The Journey of the Default Mode Network: Development, Function, and Impact on Mental Health
Simple Summary
Abstract
1. Introduction
2. Neuroanatomical Basis of the Default Mode Network
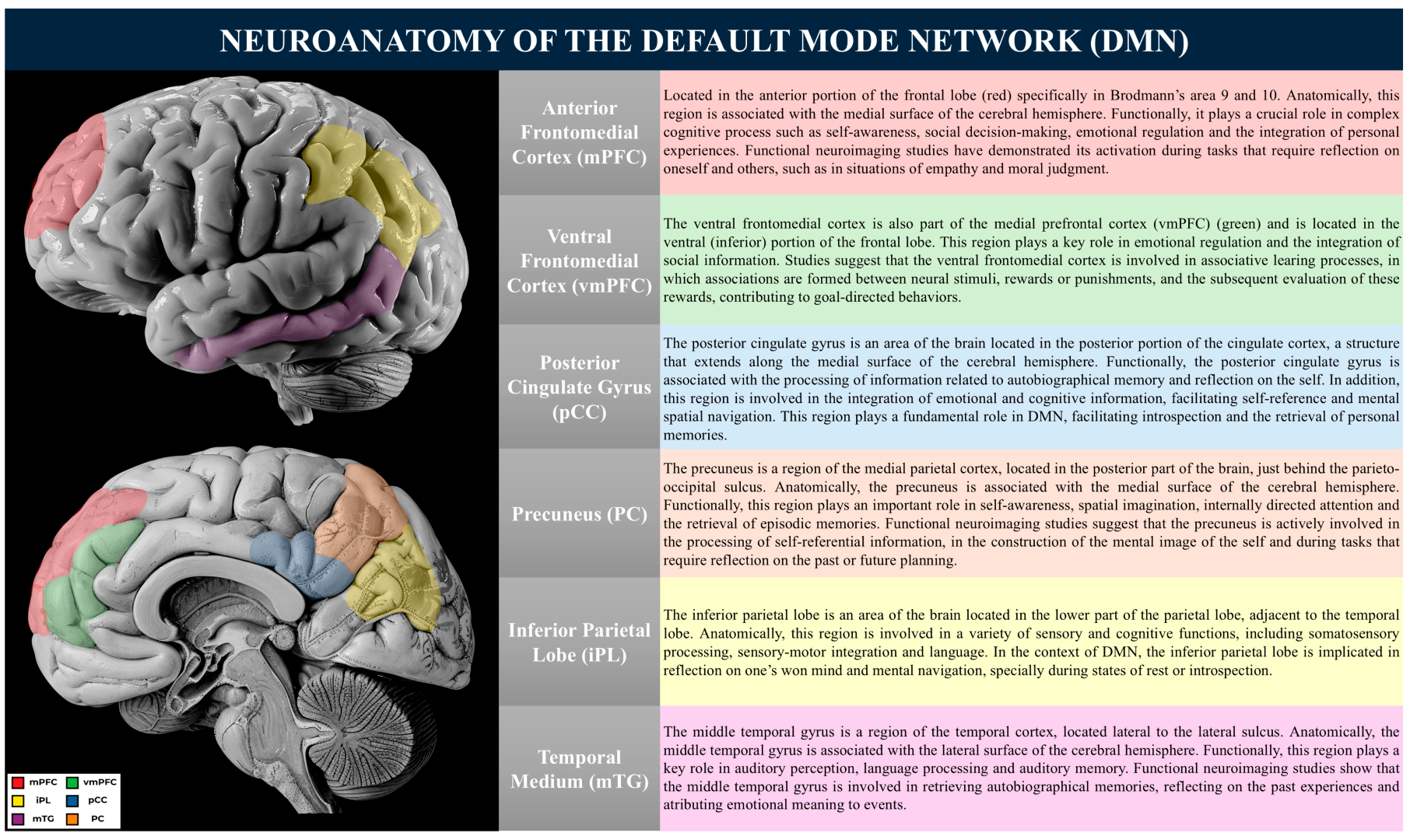
- Posterior Cingulate Cortex (PCC): The PCC functions as a highly connected node within the DMN, facilitating the communication between its subsystems. It is implicated in self-reflective processing, the monitoring of internal and external stimuli, and the integration of autobiographical information [69]. Aberrant activity within the PCC, particularly hyperactivity, has been associated with psychiatric conditions such as depression and excessive rumination [69];
- Precuneus: Situated in the medial parietal lobe, the precuneus is involved in the consolidation of episodic memory, imaginative cognition, and the simulation of future events [70]. Furthermore, it plays a role in attentional shifts and transitions between wakefulness and mind-wandering states.
- Hippocampus and Parahippocampal Cortex: Although the hippocampus is not typically considered part of the DMN, it is closely linked to it. Together, they play vital roles in human cognition, impacting memory, spatial navigation, introspection, and other cognitive processes [25,71]. The interaction between these two neuroanatomical structures has garnered increasing interest in neuroscience, with significant implications for understanding neurological disorders and developing therapeutic interventions [25,71].
- Amygdala: The amygdala is a fundamental region for processing emotions and emotional memories. Situated bilaterally in the medial temporal lobes, next to the hippocampus, it consists of several sub-regions, including the basolateral nucleus, central nucleus, medial cortical nucleus, and lateral nucleus [11,75,78].
- Orbitofrontal Cortex: This region is involved in reward evaluation and emotion-based decision-making. Its extensive connections with the limbic system allow for the regulation of emotional responses and contextual representations [90].
- Medial Prefrontal Cortex (mPFC): The mPFC is central to self-referential processing and emotional regulation, integrating social and affective information [91]. Dysfunctions in this region have been implicated in psychiatric disorders, including depression and anxiety;
- Orbitofrontal Cortex: This region is involved in reward evaluation and emotion-based decision-making. Its extensive connections with the limbic system allow for the regulation of emotional responses and motivational states, influencing decision-making processes [90].
3. Default Mode Network and Brain Networks
3.1. Attention Networks
3.2. Limbic System
3.3. Salience Network
4. Default Mode Network Investigation Tools
5. Default Mode Network Maturation
6. Default Mode Network and Social Relations
7. DMN Influencing Factors
7.1. Age
7.2. Emotional State
7.3. Cognitive States
7.4. Sensory Experiences
7.5. Social Experiences
7.6. States of Consciousness
7.7. Chronic Stress
8. Medical and Psychological Diagnoses Associated with Default Mode Network
8.1. Alzheimer’s Disease
8.2. Schizophrenia
8.3. Anxiety Disorders
8.4. Post-Traumatic Stress Disorder (PTSD)
8.5. Depression
8.6. Attention Deficit Hyperactivity Disorder (ADHD)
9. Neuroplasticity and DMN
10. Final Considerations
11. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Wang, S.; Tepfer, L.J.; Taren, A.A.; Smith, D.V. Functional Parcellation of the Default Mode Network: A Large-Scale Meta-Analysis. Sci. Rep. 2020, 10, 16096. [Google Scholar] [CrossRef] [PubMed]
- Shulman, R.G.; Hyder, F.; Rothman, D.L. Baseline Brain Energy Supports the State of Consciousness. Proc. Natl. Acad. Sci. USA 2009, 106, 11096–11101. [Google Scholar] [CrossRef] [PubMed]
- Raichle, M.E.; Snyder, A.Z. A Default Mode of Brain Function: A Brief History of an Evolving Idea. Neuroimage 2007, 37, 1083–1090. [Google Scholar] [CrossRef] [PubMed]
- Bressler, S.L.; Menon, V. Large-Scale Brain Networks in Cognition: Emerging Methods and Principles. Trends Cogn. Sci. 2010, 14, 277–290. [Google Scholar] [CrossRef]
- Diez, I.; Bonifazi, P.; Escudero, I.; Mateos, B.; Muñoz, M.A.; Stramaglia, S.; Cortes, J.M. A Novel Brain Partition Highlights the Modular Skeleton Shared by Structure and Function. Sci. Rep. 2015, 5, srep10532. [Google Scholar] [CrossRef]
- Xia, M.; He, Y. Functional Connectomics from a “Big Data” Perspective. Neuroimage 2017, 160, 152–167. [Google Scholar] [CrossRef]
- Greicius, M.D.; Srivastava, G.; Reiss, A.L.; Menon, V. Default-Mode Network Activity Distinguishes Alzheimer’s Disease from Healthy Aging: Evidence from Functional MRI. Proc. Natl. Acad. Sci. USA 2004, 101, 4637–4642. [Google Scholar] [CrossRef]
- Andrews-Hanna, J.R.; Smallwood, J.; Spreng, R.N. The Default Network and Self-Generated Thought: Component Processes, Dynamic Control, and Clinical Relevance. Ann. N. Y. Acad. Sci. 2014, 1316, 29. [Google Scholar] [CrossRef]
- Hausman, H.K.; O’Shea, A.; Kraft, J.N.; Boutzoukas, E.M.; Evangelista, N.D.; Van Etten, E.J.; Bharadwaj, P.K.; Smith, S.G.; Porges, E.; Hishaw, G.A.; et al. The Role of Resting-State Network Functional Connectivity in Cognitive Aging. Front. Aging Neurosci. 2020, 12, 177. [Google Scholar] [CrossRef]
- Chand, G.B.; Dhamala, M. Interactions Among the Brain Default-Mode, Salience, and Central-Executive Networks During Perceptual Decision-Making of Moving Dots. Brain Connect. 2016, 6, 249–254. [Google Scholar] [CrossRef]
- Šimić, G.; Tkalčić, M.; Vukić, V.; Mulc, D.; Španić, E.; Šagud, M.; Olucha-Bordonau, F.E.; Vukšić, M.; Hof, P.R. Understanding Emotions: Origins and Roles of the Amygdala. Biomolecules 2021, 11, 823. [Google Scholar] [CrossRef] [PubMed]
- Lettieri, G.; Handjaras, G.; Setti, F.; Cappello, E.M.; Bruno, V.; Diano, M.; Leo, A.; Ricciardi, E.; Pietrini, P.; Cecchetti, L. Default and Control Network Connectivity Dynamics Track the Stream of Affect at Multiple Timescales. Soc. Cogn. Affect. Neurosci. 2022, 17, 461–469. [Google Scholar] [CrossRef] [PubMed]
- Girotti, M.; Bulin, S.E.; Carreno, F.R. Effects of Chronic Stress on Cognitive Function—From Neurobiology to Intervention. Neurobiol. Stress 2024, 33, 100670. [Google Scholar] [CrossRef] [PubMed]
- Broyd, S.J.; Demanuele, C.; Debener, S.; Helps, S.K.; James, C.J.; Sonuga-Barke, E.J.S. Default-Mode Brain Dysfunction in Mental Disorders: A Systematic Review. Neurosci. Biobehav. Rev. 2009, 33, 279–296. [Google Scholar] [CrossRef]
- Farb, N.A.S.; Segal, Z.V.; Mayberg, H.; Bean, J.; Mckeon, D.; Fatima, Z.; Anderson, A.K. Attending to the Present: Mindfulness Meditation Reveals Distinct Neural Modes of Self-Reference. Soc. Cogn. Affect. Neurosci. 2007, 2, 313. [Google Scholar] [CrossRef]
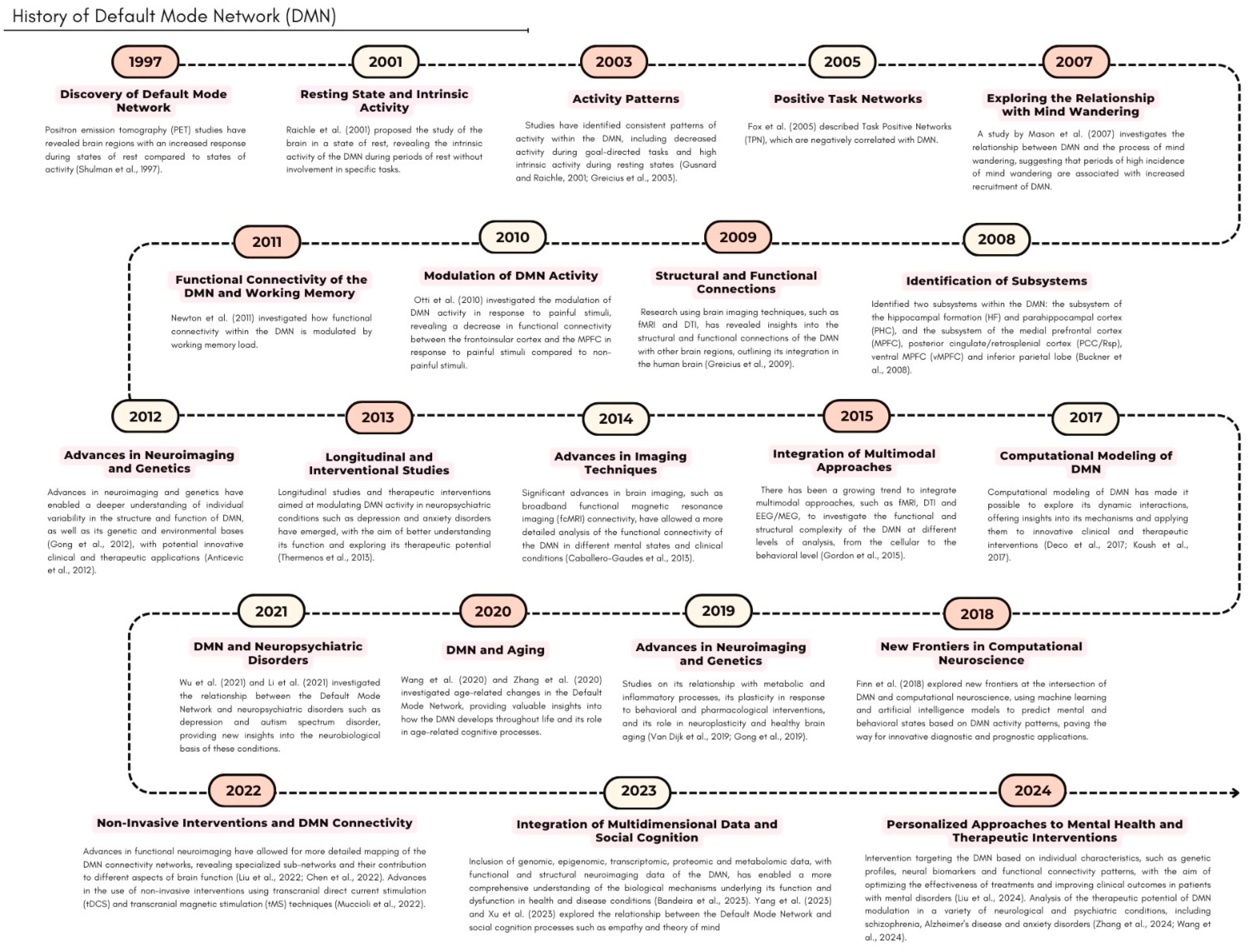
- Shulman, G.L.; Fiez, J.A.; Corbetta, M.; Buckner, R.L.; Miezin, F.M.; Raichle, M.E.; Petersen, S.E. Common Blood Flow Changes across Visual Tasks: II. Decreases in Cerebral Cortex. J. Cogn. Neurosci. 1997, 9, 648–663. [Google Scholar] [CrossRef]
- Raichle, M.E.; MacLeod, A.M.; Snyder, A.Z.; Powers, W.J.; Gusnard, D.A.; Shulman, G.L. A Default Mode of Brain Function. Proc. Natl. Acad. Sci. USA 2001, 98, 676–682. [Google Scholar] [CrossRef]
- Gusnard, D.A.; Ollinger, J.M.; Shulman, G.L.; Cloninger, C.R.; Price, J.L.; Van Essen, D.C.; Raichle, M.E. Persistence and brain circuitry. Proc. Natl. Acad. Sci. USA 2003, 100, 3479–3484. [Google Scholar]
- Greicius, M.D.; Krasnow, B.; Reiss, A.L.; Menon, V. Functional Connectivity in the Resting Brain: A Network Analysis of the Default Mode Hypothesis. Proc. Natl. Acad. Sci. USA 2003, 100, 253–258. [Google Scholar] [CrossRef]
- Fox, M.D.; Snyder, A.Z.; Vincent, J.L.; Corbetta, M.; Van Essen, D.C.; Raichle, M.E. The Human Brain Is Intrinsically Organized into Dynamic, Anticorrelated Functional Networks. Proc. Natl. Acad. Sci. USA 2005, 102, 9673–9678. [Google Scholar] [CrossRef]
- Mason, M.F.; Norton, M.I.; Van Horn, J.D.; Wegner, D.M.; Grafton, S.T.; Macrae, C.N. Wandering Minds: The Default Network and Stimulus-Independent Thought. Science 2007, 315, 393. [Google Scholar] [CrossRef] [PubMed]
