Microplastic Contamination of the Turkish Worm Lizard (Blanus strauchi Bedriaga, 1884) in Muğla Province (Türkiye)
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Studied Species
2.2. Studied Province
2.3. Characterization of Microplastics
2.4. Statistical Analyses
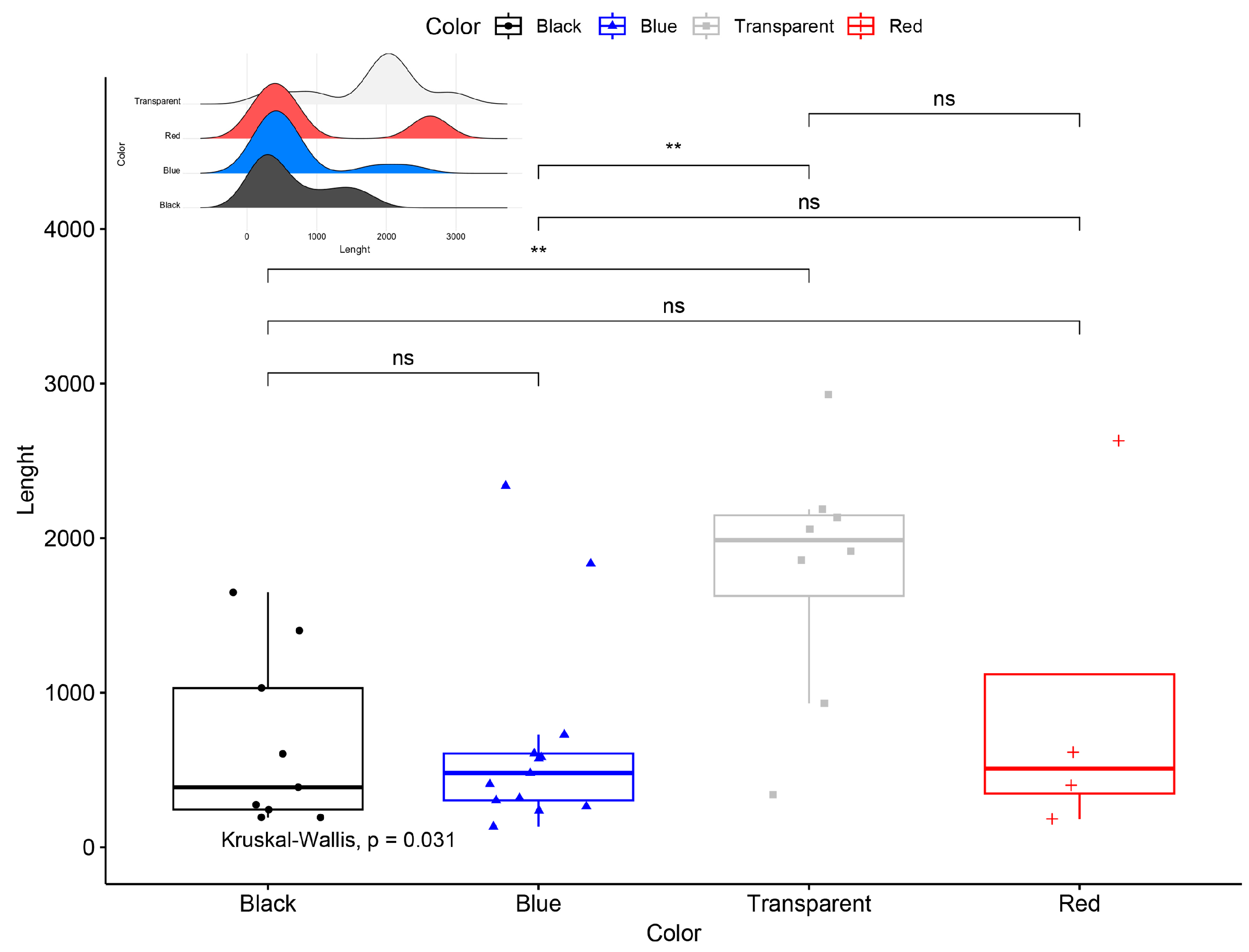
3. Results
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- An, L.; Liu, Q.; Deng, Y.; Wu, W.; Gao, Y.; Ling, W. Sources of Microplastic in the Environment. In Microplastics in Terrestrial Environments: Emerging Contaminants and Major Challenges; Springer: Cham, Switzerland, 2020; pp. 143–159. [Google Scholar]
- Rillig, M.C.; Lehmann, A. Microplastic in Terrestrial Ecosystems. Science 2020, 368, 1430–1431. [Google Scholar] [CrossRef] [PubMed]
- Dusaucy, J.; Gateuille, D.; Perrette, Y.; Naffrechoux, E. Microplastic Pollution of Worldwide Lakes. Environ. Pollut. 2021, 284, 117075. [Google Scholar] [CrossRef]
- Sait, S.T.; Sørensen, L.; Kubowicz, S.; Vike-Jonas, K.; Gonzalez, S.V.; Asimakopoulos, A.G.; Booth, A.M. Microplastic Fibres from Synthetic Textiles: Environmental Degradation and Additive Chemical Content. Environ. Pollut. 2021, 268, 115745. [Google Scholar] [CrossRef] [PubMed]
- Başaran, B.; Özçifçi, Z.; Demir Kanbur, E.; Akçay, H.T.; Gül, S.; Bektaş, Y.; Aytan, Ü. Microplastics in Honey from Türkiye: Occurrence, Characteristic, Human Exposure, and Risk Assessment. J. Food Compos. Anal. 2024, 135, 106646. [Google Scholar] [CrossRef]
- Di Renzo, L.; Mascilongo, G.; Di Giacinto, F.; Zezza, D.; Di Francesco, G.; Olivieri, V.; Ferri, N. Extraction Protocol Optimization for Detection of Microplastics in Digestive System Contents of Loggerhead Turtle (Caretta caretta). In Proceedings of the 2nd International Conference on Microplastic Pollution in the Mediterranean Sea; Springer International Publishing: Berlin/Heidelberg, Germany, 2020; pp. 205–211. [Google Scholar]
- Machovsky-Capuska, G.E.; Andrades, R.; Santos, R.G. Debris Ingestion and Nutritional Niches in Estuarine and Reef Green Turtles. Mar. Pollut. Bull. 2020, 153, 110943. [Google Scholar] [CrossRef]
- Koelmans, A.A.; Redondo-Hasselerharm, P.E.; Nor, N.H.M.; de Ruijter, V.N.; Mintenig, S.M.; Kooi, M. Risk Assessment of Microplastic Particles. Nat. Rev. Mater. 2022, 7, 138–152. [Google Scholar] [CrossRef]
