Evaluating the Effect of an Essential Oil Blend on the Growth and Fitness of Gram-Positive and Gram-Negative Bacteria
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Experimental Site
2.2. Bacteria
2.3. Preparation of the Essential Oil Working Solution
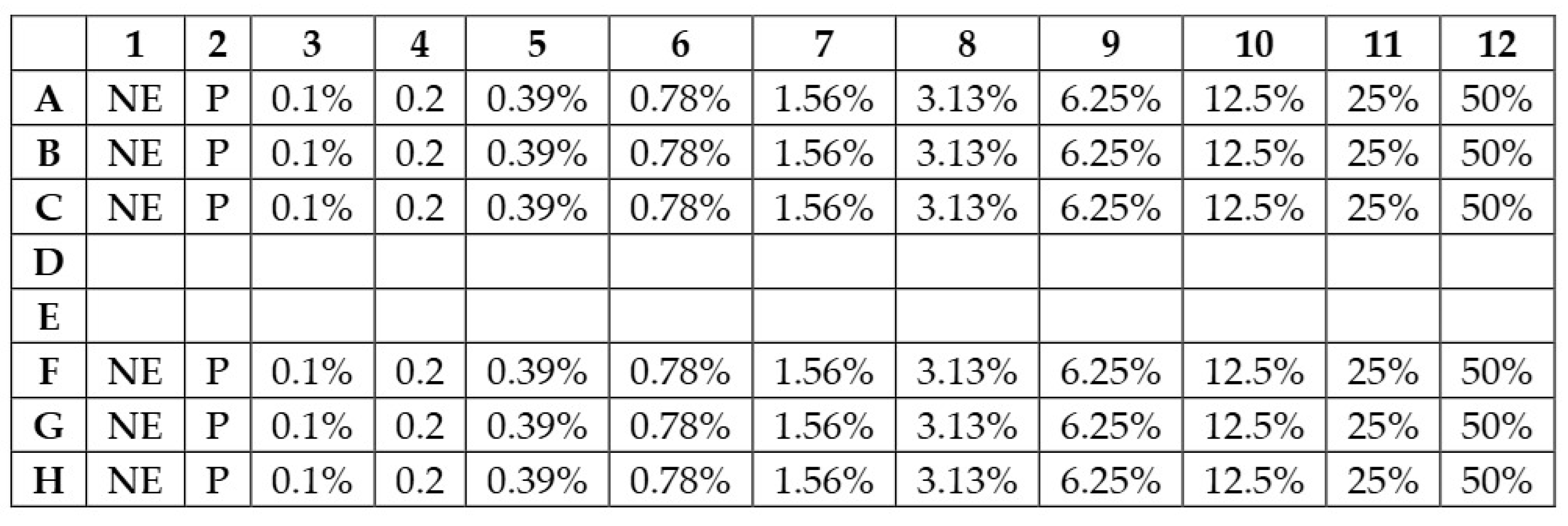
2.4. Determination of the Minimum Inhibitory Concentrations and the Growth Curve Assay
3. Results
3.1. Bioactive Compounds
3.2. Minimum Inhibitory Concentrations
3.3. Relative Fitness
3.3.1. S. epidermidis
3.3.2. E. coli
3.3.3. K. aerogenes
3.3.4. B. subtilis
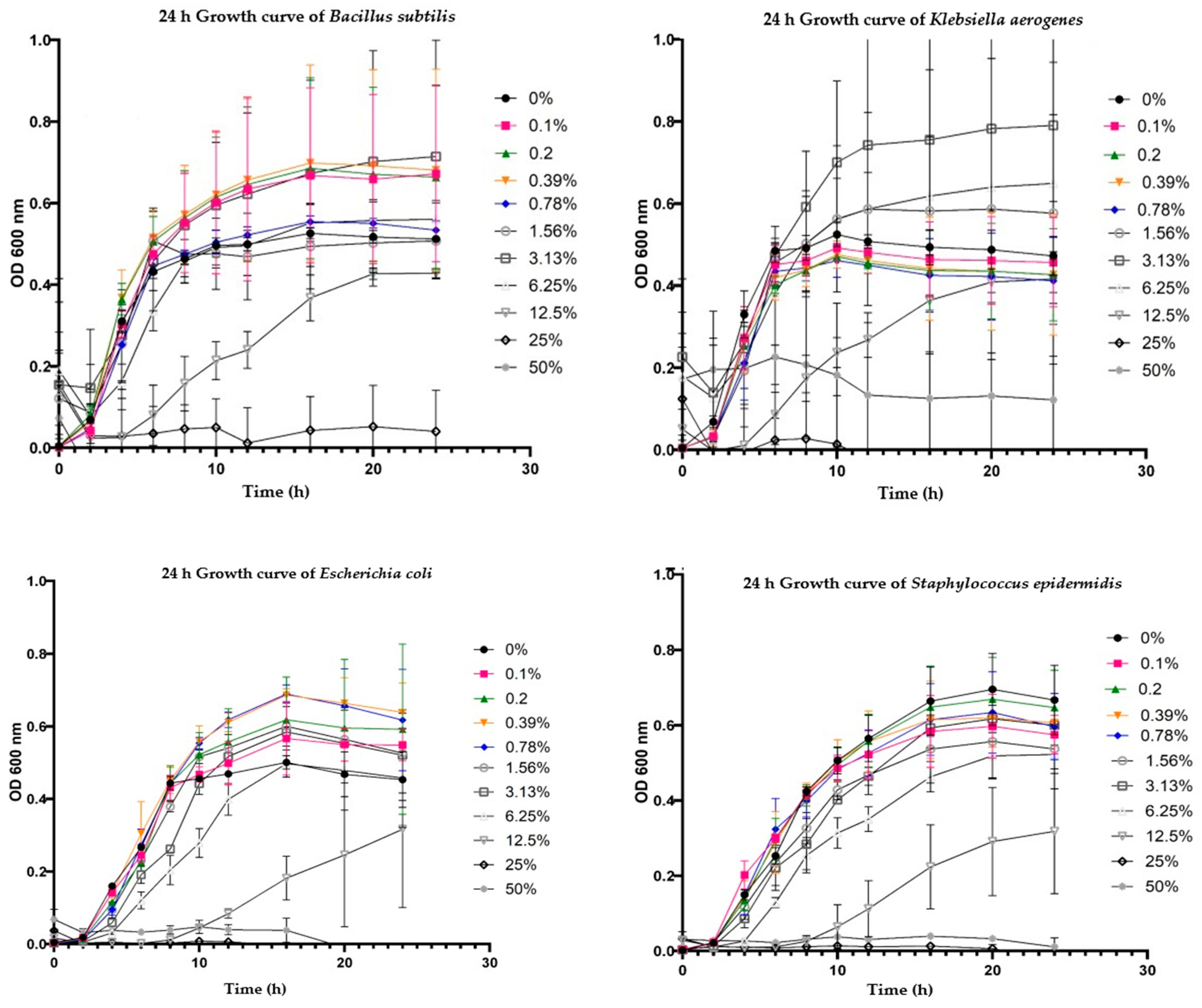
3.4. Bacterial Growth Metrics
3.4.1. Carrying Capacity (k)
3.4.2. Growth Rate (r)
3.4.3. Time at Midpoint of the Growth Curve (t_mid)
3.4.4. Generation Time (t_gen)
3.4.5. Area Under the Empirical (auc_e) and the Logistic (auc_l) Curve
4. Discussion
4.1. Growth Curves
4.2. Relative Fitness
4.3. Bacterial Growth Characteristics
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| EOB | essential oil blend |
| MICs | minimum inhibitory concentrations |
| EOs | essential oils |
| MRSA | methicillin-resistant Staphylococcus aureus |
| RF | relative fitness |
| ROS | reactive oxygen species |
References
- Prestinaci, F.; Pezzotti, P.; Pantosti, A. Antimicrobial Resistance: A Global Multifaceted Phenomenon. Pathog. Glob. Health 2015, 109, 309–318. [Google Scholar] [CrossRef] [PubMed]
- Otto, M. Staphylococcus epidermidis—the “accidental” Pathogen. Nat. Rev. Microbiol. 2009, 7, 555–567. [Google Scholar] [CrossRef] [PubMed]
- Kaper, J.B.; Nataro, J.P.; Mobley, H.L.T. Pathogenic Escherichia coli. Nat. Rev. Microbiol. 2004, 2, 123–140. [Google Scholar] [CrossRef]
- Davin-Regli, A.; Pagès, J.M. Enterobacter aerogenes and Enterobacter cloacae; Versatile Bacterial Pathogens Confronting Antibiotic Treatment. Front. Microbiol. 2015, 6, 392. [Google Scholar] [CrossRef]
- Logan, N.A. Bacillus and Relatives in Foodborne Illness. J. Appl. Microbiol. 2012, 112, 417–429. [Google Scholar] [CrossRef] [PubMed]
- Hashemi, S.R.; Davoodi, H. Herbal Plants and Their Derivatives as Growth and Health Promoters in Animal Nutrition. Vet. Res. Commun. 2011, 35, 169–180. [Google Scholar] [CrossRef]
