Rat 3D Printed Induction Device (RAPID-3D): A 3D-Printed Device for Uniform and Reproducible Scald Burn Induction in Rats with Histological and Microvascular Validation
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Device Conceptualization and 3D Modeling
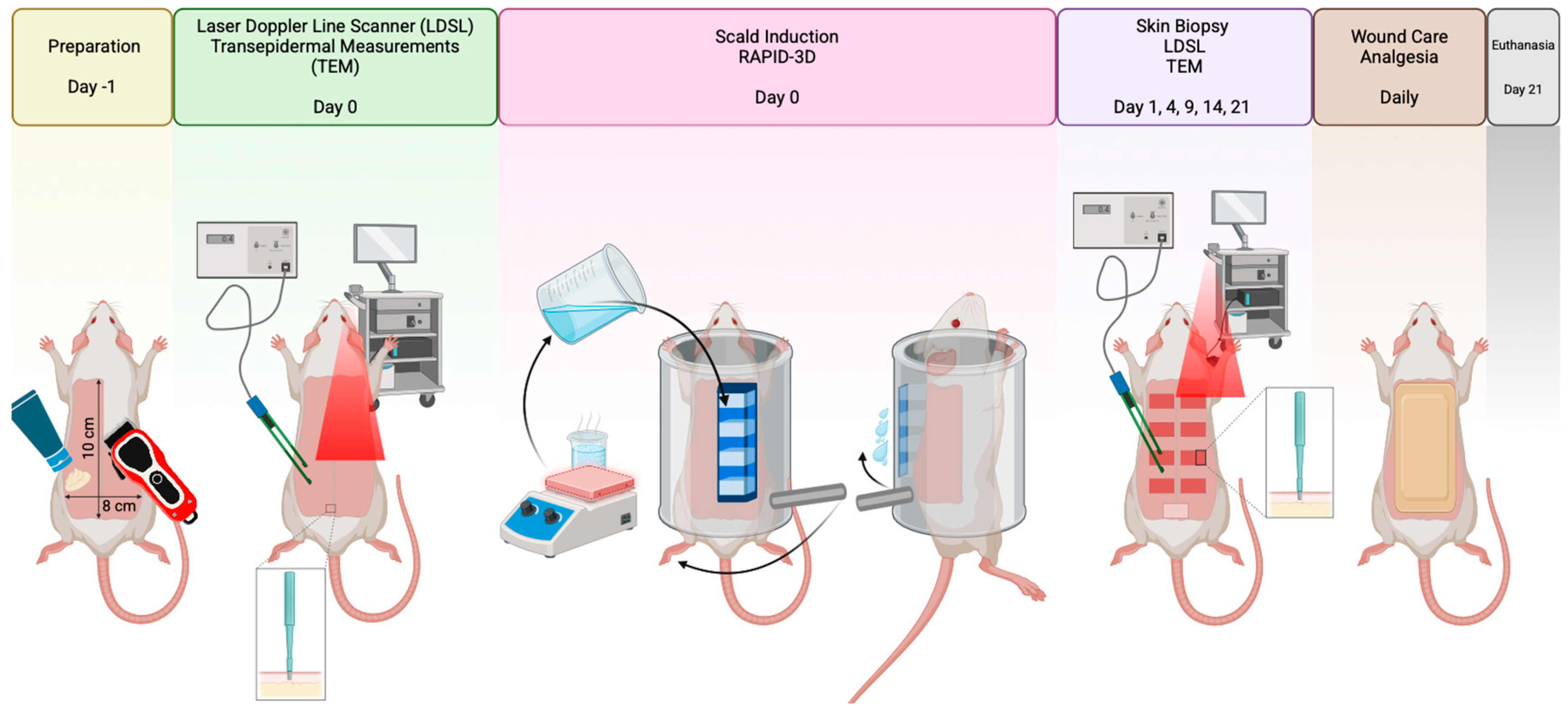
2.2. Study Design
2.3. Anesthesia and Animal Preparation
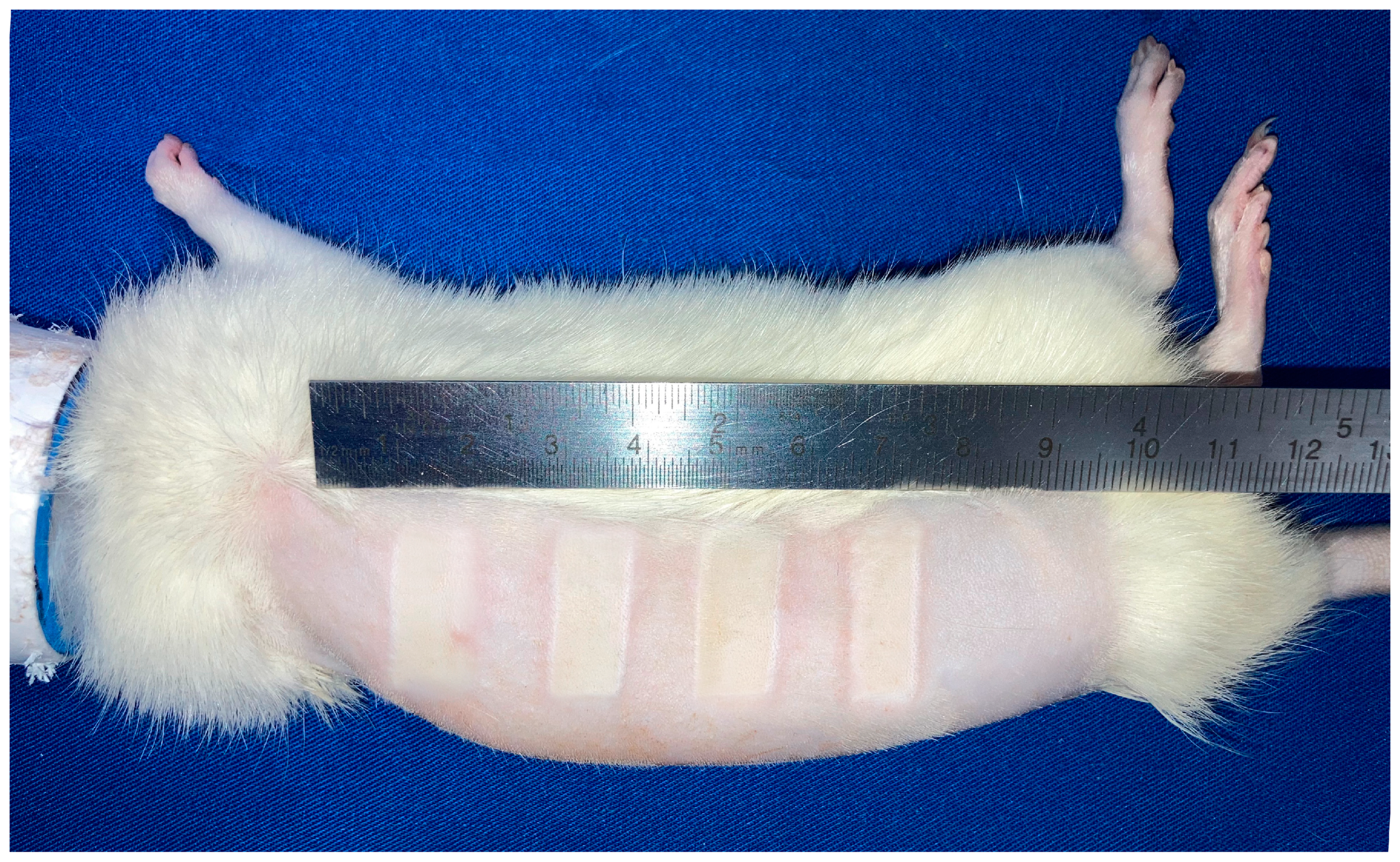
2.4. Induction of Burns
2.5. Aftercare of Experimental Animals
2.6. Morphological Analysis of Scald Burns
2.7. Transepidermal Changes of Scald Burns
2.8. Laser Doppler Line Scanner
2.9. Histology of Scald Burns
2.10. Statistical Analysis
3. Results
3.1. RAPID-3D Model
- A.
- A cuboid with four slotted bottom openings of 10 × 20 mm, spaced 10 mm apart and with 48 mL total capacity. The cuboid has an open top with a slanted edge and an evacuation overhang lip, and there are notches on the top edges for restraining and tensioning using two elastic bands.
