Simple Summary
Animals regulate their nutrient intake to meet their physiological needs, but no single diet optimizes all traits, creating the potential for nutritional trade-offs. This study analyzed how different protein-to-carbohydrate (PC) ratios affect traits in the fruit fly Drosophila melanogaster using data from the Geometric Framework of Nutrition. Three nutrient regions were identified: low PC ratios (e.g., 1:8), which support longer lifespans but hinder growth and reproduction; high PC ratios (e.g., 1:1), which enhance development, body mass, and male reproduction but reduce lifespan; and intermediate PC ratios (<1:1 to >1:8), which maximize female reproduction and larval survival. These findings highlight trade-offs between lifespan and reproduction, suggest metamorphosis may help balance nutrient needs across life stages, and point to potential genetic conflicts between males and females over metabolic traits. This research advances our understanding of how animals respond to their diets to optimize specific traits, addressing key questions in evolutionary biology and health.
Abstract
Animals often regulate their nutrient intake according to their physiological needs. There is evidence that different traits require specific nutrient blends, and that animals cannot always maximize all traits with a single diet (“nutritional trade-offs”). However, we still do not have a clear understanding of which traits might be involved in nutritional trade-offs. I compiled data from the Geometric Framework of Nutrition literature on the ratio of proteins and carbohydrates that maximize (best PC ratios) or minimize (worst PC ratios) several larval and adult traits in Drosophila melanogaster. Best and worst PC ratios clustered into three regions in the protein-carbohydrate nutrient space: (1) Low PC ratios (1:8 or higher) are best for lifespan but worst for growth or reproductive traits; (2) High PC ratios (1:1 or lower) are best for adult body mass, male reproduction, and larval developmental time but worst for lifespan; and (3) Intermediate PC ratios (<1:1 and >1:8) are best for female lifetime egg production, female reproductive rate, and larval survival. These findings support lifespan–reproduction nutritional trade-offs, highlight the potential for metamorphosis to solve nutritional trade-offs across life stages, and underscore the potential for intralocus sexual conflict to emerge over the expression of metabolic genes.
1. Introduction
Animals acquire nutrients to subsidize their metabolic demands, but the quantity and ratio of these nutrients vary [1,2,3,4]. When animals cannot acquire nutrients in the quantity and ratio for all traits, there is the potential for conflict whereby animals need to balance their nutrient intake to maximize one trait at the expense of another (i.e., “nutritional trade-offs”) [5,6,7]. Previous research has uncovered evidence to support the concept of nutritional trade-offs between reproduction and lifespan and reproduction and immune traits [5,6,7,8,9,10,11,12,13] and between reproductive traits that contribute to different sexual selection episodes [14,15,16] (see also [17]).
Nutritional trade-offs are likely ubiquitous and could play an important role in shaping animal nutrition and evolution. However, previous work has largely studied nutritional trade-offs among only a limited number of traits (usually two or three), with few exceptions (see e.g., [18]). For example, in a landmark paper, Lee et al. [6] used the Geometric Framework of Nutrition (GF) to comprehensively assess how the ratio of proteins and carbohydrates (PC ratio) in diets modulated lifespan, reproductive rate, and lifetime egg production in female Drosophila melanogaster Meigen (1830). Likewise, Maklakov et al. [7] studied PC ratio effects on the expression of three traits (lifespan and two reproductive traits) in each sex of the cricket Teleogryllus commodus Walker (1869). These examples are representative of the wider literature (e.g., [10,12,14,15,16,19,20,21] and references therein) and continue to stimulate new studies that uncover insights into animal nutritional ecology in both basic and applied sciences [22,23,24,25]. Yet, it is also important to step back to try and unify our knowledge in a more general context to gain a proper overview of nutritional trade-offs across multiple traits within and between species [10,26]. In this regard, there are unmatched advantages to working with model organisms like Drosophila melanogaster for which the nutritional effects have been mapped across several traits in high resolution using the GF. By compiling what we know about D. melanogaster responses to diets from GF studies—which to my knowledge has never been done—we will gain the much-needed general insight into nutritional trade-offs that will help us interpret current knowledge and guide future work.
Therefore, in this study, I collated the PC ratio of diets that maximize (best) or minimize (worst) a wide range of traits in D. melanogaster, using my previous analytical methods to reconstruct and analyze GF landscapes [27,28]. My main goal was to characterize the potential for nutritional trade-offs by highlighting traits that have opposing responses to the same PC ratios. To achieve this, my assumption was that the Drosophila strains used across different studies responded were comparable. This assumption was necessary because I do not have the information of the genetic architecture of all lines in the published literature. It follows from this assumption that Drosophila strains respond similarly to different diets irrespective of genetic differences, which we know is not always true at least for highly inbred lines (e.g., [24]). I discuss the implications of this in the methods and discussion sections. Nevertheless, the findings of this work highlight the potential for nutritional trade-offs in Drosophila that will stimulate future work to uncover the causes and consequences for the ecology and evolution of this and other species.
