A Study on the Community and Ecological Characteristics of Benthic Invertebrates in the Ulungu River, Xinjiang, via eDNA Metabarcoding and Morphological Methods
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Overview of the Study Area and Field Sampling
2.2. Extraction of Total DNA from Membrane Filters
2.3. COI Amplification and Sequencing
2.4. High-Throughput Sequencing Data Analysis
2.5. Construction of the eDNA Database of Local Benthic Invertebrates in the Ulungu River
2.6. Comparison of Community Structure Obtained via eDNA Metabarcoding and Traditional Morphological Methods
2.7. Comparison of EARs Between eDNA Metabarcoding Methods and Traditional Morphological Methods
3. Results
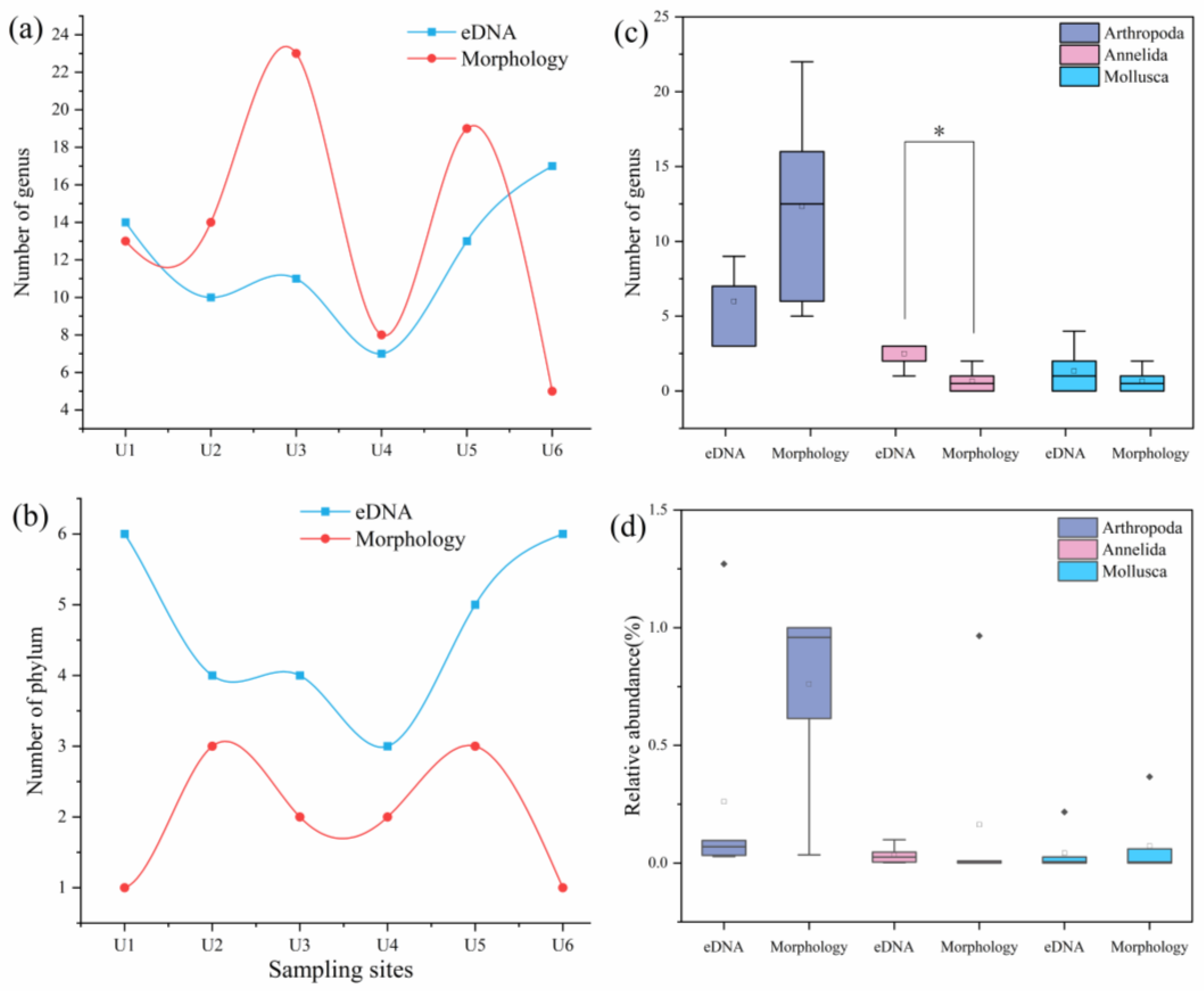
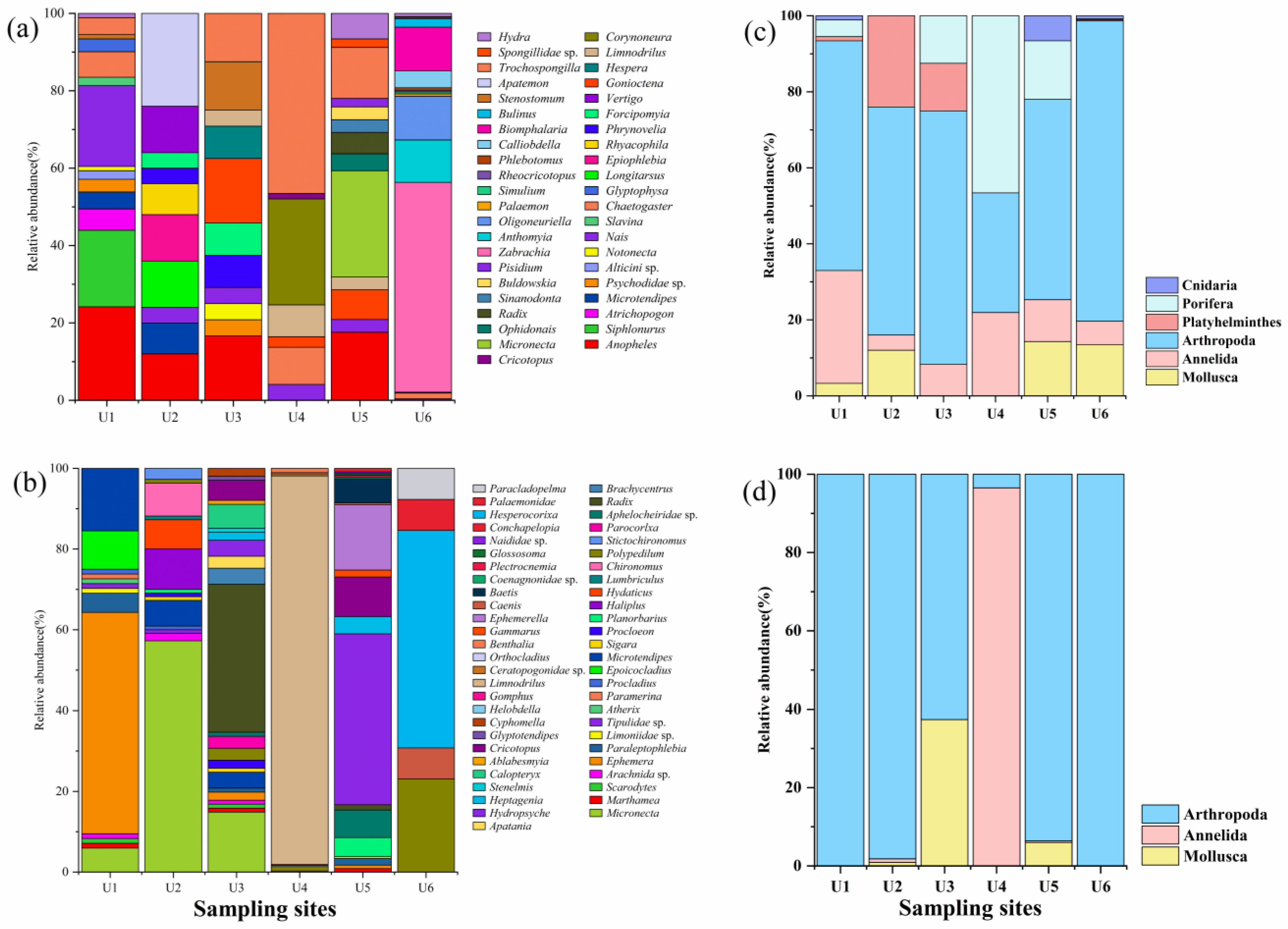
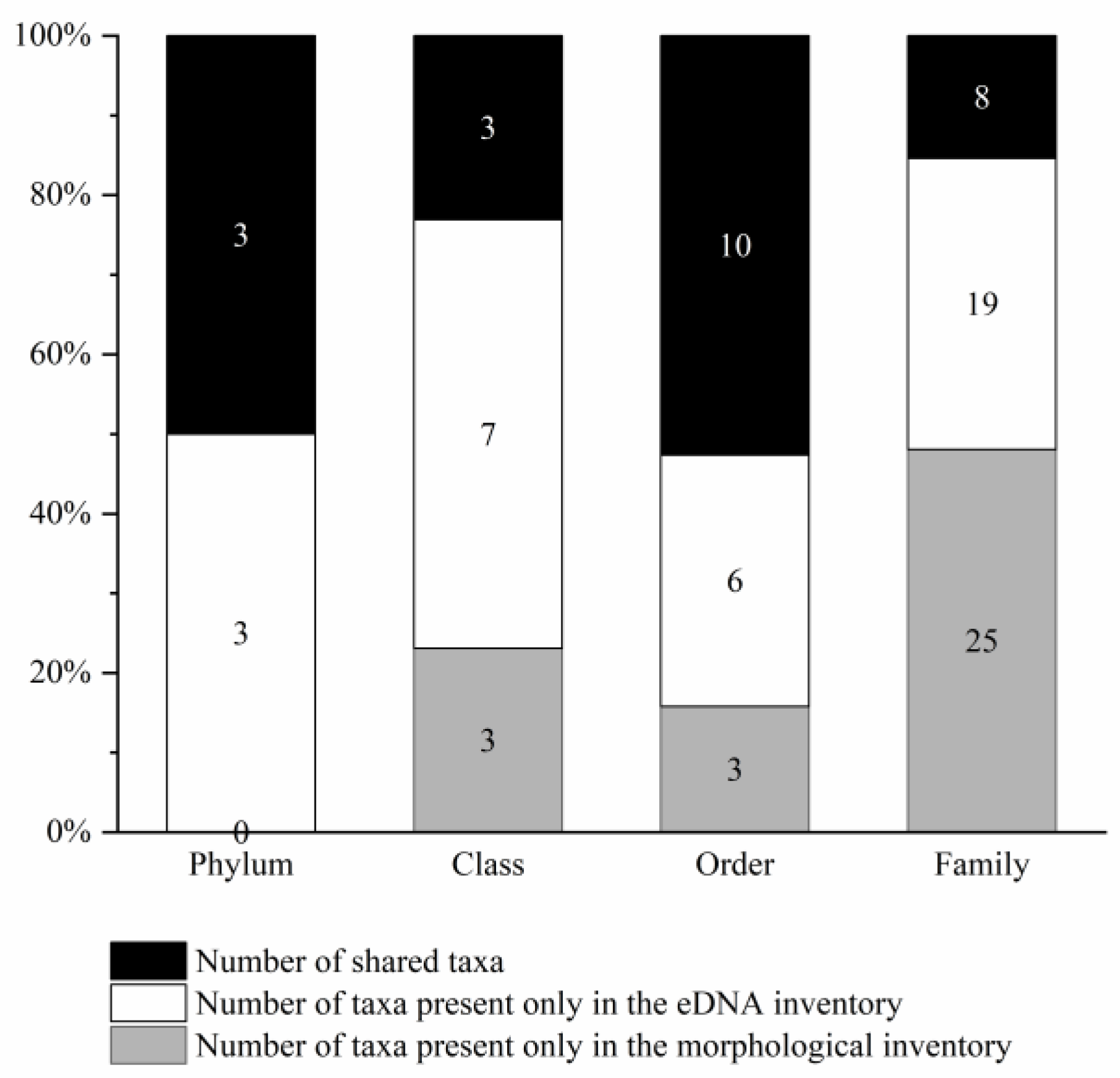
3.1. Consistency or Complementarity of the Two Methods for Monitoring Benthic Invertebrate Communities
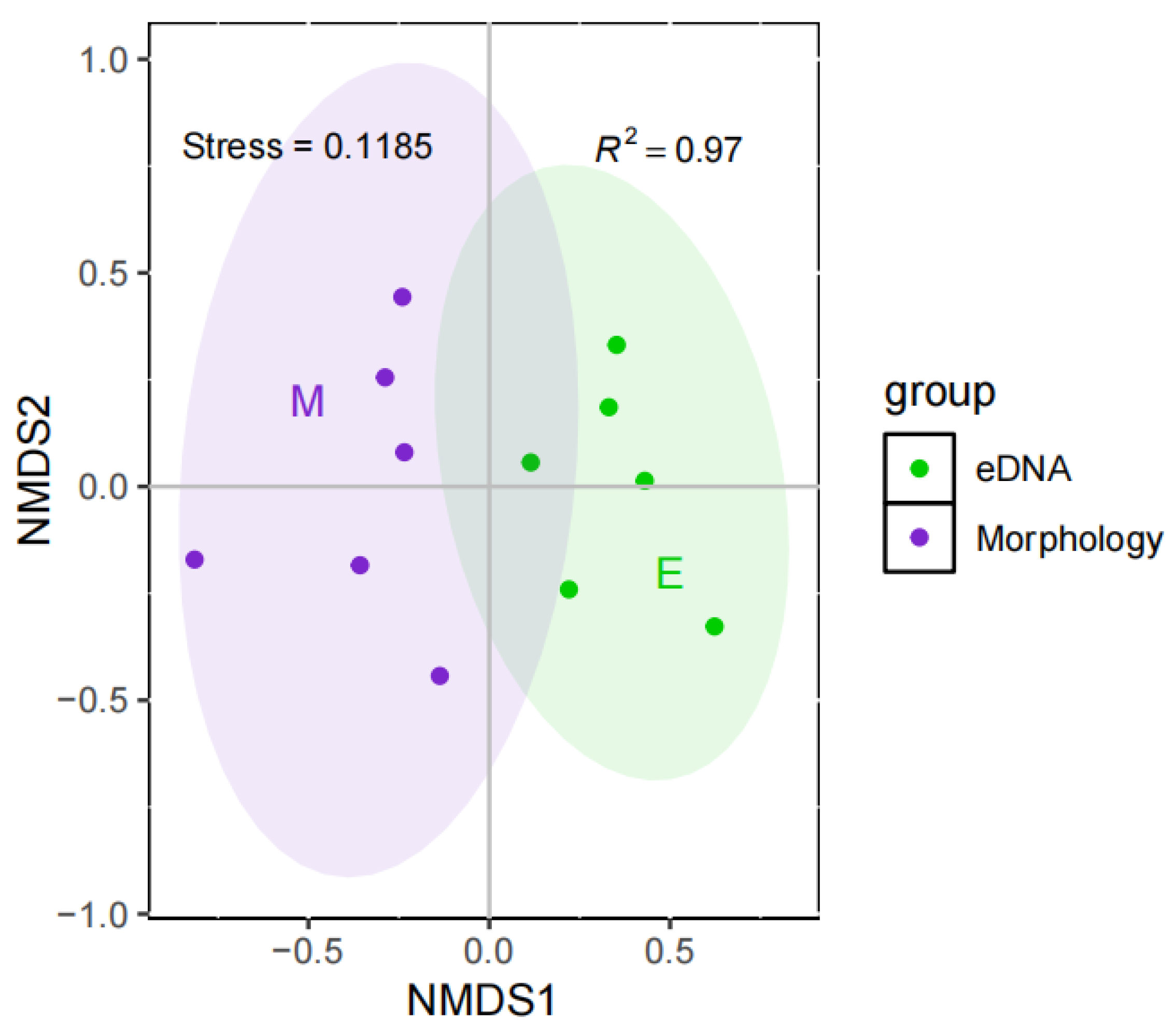
3.2. Community Correlation Analysis
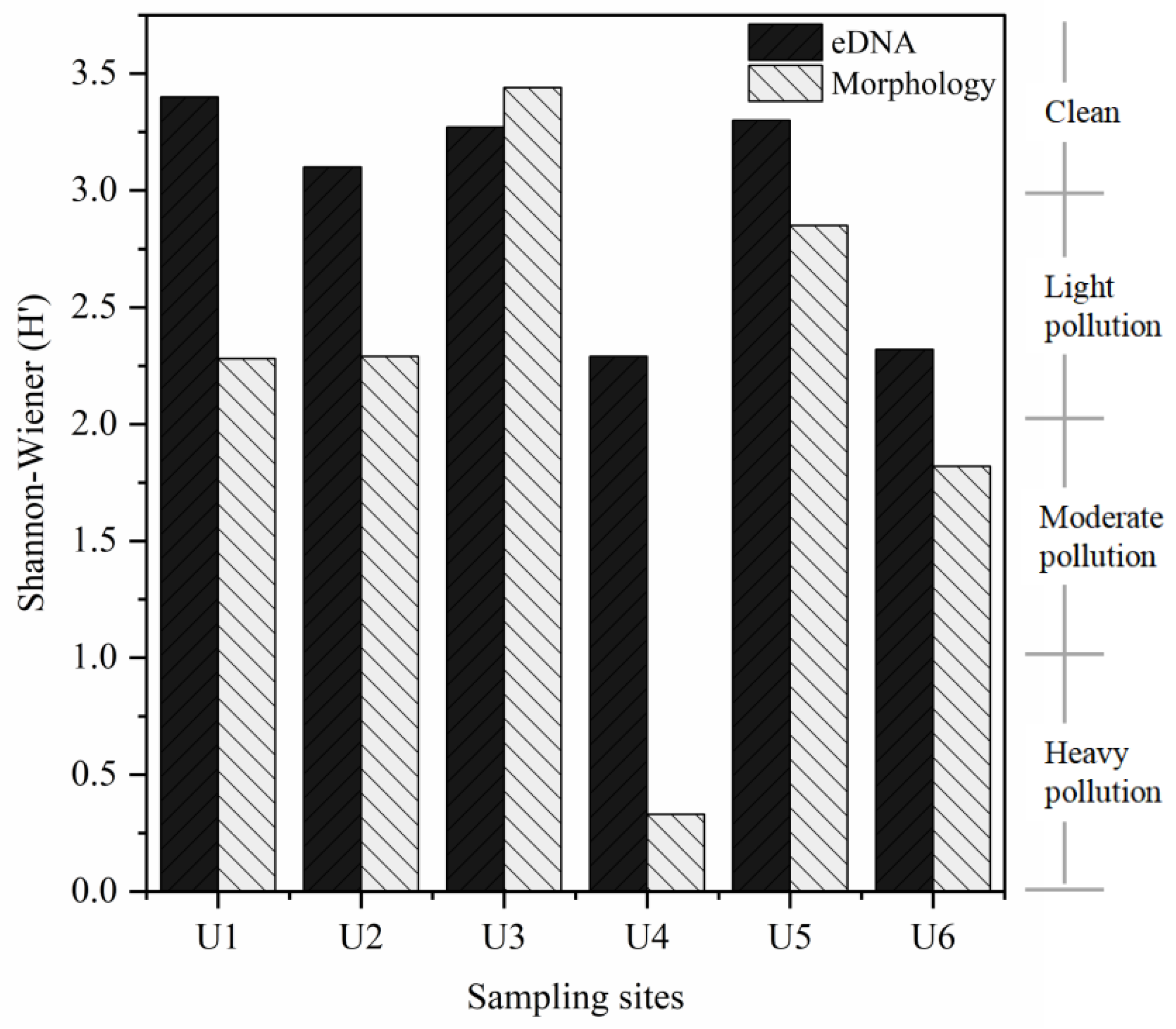
3.3. Comparison of Ecological Status Assessments
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Appendix A
| Sites | U1 | U2 | U3 | U4 | U5 | U6 | |
|---|---|---|---|---|---|---|---|
| phylum | eDNA | 6 | 4 | 4 | 3 | 5 | 6 |
| Morphology | 1 | 3 | 2 | 2 | 3 | 1 | |
| order | eDNA | 9 | 8 | 6 | 4 | 9 | 9 |
| Morphology | 5 | 6 | 8 | 5 | 9 | 4 | |
| family | eDNA | 12 | 10 | 10 | 5 | 10 | 14 |
| Morphology | 9 | 8 | 15 | 6 | 18 | 4 | |
| genus | eDNA | 14 | 10 | 11 | 7 | 13 | 17 |
| Morphology | 13 | 14 | 23 | 8 | 19 | 5 | |
| Sites | U1 | U2 | U3 | U4 | U5 | U6 | |
|---|---|---|---|---|---|---|---|
| Arthropoda | eDNA | 7 | 7 | 7 | 3 | 3 | 9 |
| Morphology | 13 | 12 | 22 | 6 | 16 | 5 | |
| Annelida | eDNA | 3 | 1 | 2 | 3 | 3 | 3 |
| Morphology | 0 | 1 | 0 | 2 | 1 | 0 | |
| Mollusca | eDNA | 1 | 1 | 0 | 0 | 4 | 2 |
| Morphology | 0 | 1 | 1 | 0 | 2 | 0 | |
| Sites | U1 | U2 | U3 | U4 | U5 | U6 | |
|---|---|---|---|---|---|---|---|
| Arthropoda | eDNA | 0.6044 | 0.6 | 0.6667 | 0.3151 | 0.5275 | 0.7900 |
| Morphology | 1 | 0.9818 | 0.6139 | 0.0349 | 0.9359 | 1 | |
| Annelida | eDNA | 0.2967 | 0.04 | 0.0833 | 0.2192 | 0.1099 | 0.0617 |
| Morphology | 0 | 0.0091 | 0 | 0.9651 | 0.0043 | 0 | |
| Mollusca | eDNA | 0.0330 | 0.12 | 0 | 0 | 0.1429 | 0.1348 |
| Morphology | 0 | 0.0091 | 0.3663 | 0 | 0.0598 | 0 | |
| Parameters/Sites | U1 | U2 | U3 | U4 | U5 | U6 | Mean |
|---|---|---|---|---|---|---|---|
| Water Temperature/°C | 7.9 | 11.4 | 11.3 | 12.6 | 14.4 | 14.1 | 12.0 |
References
- Fueyo, Á.; Sánchez, O.; Carleos, C.; Escudero, A.; Cordón, J.; Granero-Castro, J.; Borrell, Y.J. Unlocking rivers’ hidden diversity and ecological status using DNA metabarcoding in Northwest Spain. Ecol. Evol. 2024, 14, e70110. [Google Scholar] [CrossRef]