- Ramsperger, A.F.R.M.; Narayana, V.K.B.; Gross, W.; Mohanraj, J.; Thelakkat, M.; Greiner, A.; Laforsch, C. Environmental Exposure Enhances the Internalization of Microplastic Particles into Cells. Sci. Adv. 2020, 6, eabd1211. [Google Scholar] [CrossRef]
- Noventa, S.; Boyles, M.S.; Seifert, A.; Belluco, S.; Jiménez, A.S.; Johnston, H.J.; Losasso, C. Paradigms to Assess the Human Health Risks of Nano- and Microplastics. Microplast. Nanoplast. 2021, 1, 9. [Google Scholar] [CrossRef]
- Kolenda, K.; Pawlik, M.; Kuśmierek, N.; Smolis, A.; Kadej, M. Online Media Reveals a Global Problem of Discarded Containers as Deadly Traps for Animals. Sci. Rep. 2021, 11, 267. [Google Scholar] [CrossRef]
- Sindha, P.; Vyas, R.; Mistry, V. Entanglement in Fishing Nets: Deaths of Indian Rock Pythons (Python molurus). Reptiles Amphib. 2020, 26, 248–249. [Google Scholar] [CrossRef]
- Lettoof, D.; Orton, K. Evidence of Plastic Consumption by a Tiger Snake (Notechis scutatus) from a Highly Urbanised Wetland. West. Aust. Nat. 2020, 31, 187–189. [Google Scholar]
- Gül, S.; Karaoğlu, K.; Özçifçi, Z.; Candan, K.; Ilgaz, Ç.; Kumlutaş, Y. Occurrence of Microplastics in Herpetological Museum Collection: Grass Snake (Natrix natrix [Linnaeus, 1758]) and Dice Snake (Natrix tessellata [Laurenti, 1769]) as Model Organisms. Water Air Soil. Pollut. 2022, 233, 160. [Google Scholar] [CrossRef]
- Dursun, C.; Candan, K.; Karaoğlu, K.; Ilgaz, Ç.; Kumlutaş, Y.; Caynak, E.Y.; Gül, S. Microplastic Accumulation in Snake-Eyed Lizard (Ophisops elegans Menetries, 1832) after Long-Term Monitoring: Habitats Matter, Not Years. Environ. Sci. Eur. 2025, 37, 8. [Google Scholar] [CrossRef]
- Mackenzie, C.M.; Vladimirova, V. Preliminary Study and First Evidence of Presence of Microplastics in Terrestrial Herpetofauna from Southwestern Paraguay. Stud. Neotrop. Fauna Environ. 2023, 58, 16–24. [Google Scholar] [CrossRef]
- Banaee, M.; Gholamhosseini, A.; Sureda, A.; Soltanian, S.; Fereidouni, M.S.; Ibrahim, A.T.A. Effects of Microplastic Exposure on the Blood Biochemical Parameters in the Pond Turtle (Emys orbicularis). Environ. Sci. Pollut. Res. 2021, 28, 9221–9234. [Google Scholar] [CrossRef] [PubMed]
- Marn, N.; Jusup, M.; Kooijman, S.A.; Klanjscek, T. Quantifying Impacts of Plastic Debris on Marine Wildlife Identifies Ecological Breakpoints. Ecol. Lett. 2020, 23, 1479–1487. [Google Scholar] [CrossRef] [PubMed]
- Murta, A.S.N. Microplastics Go on Land: Anthropogenic and Biological Drivers of Microplastic Ingestion in Galapagos Lava Lizards. Master’s Thesis, University of Coimbra, Coimbra, Portugal, 2024; p. 63.
