Effects of Varying Dietary Concentrations of Menadione Nicotinamide Bisulphite (VK3) on Growth Performance, Muscle Composition, Liver and Muscle Menaquinone-4 Concentration, and Antioxidant Capacities of Coho Salmon (Oncorhynchus kisutch) Alevins
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Ethical Statement
2.2. Experimental Diets
2.3. Animals and Feeding Management
2.4. Euthanasia Methods for Coho Salmon Alevins
2.5. Sampling Procedures and Analytical Methods
2.5.1. Growth Performance
2.5.2. Analysis of Feed Samples and Chemical Composition of Muscles
2.5.3. MK-4 Concentration Determination in Whole-Body Muscles and Liver Tissue, and Liver Anti-Oxidative Enzyme Activity Analysis
2.5.4. Statistical Analysis
3. Results
3.1. Growth Performance
3.2. Muscle Chemical Composition
3.3. MK-4 Concentrations in Whole-Body and Liver Tissue and Liver Anti-Oxidative Enzyme Activity
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Caires, R.; Sierra-Valdez, F.J.; Millet, J.R.; Herwig, J.D.; Roan, E.; Vásquez, V.; Cordero-Morales, J.F. Omega-3 Fatty Acids Modulate TRPV4 Function through Plasma Membrane Re-modeling. Cell Rep. 2017, 21, 246–258. [Google Scholar] [CrossRef] [PubMed]
- Ullah, M.; Rasool, F.; Khan, N.; Ali, S.; Sheikh, A.A. Antibiotic resistance and its gene profile in Escherichia coli isolated from diseased farm-raised carps in Punjab, Pakistan. Pak. Vet. J. 2023, 43, saa 470–476. [Google Scholar] [CrossRef]
- Alsulami, M.N.; El-Saadony, M.T. The Enhancing Effect of Bacterial Zinc Nanoparticles on Performance, Immune Response, and Microbial Load of Nile Tilapia (Oreochromis niloticus) by Reducing the Infection by Trichodina heterodentata. Pak. Vet. J. 2024, 44, 599–610. [Google Scholar] [CrossRef]
- Andrades, J.A.; Becerra, J.; Fernández-Llebrez, P. Skeletal deformities in larval, juvenile, and adult stages of cultured gilthead seabream (Sparus aurata L.). Aquaculture 1996, 141, 1–11. [Google Scholar] [CrossRef]
- Afonso, J.M.; Montero, D.; Robaina, L.; Astorga, N.; Izquierdo, M.S.; Ginés, R. Association of a lordosis-scoliosis-kyphosis deformity in gilthead seabream (Sparus aurata) with family structure. Fish Physiol. Biochem. 2000, 22, 159–163. [Google Scholar] [CrossRef]
- Boglione, C.; Gagliardi, F.; Scardi, M.; Cataudella, S. Skeletal descriptors and quality assessment in larvae and post-larvae of wild-caught and hatchery-reared gilthead seabream (Sparus aurata L. 1758). Aquaculture 2001, 192, 1–22. [Google Scholar] [CrossRef]