- Thiele, J.; Haaren, C.V.; Albert, C. Are river landscapes outstanding in providing cultural ecosystem services? An indicator-based exploration in Germany. Ecol. Indic. 2019, 101, 31–40. [Google Scholar] [CrossRef]
- Kędzior, R.; Kłonowska, O.M.; Dumnicka, E.; Woś, A.; Wyrębek, M.; Książek, L.; Grela, J.; Madej, P.; Skalski, T. Macroinvertebrate habitat requirements in rivers: Overestimation of environmental flow calculations in incised rivers. Hydrol. Earth Syst. Sci. 2022, 26, 4109–4124. [Google Scholar] [CrossRef]
- Calapez, A.R.; Serra, S.R.Q.; Mortágua, A.; Almeida, S.F.P.; Feio, M.J. Unveiling relationships between ecosystem services and aquatic communities in urban streams. Ecol. Indic. 2023, 153, 110433. [Google Scholar] [CrossRef]
- Bohus, A.; Gál, B.; Barta, B.; Szivák, I.; Kovács, K.K.; Boda, P.; Padisák, J.; Schmera, D. Effects of urbanization-induced local alterations on the diversity and assemblage structure of macroinvertebrates in low-order streams. Hydrobiologia 2023, 850, 881–899. [Google Scholar] [CrossRef]
- Fergus, C.E.; Brooks, J.R.; Kaufmann, P.R.; Herlihy, A.T.; Hill, R.A.; Mitchell, R.M.; Ringold, P. Disentangling natural and anthropogenic effects on benthic macroinvertebrate assemblages in western US streams. Ecosphere 2023, 14, e4688. [Google Scholar] [CrossRef]
- Pinna, M.; Saccomanno, B.; Marini, G.; Zangaro, F.; Kabayeva, A.; Khalaj, M.; Shaimardan, L.; D’Attis, S.; Tzafesta, E.; Specchia, V. Testing the Influence of Incomplete DNA Barcode Libraries on Ecological Status Assessment of Mediterranean Transitional Waters. Biology 2021, 10, 1092. [Google Scholar] [CrossRef]
- Dou, Q.; Du, X.; Wang, L.; Song, D.; Zhao, C.; Huang, X.; Wang, H.; Huo, T. Effects of land use and aquatic environmental factors on secondary productivity of macroinvertebrates. J. Lake Sci. 2024, 36, 846–857. [Google Scholar] [CrossRef]
- Vezi, M.S.; Downs, C.T.; Wepener, V.; Brien, G.O. Macrobenthic Communities in Selected River-Dominated Estuaries in KwaZulu-Natal, South Africa: Effects of Contrasting Environmental Variables and Seasonal Flow Changes. J. Coastal Res. 2020, 36, 992–1004. [Google Scholar]
- Tampo, L.; Kaboré, I.; Alhassan, E.H.; Ouéda, A.; Bawa, L.M.; Boundjou, G.D. Benthic Macroinvertebrates as Ecological Indicators: Their Sensitivity to the Water Quality and Human Disturbances in a Tropical River. Front. Water 2021, 3, 662765. [Google Scholar] [CrossRef]
- Sweeney, B.W.; Battle, J.M.; Jackson, J.K.; Dapkey, T. Can DNA barcodes of stream macroinvertebrates improve descriptions of community structure and water quality? J. N. Am. Benthol. Soc. 2011, 30, 195–216. [Google Scholar] [CrossRef]
- Wang, M.; Jin, X.; Lin, X.; Du, L.; Cui, Y.; Wu, X.; Sun, H.; Xie, Z.; Wang, X.; Wang, B. Advances in the macrozoobenthos biodiversity monitoring and ecosystem assessment using environmental DNA metabarcoding. Acta Ecol. Sin. 2021, 41, 7440–7453. [Google Scholar]