- Silva, J.M.; Navoni, J.A.; Freire, E.M.X. Lizards as Model Organisms to Evaluate Environmental Contamination and Biomonitoring. Environ. Monit. Assess. 2020, 192, 454. [Google Scholar] [CrossRef]
- Fasola, E.; Biaggini, M.; Ortiz-Santaliestra, M.E.; Costa, S.; Santos, B.; Lopes, I.; Corti, C. Assessing Stress Response in Lizards from Agroecosystems with Different Management Practices. Bull. Environ. Contam. Toxicol. 2022, 108, 196–203. [Google Scholar] [CrossRef]
- Xie, M.; Lv, M.; Zhao, Z.; Li, L.; Jiang, H.; Yu, Y.; Zhang, X.; Liu, P.; Chen, J. New Insights of Bacterial and Eukaryotic Phenotypes on the Plastics Collected from the Typical Natural Habitat of the Endangered Crocodile Lizard. Ecotoxicol. Environ. Saf. 2024, 280, 116541. [Google Scholar] [CrossRef]
- Scudiero, R.; Chianese, T.; Cretì, P.; Rosati, L. Risk Assessment Arising from the Exposure of Terrestrial Vertebrates to Soil Contamination: Learning from Field Lizards of the Podarcis Genus. J. Xenobiot. 2025, 15, 21. [Google Scholar] [CrossRef]
- Wang, Q.; Adams, C.A.; Wang, F.; Sun, Y.; Zhang, S. Interactions between Microplastics and Soil Fauna: A Critical Review. Crit. Rev. Environ. Sci. Technol. 2022, 52, 3211–3243. [Google Scholar] [CrossRef]
- Wang, J.; Wang, M.; Zhou, P.; DuBay, S.; Zhang, S.; Yang, Z.; Wang, Y.; Wu, Y. Assessing Microplastic and Nanoplastic Contamination in Bird Lungs: Evidence of Ecological Risks and Bioindicator Potential. J. Hazard. Mater. 2025, 487, 137274. [Google Scholar] [CrossRef]
- Rahman, M.M.; Kim, E.S.; Sung, H.C. Microplastics as an Emerging Threat to Amphibians: Current Status and Future Perspectives. Heliyon 2024, 10, e28220. [Google Scholar] [CrossRef]
- Queiroz, L.G.; Barreto, L.M.; de Lima Júnior, J.M.; Maricato, G.; Nomura, C.S.; Pompêo, M.; Rani-Borges, B. Unveiling Microplastic and Metal Pollution in Giant Armadillos (Priodontes maximus) from Areas Impacted by Human Activities in the Rio Doce Basin, Brazil. Environ. Res. 2025, 275, 121380. [Google Scholar] [CrossRef] [PubMed]
- Sindaco, R.; Kornilios, P.; Sacchi, R.; Lymberakis, P. Taxonomic Reassessment of Blanus strauchi (Bedriaga, 1884) (Squamata: Amphisbaenia: Blanidae), with the Description of a New Species from Southeast Anatolia (Turkey). Zootaxa 2014, 3795, 311–326. [Google Scholar] [CrossRef]