- B.
- A cylinder with a slot for the cuboid and guide rails for trays that support an anesthetized rodent (rat or mouse) of variable weight/diameter and length. Stoppers on both ends of the cylinder allow a defined arc of rotation.
- C.
- Inner trays of variable sizes and lengths, running on the cylinder guides placed mm apart.
- D.
- An actioning handle attached with M5 screw to the cylinder.
- E.
- Two U-shaped supports in which the cylinder is mounted.
- F.
- An undertray in which the two U-shaped supports are fixed, and which acts as a hot water collection tray.
- G.
- Splash guards for the undertray, preventing spillage when emptying the cuboid.
3.2. Burn Area Uniformity
3.3. Wound Area Reduction over Time
3.4. Burn Depth and Histological Validation
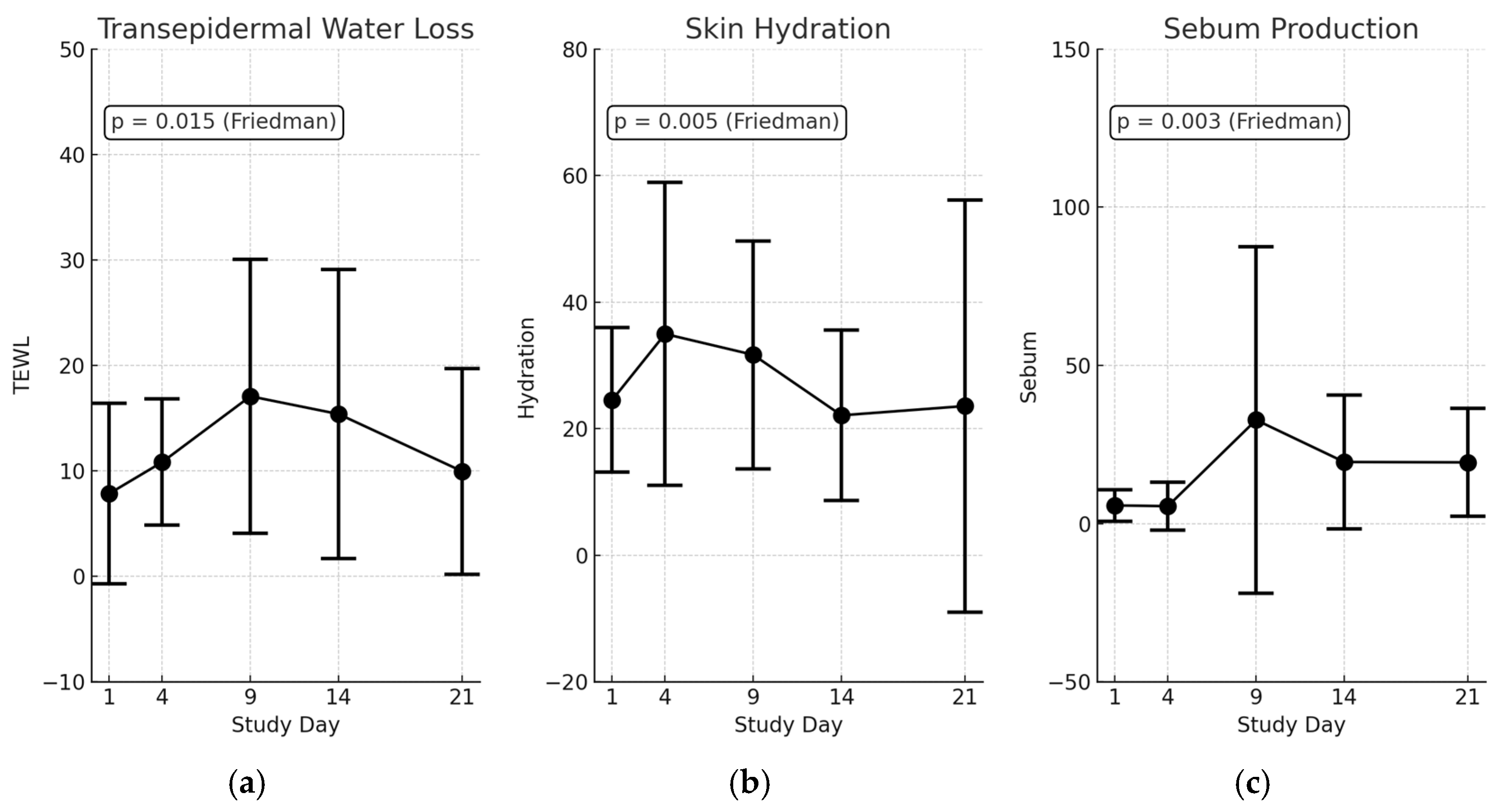
3.5. Transepidermal Changes in Scald Wounds
3.6. Microvascular Perfusion Trends (Moor LDLS Analysis)
4. Discussion
4.1. Rationale for Developing the Device
4.2. Comparison with Other Burn Inducing Models in Rodents
4.3. Limitations and Future Applications
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| RAPID-3D | Rat Printed Induction Device—3D |
| TBSA | Total Body Surface Area |
| LDLS | Laser Doppler Line Scanner |
| PLA | Polylactic acid |
| PETG | Polyethylene terephthalate glycol |
| SD | standard deviation |
| CV | coefficient of variation |
| ICC | intraclass correlation coefficient |
| SIRS | systemic inflammatory response syndrome |
| TEWL | Transepidermal Water Loss |
References
