Simple Summary
In this study, we present an analysis of SNP variants of six uncoupling protein genes in the Yakut population living in the coldest region of Siberia (minimum temperature −71.2 °C) in the context of human adaptation to cold climates. Uncoupling protein 1 (UCP1) is a transmembrane protein that may play an important role in thermogenesis; the functional role of other UCPs is clearly not known. Based on our results, we suggest that uncoupling proteins UCP1 and UCP3 are involved in human adaptation to a cold climate by increasing heat production. Thus, understanding the molecular details of the functional role of the UCPs could pave the way for the development of novel therapeutics to curb metabolic diseases such as obesity and diabetes.
Abstract
Six isoforms of uncoupling proteins (UCPs) exist, spanning from UCP1 to UCP6. A precise physiological function has only been established for UCP1, which is involved in non-shivering thermogenesis, but the functions of other UCPs are still not fully defined. Therefore, the purpose of the present study is to search for indications of the involvement of nine polymorphic variants of UCP1-6 genes in human adaptation to cold climates using four criteria: (1) the presence of associations of polymorphic variants of UCP genes with levels of thyroid-stimulating hormone, free triiodothyronine, and free thyroxine; (2) the presence of associations of polymorphic variants of UCP genes with changes in thyroid homeostasis (SPINA); (3) the presence of associations of polymorphic variants of UCP genes with body surface area; (4) the presence of signals of directional selection to cold climate for polymorphic variants of UCP genes. As a result of the evaluation, the highest scores for cold adaptation traits were recorded for polymorphic variants rs3811787 of the UCP1 gene and rs1800849 of the UCP3 gene. We suggest that the results obtained indicate the importance of uncoupling proteins UCP1 and UCP3 in human adaptation to cold through processes of non-shivering and shivering thermogenesis.
1. Introduction
Uncoupling proteins (UCPs) are transmembrane proteins that mediate specific metabolite exchange between the cell cytoplasm and mitochondrial matrix [1]. Currently, proteins of this family are represented by six isoforms (UCP1–UCP6), which are present in various tissues [2,3,4]. Among them, UCP1 is the most well-studied isoform, which is mainly expressed in brown adipose tissue (BAT) [5]. In contrast, the second isoform, UCP2, is found in a variety of tissues, including white adipose tissue, skeletal muscle, the heart, and immune cells [6,7,8,9,10,11]. UCP3 is predominantly expressed in skeletal muscle and the heart [12], while UCP4 and UCP5 are more clearly detected in brain neurons [13,14]. UCP6 mRNA was found to be expressed mainly in mouse kidneys [15]. The existence of UCP6 mRNA in humans was predicted in the seminal ducts, testis, seminal vesicles, and prostate gland [16]. A precise physiological function has only been established for UCP1, which is involved in the non-shivering thermogenesis of BAT [17,18], but the functions of other UCPs are still not fully defined.
It is known that thyroid hormones, such as triiodothyronine (T3) and thyroxine (T4), could be involved in the regulation of UCP1 gene expression during non-shivering thermogenesis of BAT [19,20,21,22]. It has been demonstrated that cold stress in brown adipocytes, through the deiodination of T4 to T3, increases concentrations of active T3, which penetrates the nucleus and forms a complex with its thyroid hormone nuclear receptors (TRs), and then this T3-TR complex binds to thyroid response elements (TRE) on the UCP1 gene stimulating its expression [19,20,21,22]. The expression of other UCP genes, such as UCP2 and UCP3, is regulated by the same mechanism. In rodents, UCP2 and UCP3 expression have been shown to be enhanced by T3 in muscles [23,24]. A direct effect of T3 on UCP2 and UCP3 mRNA expression in skeletal muscle was demonstrated in vitro in human primary cultures [25]. The results of in vivo studies by de Lange et al. [26] showed that T3 regulates UCP3 mRNA levels in mitochondria, and UCP3 could potentially act as a molecular determinant in the regulation of resting metabolic rate by T3. However, the role of thyroid hormones in the regulation of other uncoupling protein genes, UCP4, UCP5, and UCP6, is currently unknown.