- Newton, A.T.; Morgan, V.L.; Rogers, B.P.; Gore, J.C. Modulation of steady state functional connectivity in the default mode and working memory networks by cognitive load. Hum Brain Mapp. 2011, 32, 1649–1659. [Google Scholar] [CrossRef] [PubMed]
- Otti, A.; Guendel, H.; Läer, L.; Wohlschlaeger, A.M.; Lane, R.D.; Decety, J.; Zimmer, C.; Henningsen, P.; Noll-Hussong, M. I know the pain you feel-how the human brain's default mode predicts our resonance to another's suffering. Neuroscience 2010, 169, 143–148. [Google Scholar] [CrossRef] [PubMed]
- Greicius, M.D.; Supekar, K.; Menon, V.; Dougherty, R.F. Resting-State Functional Connectivity Reflects Structural Connectivity in the Default Mode Network. Cerebral Cortex 2009, 19, 72–78. [Google Scholar] [CrossRef]
- Buckner, R.L.; Andrews-Hanna, J.R.; Schacter, D.L. The brain's default network: Anatomy, function, and relevance to disease. Ann. N. Y. Acad. Sci. 2008, 1124, 1–38. [Google Scholar]
- Gong, J.; Tong, Y.; Zhang, H.M.; Wang, K.; Hu, T.; Shan, G.; Sun, J.; Guo, A.Y. Genome-wide identification of SNPs in microRNA genes and the SNP effects on microRNA target binding and biogenesis. Hum Mutat. 2012, 33, 254–263. [Google Scholar] [CrossRef]
- Anticevic, A.; Cole, M.W.; Murray, J.D.; Corlett, P.R.; Wang, X.J.; Krystal, J.H. The role of default network deactivation in cognition and disease. Trends Cogn Sci. 2012, 16, 584–592. [Google Scholar] [CrossRef]
- Thermenos, H.W.; Keshavan, M.S.; Juelich, R.J.; Molokotos, E.; Whitfield-Gabrieli, S.; Brent, B.K.; Makris, N.; Seidman, L.J. A review of neuroimaging studies of young relatives of individuals with schizophrenia: A developmental perspective from schizotaxia to schizophrenia. Am J Med Genet B Neuropsychiatr Genet. 2013, 62, 604–635. [Google Scholar] [CrossRef]
- Caballero-Gaudes, C.; Van de Ville, D.; Grouiller, F.; Thornton, R.; Lemieux, L.; Seeck, M.; Lazeyras, F.; Vulliemoz, S. Mapping interictal epileptic discharges using mutual information between concurrent EEG and fMRI. Neuroimage 2013, 68, 248–262. [Google Scholar] [CrossRef]
- Gordon, R.L.; Jacobs, M.S.; Schuele, C.M.; McAuley, J.D. Perspectives on the rhythm-grammar link and its implications for typical and atypical language development. Ann. N.Y. Acad. Sci. 2015, 1337, 16–25. [Google Scholar] [CrossRef]
- Deco, G.; Kringelbach, M.L.; Jirsa, V.K.; Ritter, P. The dynamics of resting fluctuations in the brain: Metastability and its dynamical cortical core. Sci Rep. 2017, 7, 3095. [Google Scholar] [CrossRef] [PubMed]
- Koush, Y.; Ashburner, J.; Prilepin, E.; Sladky, R.; Zeidman, P.; Bibikov, S.; Scharnowski, F.; Nikonorov, A.; Van De Ville, D. Real-time fMRI data for testing OpenNFT functionality. Data Brief 2017, 14, 344–347. [Google Scholar] [CrossRef]
- Wu, X.; Wang, L.; Geng, Z.; Wei, L.; Yan, Y.; Xie, C.; Chen, X.; Ji, G.J.; Tian, Y.; Wang, K. Improved cognitive promotion through accelerated magnetic stimulation. Eneuro 2021, 8, ENEURO.0392-20.2020. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Dai, X.; Wu, H.; Wang, L. Establishment of effective biomarkers for depression diagnosis with fusion of multiple resting-state connectivity measures. Front. Neurosci. 2021, 15, 729958. [Google Scholar]
- Wang, C.; Pan, R.; Wan, X.; Tan, Y.; Xu, L.; McIntyre, R.S.; Choo, F.N.; Tran, B.; Ho, R.; Sharma, V.K.; et al. A longitudinal study on the mental health of general population during the COVID-19 epidemic in China. Brain Behav. Immun. 2020, 87, 40–48. [Google Scholar] [CrossRef]
- Zhang, J.; Andreano, J.M.; Dickerson, B.C.; Touroutoglou, A.; Barrett, L.F. Stronger functional connectivity in the default mode and salience networks is associated with youthful memory in superaging. Cereb. Cortex 2020, 30, 72–84. [Google Scholar]
- Van Dijk, I.K.; Janssens, A.; Smith, K.R. The long harm of childhood: Childhood exposure to mortality and subsequent risk of adult mortality in Utah and the Netherlands. Eur. J. Popul. 2019, 35, 851–871. [Google Scholar]
- Gong, G.; Wang, P.; Zhou, Z.; Hu, Y. New Insights into the Role of an Interlayer for the Fabrication of Highly Selective and Permeable Thin-Film Composite Nanofiltration Membrane. ACS Appl. Mater. Interfaces 2019, 11, 7349–7356. [Google Scholar] [CrossRef]
- Finn, E.S.; Corlett, P.R.; Chen, G.; Bandettini, P.A.; Constable, R.T. Trait paranoia shapes inter-subject synchrony in brain activity during an ambiguous social narrative. Nat. Commun. 2018, 9, 2043. [Google Scholar] [CrossRef]
- Liu, L.; Li, H.; Ren, Z.; Zhou, Q.; Zhang, Y.; Lu, C.; Qiu, J.; Chen, H.; Ding, G. The “two-brain” approach reveals the active role of task-deactivated default mode network in speech comprehension. Cerebral Cortex 2022, 32, 4869–4884. [Google Scholar]
- Chen, T.; Huang, J.; Cui, J.F.; Li, Z.; Wang, Y.; Irish, M.; Chan, R.C. Functional coupling between the fronto-parietal network and default mode network is associated with balanced time perspective. Brain Sci. 2022, 12, 1201. [Google Scholar] [CrossRef] [PubMed]
- Muccioli, L.; Farolfi, A.; Pondrelli, F.; Matteo, E.; Ferri, L.; Licchetta, L.; Alvisi, L.; Tinuper, P.; Bisulli, F. FDG-PET findings and alcohol-responsive myoclonus in a patient with Unverricht-Lundborg disease. Epilepsy Behav. Rep. 2022, 19, 100551. [Google Scholar] [CrossRef] [PubMed]
- Bandeira, C.E.; Grevet, E.H.; da Silva, B.S.; Vitola, E.S.; Tavares, M.E.; Cupertino, R.B.; Rovaris, D.L.; Bau, C.H. T61. Clinical, Genetic and Neuroimaging Aspects of Attention-Deficit/Hyperactivity Disorder Courses Through Midlife. Eur. Neuropsychopharmacol. 2023, 75, S194–S195. [Google Scholar]
- Yang, H.; Zhao, X.; Wang, T.; Zhou, Z.; Cheng, Z.; Zhao, X.; Cao, Y. Hypoconnectivity within the cingulo-opercular network in patients with mild cognitive impairment in Chinese communities. Int. J. Geriatr. Psychiatry 2023, 38, e5979. [Google Scholar]
- Xu, L.; Huang, M.H.; Shang, X.; Yuan, Z.; Sun, Y.; Liu, J. Meta compositional referring expression segmentation. In Proceedings of the IEEE/CVF Conference on Computer Vision and Pattern Recognition, Vancouver, BC, Canada, 17–24 June 2023; pp. 19478–19487. [Google Scholar]
- Liu, X.; Liu, Z.; Wang, F.; Cheng, P.; Yang, J.; Tan, W.; Cheng, Y.; Huang, D.; Xiang, Z.; Zhang, J.; et al. A connectome-based model of delusion in schizophrenia using functional connectivity under working memory task. J. Psychiatr. Res. 2024, 177, 75–81. [Google Scholar]
- Zhang, J.; Guo, Y.; Zhou, L.; Wang, L.; Wu, W.; Shen, D. Constructing hierarchical attentive functional brain networks for early AD diagnosis. Med. Image Anal. 2024, 94, 103137. [Google Scholar] [CrossRef]
- Wang, X.; Krieger-Redwood, K.; Lyu, B.; Lowndes, R.; Wu, G.; Souter, N.E.; Wang, X.; Kong, R.; Shafiei, G.; Bernhardt, B.C.; et al. The brain’s topographical organization shapes dynamic interaction patterns that support flexible behavior based on rules and long-term knowledge. J. Neurosci. 2024, 44, e2223232024. [Google Scholar]
- Christoff, K.; Gordon, A.M.; Smallwood, J.; Smith, R.; Schooler, J.W. Experience Sampling during FMRI Reveals Default Network and Executive System Contributions to Mind Wandering. Proc. Natl. Acad. Sci. USA 2009, 106, 8719–8724. [Google Scholar] [CrossRef]
- Tulving, E. Episodic Memory: From Mind to Brain. Annu. Rev. Psychol. 2002, 53, 1–25. [Google Scholar] [CrossRef]
- Dennett, D.C. Facing up to the Hard Question of Consciousness. Philos. Trans. R. Soc. Lond. B Biol. Sci. 2018, 373, 20170342. [Google Scholar] [CrossRef]
- Greenberg, D.L.; Rubin, D.C. The Neuropsychology of Autobiographical Memory. Cortex 2003, 39, 687–728. [Google Scholar] [CrossRef]
- Conway, M.A. Memory and the Self. J. Mem. Lang. 2005, 53, 594–628. [Google Scholar] [CrossRef]
- Andrews-Hanna, J.R.; Reidler, J.S.; Sepulcre, J.; Poulin, R.; Buckner, R.L. Functional-Anatomic Fractionation of the Brain’s Default Network. Neuron 2010, 65, 550–562. [Google Scholar] [CrossRef] [PubMed]
- Spreng, R.N.; Stevens, W.D.; Chamberlain, J.P.; Gilmore, A.W.; Schacter, D.L. Default Network Activity, Coupled with the Frontoparietal Control Network, Supports Goal-Directed Cognition. Neuroimage 2010, 53, 303–317. [Google Scholar] [CrossRef] [PubMed]