2. Materials and Methods
2.1. The Data
I included studies from the literature which used Drosophila melanogaster as a model system and the Geometric Framework for Nutrition as the experimental design. I included studies that measured both food intake and that manipulated the macronutrients in the diets without measuring diet intake; the former approach was usually adopted by studies using adult flies while for the latter this was larvae. I also included studies with liquid diets that used the CAFÉ assay and studies with solid media (Table 1). When possible, I used studies for which raw data was made available in the original publication. When the raw data was not available for lifespan, I used my previously validated approach to reconstruct GF landscapes to extract data that could be used to estimate PC ratios [27]. Briefly, each GF landscape is segmented from an image obtained in the original publication and then reconstructed with a semi-automated algorithm capable of building a surrogate GF landscape with peaks and valleys in the same region as the original landscape. This allows us to analyze GF landscapes for which the raw data is unavailable [27]. Table 1 lists the studies which were used and the traits that were studied. Raw data are provided in Figure S1 in Supplementary Materials.

Table 1.
Literature that was included in this study.
2.2. Estimates of the Peaks and Valleys
Using R version 4.3.2 [33], I used the Nutrigonometry models to estimate the best (peaks) and worst (valleys) diets for the expression of the traits [28]. I plotted the average protein and carbohydrate estimate of peaks and valleys from different traits in the same nutrient space to aid visualization of the potential nutritional trade-offs among traits. I estimated peaks and valleys for all the studies individually and then averaged these estimates across different studies that measured the same trait to create a single estimate of PC ratios for the peak and valley (e.g., male lifespan in [19,30]). The best and worst PC ratio for male paternity share was estimated from [16] when males were the first (Paternity 1) or second (Paternity 2) to mate with females. I also estimated ‘refractoriness’ as the latency of females mated to focal males to remate with a competitor male when focal males were the first to mate (i.e., which helps increase male paternity 1) and the latency of previously mated females to remate with a focal male (‘Latency (Remate)’) when focal males were the second to mate (i.e., for males to gain paternity 2) [see [16] for details]. All estimates of peaks and valleys across traits were plotted in milligrams. There were no outliers in the data. All figures were done using the ‘ggplot2’ package version 3.5.1 [34]. As mentioned above, studies varied in multiple ways, namely the genetic background of the Drosophila melanogaster stock, diet composition (solid vs. liquid), and intake estimates. I therefore opted to not conduct statistical inferences as those would inevitably be biased. Note that although traditional GF experiments enable caloric intake and macronutrient ratio effects to be disentangled, this is not possible in this cross-study comparison due to confounding factors. Nevertheless, even if caloric intake had an influence, it would likely affect the position of the points relative to the origin along an isoline and not change the ratio of nutrients that maximize (or minimize) a particular trait [3,4].
3. Results
3.1. The Distribution of Peaks and Valleys in the Nutrient Space Created Three Regions
3.1.1. Region 1: High Carbohydrate, Low Protein Diets
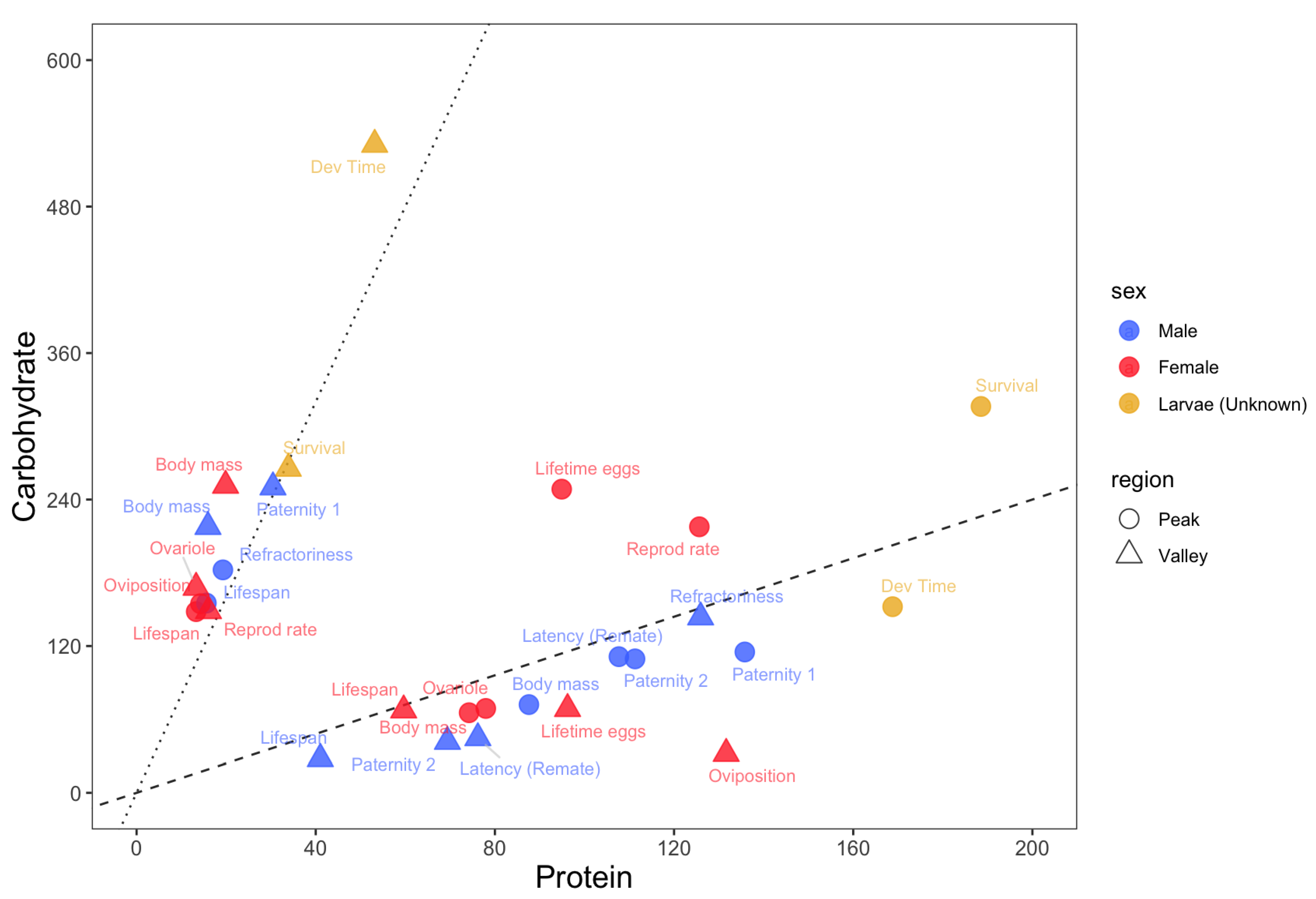
Low PC ratios (PC ratio of ~1:8 or lower) maximized lifespans for both adult males and females as well as short-term female oviposition rates and male refractoriness (Figure 1). On the other hand, low PC ratios minimized male paternity 1, male and female adult body mass, larval developmental time and survival, and female ovariole number. Overall, low PC ratios maximize lifespans at the expense of most traits related to growth and male and female reproduction.