- Berillis, P. Skeletal deformities in seabreams: Understanding the genetic origin can improve production? J. Fish Sci. 2017, 11, 57–59. [Google Scholar] [CrossRef]
- Richard, N.; Fernández, I.; Wulff, T.; Hamre, K.; Cancela, L.; Conceição, L.E.C.; Gavaia, P.J. Dietary supplementation with vitamin K affects transcriptome and proteome of Senegalese sole, improving larval performance and quality. Mar. Biotechnol. 2014, 16, 522–537. [Google Scholar] [CrossRef]
- Izquierdo, M.; Domínguez, D.; Jiménez, J.I.; Saleh, R.; Hernández-Cruz, C.M.; Zamorano, M.J.; Hamre, K. Interaction between taurine, vitamin E, and vitamin C in microdiets for gilthead seabream (Sparus aurata) larvae. Aquaculture 2019, 498, 246–253. [Google Scholar] [CrossRef]
- Izquierdo, M.; Fernandez-Palacios, H. Nutritional requirements of marine fish larvae and broodstock. Cah. Options Méditerranéennes 1997, 22, 243–264. [Google Scholar]
- Izquierdo, M.S.; Socorro, J.; Arantzamendi, L.; Hernández-Cruz, C.M. Recent advances in lipid nutrition in fish larvae. Fish Physiol. Biochem. 2000, 22, 97–107. [Google Scholar] [CrossRef]
- National Research Council (NARC). Nutrient Requirements of Fish and Shrimp; National Academies Press: Washington, DC, USA, 2011. [Google Scholar]
- Graff, I.E.; Øyen, J.; Kjellevold, M.; Frøyland, L.; Gjesdal, C.G.; Almås, B.; Rosenlund, G.; Lie, Ø. Reduced bone resorption by intake of dietary vitamin D and K from tailor-made Atlantic salmon: A randomized intervention trial. Oncotarget 2016, 7, 69200–69215. [Google Scholar] [CrossRef]
- Furukawa, N.; Chen, X.; Asano, S.; Matsumoto, M.; Wu, Y.; Murata, K.; Takeuchi, A.; Tode, C.; Homma, T.; Koharazawa, R.; et al. Synthesis of New Vitamin K Derivatives with a Ketone Group at the C-1′ Position of the Side Chain and Their Conversion to Menaquinone-4. J. Mol. Struct. 2023, 1276, 134614. [Google Scholar] [CrossRef]
- Krossøy, C.; Waagbø, R.; Ørnsrud, R. Vitamin K in fish nutrition. Aquac. Nutr. 2011, 17, 585–594. [Google Scholar] [CrossRef]
- Dominguez, D.; Castro, P.; Lall, S.; Montero, D.; Zamorano, M.J.; Fontanillas, R.; Izquierdo, M. Effects of menadione sodium bisulphite (vitamin K3) supplementation of diets based on plant feed ingredients on growth and bone health of gilthead seabream (Sparus aurata) fingerlings. Aquac. Nutr. 2022, 1613030. [Google Scholar] [CrossRef]
- Graff, I.E.; Krossøy, C.; Gjerdevik, K.; Julshamn, K. Influence of dietary menadione nicotinamide bisulphite (vitamin K3) and phylloquinone (vitamin K1) on Atlantic salmon (Salmo salar L.) tissue levels. Aquac. Nutr. 2010, 16, 637–647. [Google Scholar] [CrossRef]