- Svoboda, E.; McKinnon, M.C.; Levine, B. The Functional Neuroanatomy of Autobiographical Memory: A Meta-Analysis. Neuropsychologia 2006, 44, 2189. [Google Scholar] [CrossRef]
- Pagani, M.; Gutierrez-Barragan, D.; de Guzman, A.E.; Xu, T.; Gozzi, A. Mapping and Comparing FMRI Connectivity Networks across Species. Commun. Biol. 2023, 6, 1238. [Google Scholar] [CrossRef]
- Addis, D.R.; Wong, A.T.; Schacter, D.L. Remembering the Past and Imagining the Future: Common and Distinct Neural Substrates during Event Construction and Elaboration. Neuropsychologia 2007, 45, 1363. [Google Scholar] [CrossRef]
- Mars, R.B.; Neubert, F.X.; Noonan, M.A.P.; Sallet, J.; Toni, I.; Rushworth, M.F.S. On the Relationship between the “Default Mode Network” and the “Social Brain”. Front. Hum. Neurosci. 2012, 6, 189. [Google Scholar] [CrossRef]
- Burklund, L.J.; David Creswell, J.; Irwin, M.R.; Lieberman, M.D. The Common and Distinct Neural Bases of Affect Labeling and Reappraisal in Healthy Adults. Front. Psychol. 2014, 5, 221. [Google Scholar] [CrossRef]
- Spreng, R.N.; Mar, R.A.; Kim, A.S.N. The Common Neural Basis of Autobiographical Memory, Prospection, Navigation, Theory of Mind, and the Default Mode: A Quantitative Meta-Analysis. J. Cogn. Neurosci. 2009, 21, 489–510. [Google Scholar] [CrossRef]
- Hassabis, D.; Kumaran, D.; Maguire, E.A. Using Imagination to Understand the Neural Basis of Episodic Memory. J. Neurosci. 2007, 27, 14365. [Google Scholar] [CrossRef] [PubMed]
- Smallwood, J.; Schooler, J.W. The Restless Mind. Psychol Bull 2006, 132, 946–958. [Google Scholar] [CrossRef] [PubMed]
- Smith, V.; Mitchell, D.J.; Duncan, J. The Effect of Rule Retrieval on Activity in the Default Mode Network. Neuroimage 2019, 202, 116088. [Google Scholar] [CrossRef] [PubMed]
- Whitfield-Gabrieli, S.; Ford, J.M. Default Mode Network Activity and Connectivity in Psychopathology. Annu. Rev. Clin. Psychol. 2012, 8, 49–76. [Google Scholar] [CrossRef]
- Menon, V. Brain Networks and Cognitive Impairment in Psychiatric Disorders. World Psychiatry 2020, 19, 309–310. [Google Scholar] [CrossRef]
- Menon, V.; Uddin, L.Q. Saliency, Switching, Attention and Control: A Network Model of Insula Function. Brain Struct. Funct. 2010, 214, 655. [Google Scholar] [CrossRef]
- Van Dam, D.; Vermeiren, Y.; Dekker, A.D.; Naudé, P.J.W.; De Deyn, P.P. Neuropsychiatric Disturbances in Alzheimer’s Disease: What Have We Learned from Neuropathological Studies? Curr. Alzheimer Res. 2016, 13, 1145. [Google Scholar] [CrossRef]
- Leech, R.; Sharp, D.J. The Role of the Posterior Cingulate Cortex in Cognition and Disease. Brain 2013, 137, 12–32. [Google Scholar] [CrossRef]
- Tanaka, S.; Kirino, E. The Precuneus Contributes to Embodied Scene Construction for Singing in an Opera. Front. Hum. Neurosci. 2021, 15, 737742. [Google Scholar] [CrossRef]
- Raichle, M.E. The Brain’s Default Mode Network. Annu. Rev. Neurosci. 2015, 38, 433–447. [Google Scholar] [CrossRef]
- Ranganath, C.; Ritchey, M. Two Cortical Systems for Memory-Guided Behaviour. Nat Rev Neurosci 2012, 13, 713–726. [Google Scholar] [CrossRef]
- Squire, L.R.; Stark, C.E.L.; Clark, R.E. The Medial Temporal Lobe. Annu Rev Neurosci 2004, 27, 279–306. [Google Scholar] [CrossRef] [PubMed]
- Duncan, J.; Owen, A.M. Common Regions of the Human Frontal Lobe Recruited by Diverse Cognitive Demands. Trends Neurosci. 2000, 23, 475–483. [Google Scholar] [CrossRef] [PubMed]
- Beaty, R.E.; Kenett, Y.N.; Christensen, A.P.; Rosenberg, M.D.; Benedek, M.; Chen, Q.; Fink, A.; Qiu, J.; Kwapil, T.R.; Kane, M.J.; et al. Robust Prediction of Individual Creative Ability from Brain Functional Connectivity. Proc. Natl. Acad. Sci. USA 2018, 115, 1087–1092. [Google Scholar] [CrossRef] [PubMed]
- Maguire, E.A.; Burgess, N.; Donnett, J.G.; Frackowiak, R.S.J.; Frith, C.D.; O’Keefe, J. Knowing Where and Getting There: A Human Navigation Network. Science 1998, 280, 921–924. [Google Scholar] [CrossRef]
- Uddin, L.Q.; Supekar, K.; Lynch, C.J.; Cheng, K.M.; Odriozola, P.; Barth, M.E.; Phillips, J.; Feinstein, C.; Abrams, D.A.; Menon, V. Brain State Differentiation and Behavioral Inflexibility in Autism. Cereb. Cortex 2015, 25, 4740–4747. [Google Scholar] [CrossRef]
- Swanson, L.W. Brain Maps 4.0—Structure of the Rat Brain: An Open Access Atlas with Global Nervous System Nomenclature Ontology and Flatmaps. J. Comp. Neurol. 2018, 526, 935. [Google Scholar] [CrossRef]
- Phelps, E.A.; LeDoux, J.E. Contributions of the Amygdala to Emotion Processing: From Animal Models to Human Behavior. Neuron 2005, 48, 175–187. [Google Scholar] [CrossRef]
- Janak, P.H.; Tye, K.M. From Circuits to Behaviour in the Amygdala. Nature 2015, 517, 284–292. [Google Scholar] [CrossRef]
- Vanhaudenhuyse, A.; Noirhomme, Q.; Tshibanda, L.J.F.; Bruno, M.A.; Boveroux, P.; Schnakers, C.; Soddu, A.; Perlbarg, V.; Ledoux, D.; Brichant, J.F.; et al. Default Network Connectivity Reflects the Level of Consciousness in Non-Communicative Brain-Damaged Patients. Brain 2010, 133, 161–171. [Google Scholar] [CrossRef]
- Motzkin, J.C.; Philippi, C.L.; Wolf, R.C.; Baskaya, M.K.; Koenigs, M. Ventromedial Prefrontal Cortex Is Critical for the Regulation of Amygdala Activity in Humans. Biol. Psychiatry 2015, 77, 276–284. [Google Scholar] [CrossRef] [PubMed]
- Etkin, A.; Wager, T.D. Functional Neuroimaging of Anxiety: A Meta-Analysis of Emotional Processing in PTSD, Social Anxiety Disorder, and Specific Phobia. Am. J. Psychiatry 2007, 164, 1476. [Google Scholar] [CrossRef] [PubMed]
- Baur, V.; Hänggi, J.; Langer, N.; Jäncke, L. Resting-State Functional and Structural Connectivity within an Insula-Amygdala Route Specifically Index State and Trait Anxiety. Biol. Psychiatry 2013, 73, 85–92. [Google Scholar] [CrossRef] [PubMed]
- Medaglia, J.D.; Pasqualetti, F.; Hamilton, R.H.; Thompson-Schill, S.L.; Bassett, D.S. Brain and Cognitive Reserve: Translation via Network Control Theory. Neurosci. Biobehav. Rev. 2017, 75, 53–64. [Google Scholar] [CrossRef]
- Gabard-Durnam, L.J.; O’Muircheartaigh, J.; Dirks, H.; Dean, D.C.; Tottenham, N.; Deoni, S. Human Amygdala Functional Network Development: A Cross-Sectional Study from 3 Months to 5 Years of Age. Dev. Cogn. Neurosci. 2018, 34, 63. [Google Scholar] [CrossRef]
- Ghashghaei, H.T.; Barbas, H. Pathways for Emotion: Interactions of Prefrontal and Anterior Temporal Pathways in the Amygdala of the Rhesus Monkey. Neuroscience 2002, 115, 1261–1279. [Google Scholar] [CrossRef]
- Pessoa, L.; Adolphs, R. Emotion Processing and the Amygdala: From a “low Road” to “Many Roads” of Evaluating Biological Significance. Nat. Rev. Neurosci. 2010, 11, 773–782. [Google Scholar] [CrossRef]
- Kim, M.J.; Loucks, R.A.; Palmer, A.L.; Brown, A.C.; Solomon, K.M.; Marchante, A.N.; Whalen, P.J. The Structural and Functional Connectivity of the Amygdala: From Normal Emotion to Pathological Anxiety. Behav. Brain Res. 2011, 223, 403. [Google Scholar] [CrossRef]
- Rolls, E.T. Emotion, Motivation, Decision-Making, the Orbitofrontal Cortex, Anterior Cingulate Cortex, and the Amygdala. Brain Struct. Funct. 2023, 228, 1201. [Google Scholar] [CrossRef]
- De Pisapia, N.; Barchiesi, G.; Jovicich, J.; Cattaneo, L. The Role of Medial Prefrontal Cortex in Processing Emotional Self-Referential Information: A Combined TMS/FMRI Study. Brain Imaging Behav. 2019, 13, 603–614. [Google Scholar] [CrossRef]
- Corbetta, M.; Shulman, G.L. Control of Goal-Directed and Stimulus-Driven Attention in the Brain. Nat. Rev. Neurosci. 2002, 3, 201–215. [Google Scholar] [CrossRef] [PubMed]
- Chen, T.; Michels, L.; Supekar, K.; Kochalka, J.; Ryali, S.; Menon, V. Role of the Anterior Insular Cortex in Integrative Causal Signaling during Multisensory Auditory-Visual Attention. Eur. J. Neurosci. 2015, 41, 264–274. [Google Scholar] [CrossRef] [PubMed]
- Buckner, R.L.; Carroll, D.C. Self-Projection and the Brain. Trends Cogn. Sci. 2007, 11, 49–57. [Google Scholar] [CrossRef] [PubMed]
- Kucyi, A.; Esterman, M.; Riley, C.S.; Valera, E.M. Spontaneous Default Network Activity Reflects Behavioral Variability Independent of Mind-Wandering. Proc. Natl. Acad. Sci. USA 2016, 113, 13899–13904. [Google Scholar] [CrossRef]