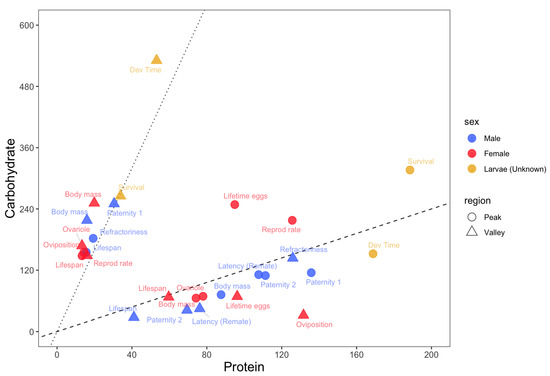
Figure 1.
Nutritional trade-offs in Drosophila melanogaster. The protein (x-axis) and carbohydrate (y-axis) estimates for the peaks (circles) and valleys (triangles) of each trait. Light blue = adult males; Red = adult females; Green = larvae. Black dashed line represents a PC ratio of 1:1.2. Black dotted line represents a PC ratio of 1:8.
3.1.2. Region 2: High Protein, Low Carbohydrate Diets
High PC ratios (PC ratios of ~1:1 or higher) maximized larval developmental time, the adult body masses of both sexes, female ovariole numbers and, for diets with a higher concentration of macronutrients, male paternity 2 and the latency of females to remate with focal males (Figure 1). On the other hand, high PC ratios minimized lifespans in both sexes as well as short-term female oviposition rates. Male paternity 2 and the latency of females to remate with focal males were also minimized in this region, but when diets had less macronutrients. Overall, high PC ratios maximized traits related to growth and reproduction at the expense of lifespan.
3.1.3. Region 3: Balanced Diets
Balanced diets with PC ratios between ~1:1 and ~1:3 maximized larval survival and female reproductive rates and lifetime egg production. No trait was minimized in this region (Figure 1).
4. Discussion
Animals must balance their nutrient intake to express fitness-related traits, which creates the potential for nutritional trade-offs among traits with competing nutritional needs [35]. Using the Nutrigonometry models on key GF studies from the Drosophila melanogaster literature, I compiled information about the optimum PC ratio for a wide range of traits across life stages of the fly D. melanogaster and found a strong potential for nutritional trade-offs among traits related to lifespans, growth and reproduction. Specifically, there were three regions in protein–carbohydrate nutrient space where peaks and valleys of traits were found. Low PC ratios, which are diets richer in carbohydrates, maximized lifespans and short-term female oviposition rates but minimized all traits related to larval growth and survival, adult body mass, and adult reproduction. High PC ratios, which are diets richer in protein, showed the opposite effect. Three traits were maximized at more intermediate PC ratios, namely female lifetime egg production (PC ~1:3), female reproductive rates (PC~1:2), and larval survival (PC ~1:1.5) (Figure 1). Flies are holometabolous insects and metamorphosis might help resolve nutritional trade-offs between life stages [36,37]. This is less clear within life stages and between sexes, as shown here for male and female reproductive and lifespan traits. Because males and females share the same genome, nutritional trade-offs could create the potential for intralocus sexual conflict [38,39,40], which might be pervasive across insects [27]. One way that intralocus sexual conflict could be resolved is through the modulation of the expression of metabolic genes in males and females [41], but we do not yet have a complete understanding of how nutritional trade-offs and sexual conflict interact to modulate organism-wide gene expression (but see discussion below).