- Lall, S.P.; Lewis-McCrea, L.M. Role of nutrients in skeletal metabolism and pathology in fish—An overview. Aquaculture 2007, 267, 3–19. [Google Scholar] [CrossRef]
- Neacsu, C.D.; Grosch, M.; Tejada, M.; Winterpacht, A.; Paulsson, M.; Wagener, R.; Tagariello, A. Ucmaa (Grp-2) is required for zebrafish skeletal development: Evidence for a functional role of its glutamate γ-carboxylation. Matrix Biol. 2011, 30, 369–378. [Google Scholar] [CrossRef] [PubMed]
- Oldenburg, J.; Marivona, M.; Müller-Reible, C.; Waltzka, M. The vitamin K cycle. Vitam. Horm. 2008, 78, 35–62. [Google Scholar] [CrossRef]
- Rishavy, M.A.; Berkner, K.L. Vitamin K oxygenation, glutamate carboxylation, and processivity: Defining the three critical facets of catalysis by the vitamin K-dependent carboxylase. Adv. Nutr. 2012, 3, 135–148. [Google Scholar] [CrossRef]
- Poston, H.A. Relative effect of two dietary water-soluble analogues of menaquinone on coagulation and packed cell volume of blood of lake trout (Salvelinus namaycush). J. Fish Res. Board Can. 1976, 33, 1791–1793. [Google Scholar] [CrossRef]
- Taveekijakarn, P.; Miyazaki, T.; Matsumoto, M.; Arai, S. Studies on vitamin K deficiency in Amago salmon, Oncorhynchus rhodurus (Jordan & McGregor). J. Fish Dis. 1996, 19, 209–214. [Google Scholar]
- Udagawa, M. The Effect of Dietary Vitamin K (Phylloquinone and Menadione) Levels on the Vertebral Formation in Mummichog Fundulus heteroclitus. Fish Sci. 2001, 67, 104–109. [Google Scholar] [CrossRef][Green Version]
- Halver, J.E.; Hardy, R.W. The Vitamins. In Fish Nutrition, 3rd ed.; Academic Press: San Diego, CA, USA, 2002; pp. 62–141. [Google Scholar]
- Roy, P.K.; Lall, S.P. Vitamin K Deficiency Inhibits Mineralization and Enhances Deformity in Vertebrae of Haddock (Melanogrammus aeglefinus L.). Comp. Biochem. Physiol. B Biochem. Mol. Biol. 2007, 148, 174–183. [Google Scholar] [CrossRef]
- Shiau, S.-Y.; Liu, J.-S. Estimation of the Dietary Vitamin K Requirement of Juvenile (Penaeus chinensis) Using Menadione. Aquaculture 1994, 126, 129–135. [Google Scholar] [CrossRef]
- Fu, J.-H.; Xu, W.; Mai, K.; Zhang, W.-B.; Feng, X.-N.; Liufu, Z.-G. Effects of Dietary Menadione on the Activity of Antioxidant Enzymes in Abalone (Haliotis discus hannai Ino). Chin. J. Oceanol. Limnol. 2012, 30, 118–123. [Google Scholar] [CrossRef]