- Wong, T.Y.; Zhang, H.; White, T.; Xu, L.; Qiu, A. Common Functional Brain Networks between Attention Deficit and Disruptive Behaviors in Youth. Neuroimage 2021, 245, 118732. [Google Scholar] [CrossRef]
- Adolphs, R. Conceptual Challenges and Directions for Social Neuroscience. Neuron 2010, 65, 752. [Google Scholar] [CrossRef]
- Davis, M.; Whalen, P.J. The Amygdala: Vigilance and Emotion. Mol. Psychiatry 2001, 6, 13–34. [Google Scholar] [CrossRef]
- Seeley, W.W.; Menon, V.; Schatzberg, A.F.; Keller, J.; Glover, G.H.; Kenna, H.; Reiss, A.L.; Greicius, M.D. Dissociable Intrinsic Connectivity Networks for Salience Processing and Executive Control. J. Neurosci. 2007, 27, 2349–2356. [Google Scholar] [CrossRef]
- Mohr, H.; Wolfensteller, U.; Betzel, R.F.; Mišić, B.; Sporns, O.; Richiardi, J.; Ruge, H. Integration and Segregation of Large-Scale Brain Networks during Short-Term Task Automatization. Nat. Commun. 2016, 7, 13217. [Google Scholar] [CrossRef]
- López-Muguruza, E.; Matute, C. Alterations of Oligodendrocyte and Myelin Energy Metabolism in Multiple Sclerosis. Int. J. Mol. Sci. 2023, 24, 12912. [Google Scholar] [CrossRef]
- Bonnelle, V.; Ham, T.E.; Leech, R.; Kinnunen, K.M.; Mehta, M.A.; Greenwood, R.J.; Sharp, D.J. Salience Network Integrity Predicts Default Mode Network Function after Traumatic Brain Injury. Proc. Natl. Acad. Sci. USA 2012, 109, 4690–4695. [Google Scholar] [CrossRef] [PubMed]
- Enriquez-Geppert, S.; Huster, R.J.; Figge, C.; Herrmann, C.S. Self-Regulation of Frontal-Midline Theta Facilitates Memory Updating and Mental Set Shifting. Front. Behav. Neurosci. 2014, 8, 420. [Google Scholar] [CrossRef]
- Power, J.D.; Cohen, A.L.; Nelson, S.M.; Wig, G.S.; Barnes, K.A.; Church, J.A.; Vogel, A.C.; Laumann, T.O.; Miezin, F.M.; Schlaggar, B.L.; et al. Functional Network Organization of the Human Brain. Neuron 2011, 72, 665. [Google Scholar] [CrossRef] [PubMed]
- Rubinov, M.; Sporns, O. Complex Network Measures of Brain Connectivity: Uses and Interpretations. Neuroimage 2010, 52, 1059–1069. [Google Scholar] [CrossRef]
- Acharya, A.; Pradeep, C.S.; Sinha, N. Localizing and Assessing Node Significance in Default Mode Network Using Sub-Community Detection in Mild Cognitive Impairment. arXiv 2023, arXiv:2312.01768. [Google Scholar]
- Brookes, M.J.; Hale, J.R.; Zumer, J.M.; Stevenson, C.M.; Francis, S.T.; Barnes, G.R.; Owen, J.P.; Morris, P.G.; Nagarajan, S.S. Measuring Functional Connectivity Using MEG: Methodology and Comparison with FcMRI. Neuroimage 2011, 56, 1082–1104. [Google Scholar] [CrossRef]
- Mulert, C.; Seifert, C.; Leicht, G.; Kirsch, V.; Ertl, M.; Karch, S.; Moosmann, M.; Lutz, J.; Möller, H.J.; Hegerl, U.; et al. Single-Trial Coupling of EEG and FMRI Reveals the Involvement of Early Anterior Cingulate Cortex Activation in Effortful Decision Making. Neuroimage 2008, 42, 158–168. [Google Scholar] [CrossRef]
- Afnan, J.; Cai, Z.; Lina, J.M.; Abdallah, C.; Delaire, E.; Avigdor, T.; Ros, V.; Hedrich, T.; von Ellenrieder, N.; Kobayashi, E.; et al. EEG/MEG Source Imaging of Deep Brain Activity within the Maximum Entropy on the Mean Framework: Simulations and Validation in Epilepsy. Hum. Brain Mapp. 2024, 45, e26720. [Google Scholar] [CrossRef]
- Raichle, M.E.; Mintun, M.A. Brain Work and Brain Imaging. Annu. Rev. Neurosci. 2006, 29, 449–476. [Google Scholar] [CrossRef]
- Buckner, R.L.; DiNicola, L.M. The Brain’s Default Network: Updated Anatomy, Physiology and Evolving Insights. Nat. Rev. Neurosci. 2019, 20, 593–608. [Google Scholar] [CrossRef]
- Crișan, G.; Moldovean-cioroianu, N.S.; Timaru, D.G.; Andrieș, G.; Căinap, C.; Chiș, V. Radiopharmaceuticals for PET and SPECT Imaging: A Literature Review over the Last Decade. Int. J. Mol. Sci. 2022, 23, 5023. [Google Scholar] [CrossRef] [PubMed]
- Rosca, E.C.; Halkiopoulos, C.; Gkintoni, E.; Aroutzidis, A.; Antonopoulou, H. Advances in Neuroimaging and Deep Learning for Emotion Detection: A Systematic Review of Cognitive Neuroscience and Algorithmic Innovations. Diagnostics 2025, 15, 456. [Google Scholar] [CrossRef] [PubMed]
- Yen, C.; Lin, C.L.; Chiang, M.C. Exploring the Frontiers of Neuroimaging: A Review of Recent Advances in Understanding Brain Functioning and Disorders. Life 2023, 13, 1472. [Google Scholar] [CrossRef] [PubMed]
- Cao, J.; Zhao, Y.; Shan, X.; Wei, H.L.; Guo, Y.; Chen, L.; Erkoyuncu, J.A.; Sarrigiannis, P.G. Brain Functional and Effective Connectivity Based on Electroencephalography Recordings: A Review. Hum. Brain Mapp. 2021, 43, 860. [Google Scholar] [CrossRef]
- Farras-Permanyer, L.; Mancho-Fora, N.; Montalà-Flaquer, M.; Bartrés-Faz, D.; Vaqué-Alcázar, L.; Peró-Cebollero, M.; Guàrdia-Olmos, J. Age-Related Changes in Resting-State Functional Connectivity in Older Adults. Neural Regen. Res. 2019, 14, 1544–1555. [Google Scholar] [CrossRef]
- Shah, C.; Liu, J.; Lv, P.; Sun, H.; Xiao, Y.; Liu, J.; Zhao, Y.; Zhang, W.; Yao, L.; Gong, Q.; et al. Age Related Changes in Topological Properties of Brain Functional Network and Structural Connectivity. Front. Neurosci. 2018, 12, 318. [Google Scholar] [CrossRef]
- Neudorf, J.; Kress, S.; Borowsky, R. Comparing Models of Information Transfer in the Structural Brain Network and Their Relationship to Functional Connectivity: Diffusion versus Shortest Path Routing. Brain Struct. Funct. 2023, 228, 651. [Google Scholar] [CrossRef]
- Perinelli, A.; Assecondi, S.; Tagliabue, C.F.; Mazza, V. Power Shift and Connectivity Changes in Healthy Aging during Resting-State EEG. Neuroimage 2022, 256, 119247. [Google Scholar] [CrossRef]
- Stark, S.M.; Frithsen, A.; Stark, C.E.L. Age-Related Alterations in Functional Connectivity along the Longitudinal Axis of the Hippocampus and Its Subfields. Hippocampus 2020, 31, 11. [Google Scholar] [CrossRef]
- Sanli, D.B.; Bilici, R.; Suner, O.; Citak, S.; Kartkaya, K.; Mutlu, F.S. Effect of Different Psychoactive Substances on Serum Biochemical Parameters. Int. J. High Risk Behav. Addict. 2015, 4, e22702. [Google Scholar] [CrossRef]
- Brewer, J.A.; Worhunsky, P.D.; Gray, J.R.; Tang, Y.Y.; Weber, J.; Kober, H. Meditation Experience Is Associated with Differences in Default Mode Network Activity and Connectivity. Proc. Natl. Acad. Sci. USA 2011, 108, 20254–20259. [Google Scholar] [CrossRef] [PubMed]
- Fateh, A.A.; Huang, W.; Hassan, M.; Zhuang, Y.; Lin, J.; Luo, Y.; Yang, B.; Zeng, H. Default Mode Network Connectivity and Social Dysfunction in Children with Attention Deficit/Hyperactivity Disorder. Int. J. Clin. Health Psychol. 2023, 23, 100393. [Google Scholar] [CrossRef] [PubMed]
- Brefczynski-Lewis, J.A.; Lutz, A.; Schaefer, H.S.; Levinson, D.B.; Davidson, R.J. Neural Correlates of Attentional Expertise in Long-Term Meditation Practitioners. Proc. Natl. Acad. Sci. USA 2007, 104, 11483–11488. [Google Scholar] [CrossRef] [PubMed]
- Bremer, B.; Wu, Q.; Mora Álvarez, M.G.; Hölzel, B.K.; Wilhelm, M.; Hell, E.; Tavacioglu, E.E.; Torske, A.; Koch, K. Mindfulness Meditation Increases Default Mode, Salience, and Central Executive Network Connectivity. Sci. Rep. 2022, 12, 13219. [Google Scholar] [CrossRef]
- Hansen, J.Y.; Shafiei, G.; Voigt, K.; Liang, E.X.; Cox, S.M.L.; Leyton, M.; Jamadar, S.D.; Misic, B. Integrating Multimodal and Multiscale Connectivity Blueprints of the Human Cerebral Cortex in Health and Disease. PLoS Biol. 2023, 21, e3002314. [Google Scholar] [CrossRef]
- Owens, M.M.; Yuan, D.K.; Hahn, S.; Albaugh, M.; Allgaier, N.; Chaarani, B.; Potter, A.; Garavan, H. Investigation of Psychiatric and Neuropsychological Correlates of Default Mode Network and Dorsal Attention Network Anticorrelation in Children. Cereb. Cortex 2020, 30, 6083. [Google Scholar] [CrossRef]
- Fair, D.A.; Cohen, A.L.; Dosenbach, N.U.F.; Church, J.A.; Miezin, F.M.; Barch, D.M.; Raichle, M.E.; Petersen, S.E.; Schlaggar, B.L. The Maturing Architecture of the Brain’s Default Network. Proc. Natl. Acad. Sci. USA 2008, 105, 4028–4032. [Google Scholar] [CrossRef]
- McLaughlin, K.A.; Sheridan, M.A. Beyond Cumulative Risk: A Dimensional Approach to Childhood Adversity. Curr. Dir. Psychol Sci. 2016, 25, 239. [Google Scholar] [CrossRef]