It would be interesting to study the molecular mechanisms and metabolic pathways which are up- and down-regulated when flies experience different diets. An ambitious but worthwhile goal is to create GF performance landscapes of genes and pathways using omics technologies to give us the necessary mechanistic insights into the drivers underlying nutritional trade-offs. The TOR and AMPK pathways are two obvious higher level regulatory pathways that control nutritional trade-offs but what are the genes that modulate nutritional responses and trade-offs downstream of these major pathways [42,43,44,45]? My previous work has raised the possibility that the uric acid pathway modulates at least some diet- and density-dependent responses during D. melanogaster larval development [46], but that study lacks the high-resolution nature of GF experiments and is by no means comprehensive. Other studies in insects have used the GF but did not gain similar levels of molecular insights in either larvae (e.g., [18,20,21,32]) or adults (e.g., [6,19,30]). Molecular insights are crucial because we are now uncovering how diet composition interacts with genes and their expression to modulate diet responses, growth, and fitness. For example, Yurkevych et al., [47] have shown changes in the expression of a wider range of genes that underpinned tolerance to high protein diets. Similarly, Francis et al. [23] have shown that genetics plays a major role in diet-dependent responses in D. melanogaster. Likewise, Havula et al. [24] have shown that genetics can strongly modulate larval development and survival, particularly in less favorable diets such as high-sugar diets. Investigating the effects of larval crowding—which is known to modulate protein availability—on D. melanogaster larval gene expression, I found transcriptomic-wide trade-offs across most major pathways including metabolism and immunity [48]. Similar findings were reported in Drosophila simulans [49]. It is possible that these transcriptomic-wide trade-offs emerge and are modulated by diets, but specifically how remains to be ascertained. Future work should take advantage of the molecular resources available for D. melanogaster to uncover further insights into gene–diet interactions.
The data collated here highlight a nutritional conundrum for adult flies: how to balance diets that maximize lifespans against those that enhance reproduction. Physiological nutrient requirements vary depending on life stage, environment, genetic background, and stress levels, making nutritional choices inherently multi-factorial [35]. However, Drosophila melanogaster possess a well-characterized molecular pathway that acts as a dietary switch: the sex peptide (SP) pathway [50]. When females mate, they receive a cocktail of seminal fluid proteins from the male’s ejaculate, the most abundant of which is SP [51]. SP binds to its receptor (sex peptide receptor or SPR) in the female reproductive tract and brain, triggering physiological and behavioral changes, including increased protein intake [52]. Thus, mating serves as a clear biological signal that reproduction should be prioritized at least partly through the physiological cascade activated by the SP–SPR interaction. Males also express SPR in the brain, but whether this influences their nutritional needs and behavior remains unclear. Drosophila melanogaster males are reproductively active even in the absence of suitable mates, which suggests that they may be inherently predisposed to prioritizing diets that support reproduction over longevity [53,54].
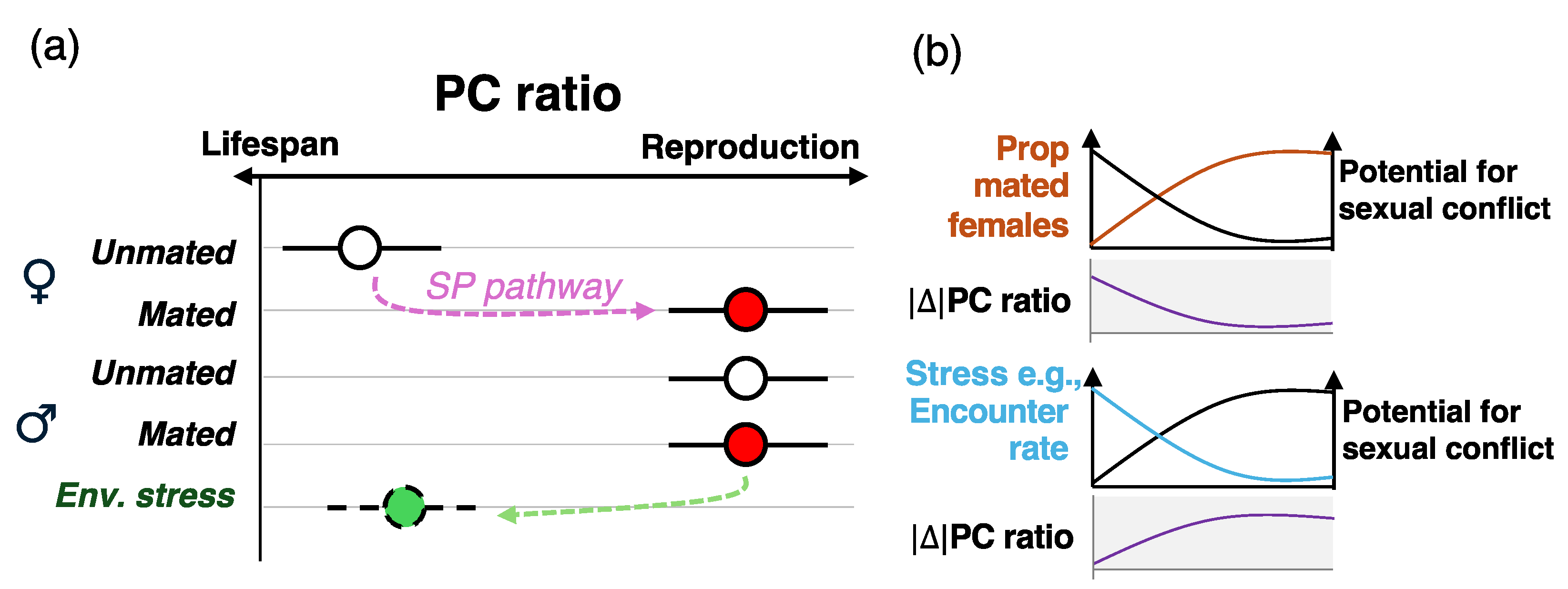
Based on this, I hypothesize that males and females have different baseline nutritional needs: unmated females require a lower PC ratio until mating triggers a shift towards higher PC ratio diets that enhance reproductive output. In contrast, males may be hardwired to consume higher PC ratio diets by default, unless environmental factors (e.g., low female encounter rates) drive a shift towards diets that favor lifespan extension (Figure 2a). This hypothesis has significant implications for sexual conflict over nutrition. In a D. melanogaster population that adheres to Bateman’s principles—which appears ubiquitous—most females are mated, with fitness constrained only by egg production, whereas a smaller proportion of males successfully mate, leaving many unmated [55]. Under these conditions, sexual conflict over nutrition is likely weak because the optimal PC ratio for both sexes tend to converge: mated females shift to higher PC ratios to maximize reproduction, while males already prioritize these diets for reproductive success (Figure 2b). Further studies are needed to test this hypothesis and, more broadly, to investigate how population structures, mating systems, and evolutionary pressures, including Bateman’s principles, shape nutritional responses and adaptation.