- Compson, A.B.Z.; Rideout, N.K.; Levenstein, B.; Kattilakoski, M.; Hajibabaei, M.; Monk, W.A.; Wright, M.T.G.; Baird, D.J. Replicate DNA metabarcoding can discriminate seasonal and spatial abundance shifts in river macroinvertebrate assemblages. Mol. Ecol. Resour. 2023, 23, 1275–1287. [Google Scholar] [CrossRef]
- Heyde, M.V.D.; White, N.E.; Nevill, P.; Austin, A.D.; Stevens, N.; Jones, M.; Guzik, M.T. Taking eDNA underground: Factors affecting eDNA detection of subterranean fauna in groundwater. Mol. Ecol. Resour. 2023, 23, 1257–1274. [Google Scholar] [CrossRef] [PubMed]
- Duarte, S.; Leite, B.R.; Feio, M.J.; Costa, F.O.; Filipe, A.F. Integration of DNA-Based Approaches in Aquatic Ecological Assessment Using Benthic Macroinvertebrates. Water 2021, 13, 331. [Google Scholar] [CrossRef]
- Thudi, M.; Li, Y.; Jackson, S.A.; May, G.D.; Varshney, R.K. Current state-of-art of sequencing technologies for plant genomics research. Brief. Funct. Genom. 2012, 11, 3–11. [Google Scholar] [CrossRef]
- Voelkerding, K.V.; Dames, S.A.; Durtschi, J.D. Next-generation sequencing: From basic research to diagnostics. Clin. Chem. 2009, 55, 641–658. [Google Scholar] [CrossRef]
- Yang, J.; Zhang, X. eDNA metabarcoding in zooplankton improves the ecological status assessment of aquatic ecosystems. Environ. Int. 2020, 134, 105230. [Google Scholar] [CrossRef]
- Lee, A.H.; Lee, J.; Noh, J.; Lee, C.; Hong, S.; Kwon, B.O.; Kim, J.J.; Khim, J.S. Characteristics of long-term changes in microbial communities from contaminated sediments along the west coast of South Korea: Ecological assessment with eDNA and physicochemical analyses. Mar. Pollut. Bull. 2020, 160, 111592. [Google Scholar] [CrossRef]
- Seymour, M.; Edwards, F.K.; Cosby, B.J.; Kelly, M.G.; Bruyn, M.D.; Carvalho, G.R.; Creer, S. Executing multi-taxa eDNA ecological assessment via traditional metrics and interactive networks. Sci. Total Environ. 2020, 729, 138801. [Google Scholar] [CrossRef]
- Wang, B.; Li, R.; Lan, X.; Kong, D.; Liu, X.; Xie, S. Benthic diatom eDNA metabarcoding for ecological assessment of an urban river: A comparison with morphological method. Ecol. Indic. 2024, 166, 112302. [Google Scholar] [CrossRef]
- Hanžek, N.; Udovič, M.G.; Kajan, K.; Borics, G.; Várbíró, G.; Stoeck, T.; Orlić, S.; Stanković, I. Comparative identification of phytoplankton taxonomic and functional group approach in karst lakes using classical microscopy and eDNA metabarcoding for ecological status assessment. Hydrobiologia 2023, 851, 1015–1034. [Google Scholar] [CrossRef]
- Yang, J.; Zhang, L.; Mu, Y.; Wang, J.; Yu, H.; Zhang, X. Unsupervised biological integrity assessment by eDNA biomonitoring of multi-trophic aquatic taxa. Environ. Int. 2023, 175, 107950. [Google Scholar] [CrossRef]
