Space and Time Dynamics of Honeybee (Apis mellifera L.)-Melliferous Resource Interactions Within a Foraging Area: A Case Study in the Banja Luka Region (Bosnia & Herzegovina)
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Site
2.2. Environmental Data
2.3. Characterisation of Land Use Using a Combined Field and GIS Approach
2.4. Analysis of Landscape Structure
2.5. Characterisation of Melliferous Flora
2.5.1. Sampling Method
2.5.2. Botanical Parameter
2.5.3. Phenological Parameter
- the early flowering phase, where less than 50% of the flowers were open (the rest were in the form of flower buds),
- the full flowering phase, where more than 50% of the flowers were open,
- the late flowering phase, where less than 50% of the flowers were open and the rest were wilted.
2.5.4. Ecological Parameter
2.6. Modelling
2.6.1. Estimation of Honey Production Potential
2.6.2. Mapping the Space and Time Evolution of Melliferous Resources
2.7. Statistical Analysis
3. Results
3.1. Landscape Characterisation
3.2. Link Between Land Use and Botanical Diversity
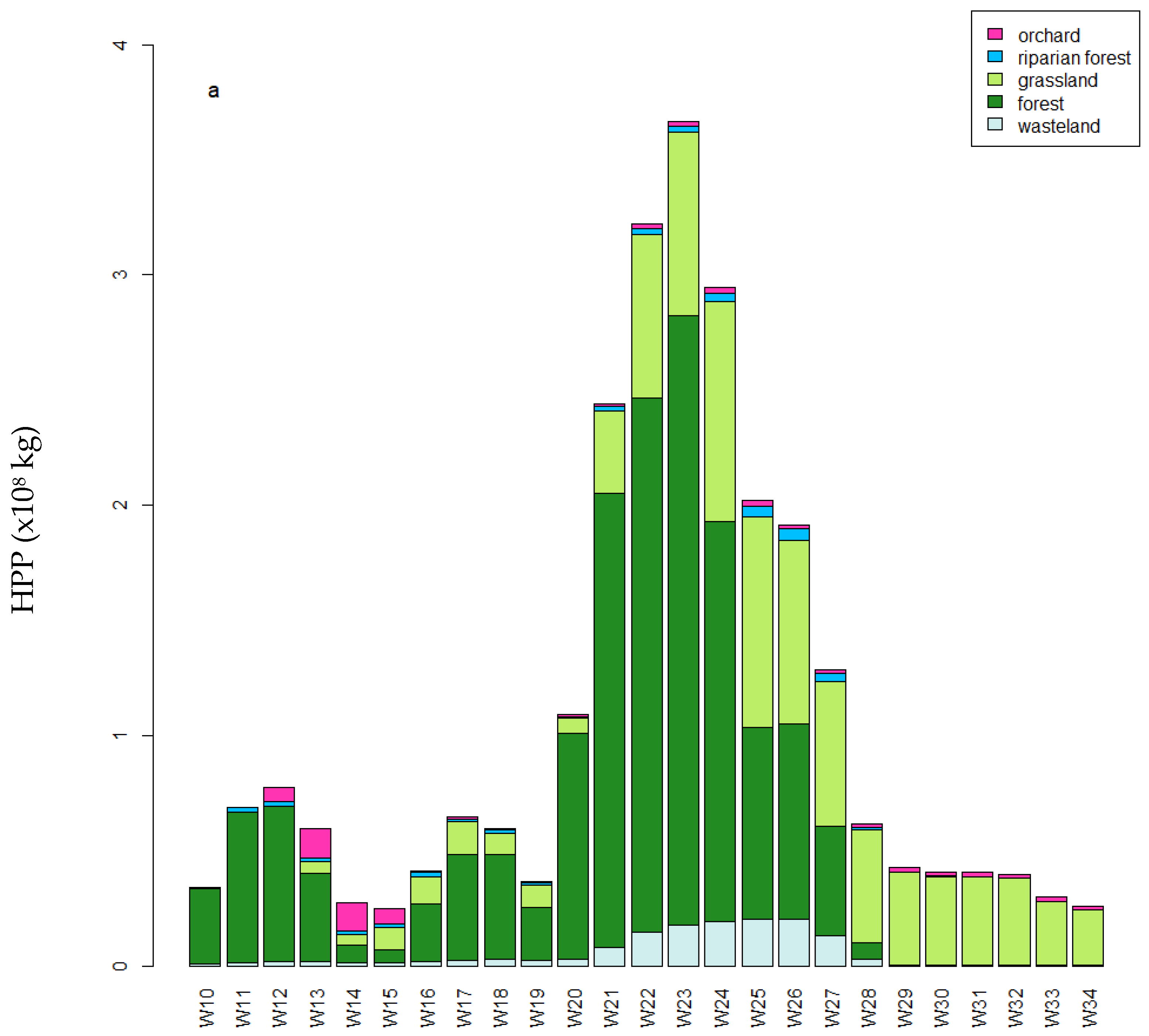
3.3. Spatial and Temporal Distribution of HPP
3.4. Temporal Variations in Cumulative Weekly HPP in the Foraging Area
3.5. Contribution of the Main Taxa to Overall HPP
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| B&H | Bosnia & Herzegovina |
| GIS | Geographical Information System |
| HPP | Honey Production Potential |
| LUS | Land Use Station |
| LUU | Land Use Unit |
| NDVI | Normalized Difference Vegetation Index |
References
- Eilers, E.J.; Kremen, C.; Smith Greenleaf, S.; Garber, A.K.; Klein, A.-M. Contribution of Pollinator-Mediated Crops to Nutrients in the Human Food Supply. PLoS ONE 2011, 6, e21363. [Google Scholar] [CrossRef] [PubMed]
- Alignier, A.; Aviron, S.; Baude, M.; Jeavons, E.; Michelot-Antalik, A.; Porcher, E. Les Plantes de Services Favorisant La Pollinisation. In Les Plantes de Services, vers de Nouveaux Agroécosystèmes; Editions Quae: Versailles, France, 2024; p. 388. [Google Scholar]
- Baveco, J.M.; Focks, A.; Belgers, D.; van der Steen, J.J.M.; Boesten, J.J.T.I.; Roessink, I. An Energetics-Based Honeybee Nectar-Foraging Model Used to Assess the Potential for Landscape-Level Pesticide Exposure Dilution. PeerJ 2016, 4, e2293. [Google Scholar] [CrossRef] [PubMed]
- Dicks, L.V.; Breeze, T.D.; Ngo, H.T.; Senapathi, D.; An, J.; Aizen, M.A.; Basu, P.; Buchori, D.; Galetto, L.; Garibaldi, L.A.; et al. A Global-Scale Expert Assessment of Drivers and Risks Associated with Pollinator Decline. Nat. Ecol. Evol. 2021, 5, 1453–1461. [Google Scholar] [CrossRef] [PubMed]