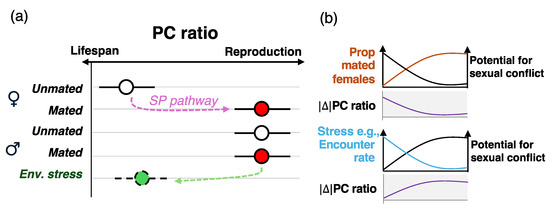
Figure 2.
Nutritional switches might assuage the potential for nutritional trade-offs. (a) Upon mating, females shift their dietary preference and consume higher PC ratio diets to support egg production, as a result of the sex peptide (SP) pathway. Males eat higher PC ratios regardless of their mating status, maximizing reproductive traits as opposed to lifespans. I hypothesize that this is the default nutritional behavior in females and males, respectively, and males only shift to PC ratios that maximize lifespans due to environmental conditions, which could lead to males surviving longer and achieving higher fitness. An example could be a population where female encounter rates by males are relatively low, and males could benefit from living longer to maximize their chances of reproduction. (b) Example of two population factors that can modulate the potential for sexual conflict over nutrition. As the proportion of mated females increases, the absolute difference of the PC ratio between males and females decreases (i.e., both eat higher PC ratio diets), in turn leading to lower potential for sexual conflict. Stress, such as for example low encouter rates, could lead males to shift their dietary intake to lower PC ratio diets to live longer and increase their chances of findings a mate, increasing and the potential for sexual conflict. This hypothesis remains untested.
Our data reflected studies that used a wide range of methodological details, such food types (solid vs. liquid), which might influence nutritional responses. For example, measuring diet intake and compensatory responses when animals are given a solid diet remains challenging. The CAFÉ assay enables a precise quantification of dietary intakes; however, it provides a highly unnatural way of feeding for the flies, which could lead to other physiological and behavioral changes that affect their response to diets. We still lack a systematic comparison of the nutritional responses of flies with similar genetic backgrounds on different diet types or different genetic backgrounds on the same diet type to properly quantify the impact of this experimental approach on nutritional responses. With a growing number of studies in the literature, it will become possible to quantify these effects and account for their consequences, leading to better interpretation of results. Nevertheless, it is worth noting that GF landscapes for lifespans and reproduction are qualitatively similar among different studies (Figure S1), which partly assuages concerns about the confounding effects of unmeasured factors on the nutritional patterns presented here.
Even though there is growing evidence that genetics play an important role in diet-dependent responses, I assumed that the nutritional responses of the different D. melanogaster strains used across studies in the literature are comparable. There are not enough studies which consistently use the same genetic strain to allow for a study such as this. Therefore, the results presented here should be interpreted with caution, as there are likely unaccounted for genetic effects underpinning the estimates for peaks and valleys. However, it is interesting that higher larval survival variability was reported in high-sugar diets across 196 D. melanogaster isolines [24]. As shown here, this could be explained by the fact that diets with low PC ratios (i.e., sugar-rich diets) represent the worst diet for larval survival and therefore impose a much harsher developmental environment that could translate into higher variability in survival. Similar effects of genetic variability in more extreme (high sugar and high protein) diets have also been reported in other studies [23]. Together, these findings agree with the argument I recently put forward that trait variability should increase when organisms feed in imbalanced diets [56]. This remains to be empirically tested.
5. Conclusions
Through data collection from a range of studies, I demonstrated that larval and adult traits in D. melanogaster have distinct—often conflicting—nutritional requirements, creating the potential for nutritional trade-offs and sexual conflict over nutrition. Specifically, PC ratios that maximized lifespans almost invariably minimized reproductive traits, particularly in females. Future studies should focus on uncovering the mechanisms that trigger and enable dietary switches that optimize different life-history strategies. Moreover, a broader taxonomic perspective is needed to understand how dietary responses are realized and whether phylogenetic patterns across taxa can provide deeper insights into the evolutionary dynamics of nutritional adaptation.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/biology14040384/s1, Data S1 excel file containing the raw data used in this work. Figure S1. Peaks for lifespan (circle) and reproduction (triangles) across studies, highlighting the remarkable qualitative consistency of effect sizes across studies varying in methodology and genetic strains [6,19,30,53].
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The data used in this paper is available in the Supplementary Materials.
Acknowledgments
I would like to acknowledge all colleagues working in the field of nutritional ecology whose work continues to inspire and underpin my own.
Conflicts of Interest
The author declares no conflicts of interest.