- McLaughlin, K.A.; Peverill, M.; Gold, A.L.; Alves, S.; Sheridan, M.A. Child Maltreatment and Neural Systems Underlying Emotion Regulation. J. Am. Acad. Child Adolesc. Psychiatry 2015, 54, 753–762. [Google Scholar] [CrossRef]
- Tost, H.; Champagne, F.A.; Meyer-Lindenberg, A. Environmental Influence in the Brain, Human Welfare and Mental Health. Nat. Neurosci. 2015, 18, 4121–4131. [Google Scholar] [CrossRef]
- Sheridan, M.A.; McLaughlin, K.A. Dimensions of Early Experience and Neural Development: Deprivation and Threat. Trends Cogn. Sci. 2014, 18, 580–585. [Google Scholar] [CrossRef] [PubMed]
- Tottenham, N.; Sheridan, M.A. A Review of Adversity, The Amygdala and the Hippocampus: A Consideration of Developmental Timing. Front. Hum. Neurosci. 2009, 3, 68. [Google Scholar] [CrossRef] [PubMed]
- Johnson, S.B.; Riis, J.L.; Noble, K.G. State of the Art Review: Poverty and the Developing Brain. Pediatrics 2016, 137, e20153075. [Google Scholar] [CrossRef] [PubMed]
- Luby, J.; Belden, A.; Botteron, K.; Marrus, N.; Harms, M.P.; Babb, C.; Nishino, T.; Barch, D. The Effects of Poverty on Childhood Brain Development: The Mediating Effect of Caregiving and Stressful Life Events. JAMA Pediatr. 2013, 167, 1135. [Google Scholar] [CrossRef]
- Hanson, J.L.; Hariri, A.R.; Williamson, D.E. Blunted Ventral Striatum Development in Adolescence Reflects Emotional Neglect and Predicts Depressive Symptoms. Biol. Psychiatry 2015, 78, 598–605. [Google Scholar] [CrossRef]
- Hair, N.L.; Hanson, J.L.; Wolfe, B.L.; Pollak, S.D. Association of Child Poverty, Brain Development, and Academic Achievement. JAMA Pediatr. 2015, 169, 822–829. [Google Scholar] [CrossRef]
- McLaughlin, K.A.; Lambert, H.K. Child Trauma Exposure and Psychopathology: Mechanisms of Risk and Resilience. Curr. Opin. Psychol. 2017, 14, 29. [Google Scholar] [CrossRef]
- Gee, D.G.; Gabard-Durnam, L.J.; Flannery, J.; Goff, B.; Humphreys, K.L.; Telzer, E.H.; Hare, T.A.; Bookheimer, S.Y.; Tottenham, N. Early Developmental Emergence of Human Amygdala-Prefrontal Connectivity after Maternal Deprivation. Proc. Natl. Acad. Sci. USA 2013, 110, 15638–15643. [Google Scholar] [CrossRef]
- Barch, D.; Pagliaccio, D.; Belden, A.; Harms, M.P.; Gaffrey, M.; Sylvester, C.M.; Tillman, R.; Luby, J. Hippocampal and Amygdala Connectivity Mediate The Relationship Between Preschool Poverty and School Aged Depression. Am. J. Psychiatry 2016, 173, 625. [Google Scholar] [CrossRef]
- Noble, K.G.; McCandliss, B.D.; Farah, M.J. Socioeconomic Gradients Predict Individual Differences in Neurocognitive Abilities. Dev. Sci. 2007, 10, 464–480. [Google Scholar] [CrossRef]
- Hackman, D.A.; Farah, M.J. Socioeconomic Status and the Developing Brain. Trends Cogn. Sci. 2009, 13, 65–73. [Google Scholar] [CrossRef] [PubMed]
- Hanson, J.L.; Chung, M.K.; Avants, B.B.; Shirtcliff, E.A.; Gee, J.C.; Davidson, R.J.; Pollak, S.D. Early Stress Is Associated with Alterations in the Orbitofrontal Cortex: A Tensor-Based Morphometry Investigation of Brain Structure and Behavioral Risk. J. Neurosci. 2010, 30, 7466. [Google Scholar] [CrossRef] [PubMed]
- Dannlowski, U.; Stuhrmann, A.; Beutelmann, V.; Zwanzger, P.; Lenzen, T.; Grotegerd, D.; Domschke, K.; Hohoff, C.; Ohrmann, P.; Bauer, J.; et al. Limbic Scars: Long-Term Consequences of Childhood Maltreatment Revealed by Functional and Structural Magnetic Resonance Imaging. Biol. Psychiatry 2012, 71, 286–293. [Google Scholar] [CrossRef] [PubMed]
- Van Der Werff, S.J.A.; Pannekoek, J.N.; Veer, I.M.; Van Tol, M.J.; Aleman, A.; Veltman, D.J.; Zitman, F.G.; Rombouts, S.A.R.B.; Elzinga, B.M.; Van Der Wee, N.J.A. Resting-State Functional Connectivity in Adults with Childhood Emotional Maltreatment. Psychol. Med. 2013, 43, 1825–1836. [Google Scholar] [CrossRef]
- van Praag, H.; Kempermann, G.; Gage, F.H. Neural Consequences of Enviromental Enrichment. Nat. Rev. Neurosci. 2000, 1, 191–198. [Google Scholar] [CrossRef]
- Farah, M.J.; Shera, D.M.; Savage, J.H.; Betancourt, L.; Giannetta, J.M.; Brodsky, N.L.; Malmud, E.K.; Hurt, H. Childhood Poverty: Specific Associations with Neurocognitive Development. Brain Res. 2006, 1110, 166–174. [Google Scholar] [CrossRef]
- Sidlauskaite, J.; Sonuga-Barke, E.; Roeyers, H.; Wiersema, J.R. Altered Intrinsic Organisation of Brain Networks Implicated in Attentional Processes in Adult Attention-Deficit/Hyperactivity Disorder: A Resting-State Study of Attention, Default Mode and Salience Network Connectivity. Eur. Arch. Psychiatry Clin. Neurosci. 2016, 266, 349–357. [Google Scholar] [CrossRef]
- Schilbach, L.; Eickhoff, S.B.; Rotarska-Jagiela, A.; Fink, G.R.; Vogeley, K. Minds at Rest? Social Cognition as the Default Mode of Cognizing and Its Putative Relationship to the “Default System” of the Brain. Conscious. Cogn. 2008, 17, 457–467. [Google Scholar] [CrossRef]
- Dunbar, R.I.M. The Social Brain Hypothesis and Its Implications for Social Evolution. Ann. Hum. Biol. 2009, 36, 562–572. [Google Scholar] [CrossRef]
- Lieberman, M.D. Social Cognitive Neuroscience: A Review of Core Processes. Annu. Rev. Psychol. 2007, 58, 259–289. [Google Scholar] [CrossRef]
- Bzdok, D.; Langner, R.; Schilbach, L.; Engemann, D.A.; Laird, A.R.; Fox, P.T.; Eickhoff, S.B. Segregation of the Human Medial Prefrontal Cortex in Social Cognition. Front. Hum. Neurosci. 2013, 7, 232. [Google Scholar] [CrossRef]
- Yang, J.; Weng, X.; Zang, Y.; Xu, M.; Xu, X. Sustained Activity within the Default Mode Network during an Implicit Memory Task. Cortex 2009, 46, 354. [Google Scholar] [CrossRef] [PubMed]
- Assaf, M.; Jagannathan, K.; Calhoun, V.D.; Miller, L.; Stevens, M.C.; Sahl, R.; O’Boyle, J.G.; Schultz, R.T.; Pearlson, G.D. Abnormal Functional Connectivity of Default Mode Sub-Networks in Autism Spectrum Disorder Patients. Neuroimage 2010, 53, 247–256. [Google Scholar] [CrossRef] [PubMed]
- Mills, K.L.; Goddings, A.L.; Herting, M.M.; Meuwese, R.; Blakemore, S.J.; Crone, E.A.; Dahl, R.E.; Güroğlu, B.; Raznahan, A.; Sowell, E.R.; et al. Structural Brain Development between Childhood and Adulthood: Convergence across Four Longitudinal Samples. Neuroimage 2016, 141, 273–281. [Google Scholar] [CrossRef]
- Sampaio, A.; Soares, J.M.; Coutinho, J.; Sousa, N.; Gonçalves, Ó.F. The Big Five Default Brain: Functional Evidence. Brain Struct. Funct. 2014, 219, 1913–1922. [Google Scholar] [CrossRef]
- Pelletier-Baldelli, A.; Andrews-Hanna, J.R.; Mittal, V.A. Resting State Connectivity Dynamics in Youth at Risk for Psychosis. J. Abnorm. Psychol. 2018, 127, 314. [Google Scholar] [CrossRef]
- Malagurski, B.; Deschwanden, P.F.; Jäncke, L.; Mérillat, S. Longitudinal Functional Connectivity Patterns of the Default Mode Network in Healthy Older Adults. Neuroimage 2022, 259, 119414. [Google Scholar] [CrossRef]
- Menardi, A.; Spoa, M.; Vallesi, A. Brain Topology Underlying Executive Functions across the Lifespan: Focus on the Default Mode Network. Front. Psychol. 2024, 15, 1441584. [Google Scholar] [CrossRef]
- Averill, C.L.; Averill, L.A.; Akiki, T.J.; Fouda, S.; Krystal, J.H.; Abdallah, C.G. Findings of PTSD-Specific Deficits in Default Mode Network Strength Following a Mild Experimental Stressor. NPP Digit. Psychiatry Neurosci. 2024, 2, 9. [Google Scholar] [CrossRef]
- Spreng, R.N.; Grady, C.L. Patterns of Brain Activity Supporting Autobiographical Memory, Prospection, and Theory of Mind, and Their Relationship to the Default Mode Network. J. Cogn. Neurosci. 2010, 22, 1112–1123. [Google Scholar] [CrossRef]
- Tillman, R.M.; Stockbridge, M.D.; Nacewicz, B.M.; Torrisi, S.; Fox, A.S.; Smith, J.F.; Shackman, A.J. Intrinsic Functional Connectivity of the Central Extended Amygdala. Hum. Brain Mapp. 2018, 39, 1291–1312. [Google Scholar] [CrossRef] [PubMed]
- Uddin, L.Q.; Kelly, A.M.C.; Biswal, B.B.; Castellanos, F.X.; Milham, M.P. Functional Connectivity of Default Mode Network Components: Correlation, Anticorrelation, and Causality. Hum. Brain Mapp. 2009, 30, 625–637. [Google Scholar] [CrossRef] [PubMed]
- Greicius, M.D.; Flores, B.H.; Menon, V.; Glover, G.H.; Solvason, H.B.; Kenna, H.; Reiss, A.L.; Schatzberg, A.F. Resting-State Functional Connectivity in Major Depression: Abnormally Increased Contributions from Subgenual Cingulate Cortex and Thalamus. Biol. Psychiatry 2007, 62, 429–437. [Google Scholar] [CrossRef]