- Chávez-González, M.L.; Rodríguez-Herrera, R.; Aguilar, C.N. Essential Oils: A Natural Alternative to Combat Antibiotics Resistance. In Antibiotic Resistance: Mechanisms and New Antimicrobial Approaches; Kon, K., Rai, M., Eds.; Academic Press: Cambridge, MA, USA, 2016; pp. 227–237. [Google Scholar]
- Nora, L.; Marcon, C.; Deolindo, G.L.; Signor, M.H.; Muniz, A.L.; Bajay, M.M.; Copetti, P.M.; Bissacotti, B.F.; Morsch, V.M.; da Silva, A.S. The Effects of a Blend of Essential Oils in the Milk of Suckling Calves on Performance, Immune and Antioxidant Systems, and Intestinal Microbiota. Animals 2024, 14, 3555. [Google Scholar] [CrossRef]
- Murugaiyan, J.; Kumar, P.A.; Rao, G.S.; Iskandar, K.; Hawser, S.; Hays, J.P.; Mohsen, Y.; Adukkadukkam, S.; Awuah, W.A.; Jose, R.A.M.; et al. Progress in Alternative Strategies to Combat Antimicrobial Resistance: Focus on Antibiotics. Antibiotics 2022, 11, 200. [Google Scholar] [CrossRef]
- Mittal, R.P.; Rana, A.; Jaitak, V. Essential Oils: An Impending Substitute of Synthetic Antimicrobial Agents to Overcome Antimicrobial Resistance. Curr. Drug Targets 2019, 20, 605–624. [Google Scholar] [CrossRef]
- Giovannini, D.; Gismondi, A.; Basso, A.; Canuti, L.; Braglia, R.; Canini, A.; Mariani, F.; Cappelli, G. Lavandula angustifolia Mill. Essential Oil Exerts Antibacterial and Anti-Inflammatory Effect in Macrophage Mediated Immune Response to Staphylococcus aureus. Immunol. Investig. 2016, 45, 11–28. [Google Scholar] [CrossRef]
- Li, D.; Wang, R.; You, M.; Chen, N.; Sun, L.; Chen, N. The Antimicrobial Effect and Mechanism of the Artemisia argyi Essential Oil against Bacteria and Fungus. Braz. J. Microbiol. 2024, 55, 727–735. [Google Scholar] [CrossRef]
- Razavi, R.; Toosi, E.; Sheikholeslami, M.; Konjedi, M.; Hajian-Tilaki, A.; Najafi, A. Effect of Origanum onites L. Essential Oil and Cold Atmospheric Plasma on Physicochemical, Microbial, and Sensory Properties of Iranian White Cheese. J. Food Qual. 2024, 2024, 2308789. [Google Scholar] [CrossRef]
- Ainseba, N.; Loukili, N.; Soulimane, A.; Bellifa, S.; Dib, M.E.A.; Muselli, A. Antimicrobial and Antifungal Effects of Essential Oils from Origanum vulgare, Lavandula officinalis, and Syzygium aromaticum on Bacterial Strains through Gaseous Contact. Antiinfect. Agents 2024, 22, 642324. [Google Scholar] [CrossRef]