- Chen, Z.; Zeng, Y.; Tian, F. Developing a third-degree burn model of rats using the Delphi method. Sci. Rep. 2022, 12, 13852. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Hao, D.; Nourbakhsh, M. Recent Advances in Experimental Burn Models. Biology 2021, 10, 526. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Brigham, P.A.; McLoughlin, E. Burn incidence and medical care use in the United States: Estimates, trends, and data sources. J. Burn. Care Rehabil. 1996, 17, 95–107. [Google Scholar] [CrossRef] [PubMed]
- Clouatre, E.; Pinto, R.; Banfield, J.; Jeschke, M.G. Incidence of hot tap water scalds after the introduction of regulations in Ontario. J. Burn. Care Res. 2013, 34, 243–248. [Google Scholar] [CrossRef]
- Jordan, K.C.; Di Gennaro, J.L.; von Saint André-von Arnim, A.; Stewart, B.T. Global trends in pediatric burn injuries and care capacity from the World Health Organization Global Burn Registry. Front. Pediatr. 2022, 10, 954995. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Lee, C.J.; Mahendraraj, K.; Houng, A.; Marano, M.; Petrone, S.; Lee, R.; Chamberlain, R.S. Pediatric Burns: A Single Institution Retrospective Review of Incidence, Etiology, and Outcomes in 2273 Burn Patients (1995–2013). J. Burn Care Res. 2016, 37, e579–e585. [Google Scholar] [CrossRef] [PubMed]
- Jeschke, M.G.; van Baar, M.E.; Choudhry, M.A.; Chung, K.K.; Gibran, N.S.; Logsetty, S. Burn injury. Nat. Rev. Dis. Primers 2020, 6, 11. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Williams, F.N.; Herndon, D.N.; Jeschke, M.G. The hypermetabolic response to burn injury and interventions to modify this response. Clin. Plast. Surg. 2009, 36, 583–596. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Revision of the ARRIVE Guidelines|NC3Rs. Available online: https://nc3rs.org.uk/our-portfolio/revision-arrive-guidelines (accessed on 1 January 2025).