- Gleason, J.E.; Elbrecht, V.; Braukmann, T.W.A.; Hanner, R.H.; Cottenie, K. Assessment of stream macroinvertebrate communities with eDNA is not congruent with tissue-based metabarcoding. Mol. Ecol. 2021, 30, 3239–3251. [Google Scholar] [CrossRef] [PubMed]
- Pawlowski, J.; Bruce, K.; Panksep, K.; Aguirre, F.I.; Amalfitano, S.; Gentil, L.A.P.; Baussant, T.; Bouchez, A.; Carugati, L.; Cermakova, K.; et al. Environmental DNA metabarcoding for benthic monitoring: A review of sediment sampling and DNA extraction methods. Sci. Total Environ. 2021, 818, 151783. [Google Scholar] [CrossRef] [PubMed]
- Marshall, N.T.; Stepien, C.A. Macroinvertebrate community diversity and habitat quality relationships along a large river from targeted eDNA metabarcode assays. Environ. DNA 2020, 2, 572–586. [Google Scholar] [CrossRef]
- Li, X.; Jia, P.; Niu, C.; Xing, Y.; Li, H.; Liu, H.; Tang, W.; Zhao, Y. Species diversity of freshwater fish and assessment on watershed health in the Irtysh River and Ulungur River basins in Xinjiang, China. Biodivers. Sci. 2020, 28, 422–434. [Google Scholar] [CrossRef]
- Gao, F.; He, B.; Xue, S.; Li, Y. Impact of landscape pattern change on runoff processes in catchment area of the Ulungur River Basin. Water Supply 2020, 20, 1046–1058. [Google Scholar] [CrossRef]
- Xin, T. Study on Hydrochemical Field and Renewal of Different Water Bodies in Ulungur River Watershed. Master’s Thesis, Xi’an University of Science and Technology, Xi’an, China, 2021. [Google Scholar] [CrossRef]
- Liu, Y. Economic Zoology of China: Freshwater Molluscs; Science Press: Beijing, China, 1979; ISBN 9787100000000. [Google Scholar]
- Wang, J.; Wang, X. Chironomid Larvae of Northern China; China Yanshi Press: Beijing, China, 2011; ISBN 7-80250-200-0. [Google Scholar]
- Hinlo, R.; Gleeson, D.; Lintermans, M.; Furlan, E. Methods to maximise recovery of environmental DNA from water samples. PLoS ONE 2017, 12, e0179251. [Google Scholar] [CrossRef]
- Zhao, Q.; Pan, F.; Li, B.; Zang, X.; Ding, S. Distribution patterns of macroinvertebrate community diversity and their impact factors analysis in mountainous rivers at lower Yellow River Basin based on environmental DNA technology. J. Lake Sci. 2024, 36, 523–536. [Google Scholar] [CrossRef]
- Jin, K.; Zhang, L.; Zhang, W.; Zhang, X.; Chen, Q.; Yang, J.; Zhang, Y.; Zhang, X. Benthic Macroinvertebrate Biomonitoring and Ecological Assessment in Taihu Lake Basin Based on Environmental DNA Metabarcoding. Environ. Monit. China 2022, 38, 175–188. [Google Scholar] [CrossRef]
- Zhao, Q.; Pan, F.; Li, B.; Jia, X.; Ding, S. Response mechanism of benthic invertebrate diversity and stability to land-use patterns in mountainous rivers. Acta Ecol. Sin. 2024, 44, 7844–7858. [Google Scholar] [CrossRef]
- Shannon, C.E.; Weaver, W. The Mathematical Theory of Communication; University of Illinois Press: Urbana, IL, USA, 1949. [Google Scholar] [CrossRef]