- Potts, S.G.; Imperatriz-Fonseca, V.; Ngo, H.T.; Aizen, M.A.; Biesmeijer, J.C.; Breeze, T.D.; Dicks, L.V.; Garibaldi, L.A.; Hill, R.; Settele, J.; et al. Safeguarding Pollinators and Their Values to Human Well-Being. Nature 2016, 540, 220–229. [Google Scholar] [CrossRef]
- Potts, S.G.; Biesmeijer, J.C.; Kremen, C.; Neumann, P.; Schweiger, O.; Kunin, W.E. Global Pollinator Declines: Trends, Impacts and Drivers. Trends Ecol. Evol. 2010, 25, 345–353. [Google Scholar] [CrossRef]
- Biesmeijer, J.C.; Roberts, S.P.M.; Reemer, M.; Ohlemüller, R.; Edwards, M.; Peeters, T.; Schaffers, A.P.; Potts, S.G.; Kleukers, R.; Thomas, C.D.; et al. Parallel Declines in Pollinators and Insect-Pollinated Plants in Britain and the Netherlands. Science 2006, 313, 351–354. [Google Scholar] [CrossRef]
- Mugnier, R. Le travail interespèces de pollinisation dans les écologies perturbées. Rev. D’anthropologie Connaiss. 2023, 17, 1. [Google Scholar] [CrossRef]
- Beekman, M.; Ratnieks, F.L.W. Long-Range Foraging by the Honey-Bee, Apis mellifera L. Funct. Ecol. 2000, 14, 490–496. [Google Scholar] [CrossRef]
- Couvillon, M.J.; Riddell Pearce, F.C.; Accleton, C.; Fensome, K.A.; Quah, S.K.L.; Taylor, E.L.; Ratnieks, F.L.W. Honey Bee Foraging Distance Depends on Month and Forage Type. Apidologie 2015, 46, 61–70. [Google Scholar] [CrossRef]
- Danner, N. Honey Bee Foraging in Agricultural Landscapes. Ph.D. Thesis, Julius-Maximilians Universität, Würzburg, Germany, 2016. [Google Scholar]
- Greenleaf, S.S.; Williams, N.M.; Winfree, R.; Kremen, C. Bee Foraging Ranges and Their Relationship to Body Size. Oecologia 2007, 153, 589–596. [Google Scholar] [CrossRef]
- Hagler, J.R.; Mueller, S.; Teuber, L.R.; Machtley, S.A.; Van Deynze, A. Foraging Range of Honey Bees, Apis Mellifera, in Alfalfa Seed Production Fields. J. Insect Sci. Online 2011, 11, 144. [Google Scholar] [CrossRef] [PubMed]
- Steffan-Dewenter, I.; Kuhn, A. Honeybee Foraging in Differentially Structured Landscapes. Proc. R. Soc. Lond. B Biol. Sci. 2003, 270, 569–575. [Google Scholar] [CrossRef] [PubMed]
- Visscher, P.K.; Seeley, T.D. Foraging Strategy of Honeybee Colonies in a Temperate Deciduous Forest. Ecology 1982, 63, 1790–1801. [Google Scholar] [CrossRef]
- Rutschmann, B.; Kohl, P.L.; Steffan-Dewenter, I. Foraging Distances, Habitat Preferences and Seasonal Colony Performance of Honeybees in Central European Forest Landscapes. J. Appl. Ecol. 2023, 60, 1056–1066. [Google Scholar] [CrossRef]