- O’Brien, K.; Bhatia, A.; Tsen, F.; Chen, M.; Wong, A.K.; Woodley, D.T.; Li, W. Identification of the critical therapeutic entity in secreted Hsp90α that promotes wound healing in newly re-standardized healthy and diabetic pig models. PLoS ONE 2014, 9, e113956. [Google Scholar] [CrossRef]
- Walker, H.L.; McLeod, C.G., Jr.; Leppla, S.H.; Mason, A.D., Jr. Evaluation of Pseudomonas aeruginosa toxin A in experimental rat burn wound sepsis. Infect. Immun. 1979, 25, 828–830. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Regas, F.C.; Ehrlich, H.P. Elucidating the vascular response to burns with a new rat model. J. Trauma Acute Care Surg. 1992, 32, 557–563. [Google Scholar] [CrossRef] [PubMed]
- Guo, H.F.; Ali, R.M.; Hamid, R.A.; Zaini, A.A.; Khaza’ai, H. A new model for studying deep partial-thickness burns in rats. Int. J. Burns Trauma 2017, 7, 107–114. [Google Scholar] [PubMed] [PubMed Central]
- Cai, E.Z.; Ang, C.H.; Raju, A.; Tan, K.B.; Hing, E.C.; Loo, Y.; Wong, Y.C.; Lee, H.; Lim, J.; Moochhala, S.M.; et al. Creation of consistent burn wounds: A rat model. Arch. Plast. Surg. 2014, 41, 317–324. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Venter, N.G.; Monte-Alto-Costa, A.; Marques, R.G. A new model for the standardization of experimental burn wounds. Burns 2015, 41, 542–547. [Google Scholar] [CrossRef] [PubMed]
- Andrade, A.L.M.; Parisi, J.R.; Brassolatti, P.; Parizotto, N.A. Alternative animal model for studies of total skin thickness burns. Acta Cir. Bras. 2017, 32, 836–842. [Google Scholar] [CrossRef] [PubMed]
- Shukla, S.K.; Sharma, A.K.; Shaw, P.; Kalonia, A.; Yashavarddhan, M.H.; Singh, S. Creation of rapid and reproducible burn in animal model with a newly developed burn device. Burns 2020, 46, 1142–1149. [Google Scholar] [CrossRef] [PubMed]
- Hu, X.; Wang, X.; Hong, X.; Fan, H.; Zhang, X.; Chen, A.; Wang, G.; Jin, J.; Xia, Z. Modification and utility of a rat burn wound model. Wound Repair. Regen. 2020, 28, 797–811. [Google Scholar] [CrossRef] [PubMed]
- Korompai, F.L.; Yuan, S.Y. Ventral burn in rats: An experimental model for intravital microscopic study of microcirculation. Burns 2002, 28, 321–327. [Google Scholar] [CrossRef] [PubMed]
- Abdullahi, A.; Amini-Nik, S.; Jeschke, M.G. Animal models in burn research. Cell. Mol. Life Sci. 2014, 71, 3241–3255. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Davenport, L.; Dobson, G.; Letson, H. A new model for standardising and treating thermal injury in the rat. MethodsX 2019, 6, 2021–2027. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Porumb, V.; Trandabăț, A.F.; Terinte, C.; Căruntu, I.D.; Porumb-Andrese, E.; Dimofte, M.G.; Pieptu, D. Design and Testing of an Experimental Steam-Induced Burn Model in Rats. BioMed Res. Int. 2017, 2017, 9878109. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Water Cooling Calculator. Available online: https://www.omnicalculator.com/food/water-cooling (accessed on 1 January 2025).






| Model | Description | Advantages | Disadvantages |
|---|---|---|---|
| Manual Immersion | Submerging a specific area of the rodent’s skin into hot water for a predetermined time. | Simple and cost-effective, widely used in various studies. | High variability due to inconsistent immersion depth and duration, operator-dependent variability, and a potential for uneven heat distribution. |
| Contact Burn | Applying a heated metal device directly to the skin to induce a burn. | Allows for control over burn size and depth, reproducible in terms of contact area and pressure. | May not accurately replicate the progressive nature of scald burns, a risk of deeper tissue damage due to prolonged heat exposure. |