- R Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2024; Available online: https://www.R-project.org/ (accessed on 2 September 2024).
- Pinna, M.; Zangaro, F.; Specchia, V. Assessing benthic macroinvertebrate communities’ spatial heterogeneity in Mediterranean transitional waters through eDNA metabarcoding. Sci. Rep. 2024, 14, 17890. [Google Scholar] [CrossRef] [PubMed]
- Tagliabue, A.; Matterson, K.O.; Ponti, M.; Turicchia, E.; Abbiati, M.; Costantini, F. Sediment and bottom water eDNA metabarcoding to support coastal management. Ocean Coast. Manag. 2023, 244, 106785. [Google Scholar] [CrossRef]
- DiBattista, J.D.; Fowler, A.M.; Riley, I.J.; Reader, S.; Hay, A.; Parkinson, K.; Hobbs, J.P.A. The use of environmental DNA to monitor impacted coastal estuaries. Mar. Pollut. Bull. 2022, 181, 113860. [Google Scholar] [CrossRef] [PubMed]
- Cordier, T.; Frontalini, F.; Cermakova, K.; Gentil, L.A.P.; Treglia, M.; Scantamburlo, E.; Bonamin, V.; Pawlowski, J. Multi-marker eDNA metabarcoding survey to assess the environmental impact of three offshore gas platforms in the North Adriatic Sea (Italy). Mar. Environ. Res. 2019, 146, 24–34. [Google Scholar] [CrossRef]
- Xu, X.; Yuan, Y.; Wang, Z.; Zheng, T.; Cai, H.; Yi, M.; Li, T.; Zhao, Z.; Chen, Q.; Sun, W. Environmental DNA metabarcoding reveals the impacts of anthropogenic pollution on multitrophic aquatic communities across an urban river of western China. Environ. Res. 2022, 216, 114512. [Google Scholar] [CrossRef]
- Beermann, A.J.; Werner, M.T.; Elbrecht, V.; Zizka, V.M.A.; Leese, F. DNA metabarcoding improves the detection of multiple stressor responses of stream invertebrates to increased salinity, fine sediment deposition and reduced flow velocity. Sci. Total Environ. 2020, 750, 141969. [Google Scholar] [CrossRef]
- Ji, F.; Han, D.; Yan, L.; Yan, S.; Zha, J.; Shen, J. Assessment of benthic invertebrate diversity and river ecological status along an urbanized gradient using environmental DNA metabarcoding and a traditional survey method. Sci. Total Environ. 2022, 806, 150587. [Google Scholar] [CrossRef]
- Ruppert, K.M.; Kline, R.J.; Rahman, M.S. Past, present, and future perspectives of environmental DNA (eDNA) metabarcoding: A systematic review in methods, monitoring, and applications of global edna. Glob. Ecol. Conserv. 2019, 17, e00547. [Google Scholar] [CrossRef]
- Elbrecht, V.; Leese, F. Primerminer: An r package for development and in silico validation of dna metabarcoding primers. Methods Ecol. Evol. 2017, 8, 622–626. [Google Scholar] [CrossRef]
- Rivera, S.F.; Vasselon, V.; Mary, N.; Monnier, O.; Rimet, F.; Bouchez, A. Exploring the capacity of aquatic biofilms to act as environmental DNA samplers: Test on macroinvertebrate communities in rivers. Sci. Total Environ. 2021, 763, 144208. [Google Scholar] [CrossRef] [PubMed]