- Hamoud, R.; Sporer, F.; Reichling, J.; Wink, M. Antimicrobial Activity of a Traditionally Used Complex Essential Oil Distillate (Olbas® Tropfen) in Comparison to Its Individual Essential Oil Ingredients. Phytomedicine 2012, 19, 969–976. [Google Scholar] [CrossRef]
- Ike, K.A.; Adelusi, O.O.; Alabi, J.O.; Olagunju, L.K.; Wuaku, M.; Anotaenwere, C.C.; Okedoyin, D.O.; Gray, D.; Dele, P.A.; Subedi, K.; et al. Effects of Different Essential Oil Blends and Fumaric Acid on in Vitro Fermentation, Greenhouse Gases, Nutrient Degradability, and Total and Molar Proportions of Volatile Fatty Acid Production in a Total Mixed Ration for Dairy Cattle. Agriculture 2024, 14, 876. [Google Scholar] [CrossRef]
- Zimmer, B.L.; Carpenter, D.E.; Esparza, G.; Alby, K.; Bhatnagar, A.; Ferrell, A.L.; Flemming, L.; Huband, M.D.; Jiménez-Pearson, A.; Kircher, S.M.; et al. CLSI M07 Methods for Dilution Antimicrobial Susceptibility Tests for Bacteria That Grow Aerobically, 11th ed.; Clinical and Laboratory Standards Institute: Berwyn, PA, USA, 2018; ISBN 9781684402267. [Google Scholar]
- Man, A.; Santacroce, L.; Jacob, R.; Mare, A.; Man, L. Antimicrobial Activity of Six Essential Oils against a Group of Human Pathogens: A Comparative Study. Pathogens 2019, 8, 15. [Google Scholar] [CrossRef]
- Man, A.; Gâz, A.Ş.; Mare, A.D.; Berţa, L. Effects of Low-Molecular Weight Alcohols on Bacterial Viability. Rev. Rom. Med. Lab. 2017, 25, 335–343. [Google Scholar] [CrossRef]
- Sprouffske, K.; Wagner, A. Growthcurver: An R Package for Obtaining Interpretable Metrics from Microbial Growth Curves. BMC Bioinform. 2016, 17, 172. [Google Scholar] [CrossRef]
- Silva-Angulo, A.B.; Zanini, S.F.; Rodrigo, D.; Rosenthal, A.; Martinez, A. Growth Kinetics of Listeria innocua and Listeria monocytogenes under Exposure to Carvacrol and the Occurrence of Sublethal Damage. Food Control 2014, 37, 336–342. [Google Scholar] [CrossRef]