| Flame Burn | Exposing the skin to an open flame for a specific duration. | Mimics certain types of thermal injuries seen in humans, useful for studying deep partial-thickness and full-thickness burns. | Difficult to control burn size and depth, safety concerns for both the animal and the researcher, and it is not representative of scald injuries. |
| Steam Exposure | Exposing the skin to steam to induce a burn. | Closer mimicry of scald injuries compared to dry heat methods, uniform heat distribution. | Challenges in controlling exposure parameters, and a potential for respiratory complications in animals due to inhalation of steam. |
| RAPID-3D Device | A 3D-printed apparatus designed to deliver controlled scald burns with precise exposure parameters. | High reproducibility and standardization, minimizes operator-dependent variability, closely mimics human scald injury characteristics, and allows for consistent burn size and depth. | Requires access to 3D printing technology, and the initial setup may be more complex compared to traditional methods. |
| Feature | Contact Burn Model | Scald Burn Model |
|---|---|---|
| Induction Method | Heated metal surface. | Hot water immersion. |
| Burn Type | Localized, well-defined. | Diffuse, large area. |
| Depth Control | Precise with controlled pressure. | It depends on water temperature and duration. |
| Use in Studies | Wound healing, topical therapy, and hypertrophic scarring. | Wound healing, topical therapy, systemic effects, infection, and immune response. |
| Systemic Effects | Minimal. | Minimal systemic response if TBSA < 10% Strong systemic response if TBSA > 30%. |
| Clinical Relevance | Burns from hot objects (e.g., metals). | Scalds from hot liquids or steam. |
| Item | Recommended Filament | Supports Required | Printing Time (min) | Filament Usage (g) | Filament Cost (US$) |
|---|---|---|---|---|---|
| 4-slotted cuboid | PETG | Yes | 60 | 25 | 0.6 |
| Cylinder | PETG | Yes | 340 | 168 | 4.21 |
| Inner trays | PLA | No | 210 | 140 | 3.51 |
| Actioning lever | PLA | No | 84 | 16 | 0.41 |
| U-shaped supports | PLA | No | 100 | 66 | 1.66 |
| Undertray | PLA | No | 220 | 168 | 4.21 |
| Splash guards | PLA | No | 83 | 44 | 1.09 |
| Total | 18 h 17 min | 459 | 15.7 |
| Experimental Model | Device Complexity 1 | Burn Type | Burn Inducing Device | Device Material | Shape of Burn Lesion | Contact Area with Skin (mm) | Area of Burn Lesion (mm2) | Number of Lesions/Rat | Total Burn Surface Area (TBSA) | Region of Burn in Rats | Application Method | Temperature at Burn Induction | Exposure Time | Burn Degree | Experimental Animals | Gender | Weight (g) | Age | Hair Removal Method |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Walker et al., 1979 [11] | ++++++ | Contact | Metal cylinder | Brass | Rectangular | 50.8 × 279.4 | 14,193.5 | 1 | 42.2% | Dorsal | Rat immersion | 100 °C | N/A | N/A | Rats | N/A | 180–220 | N/A | Shaving |
| Regas et al., 1992 [12] | + | Contact | Brass | Brass | Rectangular | 55 × 19 | 1045 | 4 | 7.8% | Dorsal | Probe water immersion + Gravitational contact | N/A | 20 s | Full thickness | Sprague–Dawley rats | males | 300–500 | N/A | Shaving |
| Guo et al., 2017 [13] | +++++ | Contact | Aluminum bar | Aluminum | Round | 20 | 314.2 | 1 | 3.2% | Dorsal | Gravitational contact | 60–70 °C | 10 s | Deep partial-thickness | Sprague–Dawley rats | males | 250 ± 50 | N/A | Shaving + Depilatory cream |
| Cai et al., 2014 [14] | ++ | Contact | Stainless steel rod | Stainless-steel | Round | 10 | 78.54 | 3 | 0.5% | Dorsal | Probe water immersion + Gravitational contact | 100 °C | 5–20 s | Full thickness | Sprague–Dawley rats | N/A | 350–400 | 6–8 weeks | Shaving |
| Venter et al., 2015 [15] | ++++++ | Contact | Aluminum bar | Aluminum | Round | 19; 23; 32 | 283.53; 415.5; 804.2 | 1 | 0.7%; 1%; 2% | Central thoracodorsal | Gravitational contact + Skin fixation with syringe needles | 60 °C, 70 °C, 80 °C | 10 s | 1. Superficial second-degree burn. 2. Deep second-degree burn. 3. Third degree-burn. | Wistar rats | males | 275–300 | N/A | Shaving + Depilatory cream |