References
- Evans, E.; Miller, D.S. Comparative Nutrition, Growth and Longevity. Proc. Nutr. Soc. 1968, 27, 121–129. [Google Scholar] [PubMed]
- Mitchell, H. Comparative Nutrition of Man and Domestic Animals; Academic Press: Cambridge, MA, USA, 1964. [Google Scholar]
- Raubenheimer, D.; Simpson, S.J. The Geometry of Compensatory Feeding in the Locust. Anim. Behav. 1993, 45, 953–964. [Google Scholar]
- Simpson, S.J.; Raubenheimer, D. A Multi-Level Analysis of Feeding Behaviour: The Geometry of Nutritional Decisions. Philos. Trans. R. Soc. Lond. B. Biol. Sci. 1993, 342, 381–402. [Google Scholar] [CrossRef]
- Fanson, B.; Yap, S.; Taylor, P.W. Geometry of Compensatory Feeding and Water Consumption in Drosophila melanogaster. J. Exp. Biol. 2012, 215, 766–773. [Google Scholar] [CrossRef] [PubMed]
- Lee, K.P.; Simpson, S.J.; Clissold, F.J.; Brooks, R.; Ballard, J.W.; Taylor, P.W.; Soran, N.; Raubenheimer, D. Lifespan and Reproduction in Drosophila: New Insights from Nutritional Geometry. Proc. Natl. Acad. Sci. USA 2008, 105, 2498–2503. [Google Scholar] [CrossRef]
- Maklakov, A.A.; Simpson, S.J.; Zajitschek, F.; Hall, M.D.; Dessmann, J.; Clissold, F.; Raubenheimer, D.; Bonduriansky, R.; Brooks, R.C. Sex-Specific Fitness Effects of Nutrient Intake on Reproduction and Lifespan. Curr. Biol. 2008, 18, 1062–1066. [Google Scholar] [CrossRef]
- Guo, J.; Cui, Y.; Lin, P.; Zhai, B.; Lu, Z.; Chapman, J.W.; Hu, G. Male Nutritional Status Does Not Impact the Reproductive Potential of Female Cnaphalocrocis Medinalis Moths under Conditions of Nutrient Shortage. Insect Sci. 2021, 29, 467–477. [Google Scholar]
- Treidel, L.A.; Clark, R.M.; Lopez, M.T.; Williams, C.M. Physiological Demands and Nutrient Intake Modulate a Trade-off between Dispersal and Reproduction Based on Age and Sex of Field Crickets. J. Exp. Biol. 2021, 224, jeb237834. [Google Scholar]
- Rapkin, J.; Jensen, K.; Archer, C.R.; House, C.M.; Sakaluk, S.K.; Castillo, E.D.; Hunt, J. The Geometry of Nutrient Space-Based Life-History Trade-Offs: Sex-Specific Effects of Macronutrient Intake on the Trade-off between Encapsulation Ability and Reproductive Effort in Decorated Crickets. Am. Nat. 2018, 191, 452–474. [Google Scholar] [CrossRef]
- Harrison, S.J.; Raubenheimer, D.; Simpson, S.J.; Godin, J.-G.J.; Bertram, S.M. Towards a Synthesis of Frameworks in Nutritional Ecology: Interacting Effects of Protein, Carbohydrate and Phosphorus on Field Cricket Fitness. Proc. R. Soc. B Biol. Sci. 2014, 281, 20140539. [Google Scholar]
- Ponton, F.; Wilson, K.; Holmes, A.; Raubenheimer, D.; Robinson, K.L.; Simpson, S.J. Macronutrients Mediate the Functional Relationship between Drosophila and Wolbachia. Proc. R. Soc. B Biol. Sci. 2015, 282, 20142029. [Google Scholar] [CrossRef] [PubMed]
- Fanson, B.; Taylor, P.W. Protein: Carbohydrate Ratios Explain Life Span Patterns Found in Queensland Fruit Fly on Diets Varying in Yeast: Sugar Ratios. Age 2012, 34, 1361–1368. [Google Scholar] [CrossRef]
- Ng, S.H.; Simpson, S.J.; Simmons, L.W. Macronutrients and Micronutrients Drive Trade-offs between Male Pre-and Postmating Sexual Traits. Funct. Ecol. 2018, 32, 2380–2394. [Google Scholar] [CrossRef]
- Bunning, H.; Rapkin, J.; Belcher, L.; Archer, C.R.; Jensen, K.; Hunt, J. Protein and Carbohydrate Intake Influence Sperm Number and Fertility in Male Cockroaches, but Not Sperm Viability. Proc. R. Soc. B Biol. Sci. 2015, 282, 20142144. [Google Scholar] [CrossRef]
- Morimoto, J.; Wigby, S. Differential Effects of Male Nutrient Balance on Pre-and Post-Copulatory Traits, and Consequences for Female Reproduction in Drosophila melanogaster. Sci. Rep. 2016, 6, 27673. [Google Scholar] [CrossRef]
- Gage, M.J.G.; Cook, P.A. Sperm Size or Numbers—Effects of Nutritional Stress upon Eupyrene and Apyrene Sperm Production Strategies in the Moth Plodia Interpunctella (Lepidoptera: Pyralidae). Funct. Ecol. 1994, 8, 594–599. [Google Scholar] [CrossRef]