- Sivagurunathan, U.; Dominguez, D.; Tseng, Y.; Zamorano, M.J.; Prabhu, A.J.; Izquierdo, M. Deficiency and Excess in Dietary Vitamin K3 Negatively Affect Gilthead Seabream (Sparus aurata) Larvae Performance and Bone Health. Aquaculture 2023, 574, 739646. [Google Scholar] [CrossRef]
- Dai, T.; Zhang, X.; Li, M.; Tao, X.; Jin, M.; Sun, P.; Jiao, L. Dietary Vitamin K3 Activates Mitophagy, Improves Antioxidant Capacity, Immunity, and Affects Glucose Metabolism in L. vannamei. Food Funct. 2022, 13, 6362–6372. [Google Scholar] [CrossRef]
- Abdelhamid, A.F.; Gewida, A.G.A.; El-Sayed, A.F.M.; Badran, M.F. Impacts of Different Levels of Vitamin K on the Growth Performance, Hematological Parameters, and Immunological Response of Juvenile Nile Tilapia (Oreochromis niloticus). Aquac. Int. 2024, 32, 477–488. [Google Scholar] [CrossRef]
- Yuan, J.; Feng, L.; Jiang, W.D.; Liu, Y.; Jiang, J.; Li, S.H.; Zhou, X.Q. Effects of Dietary Vitamin K Levels on Growth Performance, Enzyme Activities and Antioxidant Status in the Hepatopancreas and Intestine of Juvenile Jian Carp (Cyprinus carpio var. Jian). Aquac. Nutr. 2016, 22, 352–366. [Google Scholar] [CrossRef]
- Cheng, L.; Zhang, W.; Lin, S.; Xu, W.; Mai, K. Effects of Dietary Vitamin K on Growth Performances, Blood Coagulation Time, and Menaquinone-4 (MK-4) Concentration in Tissues of Juvenile Large Yellow Croaker (Pseudosciaena crocea). Aquac. Res. 2015, 46, 1269–1275. [Google Scholar] [CrossRef]
- Wei, X.; Hang, Y.; Li, X.; Hua, X.; Cong, X.; Yi, W.; Guo, X. Effects of Dietary Vitamin K3 Levels on Growth, Coagulation, Calcium Content, and Antioxidant Capacity in Largemouth Bass(Micropterus salmoides). Aquac. Fish. 2023, 8, 159–165. [Google Scholar] [CrossRef]
- Hart, J.L. Pacific Fishes of Canada. Bull. Fish. Res. Board Can. 1973, 180, 1–740, reprinted in 1975, 1980, 1988. [Google Scholar]
- FAO. Brief to The State of World Fisheries and Aquaculture 2024; Blue Transformation in Action; FAO: Rome, Italy, 2024. [Google Scholar] [CrossRef]
- Swanson, D.; Block, R.; Mousa, S.A. Omega-3 Fatty Acids EPA and DHA: Health Benefits throughout Life. Adv. Nutr. 2012, 3, 1–7. [Google Scholar] [CrossRef]
- Tsoupras, A.; Brummell, C.; Kealy, C.; Vitkaitis, K.; Redfern, S.; Zabetakis, I. Cardio-Protective Properties and Health Benefits of Fish Lipid Bioactives; The Effects of Thermal Processing. Mar. Drugs 2022, 20, 187. [Google Scholar] [CrossRef]
- Kiranpreet, K.; Trond, M.K.; Tibiabin, B.S.; Lena, B. Effects of Antarctic Krill Products on Feed Intake, Growth Performance, Fillet Quality, and Health in Salmonids. Aquac. Nutr. 2022, 2022, 3170854. [Google Scholar] [CrossRef]