- Béguin, C. Contribution à la cartographie des potentialités mellifères de Haut-Jura: Exemples de variations spatio-temporelles autour d’un rucher à Chaumont/NE. Geogr. Helvetica 1994, 49, 115–124. [Google Scholar] [CrossRef]
- Briane, G.; Cabrol, J.-L. L’abeille dans le géosystème: Essai de cartographie des ressources mellifères. Rev. Géographique Pyrén. Sud-Ouest 1986, 57, 363–373. [Google Scholar] [CrossRef]
- Timberlake, T.P.; Vaughan, I.P.; Memmott, J. Phenology of Farmland Floral Resources Reveals Seasonal Gaps in Nectar Availability for Bumblebees. J. Appl. Ecol. 2019, 56, 1585–1596. [Google Scholar] [CrossRef]
- Grüter, C.; Moore, H.; Firmin, N.; Helanterä, H.; Ratnieks, F.L.W. Flower Constancy in Honey Bee Workers (Apis mellifera) Depends on Ecologically Realistic Rewards. J. Exp. Biol. 2011, 214, 1397–1402. [Google Scholar] [CrossRef]
- von Frisch, K. The Dance Language and Orientation of Bees; Harvard Univ. Press: Cambridge, UK, 1967. [Google Scholar]
- Shackleton, K.; Balfour, N.J.; Al Toufailia, H.; James, E.; Ratnieks, F.L.W. Honey Bee Waggle Dances Facilitate Shorter Foraging Distances and Increased Foraging Aggregation. Anim. Behav. 2023, 198, 11–19. [Google Scholar] [CrossRef]
- Sherman, G.; Visscher, P.K. Honeybee Colonies Achieve Fitness through Dancing. Nature 2002, 419, 920–922. [Google Scholar] [CrossRef]
- Becher, M.A.; Osborne, J.L.; Thorbek, P.; Kennedy, P.J.; Grimm, V. REVIEW: Towards a Systems Approach for Understanding Honeybee Decline: A Stocktaking and Synthesis of Existing Models. J. Appl. Ecol. 2013, 50, 868–880. [Google Scholar] [CrossRef] [PubMed]
- Polce, C.; Maes, J.; Rotllan-Puig, X.; Michez, D.; Castro, L.; Dvorak, L.; Fitzpatrick, Ú.; Francis, F.; Neumayer, J.; Manino, A.; et al. Distribution of Bumblebees across Europe. One Ecosyst. 2018, 3, e28143. [Google Scholar] [CrossRef]
- Sárospataki, M.; Bakos, R.; Horváth, A.; Neidert, D.; Horváth, V.; Vaskor, D.; Szita, É.; Samu, F. The Role of Local and Landscape Level Factors in Determining Bumblebee Abundance and Richness. Acta Zool. Acad. Sci. Hung. 2016, 62, 387–407. [Google Scholar] [CrossRef]
- Whitehorn, P.R.; Seo, B.; Comont, R.F.; Rounsevell, M.; Brown, C. The Effects of Climate and Land Use on British Bumblebees: Findings from a Decade of Citizen-science Observations. J. Appl. Ecol. 2022, 59, 1837–1851. [Google Scholar] [CrossRef]
- Bijelčić, A.; Krajinović, B.; Hodžić, S.; Zulum, D.; Voljevica, N.; Tucaković, A. Meteorološki Godišnjak 2022; Federalni Hidrometeorološki Zavod: Sarajevo, Bosnia & Herzegovina, 2022; p. 62.