- Düşen, S.; Uğurtaş, İ.H.; Aydoğdu, A. Nematode Parasites of the Two Limbless Lizards: Turkish Worm Lizard, Blanus strauchi (Bedriaga, 1884) (Squamata: Amphisbaenidae), and Slow Worm, Anguis fragilis Linnaeus 1758 (Squamata: Anguidae), from Turkey. Helminthologia 2010, 47, 158–163. [Google Scholar] [CrossRef]
- Baran, İ.; Avcı, A.; Kumlutaş, Y.; Olgun, K.; Ilgaz, Ç. Türkiye Amfibi ve Sürüngenleri; Palme Yayınevi: Ankara, Türkiye, 2021; ISBN 978-605-282-611-619. [Google Scholar]
- Venter, O.; Sanderson, E.; Magrach, A.; Allan, J.R.; Beher, J.; Jones, K.R.; Possingham, H.P.; Laurance, W.F.; Wood, P.; Fekete, B.M.; et al. Global Terrestrial Human Footprint Maps for 1993 and 2009. Sci. Data 2016, 3, 160067. [Google Scholar] [CrossRef] [PubMed]
- Tosun, C. Challenges of sustainable tourism development in the developing world: The case of Turkey. Tour. Manag. 2001, 22, 289–303. [Google Scholar] [CrossRef]
- Güzel İzmirli, Ş.; Gökkaya, A. Microplastic Pollution and Risk Assessment in Packaged Teas in Türkiye. Water Air Soil Pollut. 2024, 235, 438. [Google Scholar] [CrossRef]
- Szkudlarek, M.; Najbar, B.; Jankowiak, Ł. Variation in Microplastic Characteristics among Amphibian Larvae: A Comparative Study across Different Species and the Influence of Human Activity. Sci. Rep. 2024, 14, 13574. [Google Scholar] [CrossRef]
- Aragón-Sánchez, J.; Quintana-Marrero, Y.; Aragón-Hernández, C.; Hernández-Herero, M.J. ImageJ: A Free, Easy, and Reliable Method to Measure Leg Ulcers Using Digital Pictures. Int. J. Low. Extrem. Wounds 2017, 16, 269–273. [Google Scholar] [CrossRef] [PubMed]
- Revelle, W. Psych: Procedures for Personality and Psychological Research; Northwestern University: Evanston, IL, USA, 2022; Available online: https://CRAN.R-project.org/package=psych (accessed on 15 March 2025).
- Hebbali, A. olsrr: Tools for Building OLS Regression Models, R Package Version 0.5.3; R Foundation for Statistical Computing: Vienna, Austria, 2020; Available online: https://CRAN.R-project.org/package=olsrr (accessed on 15 March 2025).
- R Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2022; Available online: https://www.R-project.org/ (accessed on 15 March 2025).
- Moon, K. webr: Data and Functions for Web-Based Analysis, R Package Version 0.1.6; R Foundation for Statistical Computing: Vienna, Austria, 2024; Available online: https://github.com/cardiomoon/webr (accessed on 15 March 2025).