- Maggio, F.; Rossi, C.; Chaves-López, C.; Valbonetti, L.; Desideri, G.; Paparella, A.; Serio, A. A Single Exposure to a Sublethal Concentration of Origanum vulgare Essential Oil Initiates Response against Food Stressors and Restoration of Antibiotic Susceptibility in Listeria monocytogenes. Food Control 2022, 132, 108562. [Google Scholar] [CrossRef]
- Lambert, R.J.W.; Skandamis, P.N.; Coote, P.J.; Nychas, G.-J.E. A Study of the Minimum Inhibitory Concentration and Mode of Action of Oregano Essential Oil, Thymol and Carvacrol. J. Appl. Microbiol. 2001, 91, 453–462. [Google Scholar] [CrossRef]
- Bassolé, I.H.N.; Juliani, H.R. Essential Oils in Combination and Their Antimicrobial Properties. Molecules 2012, 17, 3989–4006. [Google Scholar] [CrossRef] [PubMed]
- Zainal-Abidin, Z.; Mohd-Said, S.; Adibah, F.; Majid, A.; Mustapha, W.A.W.; Jantan, I. Anti-Bacterial Activity of Cinnamon Oil on Oral Pathogens. Open Conf. Proc. J. 2014, 4, 12–16. [Google Scholar] [CrossRef]
- Wang, D.; Fan, W.; Guan, Y.; Huang, H.; Yi, T.; Ji, J. Oxidative Stability of Sunflower Oil Flavored by Essential Oil from Coriandrum sativum L. during Accelerated Storage. LWT 2018, 98, 268–275. [Google Scholar] [CrossRef]
- Siroli, L.; Patrignani, F.; Serrazanetti, D.I.; Tabanelli, G.; Montanari, C.; Gardini, F.; Lanciotti, R. Lactic Acid Bacteria and Natural Antimicrobials to Improve the Safety and Shelf-Life of Minimally Processed Sliced Apples and Lamb’s Lettuce. Food Microbiol. 2015, 47, 74–84. [Google Scholar] [CrossRef]
- Sharifi-Rad, J.; Sureda, A.; Tenore, G.; Daglia, M.; Sharifi-Rad, M.; Valussi, M.; Tundis, R.; Sharifi-Rad, M.; Loizzo, M.; Ademiluyi, A.; et al. Biological Activities of Essential Oils: From Plant Chemoecology to Traditional Healing Systems. Molecules 2017, 22, 70. [Google Scholar] [CrossRef] [PubMed]
- Saeed, S.; Tariq, P. In Vitro Antibacterial Activity of Clove against Gram Negative Bacteria. Pak. J. Bot. 2008, 40, 2157–2160. [Google Scholar]
- Newell, R.I. A Framework for Developing “Ecological Carrying Capacity” Mathematical Models for Bivalve Mollusc Aquaculture. Bull. Fish. Res. Agency 2007, 19, 41–51. [Google Scholar]
- Chen, X.D.; Zhang, C.K.; Zhou, Z.; Gong, Z.; Zhou, J.J.; Tao, J.F.; Paterson, D.M.; Feng, Q. Stabilizing Effects of Bacterial Biofilms: EPS Penetration and Redistribution of Bed Stability Down the Sediment Profile. J. Geophys. Res. Biogeosci. 2017, 122, 3113–3125. [Google Scholar] [CrossRef]