- Bijedić, A.; Krajinović, B.; Hodžić, S.; Zulum, D.; Voljevica, N.; Tucaković, A. Meteorološki Godišnjak 2023; Federalni Hidrometeorološki Zavod: Sarajevo, Bosnia & Herzegovina, 2023; p. 56.
- World Bank Climate Change Knowledge Portal. Available online: https://climateknowledgeportal.worldbank.org/ (accessed on 26 March 2025).
- QGIS Development Team QGIS Geographic Information System; Open Source Geospatial Foundation: Beaverton, OR, USA, 2023.
- Rhoné, F. L’abeille à Travers Champs: Quelles Interactions Entre Apis mellifera L. et le Paysage Agricole (Gers 32)?: Le rôle des Structures Paysagères Ligneuses Dans L’apport de Ressources Trophiques et Leurs Répercussions sur les Traits D’histoire De vie des Colonies. Ph.D. Thesis, Université Toulouse-Jean Jaurès, Toulouse, France, 2015. [Google Scholar]
- ESA. Sentinel Copernicus. Available online: https://sentinels.copernicus.eu/web/sentinel/home (accessed on 4 May 2022).
- Chuvieco, E. Fundamentals of Satellite Remote Sensing: An Environmental Approach, 3rd ed.; CRC Press: Boca Raton, FL, USA, 2020; ISBN 978-0-429-50648-2. [Google Scholar]
- Google Earth. Available online: https://earth.google.com/web/ (accessed on 4 May 2022).
- Fields Maps. Available online: https://www.esri.com/en-us/arcgis/products/arcgis-field-maps/overview (accessed on 4 May 2022).
- Williams, D.D.; Hynes, H.B.N. The Ecology of Temporary Streams II. General Remarks on Temporary Streams. Int. Rev. Gesamten Hydrobiol. Hydrogr. 1977, 62, 53–61. [Google Scholar] [CrossRef]
- Cushman, S.A.; McGarigal, K.; Neel, M.C. Parsimony in Landscape Metrics: Strength, Universality, and Consistency. Ecol. Indic. 2008, 8, 691–703. [Google Scholar] [CrossRef]
- McGarigal, K.; Cushman, S.A.; Ene, E. FRAGSTATS, Version 4. Spatial Pattern Analysis Program for Categorical Maps. University of Massachusetts: Amherst, MA, USA, 2012.
- Decourtye, A.; Lecompte, P.; Pierre, J.; Chauzat, M.-P.; Thiébeau, P. Introduction de jachères florales en zones de grandes cultures: Comment mieux concilier agriculture, biodiversité et apiculture? Courr. Environ. INRA 2007, 16, 3. [Google Scholar]
- Ponce-Hernandez, R. Assessing Carbon Stocks and Modelling Win-Win Scenarios of Carbon Sequestration Through Land-Use Changes; Food and Agriculture Organization of the United Nations: Rome, Italy, 2004. [Google Scholar]
- Beck, G.; Maly, K.; Bjelčić, Ž. Flora Bosnae et Hercegovinae; Sympetalae; Zemaljska Štamparija: Sarajevo, Bosnia & Herzegovina, 1983. [Google Scholar]
- Euro+Med PlantBase the Information Ressource for Euro-Mediterranean Plant Diversity. 2006. Available online: https://www.emplantbase.org/home.html (accessed on 4 May 2022).
- Janjić, V.; Kojić, M. Atlas Travnih Korova; Institut za Istraživanja u Poljoprivredi SRBIJA: Beograd, Serbia, 2003. [Google Scholar]
- Kojić, M. Livadske Biljke; Naučna Knjiga: Baograd, Serbia, 1990. [Google Scholar]
- Šumatić, N.; Topalić Trivunović, L.; Komljenović, I.; Todorović, J. Najčešći Korovi Regije Banja Luka; Grafomark: Laktaši, Bosnia & Herzegovina, 2006. [Google Scholar]
- Umeljic, V. In the World of Bees and Flowers. Atlas of Melliferous Plants; part 2; Kolor Press: Kragujevac, Serbia, 2003. [Google Scholar]
- Umeljic, V. In the World of Bees and Flowers. Atlas of Melliferous Plants; part 1; Kolor Press: Kragujevac, Serbia, 1999. [Google Scholar]
- Frankl, R.; Wanning, S.; Braun, R. Quantitative Floral Phenology at the Landscape Scale: Is a Comparative Spatio-Temporal Description of “Flowering Landscapes” Possible? J. Nat. Conserv. 2005, 13, 219–229. [Google Scholar] [CrossRef]
- Braun-Blanquet, J.; Roussine, N.; Negre, R. Les Groupements Végétaux de La France Méditerranéenne; CNRS: Paris, France, 1952. [Google Scholar]
- Rodwell, J.S. National Vegetation Classification: Users’ Handbook; Joint Nature Conservation Committee: Peterborough, UK, 2006; ISBN 978-1-86107-574.