- Brunson, J.C. ggalluvial: Layered Grammar for Alluvial Plots. J. Open Source Softw. 2020, 5, 02017. [Google Scholar] [CrossRef] [PubMed]
- Kassambara, A. ggpubr: ‘ggplot2’ Based Publication Ready Plots, R Package Version 0.4.0; R Foundation for Statistical Computing: Vienna, Austria, 2020. [Google Scholar]
- Ahmad, M.; Li, J.L.; Wang, P.D.; Hozzein, W.N.; Li, W.J. Environmental Perspectives of Microplastic Pollution in the Aquatic Environment: A Review. Mar. Life Sci. Technol. 2020, 2, 414–430. [Google Scholar] [CrossRef]
- Ahmed, M.B.; Rahman, M.S.; Alom, J.; Hasan, M.S.; Johir, M.A.H.; Mondal, M.I.H.; Yoon, M.H. Microplastic Particles in the Aquatic Environment: A Systematic Review. Sci. Total Environ. 2021, 775, 145793. [Google Scholar] [CrossRef]
- Szkudlarek, M.; Najbar, B.; Jankowiak, Ł. Similarity of Microplastic Characteristics between Amphibian Larvae and Their Aquatic Environment. Animals 2024, 14, 717. [Google Scholar] [CrossRef]
- Lin, L.; Yuan, B.; Liu, H.; Ke, Y.; Zhang, W.; Li, H.; Yan, C. Microplastics Emerge as a Hotspot for Dibutyl Phthalate Sources in Rivers and Oceans: Leaching Behavior and Potential Risks. J. Hazard. Mater. 2024, 475, 134920. [Google Scholar] [CrossRef]
- Xu, B.; Liu, F.; Cryder, Z.; Huang, D.; Lu, Z.; He, Y.; Wang, H.; Lu, Z.; Brookes, P.C.; Tang, C.; et al. Microplastics in the Soil Environment: Occurrence, Risks, Interactions and Fate—A Review. Crit. Rev. Environ. Sci. Technol. 2020, 50, 2175–2222. [Google Scholar] [CrossRef]
- Chang, X.; Fang, Y.; Wang, Y.; Wang, F.; Shang, L.; Zhong, R. Microplastic Pollution in Soils, Plants, and Animals: A Review of Distributions, Effects and Potential Mechanisms. Sci. Total Environ. 2022, 850, 157857. [Google Scholar] [CrossRef]
- Wang, J.; Wu, F.; Dong, S.; Wang, X.; Ai, S.; Liu, Z.; Wang, X. Meta-analysis of the effects of microplastic on fish: Insights into growth, survival, reproduction, oxidative stress, and gut microbiota diversity. Water Res. 2024, 267, 122493. [Google Scholar] [CrossRef]
- En-Nejmy, K.; Hayany, B.E.; Al-Alawi, M.; Jemo, M.; Hafidi, M.; El Fels, L. Microplastics in Soil: A Comprehensive Review of Occurrence, Sources, Fate, Analytical Techniques and Potential Impacts. Ecotoxicol. Environ. Saf. 2024, 288, 117332. [Google Scholar] [CrossRef]
- Yabanlı, M.; Yozukmaz, A.; Şener, İ.; Ölmez, Ö.T. Microplastic Pollution at the Intersection of the Aegean and Mediterranean Seas: A Study of the Datça Peninsula (Turkey). Mar. Pollut. Bull. 2019, 145, 47–55. [Google Scholar] [CrossRef] [PubMed]
- Zhu, F.; Zhu, C.; Wang, C.; Gu, C. Occurrence and Ecological Impacts of Microplastics in Soil Systems: A Review. Bull. Environ. Contam. Toxicol. 2019, 102, 741–749. [Google Scholar] [CrossRef] [PubMed]
- Mokgalaka-Fleischmann, N.S.; Melato, F.A.; Netshiongolwe, K.; Izevbekhai, O.U.; Lepule, S.P.; Motsepe, K.; Edokpayi, J.N. Microplastic Occurrence and Fate in the South African Environment: A Review. Environ. Syst. Res. 2024, 13, 59. [Google Scholar] [CrossRef]
- Khan, A.; Qadeer, A.; Wajid, A.; Ullah, Q.; Rahman, S.U.; Ullah, K.; Horky, P. Microplastics in Animal Nutrition: Occurrence, Spread, and Hazard in Animals. J. Agric. Food Res. 2024, 100, 101258. [Google Scholar] [CrossRef]