- Tsuchiya, K.; Cao, Y.-Y.; Kurokawa, M.; Ashino, K.; Yomo, T.; Ying, B.-W. A Decay Effect of the Growth Rate Associated with Genome Reduction in Escherichia coli. BMC Microbiol. 2018, 18, 101. [Google Scholar] [CrossRef]
- Marr, A.G. Growth Rate of Escherichia coli. Microbiol. Rev. 1991, 55, 316–333. [Google Scholar] [CrossRef]
- Madigan, M.T.; Kempher, M.L.; Bender, K.S.; Sullivan, P.; Matthew Sattley, W.; Dohnalkova, A.C.; Joye, S.B. Characterization of a Cold-Active Bacterium Isolated from the South Pole “Ice Tunnel”. Extremophiles 2017, 21, 891–901. [Google Scholar] [CrossRef] [PubMed]
- Aćimović, M.; Zorić, M.; Zheljazkov, V.D.; Pezo, L.; Čabarkapa, I.; Jeremić, J.S.; Cvetković, M. Chemical Characterization and Antibacterial Activity of Essential Oil of Medicinal Plants from Eastern Serbia. Molecules 2020, 25, 5482. [Google Scholar] [CrossRef]
- Gallucci, M.N.; Oliva, M.; Casero, C.; Dambolena, J.; Luna, A.; Zygadlo, J.; Demo, M. Antimicrobial Combined Action of Terpenes against the Food-borne Microorganisms Escherichia coli, Staphylococcus aureus and Bacillus cereus. Flavour Fragr. J. 2009, 24, 348–354. [Google Scholar] [CrossRef]
- Di Pasqua, R.; Mamone, G.; Ferranti, P.; Ercolini, D.; Mauriello, G. Changes in the Proteome of Salmonella enterica Serovar Thompson as Stress Adaptation to Sublethal Concentrations of Thymol. Proteomics 2010, 10, 1040–1049. [Google Scholar] [CrossRef]
- Un Jan Contreras, S.; Redfern, L.K.; Maguire, L.W.; Promi, S.I.; Gardner, C.M. Small Interfering RNAs (SiRNAs) Negatively Impact Growth and Gene Expression of Environmentally Relevant Bacteria in In Vitro Conditions. Environ. Sci. Technol. 2024, 58, 13856–13865. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Feng, R.; Li, L.; Zhou, X.; Li, Z.; Jia, R.; Song, X.; Zou, Y.; Yin, L.; He, C.; et al. The Antibacterial Mechanism of Terpinen-4-Ol Against Streptococcus agalactiae. Curr. Microbiol. 2018, 75, 1214–1220. [Google Scholar] [CrossRef]