- Sheline, Y.I.; Price, J.L.; Yan, Z.; Mintun, M.A. Resting-State Functional MRI in Depression Unmasks Increased Connectivity between Networks via the Dorsal Nexus. Proc. Natl. Acad. Sci. USA 2010, 107, 11020. [Google Scholar] [CrossRef]
- Brewin, C.R. Understanding Cognitive Behaviour Therapy: A Retrieval Competition Account. Behav. Res. Ther. 2006, 44, 765–784. [Google Scholar] [CrossRef]
- Dörfel, D.; Lamke, J.P.; Hummel, F.; Wagner, U.; Erk, S.; Walter, H. Common and Differential Neural Networks of Emotion Regulation by Detachment, Reinterpretation, Distraction, and Expressive Suppression: A Comparative FMRI Investigation. Neuroimage 2014, 101, 298–309. [Google Scholar] [CrossRef]
- Tozzi, L.; Zhang, X.; Chesnut, M.; Holt-Gosselin, B.; Ramirez, C.A.; Williams, L.M. Reduced Functional Connectivity of Default Mode Network Subsystems in Depression: Meta-Analytic Evidence and Relationship with Trait Rumination. Neuroimage Clin. 2021, 30, 102570. [Google Scholar] [CrossRef]
- Pommy, J.; Smart, C.M.; Bryant, A.M.; Wang, Y. Three Potential Neurovascular Pathways Driving the Benefits of Mindfulness Meditation for Older Adults. Front. Aging Neurosci. 2023, 15, 1207012. [Google Scholar] [CrossRef]
- Chou, T.; Deckersbach, T.; Dougherty, D.D.; Hooley, J.M. The Default Mode Network and Rumination in Individuals at Risk for Depression. Soc. Cogn. Affect. Neurosci. 2023, 18, nsad032. [Google Scholar] [CrossRef]
- Qiu, H.; Zhang, L.; Gao, Y.; Zhou, Z.; Li, H.; Cao, L.; Wang, Y.; Hu, X.; Liang, K.; Tang, M.; et al. Functional Connectivity of the Default Mode Network in First-Episode Drug-Naïve Patients with Major Depressive Disorder. J. Affect. Disord. 2024, 361, 489–496. [Google Scholar] [CrossRef]
- Zhu, X.; Zhu, Q.; Shen, H.; Liao, W.; Yuan, F. Rumination and Default Mode Network Subsystems Connectivity in First-Episode, Drug-Naive Young Patients with Major Depressive Disorder. Sci. Rep. 2017, 7, srep43105. [Google Scholar] [CrossRef]
- Lee, D.; Lee, J.; Lee, J.E.; Jung, Y.C. Altered Functional Connectivity in Default Mode Network in Internet Gaming Disorder: Influence of Childhood ADHD. Prog. Neuropsychopharmacol. Biol. Psychiatry 2017, 75, 135–141. [Google Scholar] [CrossRef] [PubMed]
- Lyubomksky, S.; Sheldon, K.M.; Schkade, D. Pursuing Happiness: The Architecture of Sustainable Change. Rev. Gen. Psychol. 2005, 9, 111–131. [Google Scholar] [CrossRef]
- Lyubomirsky, S.; King, L.; Diener, E. The Benefits of Frequent Positive Affect: Does Happiness Lead to Success? Psychol. Bull. 2005, 131, 803–855. [Google Scholar] [CrossRef]
- Nolen-Hoeksema, S.; Wisco, B.E.; Lyubomirsky, S. Rethinking Rumination. Perspect. Psychol. Sci. 2008, 3, 400–424. [Google Scholar] [CrossRef]
- Killingsworth, M.A.; Gilbert, D.T. A Wandering Mind Is an Unhappy Mind. Science 2010, 330, 932. [Google Scholar] [CrossRef]
- Stawarczyk, D.; Majerus, S.; Van der Linden, M.; D’Argembeau, A. Using the Daydreaming Frequency Scale to Investigate the Relationships between Mind-Wandering, Psychological Well-Being, and Present-Moment Awareness. Front. Psychol. 2012, 3, 363. [Google Scholar] [CrossRef]
- Li, C.T.; Chen, M.H.; Juan, C.H.; Huang, H.H.; Chen, L.F.; Hsieh, J.C.; Tu, P.C.; Bai, Y.M.; Tsai, S.J.; Lee, Y.C.; et al. Efficacy of Prefrontal Theta-Burst Stimulation in Refractory Depression: A Randomized Sham-Controlled Study. Brain 2014, 137, 2088–2098. [Google Scholar] [CrossRef]
- Decety, J.; Svetlova, M. Putting Together Phylogenetic and Ontogenetic Perspectives on Empathy. Dev. Cogn. Neurosci. 2012, 2, 1–24. [Google Scholar] [CrossRef]
- Ringwald, W.R.; Wright, A.G.C. The Affiliative Role of Empathy in Everyday Interpersonal Interactions. Eur. J. Pers. 2020, 35, 197. [Google Scholar] [CrossRef]
- Kanske, P.; Böckler, A.; Trautwein, F.M.; Singer, T. Dissecting the Social Brain: Introducing the EmpaToM to Reveal Distinct Neural Networks and Brain-Behavior Relations for Empathy and Theory of Mind. Neuroimage 2015, 122, 6–19. [Google Scholar] [CrossRef] [PubMed]
- Kong, F.; Wang, X.; Hu, S.; Liu, J. Neural Correlates of Psychological Resilience and Their Relation to Life Satisfaction in a Sample of Healthy Young Adults. Neuroimage 2015, 123, 165–172. [Google Scholar] [CrossRef] [PubMed]
- Hoemann, K.; Xu, F.; Barrett, L.F. Emotion Words, Emotion Concepts, and Emotional Development in Children: A Constructionist Hypothesis. Dev. Psychol. 2019, 55, 1830. [Google Scholar] [CrossRef] [PubMed]
- Vincent, J.L.; Kahn, I.; Snyder, A.Z.; Raichle, M.E.; Buckner, R.L. Evidence for a Frontoparietal Control System Revealed by Intrinsic Functional Connectivity. J. Neurophysiol. 2008, 100, 3328–3342. [Google Scholar] [CrossRef]
- Mantini, D.; Gerits, A.; Nelissen, K.; Durand, J.B.; Joly, O.; Simone, L.; Sawamura, H.; Wardak, C.; Orban, G.A.; Buckner, R.L.; et al. Default Mode of Brain Function in Monkeys. J. Neurosci. 2011, 31, 12954–12962. [Google Scholar] [CrossRef]
- Rauch, S.L.; Whalen, P.J.; Shin, L.M.; McInerney, S.C.; MacKlin, M.L.; Lasko, N.B.; Orr, S.P.; Pitman, R.K. Exaggerated Amygdala Response to Masked Facial Stimuli in Posttraumatic Stress Disorder: A Functional MRI Study. Biol. Psychiatry 2000, 47, 769–776. [Google Scholar] [CrossRef]
- Etkin, A.; Schatzberg, A.F. Common Abnormalities and Disorder-Specific Compensation during Implicit Regulation of Emotional Processing in Generalized Anxiety and Major Depressive Disorders. Am. J. Psychiatry 2011, 168, 968–978. [Google Scholar] [CrossRef]
- Xiao, M.; Chen, X.; Yi, H.; Luo, Y.; Yan, Q.; Feng, T.; He, Q.; Lei, X.; Qiu, J.; Chen, H. Stronger Functional Network Connectivity and Social Support Buffer against Negative Affect during the COVID-19 Outbreak and after the Pandemic Peak. Neurobiol. Stress 2021, 15, 100418. [Google Scholar] [CrossRef]
- Sinha, R. Chronic Stress, Drug Use, and Vulnerability to Addiction. Ann. N. Y. Acad. Sci. 2008, 1141, 105. [Google Scholar] [CrossRef]
- Lombardo, M.V.; Chakrabarti, B.; Bullmore, E.T.; Wheelwright, S.J.; Sadek, S.A.; Suckling, J.; Baron-Cohen, S.; Bailey, A.J.; Bolton, P.F.; Carrington, S.; et al. Shared Neural Circuits for Mentalizing about the Self and Others. J. Cogn. Neurosci. 2010, 22, 1623–1635. [Google Scholar] [CrossRef]
- Satpute, A.B.; Lindquist, K.A. The Default Mode Network’s Role in Discrete Emotion. Trends Cogn. Sci. 2019, 23, 851. [Google Scholar] [CrossRef] [PubMed]
- Smith, V.; Mitchell, D.J.; Duncan, J. Role of the Default Mode Network in Cognitive Transitions. Cereb. Cortex 2018, 28, 3685. [Google Scholar] [CrossRef]
- Jackson, R.L.; Humphreys, G.F.; Rice, G.E.; Binney, R.J.; Lambon Ralph, M.A. A Network-Level Test of the Role of the Co-Activated Default Mode Network in Episodic Recall and Social Cognition. Cortex 2023, 165, 141–159. [Google Scholar] [CrossRef] [PubMed]
- Eisenberger, N.I.; Lieberman, M.D.; Williams, K.D. Does Rejection Hurt? An FMRI Study of Social Exclusion. Science 2003, 302, 290–292. [Google Scholar] [CrossRef] [PubMed]
- Cacioppo, J.T.; Cacioppo, S. Social Relationships and Health: The Toxic Effects of Perceived Social Isolation. Soc. Pers. Psychol. Compass 2014, 8, 58. [Google Scholar] [CrossRef]
- Yeshurun, Y.; Nguyen, M.; Hasson, U. The Default Mode Network: Where the Idiosyncratic Self Meets the Shared Social World. Nat. Rev. Neurosci. 2021, 22, 181. [Google Scholar] [CrossRef]
- Schimmelpfennig, J.; Topczewski, J.; Zajkowski, W.; Jankowiak-Siuda, K. The Role of the Salience Network in Cognitive and Affective Deficits. Front. Hum. Neurosci. 2023, 17, 1133367. [Google Scholar] [CrossRef]
- Horovitz, S.G.; Braun, A.R.; Carr, W.S.; Picchioni, D.; Balkin, T.J.; Fukunaga, M.; Duyn, J.H. Decoupling of the Brain’s Default Mode Network during Deep Sleep. Proc. Natl. Acad. Sci. USA 2009, 106, 11376–11381. [Google Scholar] [CrossRef]
- Horovitz, S.G.; Fukunaga, M.; De Zwart, J.A.; Van Gelderen, P.; Fulton, S.C.; Balkin, T.J.; Duyn, J.H. Low Frequency BOLD Fluctuations during Resting Wakefulness and Light Sleep: A Simultaneous EEG-FMRI Study. Hum. Brain Mapp. 2008, 29, 671–682. [Google Scholar] [CrossRef]
