Hrushetska, I.; Diescher, S.; Johne, A.; Richter, M.H.; Bier, N.S. Comparison of Direct and Indirect Toxoplasma gondii Detection and Genotyping in Game: Relationship and Challenges. Microorganisms 2021, 9, 1663. [Google Scholar] [CrossRef]
- Castro-Scholten, S.; Cano-Terriza, D.; Jiménez-Ruiz, S.; Almería, S.; Risalde, M.A.; Vicente, J.; Acevedo, P.; Arnal, M.C.; Balseiro, A.; Gómez-Guillamón, F.; et al. Seroepidemiology of Toxoplasma gondii in wild ruminants in Spain. Zoonoses Public Health 2021, 68, 884–895. [Google Scholar] [CrossRef] [PubMed]
- Barroso, P.; García-Bocanegra, I.; Acevedo, P.; Palencia, P.; Carro, F.; Jiménez-Ruiz, S.; Almería, S.; Dubey, J.P.; Cano-Terriza, D.; Vicente, J. Long-Term Determinants of the Seroprevalence of Toxoplasma gondii in a Wild Ungulate Community. Animals 2020, 10, 2349. [Google Scholar] [CrossRef]
- Rocchigiani, G.; Nardoni, S.; D’Ascenzi, C.; Nicoloso, S.; Picciolli, F.; Papini, R.A.; Mancianti, F. Seroprevalence of Toxoplasma gondii and Neospora caninum in red deer from Central Italy. Ann. Agric. Environ. Med. 2016, 23, 699–701. [Google Scholar] [CrossRef]
- Almazán, C.; Scimeca, R.C.; Reichard, M.V.; Mosqueda, J. Babesiosis and Theileriosis in North America. Pathogens 2022, 11, 168. [Google Scholar] [CrossRef]
- Goddard, J.; Varela-Stokes, A.S. Role of the lone star tick, Amblyomma americanum (L.), in human and animal diseases. Vet. Parasitol. 2009, 160, 1–12. [Google Scholar] [CrossRef]
- Pavón-Rocha, A.J.; Cárdenas-Flores, A.; Rábago-Castro, J.L.; Barrón-Vargas, C.A.; Mosqueda, J. First molecular evidence of Theileria cervi infection in white-tailed deer (Odocoileus virginianus) in Mexico. Vet. Parasitol. Reg. Stud. Reports 2020, 22, 100482. [Google Scholar] [CrossRef] [PubMed]
- Bravo-Ramos, J.L.; Sánchez-Montes, S.; Sánchez-Otero, M.G.; Ballados-Gonzalez, G.G.; Gamboa-Prieto, J.; Romero-Salas, D.; Olivares-Muñoz, A. Molecular detection of Theileria cervi in equids from México. Res. Vet. Sci. 2023, 164, 105017. [Google Scholar] [CrossRef]
- Da Silveira, J.A.; Rabelo, E.M.; Lacerda, A.C.; Borges, P.A.L.; Tomás, W.M.; Pellegrin, A.O.; Tomich, R.G.P.; Ribeiro, M.F.B. Molecular detection and identification of hemoparasites in pampas deer (Ozotoceros bezoarticus Linnaeus, 1758) from the Pantanal Brazil. Ticks Tick Borne Dis. 2013, 4, 341–345. [Google Scholar] [CrossRef]
- Sebastian, P.S.; Falzone, M.P.; Lois, M.F.; Sartori, R.; Zimmerman, J.; Tarragona, E.L.; Nava, S. Phylogenetic position of Theileria cervi detected in Blastocerus dichotomus (Artiodactyla: Cervidae) with clinical symptoms from Argentina. Emerg. Anim. Species 2022, 5, 100014. [Google Scholar] [CrossRef]
- Zanet, S.; Battisti, E.; Pepe, P.; Ciuca, L.; Colombo, L.; Trisciuoglio, A.; Ferroglio, E.; Cringoli, G.; Rinaldi, L.; Maurelli, M.P. Tick-borne pathogens in Ixodidae ticks collected from privately-owned dogs in Italy: A country-wide molecular survey. BMC Vet. Res. 2020, 16, 46. [Google Scholar] [CrossRef] [PubMed]
- Hofmeester, T.R.; Sprong, H.; Jansen, P.A.; Prins, H.H.T.; van Wieren, S.E. Deer presence rather than abundance determines the population density of the sheep tick, Ixodes ricinus, in Dutch forests. Parasit. Vectors 2017, 10, 433. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).