- Múrria, C.; Wangensteen, O.S.; Somma, S.; Väisänen, L.; Fortuño, P.; Arnedo, M.A.; Prat, N. Taxonomic accuracy and complementarity between bulk and eDNA metabarcoding provides an alternative to morphology for biological assessment of freshwater macroinvertebrates. Sci. Total Environ. 2024, 935, 173243. [Google Scholar] [CrossRef]
- Lee, G.H.V.D.; Polling, M.; Laan, I.V.D.; Kodde, L.; Verdonschot, R.C.M. From DNA to diagnostics: A case study using macroinvertebrate metabarcoding to assess the effectiveness of restoration measures in a Dutch stream. Sci. Total Environ. 2024, 923, 171413. [Google Scholar] [CrossRef]
- Antich, A.; Palacín, C.; Cebrian, E.; Golo, R.; Wangensteen, O.S.; Turon, X. Marine biomonitoring with eDNA: Can metabarcoding of water samples cut it as a tool for surveying benthic communities? Mol. Ecol. 2020, 30, 3175–3188. [Google Scholar] [CrossRef] [PubMed]
- Willassen, E.; Westgaard, J.; Kongsrud, J.A.; Hanebrekke, T.; Mortensen, P.B.; Holte, B. Benthic invertebrates in Svalbard fjords-when metabarcoding does not outperform traditional biodiversity assessment. PeerJ 2022, 10, e14321. [Google Scholar] [CrossRef]
- Mauvisseau, Q.; Burian, A.; Gibson, C.; Brys, R.; Ramsey, A.; Sweet, M. Influence of accuracy repeatability and detection probability in the reliability of speciesspecific eDNA based approaches. Sci. Rep. 2019, 9, 580. [Google Scholar] [CrossRef]
- Tzafesta, E.; Zangaro, F.; Specchia, V.; Pinna, M. An Overview of DNA-Based Applications for the Assessment of Benthic Macroinvertebrates Biodiversity in Mediterranean Aquatic Ecosystems. Diversity 2021, 13, 112. [Google Scholar] [CrossRef]
- Serrana, J.M.; Miyake, Y.; Gamboa, M.; Watanabe, K. Comparison of DNA metabarcoding and morphological identification for stream macroinvertebrate biodiversity assessment and monitoring. Ecol. Indic. 2019, 101, 963–972. [Google Scholar] [CrossRef]
- Jo, T.S.; Tsuri, K.; Yamanaka, H. Can nuclear aquatic environmental DNA be a genetic marker for the accurate estimation of species abundance? Sci. Total Environ. 2022, 109, 38. [Google Scholar] [CrossRef]
- Rees, H.C.; Gough, K.C.; Middleditch, D.J.; Patmore, J.R.M.; Maddison, B.C. Applications and limitations of measuring environmental DNA as indicators of the presence of aquatic animals. J. Appl. Ecol. 2015, 52, 827–831. [Google Scholar] [CrossRef]
- Curtis, A.N.; Tiemann, J.S.; Douglass, S.A.; Davis, M.A.; Larson, E.R. High stream flows dilute environmental DNA (eDNA) concentrations and reduce detectability. Divers. Distrib. 2021, 27, 1918–1931. [Google Scholar] [CrossRef]
- Roussel, J.M.; Paillisson, J.M.; Tréguier, A.; Petit, E. The downside of eDNA as a survey tool in water bodies. J. Appl. Ecol. 2015, 52, 823–826. [Google Scholar] [CrossRef]