- Diekelmann, S.; Born, J. The Memory Function of Sleep. Nat. Rev. Neurosci. 2010, 11, 114–126. [Google Scholar] [CrossRef]
- Carhart-Harris, R.L.; Leech, R.; Hellyer, P.J.; Shanahan, M.; Feilding, A.; Tagliazucchi, E.; Chialvo, D.R.; Nutt, D. The Entropic Brain: A Theory of Conscious States Informed by Neuroimaging Research with Psychedelic Drugs. Front. Hum. Neurosci. 2014, 8, 20. [Google Scholar] [CrossRef]
- Khelfaoui, H.; Ibaceta-Gonzalez, C.; Angulo, M.C. Functional Myelin in Cognition and Neurodevelopmental Disorders. Cell. Mol. Life Sci. 2024, 81, 181. [Google Scholar] [CrossRef] [PubMed]
- Ibrahim, P.; Almeida, D.; Nagy, C.; Turecki, G. Molecular Impacts of Childhood Abuse on the Human Brain. Neurobiol. Stress 2021, 15, 100343. [Google Scholar] [CrossRef] [PubMed]
- McLaughlin, K.A.; Weissman, D.; Bitrán, D. Childhood Adversity and Neural Development: A Systematic Review. Annu. Rev. Dev. Psychol. 2019, 1, 277. [Google Scholar] [CrossRef]
- Nickel, M.; Gu, C. Regulation of Central Nervous System Myelination in Higher Brain Functions. Neural Plast. 2018, 2018, 6436453. [Google Scholar] [CrossRef]
- McCarthy-Jones, S.; Oestreich, L.K.L.; Lyall, A.E.; Kikinis, Z.; Newell, D.T.; Savadjiev, P.; Shenton, M.E.; Kubicki, M.; Pasternak, O.; Whitford, T.J. Childhood Adversity Associated with White Matter Alteration in the Corpus Callosum, Corona Radiata, and Uncinate Fasciculus of Psychiatrically Healthy Adults. Brain Imaging Behav. 2018, 12, 449. [Google Scholar] [CrossRef]
- McEwen, B.S. Physiology and Neurobiology of Stress and Adaptation: Central Role of the Brain. Physiol. Rev. 2007, 87, 873–904. [Google Scholar] [CrossRef]
- Breton, J.M.; Barraza, M.; Hu, K.Y.; Frias, S.J.; Long, K.L.P.; Kaufer, D. Juvenile Exposure to Acute Traumatic Stress Leads to Long-Lasting Alterations in Grey Matter Myelination in Adult Female but Not Male Rats. Neurobiol. Stress 2021, 14, 100319. [Google Scholar] [CrossRef]
- Hernández-Álvarez, D.M.; Pacheco, L.; Velasco-Segura, R.; Pérez de la Mora, M.; Tejeda-Romero, C.; González-García, N. Default Mode Network Efficiency Is Correlated With Deficits in Inhibition in Adolescents With Inhalant Use Disorder. Front. Psychiatry 2020, 11, 504333. [Google Scholar] [CrossRef]
- Abellaneda-Pérez, K.; Vaqué-Alcázar, L.; Vidal-Piñeiro, D.; Jannati, A.; Solana, E.; Bargalló, N.; Santarnecchi, E.; Pascual-Leone, A.; Bartrés-Faz, D. Age-Related Differences in Default-Mode Network Connectivity in Response to Intermittent Theta-Burst Stimulation and Its Relationships with Maintained Cognition and Brain Integrity in Healthy Aging. Neuroimage 2018, 188, 794. [Google Scholar] [CrossRef]
- Marques, R.C.; Vieira, L.; Marques, D.; Cantilino, A. Transcranial Magnetic Stimulation of the Medial Prefrontal Cortex for Psychiatric Disorders: A Systematic Review. Rev. Bras. Psiquiatr. 2019, 41, 447. [Google Scholar] [CrossRef] [PubMed]
- Sala-Llonch, R.; Idland, A.V.; Borza, T.; Watne, L.O.; Wyller, T.B.; Brækhus, A.; Zetterberg, H.; Blennow, K.; Walhovd, K.B.; Fjell, A.M. Inflammation, Amyloid, and Atrophy in The Aging Brain: Relationships with Longitudinal Changes in Cognition. J Alzheimers Dis 2017, 58, 829–840. [Google Scholar] [CrossRef] [PubMed]
- Chhatwal, J.P.; Schultz, A.P.; Johnson, K.A.; Hedden, T.; Jaimes, S.; Benzinger, T.L.S.; Jack, C.; Ances, B.M.; Ringman, J.M.; Marcus, D.S.; et al. Preferential Degradation of Cognitive Networks Differentiates Alzheimer’s Disease from Ageing. Brain 2018, 141, 1486–1500. [Google Scholar] [CrossRef] [PubMed]
- Franzmeier, N.; Göttler, J.; Grimmer, T.; Drzezga, A.; Áraque-Caballero, M.A.; Simon-Vermot, L.; Taylor, A.N.W.; Bürger, K.; Catak, C.; Janowitz, D.; et al. Resting-State Connectivity of the Left Frontal Cortex to the Default Mode and Dorsal Attention Network Supports Reserve in Mild Cognitive Impairment. Front. Aging Neurosci. 2017, 9, 264. [Google Scholar] [CrossRef]
- Al-Ezzi, A.; Arechavala, R.J.; Butler, R.; Nolty, A.; Kang, J.J.; Shimojo, S.; Wu, D.A.; Fonteh, A.N.; Kleinman, M.T.; Kloner, R.A.; et al. Disrupted Brain Functional Connectivity as Early Signature in Cognitively Healthy Individuals with Pathological CSF Amyloid/Tau. Commun. Biol. 2024, 7, 1037. [Google Scholar] [CrossRef]
- Bullmore, E.; Sporns, O. Complex Brain Networks: Graph Theoretical Analysis of Structural and Functional Systems. Nat. Rev. Neurosci. 2009, 10, 186–198. [Google Scholar] [CrossRef]
- Zhu, T.; Wang, Z.; Wu, W.; Ling, Y.; Wang, Z.; Zhou, C.; Fang, X.; Huang, C.; Xie, C.; Chen, J.; et al. Altered Brain Functional Networks in Schizophrenia with Persistent Negative Symptoms: An Activation Likelihood Estimation Meta-Analysis. Front Hum. Neurosci. 2023, 17, 1204632. [Google Scholar] [CrossRef]
- Roig-Herrero, A.; Planchuelo-Gómez, Á.; Hernández-García, M.; de Luis-García, R.; Fernández-Linsenbarth, I.; Beño-Ruiz-de-la-Sierra, R.M.; Molina, V. Default Mode Network Components and Its Relationship with Anomalous Self-Experiences in Schizophrenia: A Rs-FMRI Exploratory Study. Psychiatry Res. Neuroimaging 2022, 324, 111495. [Google Scholar] [CrossRef]
- Mehta, U.M.; Ibrahim, F.A.; Sharma, M.S.; Venkatasubramanian, G.; Thirthalli, J.; Bharath, R.D.; Bolo, N.R.; Gangadhar, B.N.; Keshavan, M.S. Resting-State Functional Connectivity Predictors of Treatment Response in Schizophrenia—A Systematic Review and Meta-Analysis. Schizophr. Res. 2021, 237, 153–165. [Google Scholar] [CrossRef]
- Lebedeva, I.S.; Panikratova, Y.R.; Abdullina, E.G.; Migalina, V.V.; Tikhonov, D.V.; Omelchenko, M.A.; Kaleda, V.G. Neuroimaging (Resting-State FMRI) and Neuropsychological Characteristics of Non-Converted Patients from a Group at Clinical High Risk for Schizophrenia. Neurosci. Behav. Physiol. 2023, 53, 1449–1458. [Google Scholar] [CrossRef]
- Wang, Y.M.; Zou, L.Q.; Xie, W.L.; Yang, Z.Y.; Zhu, X.Z.; Cheung, E.F.C.; Sorensen, T.A.; Moller, A.; Chan, R.C.K. Altered Functional Connectivity of the Default Mode Network in Patients With Schizo-Obsessive Comorbidity: A Comparison Between Schizophrenia and Obsessive-Compulsive Disorder. Schizophr. Bull. 2018, 45, 199. [Google Scholar] [CrossRef] [PubMed]
- Garin, C.M.; Hori, Y.; Everling, S.; Whitlow, C.T.; Calabro, F.J.; Luna, B.; Froesel, M.; Gacoin, M.; Ben Hamed, S.; Dhenain, M.; et al. An Evolutionary Gap in Primate Default Mode Network Organization. Cell Rep. 2022, 39, 110669. [Google Scholar] [CrossRef] [PubMed]
- Qi, X.; Xu, W.; Li, G. Neuroimaging Study of Brain Functional Differences in Generalized Anxiety Disorder and Depressive Disorder. Brain Sci. 2023, 13, 1282. [Google Scholar] [CrossRef] [PubMed]
- Tromp, D.P.M.; Grupe, D.W.; Oathes, D.J.; McFarlin, D.R.; Hernandez, P.J.; Kral, T.R.A.; Lee, J.E.; Adams, M.; Alexander, A.L.; Nitschke, J.B. Reduced Structural Connectivity of a Major Frontolimbic Pathway in Generalized Anxiety Disorder. Arch. Gen. Psychiatry 2012, 69, 925–934. [Google Scholar] [CrossRef]
- Korgaonkar, M.S.; Felmingham, K.L.; Malhi, G.S.; Williamson, T.H.; Williams, L.M.; Bryant, R.A. Changes in Neural Responses during Affective and Non-Affective Tasks and Improvement of Posttraumatic Stress Disorder Symptoms Following Trauma-Focused Psychotherapy. Transl. Psychiatry 2023, 13, 85. [Google Scholar] [CrossRef]
- Chaposhloo, M.; Nicholson, A.A.; Becker, S.; McKinnon, M.C.; Lanius, R.; Shaw, S.B. Altered Resting-State Functional Connectivity in the Anterior and Posterior Hippocampus in Post-Traumatic Stress Disorder: The Central Role of the Anterior Hippocampus. Neuroimage Clin. 2023, 38, 103417. [Google Scholar] [CrossRef]
- Lanius, R.A.; Bluhm, R.L.; Coupland, N.J.; Hegadoren, K.M.; Rowe, B.; Théberge, J.; Neufeld, R.W.J.; Williamson, P.C.; Brimson, M. Default Mode Network Connectivity as a Predictor of Post-Traumatic Stress Disorder Symptom Severity in Acutely Traumatized Subjects. Acta Psychiatr. Scand. 2010, 121, 33–40. [Google Scholar] [CrossRef]
- Lee, C.W.; Cuijpers, P. A Meta-Analysis of the Contribution of Eye Movements in Processing Emotional Memories. J. Behav. Ther. Exp. Psychiatry 2013, 44, 231–239. [Google Scholar] [CrossRef]