- Rodrigues, M.A.; Martins, N.E.; Balancé, L.F.; Broom, L.N.; Dias, A.J.S.; Fernandes, A.S.D.; Rodrigues, F.; Sucena, É.; Mirth, C.K. Drosophila melanogaster Larvae Make Nutritional Choices That Minimize Developmental Time. J. Insect Physiol. 2015, 81, 69–80. [Google Scholar] [CrossRef] [PubMed]
- Jensen, K.; McClure, C.; Priest, N.K.; Hunt, J. Sex-Specific Effects of Protein and Carbohydrate Intake on Reproduction but Not Lifespan in Drosophila Melanogaster. Aging Cell 2015, 14, 605–615. [Google Scholar] [CrossRef]
- Pascacio-Villafán, C.; Righini, N.; Nestel, D.; Birke, A.; Guillén, L.; Aluja, M. Diet Quality and Conspecific Larval Density Predict Functional Trait Variation and Performance in a Polyphagous Frugivorous Fly. Funct. Ecol. 2022, 36, 1163–1176. [Google Scholar] [CrossRef]
- Zanco, B.; Morimoto, J.; Cockerell, F.; Mirth, C.K.; Sgro, C.M. Fluctuating Temperatures Exacerbate Nutritional Stress during Development in Drosophila melanogaster. bioRxiv 2023. [Google Scholar] [CrossRef]
- Barragan-Fonseca, K.B.; Gort, G.; Dicke, M.; Van Loon, J.J.A. Effects of Dietary Protein and Carbohydrate on Life-history Traits and Body Protein and Fat Contents of the Black Soldier Fly Hermetia illucens. Physiol. Entomol. 2019, 44, 148–159. [Google Scholar] [CrossRef]
- Francis, D.; Ghazanfar, S.; Havula, E.; Krycer, J.R.; Strbenac, D.; Senior, A.; Minard, A.Y.; Geddes, T.; Nelson, M.E.; Weiss, F. Genome-Wide Analysis in Drosophila Reveals Diet-by-Gene Interactions and Uncovers Diet-Responsive Genes. G3 2021, 11, jkab171. [Google Scholar] [PubMed]
- Havula, E.; Ghazanfar, S.; Lamichane, N.; Francis, D.; Hasygar, K.; Liu, Y.; Alton, L.A.; Johnstone, J.; Needham, E.J.; Pulpitel, T. Genetic Variation of Macronutrient Tolerance in Drosophila melanogaster. Nat. Commun. 2022, 13, 1637. [Google Scholar] [PubMed]
- Solon-Biet, S.M.; Cogger, V.C.; Pulpitel, T.; Wahl, D.; Clark, X.; Bagley, E.E.; Gregoriou, G.C.; Senior, A.M.; Wang, Q.-P.; Brandon, A.E. Branched-Chain Amino Acids Impact Health and Lifespan Indirectly via Amino Acid Balance and Appetite Control. Nat. Metab. 2019, 1, 532–545. [Google Scholar]
- Morimoto, J.; Lihoreau, M. Quantifying Nutritional Trade-Offs across Multidimensional Performance Landscapes. Am. Nat. 2019, 193, E168–E181. [Google Scholar] [CrossRef]
- Morimoto, J. Optimum Ratio of Dietary Protein and Carbohydrate That Maximises Lifespan Is Shared among Related Insect Species. Aging Cell 2024, 23, e14067. [Google Scholar] [CrossRef]
- Morimoto, J.; Conceição, P.; Mirth, C.; Lihoreau, M. Nutrigonometry I: Using Right-Angle Triangles to Quantify Nutritional Trade-Offs in Performance Landscapes. Am. Nat. 2023, 201, 725–740. [Google Scholar] [CrossRef] [PubMed]
- Semaniuk, U.; Feden’ko, K.; Yurkevych, I.S.; Storey, K.B.; Simpson, S.J.; Lushchak, O. Within-diet Variation in Rates of Macronutrient Consumption and Reproduction Does Not Accompany Changes in Lifespan in Drosophila melanogaster. Entomol. Exp. Appl. 2017, 166, 74–80. [Google Scholar] [CrossRef]
- Carey, M.R.; Archer, C.R.; Rapkin, J.; Castledine, M.; Jensen, K.; House, C.M.; Hosken, D.J.; Hunt, J. Mapping Sex Differences in the Effects of Protein and Carbohydrates on Lifespan and Reproduction in Drosophila melanogaster: Is Measuring Nutrient Intake Essential? Biogerontology 2022, 23, 129–144. [Google Scholar] [CrossRef]
- Lihoreau, M.; Poissonnier, L.-A.; Isabel, G.; Dussutour, A. Drosophila Females Trade off Good Nutrition with High-Quality Oviposition Sites When Choosing Foods. J. Exp. Biol. 2016, 219, 2514–2524. [Google Scholar]
- Kutz, T.C.; Sgrò, C.M.; Mirth, C.K. Interacting with Change: Diet Mediates How Larvae Respond to Their Thermal Environment. Funct. Ecol. 2019, 33, 1940–1951. [Google Scholar] [CrossRef]
- R Core Team. R: A Language and Environment for Statistical Computing, Version 4.3.2; R Core Team: Vienna, Austria, 2023. [Google Scholar]
- Wickham, H. Ggplot2. WIREs Comput. Stat. 2011, 3, 180–185. [Google Scholar] [CrossRef]
- Simpson, S.J.; Raubenheimer, D. The Nature of Nutrition: A Unifying Framework from Animal Adaptation to Human Obesity; Princeton University Press: Princeton, NJ, USA, 2012; ISBN 978-1-4008-4280-3. [Google Scholar]