- Shogren, A.J.; Tank, J.L.; Egan, S.P.; August, O.; Rosi, E.J.; Hanrahan, B.R.; Renshaw, M.A.; Gantz, C.A.; Bolster, D. Water Flow and Biofilm Cover Influence Environmental DNA Detection in Recirculating Streams. Environ. Sci. Technol. 2018, 52, 8530–8537. [Google Scholar] [CrossRef]
- Curtis, A.N.; Larson, E.R.; Davis, M.A. Field storage of water samples affects measured environmental DNA concentration and detection. Limnology 2021, 22, 1–4. [Google Scholar] [CrossRef]
- Yamanaka, H.; Motozawa, H.; Tsuji, S.; Miyazawa, R.C.; Takahara, T.; Minamoto, T. On-site filtration of water samples for environmental DNA analysis to avoid DNA degradation during transportation. Ecol. Res. 2016, 31, 963–967. [Google Scholar] [CrossRef]
- Joseph, C.; Faiq, M.E.; Li, Z.; Chen, G. Persistence and degradation dynamics of eDNA affected by environmental factors in aquatic ecosystems. Hydrobiologia 2022, 849, 4119–4133. [Google Scholar] [CrossRef]
- Troth, C.R.; Sweet, M.J.; Nightingale, J.; Burian, A. Seasonality, DNA degradation and spatial heterogeneity as drivers of eDNA detection dynamics. Sci. Total Environ. 2021, 768, 144466. [Google Scholar] [CrossRef] [PubMed]
- Ge, Y.; Xia, C.; Wang, J.; Zhang, X.; Ma, X.; Zhou, Q. The efficacy of DNA barcoding in the classification, genetic differentiation, and biodiversity assessment of benthic macroinvertebrates. Ecol. Evol. 2021, 11, 5669–5681. [Google Scholar] [CrossRef]
- Leese, F.; Sander, M.; Buchner, D.; Elbrecht, V.; Haase, P.; Zizka, V.M.A. Improved freshwater macroinvertebrate detection from environmental DNA through minimized nontarget amplification. Environ. DNA 2021, 3, 261–276. [Google Scholar] [CrossRef]
- Meyer, A.; Boyer, F.; Valentini, A.; Bonin, A.; Ficetola, G.F.; Beisel, J.N.; Bouquerel, J.; Wagner, P.; Gaboriaud, C.; Leese, F.; et al. Morphological vs. DNA metabarcoding approaches for the evaluation of stream ecological status with benthic invertebrates: Testing different combinations of markers and strategies of data filtering. Mol. Ecol. 2020, 30, 3203–3220. [Google Scholar] [CrossRef] [PubMed]
- Fukuzawa, T.; Shirakura, H.; Nishizawa, N.F.; Nagata, H.; Kameda, Y.; Doi, H. Environmental DNA extraction method from water for a high and consistent DNA yield. Environ. DNA 2023, 5, 627–633. [Google Scholar] [CrossRef]
- Vourka, A.; Karaouzas, I.; Parmakelis, A. River benthic macroinvertebrates and environmental DNA metabarcoding: A scoping review of eDNA sampling, extraction, amplification and sequencing methods. Biodivers. Conserv. 2023, 32, 4221–4238. [Google Scholar] [CrossRef]
- Schabacker, J.C.; Amish, S.J.; Ellis, B.K.; Gardner, B.; Miller, D.L.; Rutledge, E.A.; Sepulveda, A.J.; Luikart, G. Increased eDNA detection sensitivity using a novel high-volume water sampling method. Environ. DNA 2020, 2, 244–251. [Google Scholar] [CrossRef]