- Akarchariya, N.; Sirilun, S.; Julsrigival, J.; Chansakaowa, S. Chemical Profiling and Antimicrobial Activity of Essential Oil from Curcuma aeruginosa Roxb., Curcuma glans K. Larsen & J. Mood and Curcuma cf. xanthorrhiza Roxb. Collected in Thailand. Asian Pac. J. Trop. Biomed. 2017, 7, 881–885. [Google Scholar] [CrossRef]
- Ren, S.; Wang, B.; Qiu, X.; Wang, S.; Huang, G.; Wang, Y. Unveiling Antibacterial and Antibiofilm Mechanisms of Methyleugenol: Implications for Ecomaterial Functionalization. ACS EST Eng. 2024, 4, 1206–1217. [Google Scholar] [CrossRef]
- Merghni, A.; Belmamoun, A.R.; Urcan, A.C.; Bobiş, O.; Lassoued, M.A. 1,8-Cineol (Eucalyptol) Disrupts Membrane Integrity and Induces Oxidative Stress in Methicillin-Resistant Staphylococcus aureus. Antioxidants 2023, 12, 1388. [Google Scholar] [CrossRef]
- Sujithra, K.; Srinivasan, S.; Indumathi, D.; Vinothkumar, V. Allyl Methyl Sulfide, an Organosulfur Compound Alleviates Hyperglycemia Mediated Hepatic Oxidative Stress and Inflammation in Streptozotocin-Induced Experimental Rats. Biomed. Pharmacother. 2018, 107, 292–302. [Google Scholar] [CrossRef] [PubMed]
- Agathokleous, E.; Wang, Q.; Iavicoli, I.; Calabrese, E.J. The Relevance of Hormesis at Higher Levels of Biological Organization: Hormesis in Microorganisms. Curr. Opin. Toxicol. 2022, 29, 1–9. [Google Scholar] [CrossRef]
- Yang, Y.; Chen, Y.; Zhang, G.; Sun, J.; Guo, L.; Jiang, M.; Ou, B.; Zhang, W.; Si, H. Transcriptomic Analysis of Staphylococcus aureus Under the Stress Condition Caused by Litsea cubeba L. Essential Oil via RNA Sequencing. Front. Microbiol. 2020, 11, 540558. [Google Scholar] [CrossRef] [PubMed]

| Compound Name | RT 1 | Formula | Concentration (%) 2 |
|---|---|---|---|
| 2-Propanamine, 2-methyl- | 8.78 | C4H11N | 1.13 |
| Sulfide, allyl methyl | 9.43 | C4H8S | 2.03 |
| Bicyclo[2.2.1]heptane, 2,2-dimethyl-3-methylene-, (1S)- | 10.06 | C10H16 | 6.77 |
| Beta-Phellandrene | 10.87 | C10H16 | 20.3 |
| Beta-Myrcene | 11.14 | C10H16 | 12.1 |
| 1,3-Cyclohexadiene, 1-methyl-4-(1-methylethyl)- | 12.75 | C10H16 | 6.45 |
| Eucalyptol | 12.86 | C10H18O | 6.24 |
| 4-Nonanone | 15.43 | C9H18O | 5.18 |
| 5-Hepten-2-one, 6-methyl- | 15.7 | C8H14O | 3.00 |
| Benzene, 1-methyl-3-(1-methylethenyl)- | 16.18 | C10H12 | 2.93 |