- Yu, H.-R.; Guo, M.-J.; Yu, L.-Y.; Li, L.-Y.; Wang, Q.-H.; Li, F.-H.; Zhang, Y.-Z.; Zhang, J.-Y.; Hou, J.-Y. Effects of Dietary Riboflavin Supplementation on the Growth Performance, Muscle composition and Anti-Oxidative Capacity of Coho Salmon (Oncorhynchus kisutch) Post-Smolts. Animals 2022, 12, 3218. [Google Scholar] [CrossRef] [PubMed]
- Paulus, M.C.; Drent, M.; Kouw, I.W.K.; Balvers, M.G.J.; Bast, A.; van Zanten, A.R.H. Vitamin K: A Potential Missing Link in Critical Illness—A Scoping Review. Crit. Care 2024, 28, 212. [Google Scholar] [CrossRef]
- Yu, H.; Sattanathan, G.; Yu, L.; Li, L.; Xiao, Y. Impact of Nutritional Tea Polyphenols on Growth, Feed Efficiency, Biochemical Traits, Antioxidant Capacity, Haematological Parameters and Immunity in Coho Salmon (Oncorhynchus kisutch). Animals 2024, 14, 2104. [Google Scholar] [CrossRef]
- AOAC (Association of Official Analytical Chemists). Official Methods of Analysis, 15th ed.; AOAC: Arlington, VA, USA, 1990. [Google Scholar]
- Ostermeyer, U.; Schmidt, T. Determination of vitamin K in edible part of fish by high-performance liquid chromatography. Eur. Food Res. Technol. 2001, 212, 518–528. [Google Scholar] [CrossRef]
- Grahl-Madsen, E.; Lie, Ø. Effects of different levels of vitamin K in diets for cod (Gadus morhua). Aquaculture 1997, 151, 269–274. [Google Scholar] [CrossRef]
- Duan, Y.H.; Zhu, X.M.; Han, D.; Yang, Y.X.; Jin, J.Y.; Xie, S.Q. Dietary vitamin K requirement of juvenile gibel carp (Carassius auratus gibelio). Acta Hydrobiol. Sin. 2013, 37, 8–15. [Google Scholar]
- Grisdale-Helland, B.; Helland, S.J.; Åsgård, T. Problems associated with the present use of menadione sodium bisulfite and vitamin A in diets for Atlantic salmon. Aquaculture 1991, 92, 351–358. [Google Scholar] [CrossRef]
- Krossøy, C.; Waagbø, R.; Fjelldal, P.-G.; Wargelius, A.; Lock, E.-J.; Graff, I.E.; Ørnsrud, R. Dietary menadione nicotinamide bisulphite (vitamin K3) does not affect growth or bone health in first-feeding fry of Atlantic salmon (Salmo salar L.). Aquac. Nutr. 2009, 15, 532–540. [Google Scholar] [CrossRef]
- Graff, I.E.; Waagbø, R.; Fivelstad, S.; Vermeer, C.; Lie, Ø.; Lundebye, A.K. A multivariate study on the effects of dietary vitamin K, vitamin D3, and calcium, and dissolved carbon dioxide on growth, bone minerals, vitamin status, and health performance in smolting Atlantic salmon (Salmo salar L.). J. Fish Dis. 2002, 25, 93–106. [Google Scholar] [CrossRef]