- Sanchez, L.; Boulanger, E.; Arnal, V.; Boissery, P.; Dalongeville, A.; Dejean, T.; Deter, J.; Guellati, N.; Holon, F.; Juhel, J.B.; et al. Ecological indicators based on quantitative eDNA metabarcoding: The case of marine reserves. Ecol. Indic. 2022, 140, 108966. [Google Scholar] [CrossRef]
- Wang, P.; Yan, Z.; Yang, S.; Wang, S.; Zheng, X.; Fan, J.; Zhang, T. Environmental DNA: An Emerging Tool in Ecological Assessment. B. Environ. Contam. Tox. 2019, 103, 651–656. [Google Scholar] [CrossRef]
- Hering, D.; Borja, A.; Jones, J.I.; Pont, D.; Boets, P.; Bouchez, A.; Bruce, K.; Drakare, S.; Hänfling, B.; Kahlert, M.; et al. Implementation options for DNA-based identification into ecological status assessment under the European Water Framework Directive. Water Res. 2018, 138, 192–205. [Google Scholar] [CrossRef]
- Aunins, A.A.; Mueller, S.J.; Fike, J.A.; Cornman, R.S. Assessing arthropod diversity metrics derived from stream environmental DNA: Spatiotemporal variation and paired comparisons with manual sampling. PeerJ 2023, 11, e15163. [Google Scholar] [CrossRef]
- Keck, F.; Brantschen, J.; Altermatt, F. A combination of machine-learning and eDNA reveals the genetic signature of environmental change at the landscape levels. Mol. Ecol. 2023, 32, 4791–4800. [Google Scholar] [CrossRef]
- Brantschen, J.; Blackman, R.C.; Walser, J.C.; Altermatt, F. Environmental DNA gives comparable results to morphology-based indices of macroinvertebrates in a large-scale ecological assessment. PLoS ONE 2021, 16, e0257510. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Huo, Q.; Ma, Y.; Hu, L.; Liu, Q.; Wang, C.; Liu, J.; Ren, D.; Wang, Z.; Wang, B.; Zeng, H.; et al. A Study on the Community and Ecological Characteristics of Benthic Invertebrates in the Ulungu River, Xinjiang, via eDNA Metabarcoding and Morphological Methods. Biology 2025, 14, 410. https://doi.org/10.3390/biology14040410
Huo Q, Ma Y, Hu L, Liu Q, Wang C, Liu J, Ren D, Wang Z, Wang B, Zeng H, et al. A Study on the Community and Ecological Characteristics of Benthic Invertebrates in the Ulungu River, Xinjiang, via eDNA Metabarcoding and Morphological Methods. Biology. 2025; 14(4):410. https://doi.org/10.3390/biology14040410
Chicago/Turabian StyleHuo, Qiang, Yuying Ma, Linghui Hu, Qi Liu, Chengxin Wang, Jiaxuan Liu, Daoquan Ren, Zhichao Wang, Baoqiang Wang, Honghui Zeng, and et al. 2025. "A Study on the Community and Ecological Characteristics of Benthic Invertebrates in the Ulungu River, Xinjiang, via eDNA Metabarcoding and Morphological Methods" Biology 14, no. 4: 410. https://doi.org/10.3390/biology14040410
APA StyleHuo, Q., Ma, Y., Hu, L., Liu, Q., Wang, C., Liu, J., Ren, D., Wang, Z., Wang, B., Zeng, H., Song, Y., & Chen, S. (2025). A Study on the Community and Ecological Characteristics of Benthic Invertebrates in the Ulungu River, Xinjiang, via eDNA Metabarcoding and Morphological Methods. Biology, 14(4), 410. https://doi.org/10.3390/biology14040410