- Janssens, X.; Bruneau, É.; Lebrun, P. Prévision des potentialités de production de miel à l’échelle d’un rucher au moyen d’un système d’information géographique. Apidologie 2006, 37, 351–365. [Google Scholar] [CrossRef]
- Timberlake, T. Mind the Gap: The Importance of FLowering Phenology in Pollinator Conservation. Ph.D. Thesis, University of Bristol, Bristol, UK, 2019. [Google Scholar]
- Guerriat, H. Valeur Apicole Des Haies Dans l’Entre-Sambre et Meuse. Abeille Cie 1999, 73, 24–28. [Google Scholar]
- Mačukanović-Jocić, M.; Jarić, S. The melliferous potential of Apiflora of south Western Vojvodina (Serbia). Arch. Biol. Sci. 2016, 68, 130. [Google Scholar] [CrossRef]
- R Core Team. R: A Language and Environement for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2021. [Google Scholar]
- Hendriksma, H.P.; Shafir, S. Honey Bee Foragers Balance Colony Nutritional Deficiencies. Behav. Ecol. Sociobiol. 2016, 70, 509–517. [Google Scholar] [CrossRef]
- Schmidt, J.O. Feeding Preferences of Apis mellifera L. (Hymenoptera: Apidae): Individual versus Mixed Pollen Species. J. Kans. Entomol. Soc. 1984, 572, 323–327. [Google Scholar]
- Boccara, N. Modeling Complex Systems. In Graduate Texts in Contemporary Physics; Springer: New York, NY, USA, 2004; ISBN 978-0-387-40462-2. [Google Scholar]
- Hanley, M.E.; Franco, M.; Pichon, S.; Darvill, B.; Goulson, D. Breeding System, Pollinator Choice and Variation in Pollen Quality in British Herbaceous Plants. Funct. Ecol. 2008, 22, 592–598. [Google Scholar] [CrossRef]
- Avni, D.; Dag, A.; Shafir, S. The Effect of Surface Area of Pollen Patties Fed to Honey Bee (Apis mellifera) Colonies on Their Consumption, Brood Production and Honey Yields. J. Apic. Res. 2009, 48, 23–28. [Google Scholar] [CrossRef]
- Requier, F.; Odoux, J.-F.; Tamic, T.; Moreau, N.; Henry, M.; Decourtye, A.; Bretagnolle, V. Honey Bee Diet in Intensive Farmland Habitats Reveals an Unexpectedly High Flower Richness and a Major Role of Weeds. Ecol. Appl. 2015, 25, 881–890. [Google Scholar] [CrossRef]
- Bailey, S.; Requier, F.; Nusillard, B.; Roberts, S.P.M.; Potts, S.G.; Bouget, C. Distance from Forest Edge Affects Bee Pollinators in Oilseed Rape Fields. Ecol. Evol. 2014, 4, 370–380. [Google Scholar] [CrossRef]
- Decourtye, A.A.; Alaux, C.; Odoux, J.F.; Henry, M.; Vaissière, B.; Conte, Y.L. Why Enhancement of Floral Resources in Agro-Ecosystems Benefit Honeybees and Beekeepers? Ecosyst. Biodivers. 2011, 19, 371–388. [Google Scholar]
- Danner, N.; Keller, A.; Härtel, S.; Steffan-Dewenter, I. Honey Bee Foraging Ecology: Season but Not Landscape Diversity Shapes the Amount and Diversity of Collected Pollen. PLoS ONE 2017, 12, e0183716. [Google Scholar] [CrossRef]
- Garbuzov, M.; Balfour, N.J.; Shackleton, K.; Al Toufailia, H.; Scandian, L.; Ratnieks, F.L.W. Multiple Methods of Assessing Nectar Foraging Conditions Indicate Peak Foraging Difficulty in Late Season. Insect Conserv. Divers. 2020, 13, 532–542. [Google Scholar] [CrossRef]
- Köppler, K.; Vorwohl, G.; Koeniger, N. Comparison of Pollen Spectra Collected by Four Different Subspecies of the Honey Bee Apis Mellifera. Apidologie 2007, 38, 341–353. [Google Scholar] [CrossRef]