- Vera, L.M.; Hamre, K.; Espe, M.; Hemre, G.I.; Skjærven, K.; Lock, E.J.; Prabhu, A.J.; Leeming, D.; Migaud, H.; Tocher, D.R.; et al. Higher dietary micronutrients are required to maintain optimal performance of Atlantic salmon (Salmo salar) fed a high plant material diet during the full production cycle. Aquaculture 2020, 528, 735551. [Google Scholar] [CrossRef]
- Tampo, Y.; Yonaha, M. Enzymatic and molecular aspects of the antioxidant effect of menadione in hepatic microsomes. Arch. Biochem. Biophys. 1996, 334, 163–174. [Google Scholar] [CrossRef]
- Kohen, R.; Nyska, A. Oxidation of biological systems: Oxidative stress phenomena, antioxidants, redox reactions, and methods for their quantification. Toxicol. Pathol. 2002, 30, 620–650. [Google Scholar] [CrossRef]
- Fang, Y.Z.; Yang, S.; Wu, G. Free radicals, antioxidants, and nutrition. Nutrition 2002, 18, 872–879. [Google Scholar] [CrossRef]
- Mukai, K.; Morimoto, H.; Okauchi, Y.; Nagaoka, S.-I. Kinetic study of reactions between tocopheroxyl radicals and fatty acids. Lipids 1993, 28, 753–756. [Google Scholar] [CrossRef]
- Sahin, M.A.; Yucel, O.; Guler, A.; Doganci, S.; Jahollari, A.; Cingoz, F.; Arslan, S.; Gamsizkan, M.; Yaman, H.; Demirkilic, U. Is there any cardioprotective role of taurine during cold ischemic period following global myocardial ischemia? J. Cardiothorac. Surg. 2011, 6, 31. [Google Scholar] [CrossRef] [PubMed]



| Ingredients | Dietary Vitamin K3 Levels (mg/kg) | ||||||
|---|---|---|---|---|---|---|---|
| 0.16 | 5.25 | 10.22 | 14.93 | 20.51 | 40.09 | 59.87 | |
| Casein 1 | 38.00 | 38.00 | 38.00 | 38.00 | 38.00 | 38.00 | 38.00 |
| Gelatin 1 | 12.00 | 12.00 | 12.00 | 12.00 | 12.00 | 12.00 | 12.00 |
| Dextrin 1 | 28.00 | 28.00 | 28.00 | 28.00 | 28.00 | 28.00 | 28.00 |
| Corn oil 1 | 6.00 | 6.00 | 6.00 | 6.00 | 6.00 | 6.00 | 6.00 |
| Fish oil 1 | 3.00 | 3.00 | 3.00 | 3.00 | 3.00 | 3.00 | 3.00 |
| α-cellulose 1 | 8.00 | 8.00 | 8.00 | 8.00 | 8.00 | 8.00 | 8.00 |
| Mono-calcium phosphate 1 | 1.50 | 1.50 | 1.50 | 1.50 | 1.50 | 1.50 | 1.50 |
| Mineral premix 2 | 2.50 | 2.50 | 2.50 | 2.50 | 2.50 | 2.50 | 2.50 |
| Vitamin premix (vitamin K3 free) 3 | 1.00 | 1.00 | 1.00 | 1.00 | 1.00 | 1.00 | 1.00 |
| Vitamin K3 (mg/kg) | 0.00 | 5.00 | 10.00 | 15.00 | 20.00 | 40.00 | 60.00 |
| Proximate nutrient composition | |||||||
| Moisture | 10.37 | 10.28 | 10.49 | 10.22 | 9.91 | 10.44 | 10.31 |
| Crude protein | 45.12 | 45.15 | 44.90 | 44.97 | 45.02 | 44.87 | 45.23 |
| Crude lipid | 10.32 | 10.17 | 10.45 | 10.27 | 10.51 | 10.39 | 10.25 |
| Ash | 5.53 | 5.67 | 5.60 | 5.59 | 5.66 | 5.62 | 5.71 |