- Beigzadeh, K.; Rieland, J.M.; Eastman, C.B.; Duffy, D.J.; Love, B.J. Characterization of Ingested Plastic Microparticles Extracted from Sea Turtle Post-Hatchlings at Necropsy. Microplastics 2022, 1, 254–262. [Google Scholar] [CrossRef]
- Fuentes, M.M.; Beckwidth, V.; Ware, M. The Effects of Microplastic on the Thermal Profile of Sand: Implications for Marine Turtle Nesting Grounds. Front. Mar. Sci. 2023, 10, 1146556. [Google Scholar] [CrossRef]
- Arat, S.A. An Overview of Microplastic in Marine Waters: Sources, Abundance, Characteristics and Negative Effects on Various Marine Organisms. Desalination Water Treat. 2024, 317, 100138. [Google Scholar] [CrossRef]
- Aranda, D.A.; Sindou, P.; Rodriguez, J.V.C.; Saldaña, G.M.; Coronado, R.F.V.; González, W.D.N.; Escalante, V.C. A Non-Invasive Method of Microplastics Pollution Quantification in Green Sea Turtle Chelonia mydas of the Mexican Caribbean. Mar. Pollut. Bull. 2024, 200, 116092. [Google Scholar] [CrossRef]
- Lu, S.; Qiu, R.; Hu, J.; Li, X. Prevalence of Microplastics in Animal-Based Traditional Medicinal Materials: Widespread Pollution in Terrestrial Environments. Sci. Total Environ. 2020, 709, 136214. [Google Scholar] [CrossRef]
- Teampanpong, J.; Duengkae, P. Terrestrial Wildlife as Indicators of Microplastic Pollution in Western Thailand. PeerJ 2024, 12, e17384. [Google Scholar] [CrossRef]
- Teampanpong, J.; Duengkae, P. Using Feces to Indicate Plastic Pollution in Terrestrial Vertebrate Species in Western Thailand. PeerJ 2024, 12, e17596. [Google Scholar] [CrossRef] [PubMed]
- Borroto-Paez, R.; León, O.E.S.; Fabres, B.A. Microplastics in Invasive Geckos (Hemidactylus mabouia and H. angulatus): First Evidence in Cuba and the Caribbean, and Transfer Pathways of Concern. Reptiles Amphib. 2024, 31, e22751. [Google Scholar] [CrossRef]
- Digka, N.; Bray, L.; Tsangaris, C.; Andreanidou, K.; Kasimati, E.; Kofidou, E.; Komnenou, A.; Kaberi, H. Evidence of ingested plastics in stranded loggerhead sea turtles along the Greek coastline, East Mediterranean Sea. Environ. Pollut. 2020, 263, 114596. [Google Scholar] [CrossRef] [PubMed]
- Eastman, C.B.; Farrell, J.A.; Whitmore, L.; Rollinson Ramia, D.R.; Thomas, R.S.; Prine, J.; Eastman, S.F.; Osborne, T.Z.; Martindale, M.Q.; Duffy, D.J. Plastic Ingestion in Post-hatchling Sea Turtles: Assessing a Major Threat in Florida Near Shore Waters. Front. Mar. Sci. 2020, 7, 693. [Google Scholar] [CrossRef]
- Jung, M.R.; Horgen, F.D.; Orski, S.V.; Rodriguez, C.V.; Beers, K.L.; Balazs, G.H.; Jones, T.T.; Work, T.M.; Brignac, K.C.; Royer, S.J.; et al. Validation of ATR FT-IR to identify polymers of plastic marine debris, including those ingested by marine organisms. Mar. Pollut. Bull. 2018, 127, 704–716. [Google Scholar] [CrossRef]
- Caron, A.G.; Thomas, C.R.; Berry, K.L.; Motti, C.A.; Ariel, E.; Brodie, J.E. Ingestion of Microplastic Debris by Green Sea Turtles (Chelonia mydas) in the Great Barrier Reef: Validation of a Sequential Extraction Protocol. Mar. Pollut. Bull. 2018, 127, 743–751. [Google Scholar] [CrossRef]