- Collet, J.; Fellous, S. Do Traits Separated by Metamorphosis Evolve Independently? Concepts and Methods. Proc. R. Soc. B Biol. Sci. 2019, 286, 20190445. [Google Scholar] [CrossRef]
- Collet, J.M.; Nidelet, S.; Fellous, S. Genetic Independence between Traits Separated by Metamorphosis Is Widespread but Varies with Biological Function. Proc. R. Soc. B Biol. Sci. 2023, 290, 20231784. [Google Scholar] [CrossRef] [PubMed]
- Berger, D.; Grieshop, K.; Lind, M.I.; Goenaga, J.; Maklakov, A.A.; Arnqvist, G. Intralocus Sexual Conflict and Environmental Stress. Evolution 2014, 68, 2184–2196. [Google Scholar] [CrossRef]
- Bonduriansky, R.; Chenoweth, S.F. Intralocus Sexual Conflict. Trends Ecol. Evol. 2009, 24, 280–288. [Google Scholar]
- Pennell, T.M.; Morrow, E.H. Two Sexes, One Genome: The Evolutionary Dynamics of Intralocus Sexual Conflict. Ecol. Evol. 2013, 3, 1819–1834. [Google Scholar] [CrossRef]
- Harano, T.; Okada, K.; Nakayama, S.; Miyatake, T.; Hosken, D.J. Intralocus Sexual Conflict Unresolved by Sex-Limited Trait Expression. Curr. Biol. 2010, 20, 2036–2039. [Google Scholar] [CrossRef]
- Simpson, S.J.; Raubenheimer, D. Macronutrient Balance and Lifespan. Aging 2009, 1, 875. [Google Scholar]
- Solon-Biet, S.M.; Wahl, D.; Raubenheimer, D.; Cogger, V.C.; Le Couteur, D.G.; Simpson, S.J. The Geometric Framework: An Approach for Studying the Impact of Nutrition on Healthy Aging. Drug Discov. Today Dis. Models 2018, 27, 61–68. [Google Scholar]
- Speakman, J.R.; Mitchell, S.E. Caloric Restriction. Mol. Asp. Med. 2011, 32, 159–221. [Google Scholar]
- Sperfeld, E.; Wagner, N.D.; Halvorson, H.M.; Malishev, M.; Raubenheimer, D. Bridging Ecological Stoichiometry and Nutritional Geometry with Homeostasis Concepts and Integrative Models of Organism Nutrition. Funct. Ecol. 2017, 31, 286–296. [Google Scholar]
- Morimoto, J. Uric Acid Metabolism Modulates Diet-Dependent Responses to Intraspecific Competition in Drosophila Larvae. iScience 2022, 25, 105598. [Google Scholar] [PubMed]
- Yurkevych, I.S.; Gray, L.J.; Gospodaryov, D.V.; Burdylyuk, N.I.; Storey, K.B.; Simpson, S.J.; Lushchak, O. Development of Fly Tolerance to Consuming a High-Protein Diet Requires Physiological, Metabolic and Transcriptional Changes. Biogerontology 2020, 21, 619–636. [Google Scholar] [CrossRef]
- Morimoto, J.; Wenzel, M.; Derous, D.; Henry, Y.; Colinet, H. The Transcriptomic Signature of Responses to Larval Crowding in Drosophila melanogaster. Insect Sci. 2023, 30, 539–554. [Google Scholar] [CrossRef]
- Buchner, S.; Hsu, S.-K.; Nolte, V.; Otte, K.A.; Schlötterer, C. Effects of Larval Crowding on the Transcriptome of Drosophila Simulans. Evol. Appl. 2023, 16, 1671–1679. [Google Scholar] [CrossRef]
- Gioti, A.; Wigby, S.; Wertheim, B.; Schuster, E.; Martinez, P.; Pennington, C.J.; Partridge, L.; Chapman, T. Sex peptide of Drosophila melanogaster males is a global regulator of reproductive processes in females. Proc. R. Soc. B Biol. Sci. 2012, 279, 4423–4432. [Google Scholar]
- Perry, J.C.; Sirot, L.; Wigby, S. The seminal symphony: How to compose an ejaculate. Trends Ecol. Evol. 2013, 28, 414–422. [Google Scholar]
- Ribeiro, C.; Dickson, B.J. Sex peptide receptor and neuronal TOR/S6K signaling modulate nutrient balancing in Drosophila. Curr. Biol. 2010, 20, 1000–1005. [Google Scholar]
- Wang, K.; Guo, Y.; Wang, F.; Wang, Z. Drosophila TRPA channel painless inhibits male–male courtship behavior through modulating olfactory sensation. PLoS ONE 2011, 6, e25890. [Google Scholar]
- Ueda, A.; Berg, A.; Khan, T.; Ruzicka, M.; Li, S.; Cramer, E.; Iyengar, A.; Wu, C.F. Intense light unleashes male–male courtship behaviour in wild-type Drosophila. Open Biol. 2023, 13, 220233. [Google Scholar]
- Morimoto, J. Bateman (1948): Was it all wrong? A comment on Hoquet. Anim. Behav. 2020, 168, e1–e4. [Google Scholar]
- Morimoto, J. A reply to: Reply to: A caveat about the use of trigonometric functions in statistical tests of Nutritional Geometry models. Sci. Rep. 2025, 15, 8322. [Google Scholar]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).