- Kennis, M.; Rademaker, A.R.; van Rooij, S.J.H.; Kahn, R.S.; Geuze, E. Resting State Functional Connectivity of the Anterior Cingulate Cortex in Veterans with and without Post-traumatic Stress Disorder. Hum. Brain Mapp. 2015, 36, 99. [Google Scholar] [CrossRef]
- Okon-Singer, H.; Hendler, T.; Pessoa, L.; Shackman, A.J. The Neurobiology of Emotion–Cognition Interactions: Fundamental Questions and Strategies for Future Research. Front. Hum. Neurosci. 2015, 9, 58. [Google Scholar] [CrossRef]
- Miller, D.R.; Hayes, S.M.; Hayes, J.P.; Spielberg, J.M.; Lafleche, G.; Verfaellie, M. Default Mode Network Subsystems Are Differentially Disrupted in Posttraumatic Stress Disorder. Biol. Psychiatry Cogn. Neurosci. Neuroimaging 2017, 2, 363–371. [Google Scholar] [CrossRef] [PubMed]
- Javaheripour, N.; Li, M.; Chand, T.; Krug, A.; Kircher, T.; Dannlowski, U.; Nenadić, I.; Hamilton, J.P.; Sacchet, M.D.; Gotlib, I.H.; et al. Altered Resting-State Functional Connectome in Major Depressive Disorder: A Mega-Analysis from the PsyMRI Consortium. Transl. Psychiatry 2021, 11, 511. [Google Scholar] [CrossRef] [PubMed]
- Bao, W.; Gao, Y.; Cao, L.; Li, H.; Liu, J.; Liang, K.; Hu, X.; Zhang, L.; Hu, X.; Gong, Q.; et al. Alterations in Large-Scale Functional Networks in Adult Posttraumatic Stress Disorder: A Systematic Review and Meta-Analysis of Resting-State Functional Connectivity Studies. Neurosci. Biobehav. Rev. 2021, 131, 1027–1036. [Google Scholar] [CrossRef] [PubMed]
- Sun, J.; Du, Z.; Ma, Y.; Chen, L.; Wang, Z.; Guo, C.; Luo, Y.; Gao, D.; Hong, Y.; Zhang, L.; et al. Altered Functional Connectivity in First-Episode and Recurrent Depression: A Resting-State Functional Magnetic Resonance Imaging Study. Front. Neurol. 2022, 13, 922207. [Google Scholar] [CrossRef]
- Liu, L.; Chen, D.; Huang, F.; Jia, T.; Cheng, W.; Pan, M.; Zhao, M.; Bu, X.; Liao, X.; Wang, Y.; et al. Interference of Default Mode on Attention Networks in Adults with Attention-deficit/Hyperactivity Disorder and Its Association with Genetic Variants and Treatment Outcomes. CNS Neurosci. Ther. 2024, 30, e14900. [Google Scholar] [CrossRef]
- Cortese, S.; Kelly, C.; Chabernaud, C.; Proal, E.; Di Martino, A.; Milham, M.P.; Castellanos, F.X. Toward Systems Neuroscience of ADHD: A Meta-Analysis of 55 FMRI Studies. Am. J. Psychiatry 2012, 169, 1038–1055. [Google Scholar] [CrossRef]
- Soman, S.M.; Vijayakumar, N.; Thomson, P.; Ball, G.; Hyde, C.; Silk, T.J. Functional and Structural Brain Network Development in Children with Attention Deficit Hyperactivity Disorder. Hum. Brain Mapp. 2023, 44, 3394. [Google Scholar] [CrossRef]
- Shaw, P.; Stringaris, A.; Nigg, J.; Leibenluft, E. Emotion Dysregulation in Attention Deficit Hyperactivity Disorder. Am. J. Psychiatry 2014, 171, 276–293. [Google Scholar] [CrossRef]
- Tomasi, D.; Volkow, N.D.; Wang, R.; Telang, F.; Wang, G.J.; Chang, L.; Ernst, T.; Fowler, J.S. Dopamine Transporters in Striatum Correlate with Deactivation in the Default Mode Network during Visuospatial Attention. PLoS ONE 2009, 4, e6102. [Google Scholar] [CrossRef]
- Karnath, H.O.; Sperber, C.; Rorden, C. Mapping Human Brain Lesions and Their Functional Consequences. Neuroimage 2017, 165, 180. [Google Scholar] [CrossRef]
- Park, K.; Chang, I.; Kim, S. Resting State of Human Brain Measured by FMRI Experiment Is Governed More Dominantly by Essential Mode as a Global Signal Rather than Default Mode Network. Neuroimage 2024, 301, 120884. [Google Scholar] [CrossRef] [PubMed]
- Tang, Y.Y.; Hölzel, B.K.; Posner, M.I. The Neuroscience of Mindfulness Meditation. Nat. Rev. Neurosci. 2015, 16, 213–225. [Google Scholar] [CrossRef] [PubMed]
- Goldin, P.R.; Gross, J.J. Effects of Mindfulness-Based Stress Reduction (MBSR) on Emotion Regulation in Social Anxiety Disorder. Emotion 2010, 10, 83–91. [Google Scholar] [CrossRef] [PubMed]
- Schurz, M.; Maliske, L.; Kanske, P. Cross-Network Interactions in Social Cognition: A Review of Findings on Task Related Brain Activation and Connectivity. Cortex 2020, 130, 142–157. [Google Scholar] [CrossRef]
- Poerio, G.L.; Sormaz, M.; Wang, H.T.; Margulies, D.; Jefferies, E.; Smallwood, J. The Role of the Default Mode Network in Component Processes Underlying the Wandering Mind. Soc. Cogn. Affect. Neurosci. 2017, 12, 1047. [Google Scholar] [CrossRef]
| Lines of Study and Research on DMN | |
|---|---|
| Basal Activation for Healthy Function | Findings have led to the hypothesis that DMN activity and connectivity play a crucial role in “offline” intrinsic activity, which is necessary to maintain balanced and stable internal states during periods of rest [4,25]. |
| Reveries | It has been observed that DMN activity increases during periods of “daydreaming” and that an individual’s propensity to mental wandering is correlated with the DMN response [21]. In addition, the content of daydreams can modulate the activity and connectivity of the DMN, varying according to the different components of daydreams [54]. |
| Autobiographical Memory | Several neuroimaging studies have shown that DMN activity and connectivity are modulated by tasks that require the retrieval of past events and by spontaneous autobiographical memory [55]. It has been suggested that autobiographical memory is one of the prominent processes showing the involvement of the DMN in mental representations generated using intrinsic information, independently of extrinsic information [56,57]. |
| Prospective Memory | Memory “for the future”, like memory for the past, activates the DMN and shapes its connectivity [56,57,58]. |
| Social Cognition | Many studies have found extensive overlap between the DMN and regions involved in social cognition, collectively known as the “social brain” [59]. In addition to being involved in self-referential processes, such as thinking about one’s own mental states, the DMN is involved in thinking about other people’s beliefs, intentions, and motivations and in preparing the intentional stance [60]. Importantly, thinking about other people is not confined to moments of disengagement but is also, and perhaps primarily, evident when people are involved with other social agents in the real world [21]. |
| Meeting of the Intrinsic and Extrinsic | Taken together, the studies described here suggest that, on the one hand, the DMN is active during internally related thoughts that are generated by the individual themselves in the absence of external stimuli, as in the case of daydreaming, and can therefore be considered intrinsic, and, on the other hand, the DMN response is linked to external stimuli, especially during social interactions, and can therefore be considered extrinsic [61]. Thus, in this perspective, we combine many of these findings to suggest that the DMN is at the center of the interaction between the external and internal worlds [62]. |
| Technique | Advantages | Limitations |
|---|---|---|
| Functional Magnetic Resonance Imaging (fMRI) |
|
|
|
| |
|
| |
| Diffusion Tensor Imaging (DTI) |
|
|
|
| |
| ||
| Structural Magnetic Resonance Imaging (sMRI) |
|
|
|
| |
| Magnetoencephalography (MEG) |
|
|
|
| |
| Electroencephalography (EEG) |
|
|
|
| |
| Positron Emission Tomography (PET) |
|
|
|
| |
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Azarias, F.R.; Almeida, G.H.D.R.; de Melo, L.F.; Rici, R.E.G.; Maria, D.A. The Journey of the Default Mode Network: Development, Function, and Impact on Mental Health. Biology 2025, 14, 395. https://doi.org/10.3390/biology14040395
Azarias FR, Almeida GHDR, de Melo LF, Rici REG, Maria DA. The Journey of the Default Mode Network: Development, Function, and Impact on Mental Health. Biology. 2025; 14(4):395. https://doi.org/10.3390/biology14040395
Chicago/Turabian StyleAzarias, Felipe Rici, Gustavo Henrique Doná Rodrigues Almeida, Luana Félix de Melo, Rose Eli Grassi Rici, and Durvanei Augusto Maria. 2025. "The Journey of the Default Mode Network: Development, Function, and Impact on Mental Health" Biology 14, no. 4: 395. https://doi.org/10.3390/biology14040395
APA StyleAzarias, F. R., Almeida, G. H. D. R., de Melo, L. F., Rici, R. E. G., & Maria, D. A. (2025). The Journey of the Default Mode Network: Development, Function, and Impact on Mental Health. Biology, 14(4), 395. https://doi.org/10.3390/biology14040395