- Vecchi, S.; Bianchi, J.; Scalici, M.; Fabroni, F.; Tomassetti, P. Field Evidence for Microplastic Interactions in Marine Benthic Invertebrates. Sci. Rep. 2021, 11, 20900. [Google Scholar] [CrossRef]
- Zazouli, M.; Nejati, H.; Hashempour, Y.; Dehbandi, R.; Nam, V.T.; Fakhri, Y. Occurrence of Microplastics (MPs) in the Gastrointestinal Tract of Fishes: A Global Systematic Review and Meta-Analysis and Meta-Regression. Sci. Total Environ. 2022, 815, 152743. [Google Scholar] [CrossRef]
- Bilal, M.; Yaqub, A.; Hassan, H.U.; Akhtar, S.; Rafiq, N.; Ali Shah, M.I.; Ríos-Escalante, P.D.L. Microplastic Quantification in Aquatic Birds: Biomonitoring the Environmental Health of the Panjkora River Freshwater Ecosystem in Pakistan. Toxics 2023, 11, 972. [Google Scholar] [CrossRef]
- Onay, H.; Minaz, M.; Ak, K.; Er, A.; Emanet, M.; Karslı, B.; Bilgin, S. Decade of microplastic alteration in the southeastern black sea: An example of seahorse gastrointestinal tracts. Environ. Res. 2023, 218, 115001. [Google Scholar] [CrossRef]
- Kuranova, V.N.; Frank, Y.A.; Rakhmatullina, S.N.; Epova, L.A. Accumulation of Microplastics by the Siberian Wood Frog Rana amurensis (Anura, Amphibia) in the Western Baikal Region. Inland Water Biol. 2024, 17, 345–353. [Google Scholar] [CrossRef]
- Bahrani, F.; Mohammadi, A.; Dobaradaran, S.; De-la-Torre, G.E.; Arfaeinia, H.; Ramavandi, B.; Tekle-Röttering, A. Occurrence of Microplastics in Edible Tissues of Livestock (Cow and Sheep). Environ. Sci. Pollut. Res. 2024, 31, 22145–22157. [Google Scholar] [CrossRef]
- Gliaudelytė, U.; Persson, M.; Daukantienė, V. Impact of Textile Composition, Structure, and Treatment on Microplastic Release During Washing: A Review. Text. Res. J. 2025, 95, 220–232. [Google Scholar] [CrossRef]
- Bhatt, V.; Chauhan, J.S. Microplastic in Freshwater Ecosystem: Bioaccumulation, Trophic Transfer, and Biomagnification. Environ. Sci. Pollut. Res. 2023, 30, 9389–9400. [Google Scholar] [CrossRef] [PubMed]
- Bansal, O.P.; Singh, A. A Review on Microplastic in the Soils and Their Impact on Soil Microbes, Crops and Humans. Int. J. Res. 2022, 10, 245–273. [Google Scholar] [CrossRef]
- Prata, J.C.; Silva, A.L.P.; da Costa, J.P.; Dias-Pereira, P.; Carvalho, A.; Fernandes, A.J.S.; Rocha-Santos, T. Microplastics in Internal Tissues of Companion Animals from Urban Environments. Animals 2022, 12, 1979. [Google Scholar] [CrossRef]
- Dursun, C.; Karaoğlu, K.; Avcı, A.; Gül, S.; Özdemir, N.; Üzüm, N.; Olgun, K. The presence of microplastics in the Baran’s Newt (Neurergus barani Öz, 1994) and the Spotted Newt (Neurergus strauchii Steindachner, 1887). Environ. Sci. Pollut. Res. 2024, 31, 55974–55983. [Google Scholar] [CrossRef]
- Asani, P.C.; Alam, Z.; Poddar, R. Exploring the Impact of PVC and PVA Microplastics on Zebrafish Tissue Using Multi-Spectral Imaging, Optical Coherence Tomography (OCT) and Biospeckle OCT (bOCT). Chemosphere 2023, 341, 140088. [Google Scholar] [CrossRef] [PubMed]
- Ding, J.; Ju, P.; Ran, Q.; Li, J.; Jiang, F.; Cao, W.; Sun, C. Elder Fish Means More Microplastics? Alaska Pollock Microplastic Story in the Bering Sea. Sci. Adv. 2023, 9, eadf5897. [Google Scholar] [CrossRef]