- Alaux, C.; Ducloz, F.; Crauser, D.; Le Conte, Y. Diet Effects on Honeybee Immunocompetence. Biol. Lett. 2010, 6, 562–565. [Google Scholar] [CrossRef] [PubMed]
- Derioz, P. Arrière-pays méditerranéen entre déprise et reprise: l’Exemple du Haut-Languedoc Occidental. Econ. Rurale 1994, 223, 32–38. [Google Scholar] [CrossRef]
- Leponiemi, M.; Freitak, D.; Moreno-Torres, M.; Pferschy-Wenzig, E.-M.; Becker-Scarpitta, A.; Tiusanen, M.; Vesterinen, E.J.; Wirta, H. Honeybees’ Foraging Choices for Nectar and Pollen Revealed by DNA Metabarcoding. Sci. Rep. 2023, 13, 14753. [Google Scholar] [CrossRef]
- Radhika, V.; Kost, C.; Boland, W.; Heil, M. The Role of Jasmonates in Floral Nectar Secretion. PLoS ONE 2010, 5, e9265. [Google Scholar] [CrossRef]
- Nielsen, A.; Reitan, T.; Rinvoll, A.W.; Brysting, A.K. Effects of Competition and Climate on a Crop Pollinator Community. Agric. Ecosyst. Environ. 2017, 246, 253–260. [Google Scholar] [CrossRef]
- Pacini, E.; Nepi, M.; Vesprini, J.L. Nectar Biodiversity: A Short Review. Plant Syst. Evol. 2003, 238, 7–21. [Google Scholar] [CrossRef]
- Baude, M.; Kunin, W.E.; Boatman, N.D.; Conyers, S.; Davies, N.; Gillespie, M.A.K.; Morton, R.D.; Smart, S.M.; Memmott, J. Historical Nectar Assessment Reveals the Fall and Rise of Britain in Bloom. Nature 2016, 535, 85–88. [Google Scholar] [CrossRef]
- Power, E.F.; Stabler, D.; Borland, A.M.; Barnes, J.; Wright, G.A. Analysis of Nectar from Low-volume Flowers: A Comparison of Collection Methods for Free Amino Acids. Methods Ecol. Evol. 2018, 9, 734–743. [Google Scholar] [CrossRef]
- Schmickl, T.; Crailsheim, K. HoPoMo: A Model of Honeybee Intracolonial Population Dynamics and Resource Management. Ecol. Model. 2007, 204, 219–245. [Google Scholar] [CrossRef]
- Arundel, J.; Winter, S.; Gui, G.; Keatley, M. A Web-Based Application for Beekeepers to Visualise Patterns of Growth in Floral Resources Using MODIS Data. Environ. Model. Softw. 2016, 83, 116–125. [Google Scholar] [CrossRef]
- Campbell, T.; Fearns, P. Honey Crop Estimation from Space: Detection of Large Flowering Events in Western Australian Forests. Int. Arch. Photogramm. Remote Sens. Spat. Inf. Sci. 2018, XLII–1, 79–86. [Google Scholar] [CrossRef]
- Makori, D.M.; Abdel-Rahman, E.M.; Landmann, T.; Mutanga, O.; Odindi, J.; Nguku, E.; Tonnang, H.E.; Raina, S. Suitability of Resampled Multispectral Datasets for Mapping Flowering Plants in the Kenyan Savannah. PLoS ONE 2020, 15, e0232313. [Google Scholar] [CrossRef] [PubMed]
- Chen, B.; Jin, Y.; Brown, P. An Enhanced Bloom Index for Quantifying Floral Phenology Using Multi-Scale Remote Sensing Observations. ISPRS J. Photogramm. Remote Sens. 2019, 156, 108–120. [Google Scholar] [CrossRef]
- Nicholls, E.; Hempel de Ibarra, N. Assessment of Pollen Rewards by Foraging Bees. Funct. Ecol. 2017, 31, 76–87. [Google Scholar] [CrossRef]
- Nepi, M. Beyond Nectar Sweetness: The Hidden Ecological Role of Non-Protein Amino Acids in Nectar. J. Ecol. 2014, 102, 108–115. [Google Scholar] [CrossRef]
- Nepi, M.; Grasso, D.A.; Mancuso, S. Nectar in Plant–Insect Mutualistic Relationships: From Food Reward to Partner Manipulation. Front. Plant Sci. 2018, 9, 01063. [Google Scholar] [CrossRef]