| Andrade et al., 2017 [16] | +++ | Contact | Aluminum plate | Aluminum | Round | 30 | 706.9 | 1 | 2% | Central thoracodorsal | Gravitational contact | 150 °C | 5–15 s | Full thickness burn | Wistar rats | males | 200–250 | N/A | Shaving + Depilatory cream |
| Shukla et al., 2020 [17] | +++ | Contact | Aluminum probe | Aluminum | Round | 10 | 78.54 | N/A | 0.9% | Dorsal | Gravitational contact | 60 °C, 80 °C, 100 °C | 40 s | 1. Superficial thickness. 2. Partial thickness. 3. Full thickness burn | Mice | females | 25–30 | 10–12 weeks | Shaving |
| Hu et al., 2020 [18] | +++ | Contact | Brass comb | Brass | Rectangular | 10 × 20 | 200 | 8 | 5% | Dorsal | Probe water immersion + Gravitational contact | 100 °C | 8 s | Partial thickness | Sprague–Dawley rats | N/A | 180–220 | 6–8 weeks | Shaving |
| Korompai et al., 2002 [19] | ++++ | Scald | Plastic | Polyethylene | Oval | N/A | Width of animal; axial length of planned burn | 1 | 15–25% | 1. Ventral; 2. Chest wall 3. Flanks | Gravitational contact | 96 °C | 10–15 s | 1. Deep partial thickness burn. 2. Full thickness burns | Sprague–Dawley rats | males | 85–142 | 4.2–5 weeks | Shaving |
| Abdullahi et al., 2014 [20] | ++ | Scald | Plastic template | Plastic | Rectangular | N/A | N/A | 1 | 30% | Dorsal thorax, ventral lumbar | Probe immersion | 100 °C | 8 s | Full-thickness burn | Mice | N/A | N/A | 6–8 weeks | Shaving |
| Davenport et al., 2019 [21] | +++ | Scald | Cradle | PVC | Rectangular | 50 × 136 | 6800 | 2 | 30.4% | Both lateral dorsal side | Rat immersion | 96 °C | 8 s | Full thickness burn | Sprague–Dawley rats | males | 320–340 | adult | Shaving + Depilatory cream |
| Porumb et al., 2017 [22] | ++++ | Scald | Steam application | Steam | Round | N/A | 1256.6 | 1 | 3% | Dorsal thorax | Rat immersion | 94 °C | 1–7 s | 1. Superficial burn wound. 2. Partial thickness. 3. Full thickness | Wistar rats | males | 300 ± 20 | N/A | Shaving + Depilatory cream |
| RAPID-3D | +++ | Scald | 3D printed cuboid | PETG/PLA | Rectangular | 20 × 10 | 1600 | 8 | 3.8% | Dorsal thorax, dorsal lumbar | Controlled contact with water | 98–100 °C | 8 s | Full-thickness burn | Wistar rats | females | 278 ± 23 | 6 months | Shaving + Depilatory cream |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Roșca, O.-J.; Nistor, A.; Brandabur, C.; Heredea, R.E.; Hoinoiu, B.; Șoica, C. Rat 3D Printed Induction Device (RAPID-3D): A 3D-Printed Device for Uniform and Reproducible Scald Burn Induction in Rats with Histological and Microvascular Validation. Biology 2025, 14, 378. https://doi.org/10.3390/biology14040378
Roșca O-J, Nistor A, Brandabur C, Heredea RE, Hoinoiu B, Șoica C. Rat 3D Printed Induction Device (RAPID-3D): A 3D-Printed Device for Uniform and Reproducible Scald Burn Induction in Rats with Histological and Microvascular Validation. Biology. 2025; 14(4):378. https://doi.org/10.3390/biology14040378
Chicago/Turabian StyleRoșca, Oana-Janina, Alexandru Nistor, Călin Brandabur, Rodica Elena Heredea, Bogan Hoinoiu, and Codruța Șoica. 2025. "Rat 3D Printed Induction Device (RAPID-3D): A 3D-Printed Device for Uniform and Reproducible Scald Burn Induction in Rats with Histological and Microvascular Validation" Biology 14, no. 4: 378. https://doi.org/10.3390/biology14040378
APA StyleRoșca, O.-J., Nistor, A., Brandabur, C., Heredea, R. E., Hoinoiu, B., & Șoica, C. (2025). Rat 3D Printed Induction Device (RAPID-3D): A 3D-Printed Device for Uniform and Reproducible Scald Burn Induction in Rats with Histological and Microvascular Validation. Biology, 14(4), 378. https://doi.org/10.3390/biology14040378