- Dursun, C.; Karaoğlu, K.; Özdemir, N.; Candan, K.; Kumlutaş, Y.; Ilgaz, Ç.; Gül, S. Spatiotemporal Distribution of Microplastics in True Frogs (Ranidae: Pelophylax) Populations from Türkiye. Environ. Res. 2023, 236, 116774. [Google Scholar] [CrossRef]
- Deoniziak, K.; Cichowska, A.; Niedźwiecki, S.; Pol, W. Thrushes (Aves: Passeriformes) as Indicators of Microplastic Pollution in Terrestrial Environments. Sci. Total Environ. 2022, 853, 158621. [Google Scholar] [CrossRef] [PubMed]
- Nesterovschi, I.; Marica, I.; Levei, E.A.; Angyus, S.B.; Kenesz, M.; Moldovan, O.T.; Pînzaru, S.C. Subterranean transport of microplastics as evidenced in karst springs and their characterization using Raman spectroscopy. Spectrochim. Acta - A Mol. Biomol. Spectrosc. 2023, 298, 122811. [Google Scholar] [CrossRef] [PubMed]
- Mutshekwa, T.; Motitsoe, S.N.; Naidoo, T.; Majingo, Z.; Mlambo, M.C. Plastics underground: Microplastic pollution in South African freshwater caves and associated biota. Hydrobiologia 2025, 1–19. [Google Scholar] [CrossRef]
- Balestra, V.; Galbiati, M.; Lapadula, S.; Zampieri, V.; Cassarino, F.; Gajdošová, M.; Barzaghi, B.; Manenti, R.; Ficoleta, G.F.; Bellopede, R. Microplastic pollution calls for urgent investigations in stygobiont habitats: A case study from Classical karst. J. Environ. Manag. 2024, 356, 120672. [Google Scholar] [CrossRef] [PubMed]
- Al Malki, J.S.; Hussien, N.A.; Tantawy, E.M.; Khattab, Y.; Mohammadein, A. Terrestrial biota as bioindicators for microplastics and potentially toxic elements. Coatings 2021, 11, 1152. [Google Scholar] [CrossRef]
- Richard, C.M.; Dejoie, E.; Wiegand, C.; Gouesbet, G.; Colinet, H.; Balzani, P.; Siaussat, D.; Renault, D. Plastic pollution in terrestrial ecosystems: Current knowledge on impacts of micro and nano fragments on invertebrates. J. Hazard. Mater. 2024, 477, 135299. [Google Scholar] [CrossRef]



Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dursun, C.; Demirci, N.; Candan, K.; Yıldırım Caynak, E.; Kumlutaş, Y.; Ilgaz, Ç.; Gül, S. Microplastic Contamination of the Turkish Worm Lizard (Blanus strauchi Bedriaga, 1884) in Muğla Province (Türkiye). Biology 2025, 14, 441. https://doi.org/10.3390/biology14040441
Dursun C, Demirci N, Candan K, Yıldırım Caynak E, Kumlutaş Y, Ilgaz Ç, Gül S. Microplastic Contamination of the Turkish Worm Lizard (Blanus strauchi Bedriaga, 1884) in Muğla Province (Türkiye). Biology. 2025; 14(4):441. https://doi.org/10.3390/biology14040441
Chicago/Turabian StyleDursun, Cantekin, Nagihan Demirci, Kamil Candan, Elif Yıldırım Caynak, Yusuf Kumlutaş, Çetin Ilgaz, and Serkan Gül. 2025. "Microplastic Contamination of the Turkish Worm Lizard (Blanus strauchi Bedriaga, 1884) in Muğla Province (Türkiye)" Biology 14, no. 4: 441. https://doi.org/10.3390/biology14040441
APA StyleDursun, C., Demirci, N., Candan, K., Yıldırım Caynak, E., Kumlutaş, Y., Ilgaz, Ç., & Gül, S. (2025). Microplastic Contamination of the Turkish Worm Lizard (Blanus strauchi Bedriaga, 1884) in Muğla Province (Türkiye). Biology, 14(4), 441. https://doi.org/10.3390/biology14040441