- Nicholls, C.; Altieri, M. Plant Biodiversity Enhances Bees and Other Insect Pollinators in Agroecosystems. A Review. Agron. Sustain. Dev. 2013, 33, 257–274. [Google Scholar] [CrossRef]
- Lonsdorf, E.; Kremen, C.; Ricketts, T.; Winfree, R.; Williams, N.; Greenleaf, S. Modelling Pollination Services across Agricultural Landscapes. Ann. Bot. 2009, 103, 1589–1600. [Google Scholar] [CrossRef]
- Seeley, T.D.; Camazine, S.; Sneyd, J. Collective Decision-Making in Honey Bees: How Colonies Choose among Nectar Sources. Behav. Ecol. Sociobiol. 1991, 28, 277–290. [Google Scholar] [CrossRef]
- Kjøhl, M.; Nielsen, A.; Stenseth, N.C. Potential Effects of Climate Change on Crop Pollination: Extension of Knowledge Base, Adaptive Management, Capacity Building, Mainstreaming. In Pollitnation Services for Sustainable Agriculture; Food and Agriculture Organization of the United Nations: Rome, Italy, 2011; ISBN 978-92-5-106878-6. [Google Scholar]
- Harris, C.; Balfour, N.J.; Ratnieks, F.L.W. Seasonal Variation in the General Availability of Floral Resources for Pollinators in Northwest Europe: A Review of the Data. Biol. Conserv. 2024, 298, 110774. [Google Scholar] [CrossRef]
- Karbassioon, A.; Yearlsey, J.; Dirilgen, T.; Hodge, S.; Stout, J.C.; Stanley, D.A. Responses in Honeybee and Bumblebee Activity to Changes in Weather Conditions. Oecologia 2023, 201, 689–701. [Google Scholar] [CrossRef] [PubMed]
- Rivière, J.; Alves, T.; Alaux, C.; Conte, Y.L.; Layec, Y.; Lozac’h, A.; Singhoff, F.; Rodin, V. Modèle multi-agent d’auto-organisation pour le butinage au sein d’une colonie d’abeilles. Rev. Ouverte Intell. Artif. 2022, 3, 5–6. [Google Scholar] [CrossRef]
- Russell, S.; Barron, A.B.; Harris, D. Dynamic Modelling of Honey Bee (Apis mellifera) Colony Growth and Failure. Ecol. Model. 2013, 265, 158–169. [Google Scholar] [CrossRef]
- Betti, M.; LeClair, J.; Wahl, L.; Zamir, M. Bee++: An Object-Oriented, Agent-Based Simulator for Honey Bee Colonies. Insects 2017, 8, 31. [Google Scholar] [CrossRef]
- Arroyo-Correa, B.; Bartomeus, I.; Jordano, P. Individual-based Plant–Pollinator Networks Are Structured by Phenotypic and Microsite Plant Traits. J. Ecol. 2021, 109, 2832–2844. [Google Scholar] [CrossRef]
- Torné-Noguera, A.; Rodrigo, A.; Osorio, S.; Bosch, J. Collateral Effects of Beekeeping: Impacts on Pollen-Nectar Resources and Wild Bee Communities. Basic Appl. Ecol. 2016, 17, 199–209. [Google Scholar] [CrossRef]
- Atanasov, A.Z.; Georgiev, I.R. A Multicriteria Model for Optimal Location of Honey Bee Colonies in Regions without Overpopulation. In Proceedings of the AIP Conference Proceedings, Sofia, Bulgaria, 8 March 2021; p. 090008. [Google Scholar]
- Komasilova, O.; Komasilovs, V.; Kviesis, A.; Zacepins, A. Model for Finding the Number of Honey Bee Colonies Needed for the Optimal Foraging Process in a Specific Geographical Location. PeerJ 2021, 9, e12178. [Google Scholar] [CrossRef]





| Month | Jan. | Feb. | Mar. | Apr. | May | June | Jul. | Aug. | Sept. | Oct. | Nov. | Dec. | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Temperature (°C) | Mean monthly in 2022 | 0.60 | 5.00 | 4.90 | 10.3 | 17.8 | 22.7 | 22.4 | 21.9 | 16.3 | 13.5 | 7.70 | 5.30 |
| Mean monthly in 2023 | 3.80 | 3.20 | 8.70 | 10.3 | 15.6 | 20.2 | 22.7 | 21.6 | 18.8 | 15.6 | 8.40 | 4.90 | |