| Gross energy (MJ/kg) | 20.86 | 21.01 | 21.11 | 21.23 | 20.97 | 21.20 | 21.15 |
| Vitamin K3 (mg/kg) | 0.16 | 5.25 | 10.22 | 14.93 | 20.51 | 40.09 | 59.87 |
| Diet Groups | Dietary Vitamin K3 Levels (mg/kg) | p-Value | ||||||
|---|---|---|---|---|---|---|---|---|
| 0.16 | 5.25 | 10.22 | 14.93 | 20.51 | 40.09 | 59.87 | ||
| SR (%) | 95.33 c ± 0.88 | 96.00 bc ± 0.58 | 96.67 abc ± 0.88 | 97.33 abc ± 0.88 | 98.33 a ± 0.33 | 97.67 ab ± 0.67 | 98.33 a ± 0.33 | 0.05 |
| IBW (g) | 0.32 ± 0.03 | 0.33 ± 0.04 | 0.32 ± 0.02 | 0.32 ± 0.04 | 0.33 ± 0.03 | 0.33 ± 0.02 | 0.33 ± 0.03 | 0.53 |
| FBW (g) | 4.32 e ± 0.03 | 4.44 d± 0.04 | 4.59 c ± 0.02 | 4.90 a ± 0.04 | 4.87 a ± 0.03 | 4.83 ab ± 0.02 | 4.76 b ± 0.03 | <0.001 |
| WG (g) | 4.00 e ±0.03 | 4.12 d ±0.04 | 4.27 c ±0.02 | 4.58 a ± 0.04 | 4.55 a ±0.03 | 4.51 ab ±0.02 | 4.44 b ± 0.03 | <0.001 |
| SGR (%/d) | 3.09 f ± 0.01 | 3.11 e± 0.01 | 3.16 d ± 0.01 | 3.24 a ± 0.01 | 3.22 ab ± 0.01 | 3.21 bc ± 0.01 | 3.19 c ± 0.01 | <0.001 |
| FCR (%) | 1.27 a ± 0.02 | 1.22 b ± 0.01 | 1.17 c ± 0.01 | 1.08 d ± 0.01 | 1.08 d ± 0.01 | 1.10 d ± 0.01 | 1.11 d ± 0.01 | <0.001 |
| K (%) | 1.66 ± 0.05 | 1.71 ± 0.03 | 1.62 ± 0.04 | 1.60 ± 0.04 | 1.61 ± 0.05 | 1.65 ± 0.04 | 1.73 ± 0.03 | 0.28 |
| HSI (%) | 1.16 ± 0.03 | 1.10 ± 0.04 | 1.14 ± 0.03 | 1.08 ± 0.04 | 1.15 ± 0.07 | 1.16 ± 0.04 | 1.11 ± 0.05 | 0.79 |
| VSI (%) | 7.68 ± 0.07 | 7.86 ± 0.06 | 7.69 ± 0.09 | 7.74 ± 0.04 | 7.78 ± 0.07 | 7.49 ± 0.11 | 7.63 ± 0.09 | 0.09 |
| Dietary Vitamin K3 Levels (mg/kg) | Moisture | Crude Protein | Crude Lipid | Ash |
|---|---|---|---|---|
| 0.16 | 73.13 ± 0.26 | 19.65 ± 0.12 | 3.66 ± 0.12 | 3.28 ± 0.10 |
| 5.25 | 72.93 ± 0.34 | 19.72 ± 0.18 | 3.61 ± 0.15 | 3.36 ± 0.09 |
| 10.22 | 73.03 ± 0.42 | 19.74 ± 0.14 | 3.55 ± 0.15 | 3.39 ± 0.12 |
| 14.93 | 72.74 ± 0.57 | 19.55 ± 0.19 | 3.63 ± 0.10 | 3.48 ± 0.07 |
| 20.51 | 73.01 ± 0.50 | 19.39 ± 0.26 | 3.54 ± 0.11 | 3.51 ± 0.07 |
| 40.09 | 73.23 ± 0.53 | 19.58 ± 0.21 | 3.75 ± 0.09 | 3.56 ± 0.10 |
| 59.87 | 72.88 ± 0.32 | 19.53 ± 0.17 | 3.71 ± 0.11 | 3.54 ± 0.08 |
| p-Value | 0.99 | 0.86 | 0.86 | 0.33 |
| Dietary Vitamin K3 Levels (mg/kg) | Whole-Body MK-4 Concentration (ng/g) | Liver MK-4 Concentration (ng/g) |
|---|---|---|
| 0.16 | 5.14 g ± 0.17 | 33.76 d ± 0.73 |
| 5.25 | 21.97 f ± 0.39 | 257.65 c ± 4.29 |
| 10.22 | 33.58 e ± 0.74 | 362.58 b ± 6.41 |
| 14.93 | 42.99 d ± 0.70 | 423.88 a ± 3.22 |
| 20.51 | 47.65 c ± 0.41 | 433.07 a ± 4.82 |
| 40.09 | 64.12 b ± 0.73 | 437.05 a ± 3.72 |