| Bicyclo[3.1.0]hexan-2-ol, 2-methyl-5-(1-methylethyl)-, (1α,2α,5α)- | 16.75 | C10H18O | 1.91 |
| Bicyclo[7.2.0]undec-4-ene, 4,11,11-trimethyl-8-methylene-,[1R-(1R*,4Z,9S*)]- | 17.67 | C15H24 | 0.87 |
| Bicyclo[3.1.0]hexan-2-ol, 2-methyl-5-(1-methylethyl)-, (1α,2α,5α)- | 18.13 | C10H18O | 1.25 |
| Terpinen-4-ol | 18.67 | C10H18O | 10.53 |
| Geranyl formate | 19.5 | C11H18O2 | 9.36 |
| 3-Furaldehyde | 22.0 | C5H4O2 | 0.46 |
| Methyleugenol | 24.39 | C11H14O2 | 1.62 |
| Caryophyllene oxide | 24.68 | C15H24O | 1.13 |
| p-Cymen-7-ol | 25.27 | C10H14O | 6.71 |
| Assess Details | Mean 0% (Positive Control) | Mean Concentrations | Standard Error | Adjusted p-Value |
|---|---|---|---|---|
| S. epidermidis | ||||
| 0% vs. 0.1% | 0.8509 | 0.8512 | 0.06542 | >0.9999 |
| 0% vs. 0.2% | 0.8509 | 0.8491 | 0.06542 | >0.9999 |
| 0% vs. 0.39% | 0.8509 | 0.8354 | 0.06542 | >0.9999 |
| 0% vs. 0.78% | 0.8509 | 0.8248 | 0.06542 | >0.9999 |
| 0% vs. 1.56% | 0.8509 | 0.7180 | 0.06542 | 0.6275 |
| 0% vs. 3.13% | 0.8509 | 0.6715 | 0.06542 | 0.1987 |
| 0% vs. 6.25% | 0.8509 | 0.5104 | 0.06542 | <0.0001 |
| 0% vs. 12.50% | 0.8509 | 0.2137 | 0.06542 | <0.0001 |
| 0% vs. 25% | 0.8509 | 0.05653 | 0.06542 | <0.0001 |
| 0% vs. 50% | 0.8509 | 0.1495 | 0.06542 | <0.0001 |
| E. coli | ||||
| 0% vs. 0.1% | 0.7647 | 0.7861 | 0.07988 | >0.9999 |
| 0% vs. 0.2% | 0.7647 | 0.79 | 0.07988 | >0.9999 |
| 0% vs. 0.39% | 0.7647 | 0.8929 | 0.07988 | 0.8759 |
| 0% vs. 0.78% | 0.7647 | 0.8262 | 0.07988 | 0.9995 |
| 0% vs. 1.56% | 0.7647 | 0.732 | 0.07988 | >0.9999 |
| 0% vs. 3.13% | 0.7647 | 0.6288 | 0.07988 | 0.8313 |
| 0% vs. 6.25% | 0.7647 | 0.4771 | 0.07988 | 0.0212 |
| 0% vs. 12.50% | 0.7647 | 0.1551 | 0.07988 | <0.0001 |
| 0% vs. 25% | 0.7647 | 0.01499 | 0.07988 | <0.0001 |
| 0% vs. 50% | 0.7647 | 0.1863 | 0.07988 | <0.0001 |
| K. aerogenes | ||||
| 0% vs. 0.1% | 0.6912 | 0.6172 | 0.08149 | 0.9979 |
| 0% vs. 0.2% | 0.6912 | 0.5767 | 0.08149 | 0.9440 |
| 0% vs. 0.39% | 0.6912 | 0.5777 | 0.08149 | 0.9472 |
| 0% vs. 0.78% | 0.6912 | 0.5652 | 0.08149 | 0.8995 |
| 0% vs. 1.56% | 0.6912 | 0.6492 | 0.08149 | >0.9999 |
| 0% vs. 3.13% | 0.6912 | 0.8422 | 0.08149 | 0.745 |
| 0% vs. 6.25% | 0.6912 | 0.7325 | 0.08149 | >0.9999 |