| 1991–2020 mean | 0.64 | 1.95 | 5.73 | 10.2 | 14.7 | 18.4 | 20.4 | 20.6 | 15.9 | 11.0 | 6.03 | 1.09 | |
| Mean monthly minimum in 2022 | −4.00 | −0.60 | −2.00 | 3.70 | 10.8 | 15.2 | 14.6 | 15.8 | 11.0 | 8.50 | 4.20 | 2.10 | |
| Mean monthly minimum in 2023 | 0.30 | −2.30 | 2.40 | 5.00 | 11.4 | 14.4 | 16.4 | 15.4 | 12.7 | 9.80 | 3.30 | 0.40 | |
| Mean 1991–2020 minimum | −2.70 | −1.97 | 0.94 | 5.10 | 9.23 | 12.8 | 14.3 | 14.4 | 10.1 | 6.09 | 2.26 | −2.03 | |
| Mean monthly maximum in 2022 | 6.30 | 12.6 | 13.1 | 17.6 | 25.2 | 29.8 | 30.6 | 28.9 | 23.8 | 21.4 | 12.3 | 9.20 | |
| Mean monthly maximum in 2023 | 8.40 | 10.0 | 16.1 | 16.2 | 20.8 | 26.6 | 30.4 | 29.3 | 26.8 | 23.4 | 14.0 | 10.9 | |
| Mean 1991–2020 maximum | 3.98 | 5.89 | 10.6 | 15.4 | 20.2 | 23.9 | 26.6 | 26.9 | 21.8 | 16.0 | 9.84 | 4.21 |
| Type of Land Use | Number of LUUs | (ha) | (LUU/100 ha) | (m) |
|---|---|---|---|---|
| Cropfield excluding corn | 43 | 0.685 ± 0.52 ab | 6.08 | 118 ± 110 a |
| Forest | 40 | 6.41 ± 11.0 a | 5.66 | 51.4 ± 51.7 bc |
| Wasteland | 38 | 1.68 ± 2.95 a | 5.38 | 81.9 ± 112 abcd |
| Maize | 49 | 0.583 ± 0.50 b | 6.93 | 99.7 ± 106 ab |
| Grassland | 114 | 1.62 ± 3.44 ab | 16.1 | 24.7 ± 28.7 d |
| Riparian forest | 13 | 0.929 ± 1.28 abc | 1.84 | 72.3 ± 142 abcd |
| Orchard | 132 | 0.327 ± 0.545 c | 18.7 | 29.5 ± 45.5 cd |
| Statistical analysis | - | Χ2 = 116.84, df = 6, p < 0.001 | - | Χ2 = 71.35, df = 6, p < 0.001 |
| Land Use Type | Total Plant Species | Average Plant Species Number per Observation Plot |
|---|---|---|
| Forest | 66 | 5.39 ± 3.07 a |
| Wasteland | 50 | 5.78 ± 2.65 a |
| Grassland | 84 | 14.4 ± 6.05 b |
| Riparian forest | 69 | 16.7 ± 5.88 b |
| Orchard | 45 | - |
| Statistical analysis | - | Χ2 = 59.12, df = 3, p < 0.001 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Laboisse, S.; Vaillant, M.; Cazenave, C.; Kelečević, B.; Chevalier, I.; Andres, L. Space and Time Dynamics of Honeybee (Apis mellifera L.)-Melliferous Resource Interactions Within a Foraging Area: A Case Study in the Banja Luka Region (Bosnia & Herzegovina). Biology 2025, 14, 422. https://doi.org/10.3390/biology14040422
Laboisse S, Vaillant M, Cazenave C, Kelečević B, Chevalier I, Andres L. Space and Time Dynamics of Honeybee (Apis mellifera L.)-Melliferous Resource Interactions Within a Foraging Area: A Case Study in the Banja Luka Region (Bosnia & Herzegovina). Biology. 2025; 14(4):422. https://doi.org/10.3390/biology14040422
Chicago/Turabian StyleLaboisse, Samuel, Michel Vaillant, Clovis Cazenave, Biljana Kelečević, Iris Chevalier, and Ludovic Andres. 2025. "Space and Time Dynamics of Honeybee (Apis mellifera L.)-Melliferous Resource Interactions Within a Foraging Area: A Case Study in the Banja Luka Region (Bosnia & Herzegovina)" Biology 14, no. 4: 422. https://doi.org/10.3390/biology14040422
APA StyleLaboisse, S., Vaillant, M., Cazenave, C., Kelečević, B., Chevalier, I., & Andres, L. (2025). Space and Time Dynamics of Honeybee (Apis mellifera L.)-Melliferous Resource Interactions Within a Foraging Area: A Case Study in the Banja Luka Region (Bosnia & Herzegovina). Biology, 14(4), 422. https://doi.org/10.3390/biology14040422