| 59.87 | 75.24 a ± 0.95 | 435.85 a ± 7.33 |
| p-Value | <0.001 | <0.001 |
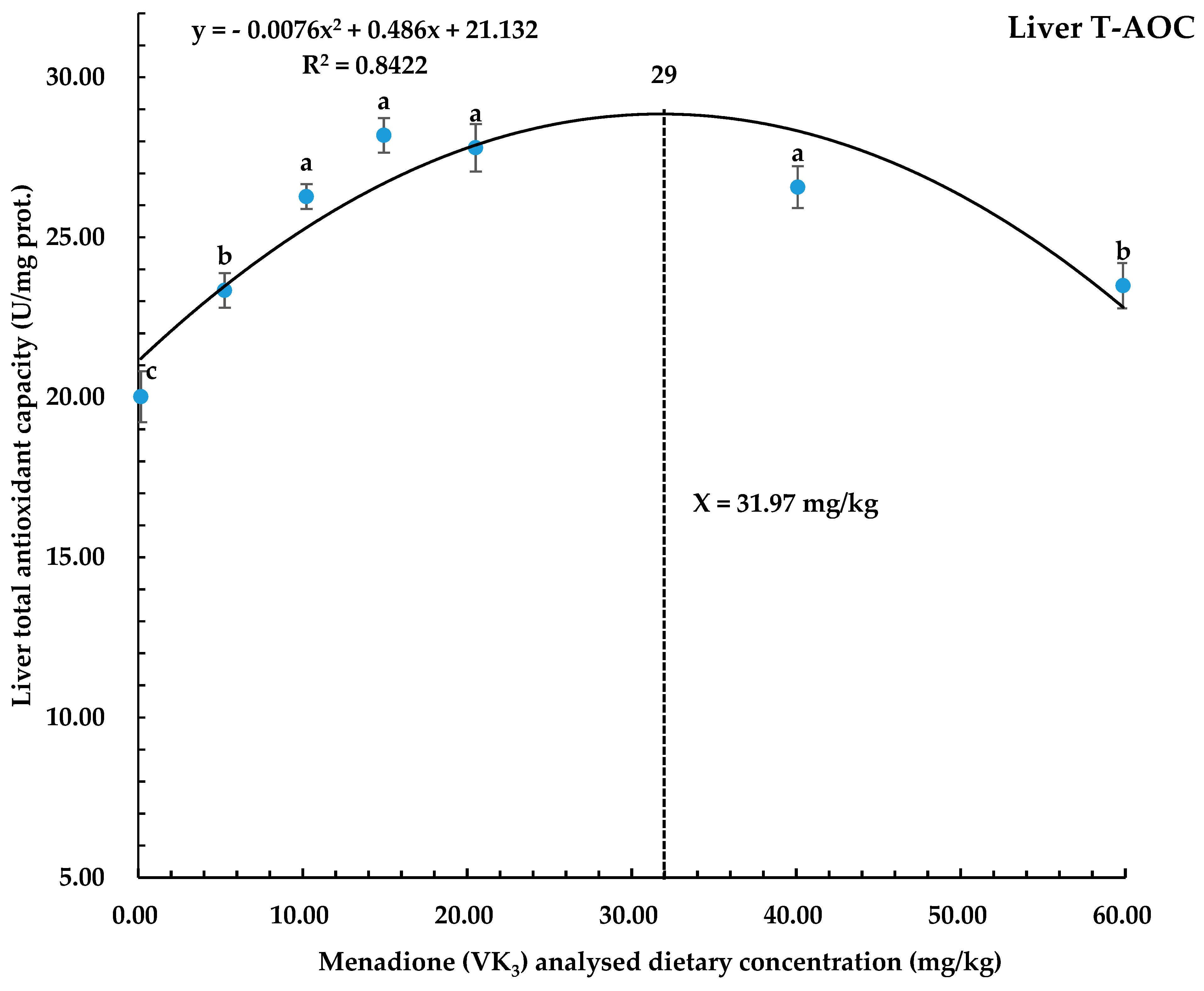
| Dietary Vitamin K3 Levels (mg/kg) | T-AOC (U/mg prot.) | T-SOD (U/mg prot.) | CAT (U/mg prot.) | MDA (nmol/mg prot.) |
|---|---|---|---|---|
| 0.16 | 20.02 c ± 0.80 | 118.90 d ± 5.01 | 7.38 f ± 0.10 | 11.52 a ± 0.17 |
| 5.25 | 23.34 b ± 0.54 | 143.86 c ± 7.34 | 9.50 e ± 0.16 | 9.46 b ± 0.14 |
| 10.22 | 26.27 a ± 0.39 | 173.77 ab ± 5.44 | 12.07 b ± 0.15 | 7.71 d ± 0.15 |
| 14.93 | 28.19 a ± 0.54 | 182.22 a ± 5.95 | 13.33 a ± 0.18 | 6.65 e ± 0.09 |
| 20.51 | 27.80 a ± 0.74 | 176.70 ab ± 6.05 | 11.23 c± 0.29 | 6.80 e ± 0.10 |
| 40.09 | 26.57 a ± 0.66 | 168.30 ab ± 6.37 | 10.50 d ± 0.16 | 7.35 d ± 0.12 |
| 59.87 | 23.49 b ± 0.71 | 156.68 bc ± 6.97 | 10.23 d ± 0.23 | 8.63 c ± 0.11 |
| p-Value | <0.001 | <0.001 | <0.001 | <0.001 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, H.; Yu, L.; Rahman, A.; Govindharajan, S.; Li, L.; Yu, H.; Waqas, M. Effects of Varying Dietary Concentrations of Menadione Nicotinamide Bisulphite (VK3) on Growth Performance, Muscle Composition, Liver and Muscle Menaquinone-4 Concentration, and Antioxidant Capacities of Coho Salmon (Oncorhynchus kisutch) Alevins. Biology 2025, 14, 447. https://doi.org/10.3390/biology14040447
Zhang H, Yu L, Rahman A, Govindharajan S, Li L, Yu H, Waqas M. Effects of Varying Dietary Concentrations of Menadione Nicotinamide Bisulphite (VK3) on Growth Performance, Muscle Composition, Liver and Muscle Menaquinone-4 Concentration, and Antioxidant Capacities of Coho Salmon (Oncorhynchus kisutch) Alevins. Biology. 2025; 14(4):447. https://doi.org/10.3390/biology14040447
Chicago/Turabian StyleZhang, Han, Leyong Yu, Abdur Rahman, Sattanathan Govindharajan, Lingyao Li, Hairui Yu, and Muhammad Waqas. 2025. "Effects of Varying Dietary Concentrations of Menadione Nicotinamide Bisulphite (VK3) on Growth Performance, Muscle Composition, Liver and Muscle Menaquinone-4 Concentration, and Antioxidant Capacities of Coho Salmon (Oncorhynchus kisutch) Alevins" Biology 14, no. 4: 447. https://doi.org/10.3390/biology14040447
APA StyleZhang, H., Yu, L., Rahman, A., Govindharajan, S., Li, L., Yu, H., & Waqas, M. (2025). Effects of Varying Dietary Concentrations of Menadione Nicotinamide Bisulphite (VK3) on Growth Performance, Muscle Composition, Liver and Muscle Menaquinone-4 Concentration, and Antioxidant Capacities of Coho Salmon (Oncorhynchus kisutch) Alevins. Biology, 14(4), 447. https://doi.org/10.3390/biology14040447