| 0% vs. 12.50% | 0.6912 | 0.317 | 0.08149 | 0.0007 |
| 0% vs. 25% | 0.6912 | 0.07049 | 0.08149 | <0.0001 |
| 0% vs. 50% | 0.6912 | 0.3202 | 0.08149 | 0.0008 |
| B. subtilis | ||||
| 0% vs. 0.1% | 0.6669 | 0.749 | 0.05662 | 0.9320 |
| 0% vs. 0.2% | 0.6669 | 0.8207 | 0.05662 | 0.2098 |
| 0% vs. 0.39% | 0.6669 | 0.8155 | 0.05662 | 0.2531 |
| 0% vs. 0.78% | 0.6669 | 0.6575 | 0.05662 | >0.9999 |
| 0% vs. 1.56% | 0.6669 | 0.6666 | 0.05662 | >0.9999 |
| 0% vs. 3.13% | 0.6669 | 0.8017 | 0.05662 | 0.3913 |
| 0% vs. 6.25% | 0.6669 | 0.6134 | 0.05662 | 0.997 |
| 0% vs. 12.50% | 0.6669 | 0.3319 | 0.05662 | <0.0001 |
| 0% vs. 25% | 0.6669 | 0.1009 | 0.05662 | <0.0001 |
| 0% vs. 50% | 0.6669 | 0.000157 | 0.05662 | <0.0001 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ike, K.A.; Omaliko, P.C.; Fernander, M.C.; Sanders, B.M.; Enikuomehin, J.M.; Alabi, J.O.; Adelusi, O.O.; Kholif, A.E.; Thomas, M.D.; Anele, U.Y. Evaluating the Effect of an Essential Oil Blend on the Growth and Fitness of Gram-Positive and Gram-Negative Bacteria. Biology 2025, 14, 437. https://doi.org/10.3390/biology14040437
Ike KA, Omaliko PC, Fernander MC, Sanders BM, Enikuomehin JM, Alabi JO, Adelusi OO, Kholif AE, Thomas MD, Anele UY. Evaluating the Effect of an Essential Oil Blend on the Growth and Fitness of Gram-Positive and Gram-Negative Bacteria. Biology. 2025; 14(4):437. https://doi.org/10.3390/biology14040437
Chicago/Turabian StyleIke, Kelechi A., Paul C. Omaliko, Mizpha C. Fernander, Brittany M. Sanders, James M. Enikuomehin, Joel O. Alabi, Oludotun O. Adelusi, Ahmed E. Kholif, Misty D. Thomas, and Uchenna Y. Anele. 2025. "Evaluating the Effect of an Essential Oil Blend on the Growth and Fitness of Gram-Positive and Gram-Negative Bacteria" Biology 14, no. 4: 437. https://doi.org/10.3390/biology14040437
APA StyleIke, K. A., Omaliko, P. C., Fernander, M. C., Sanders, B. M., Enikuomehin, J. M., Alabi, J. O., Adelusi, O. O., Kholif, A. E., Thomas, M. D., & Anele, U. Y. (2025). Evaluating the Effect of an Essential Oil Blend on the Growth and Fitness of Gram-Positive and Gram-Negative Bacteria. Biology, 14(4), 437. https://doi.org/10.3390/biology14040437