On the other hand, it is known that thyroid hormones play an important role in regulating the basal metabolic rate [27]. Previous studies have shown that the basal metabolic rates of indigenous peoples in Siberia, such as the Yakuts, Evenks, and Buryats, are higher than predicted values for European populations [28,29,30,31]. This is thought to be due to thyroid hormones [32]. It is assumed that for the survival of the organism during cold stress, the dynamics of thyroid hormones may shift towards a type 2 allostasis, characterized by an increase in circulating T3 levels, leading to a higher basal metabolic rate to maintain thermogenic mechanisms [33,34]. Currently, it is proposed to use the parameters of thyroid homeostasis, SPINA (SPINA-GD and SPINA-GT), for the differential diagnosis of an allostasis reaction, including cold stress [35]. SPINA-GD indicates the total activity of peripheral deiodinases, while SPINA-GT shows the maximum production of T4 by the thyroid gland per unit of time [36]. Previously, changes in the homeostasis of the pituitary–thyroid axis (type 2 allostasis) at low atmospheric temperatures (−47 °C to −11 °C) were detected in 70% of the individuals living in the extremely cold climatic conditions of Eastern Siberia [37].
Additionally, indigenous northern populations in Eurasia and North America tend to have more compact physiques, with relatively longer torsos and shorter limbs, as well as a larger body weight compared to their shorter height, i.e., smaller body surface area (BSA) [38,39,40,41,42]. Based on studies of the effects of cold air on humans, common morphological features associated with adaptation to cold have been identified among indigenous people of temperate and polar climates [30,43,44,45]. These morphological features of the physique allow the body to keep warm for a longer time, since at a lower BSA-to-weight ratio, a slower decrease in body temperature is observed during exposure to cold [46,47].
Recently, a number of studies have been conducted aiming to discover genes with adaptive variants for cold climate tolerance. Several studies have identified associations of some variants of UCP genes with human adaptation to cold climates. Hancock et al. [48] hypothesized that such polymorphic variants as UCP1-rs1800592, UCP2-rs659366, and UCP3-rs1800849, which are associated with increased expression of these genes, may be involved in human adaptation to cold climates. Based on the analysis of the correlation of allele frequencies in 52 world populations with winter climate variables for polymorphic variants, they concluded that the rs1800592 UCP1 and rs1800849 UCP3 variants have a significant correlation with winter climate [48]. Another group of researchers studied allele frequencies of 28 genes potentially associated with adaptation to cold climate in populations of Northern Eurasia [49]. As a result of these studies, a significant association of the rs1800592 polymorphism of the UCP1 gene with climatic (temperature) and with geographic (latitude and longitude) variables was established, but no signals of directional selection were found [49]. A study focused on identifying cold adaptation traits in ancient hunter-gatherers in Japan (the Jomon people) revealed associations between four SNPs in the UCP1 gene (rs3113195, rs12502572, rs1800592, rs4956451) and the non-shivering thermogenesis phenotype [50]. It has been hypothesized that East Eurasian hunter-gatherers adapted to cold climates through BAT thermogenesis mediated by UCP1 [50]. In addition, associations of polymorphic variants of UCP1 (rs3811787) and UCP3 (rs1800849) genes with the levels of circulating blood hormones leptin and irisin were previously found in residents of Eastern Siberia [51,52]. Based on the association analysis, a potential role of the UCP1 gene in the leptin-mediated non-shivering thermogenesis pathway, as well as a role of the UCP3 gene in shivering thermogenesis, was suggested [51,52].
In this regard, in the present work, for the first time, the search for signs of participation of nine polymorphic variants of UCP1, UCP2, UCP3, UCP4, UCP5, and UCP6 genes in human adaptation to cold climate was carried out by evaluating the strength of influence using four different criteria: (1) the presence of associations between polymorphic variants of UCP genes and levels of hormones of the pituitary–thyroid axis (thyroid-stimulating hormone—TSH, free triiodothyronine—FT3, and free thyroxine—FT4); (2) the presence of associations between polymorphic variants of UCP genes and changes in thyroid homeostasis (SPINA); (3) the presence of associations between polymorphic variants of UCP genes and BSA; (4) the presence of signals of directional selection for cold climate for polymorphic variants of UCP genes.
2. Materials and Methods
2.1. Subjects
The sample consisted of 279 individuals (185 females and 94 males), and the mean age of the participants was 19.73 ± 1.99 years. No participants had any health complaints at the time of the study, and they filled out a questionnaire themselves, in which they indicated their sex, ethnicity, age, chronic diseases, and experience of taking antidepressants. All participants provided written informed consent to participate in the study. The study was approved by the local biomedical ethics committee of the Yakutsk Scientific Center for Complex Medical Problems of the Siberian Branch of the Russian Academy of Medical Sciences, Yakutsk, Russia (Protocol No. 45, 12 October 2017).
2.2. Anthropometric Parameters
Anthropometric parameters (body weight in kilograms and height in centimeters) were measured in all participants (n = 279) using standardized methods. The body mass index was calculated by dividing body weight by the square of height. For association analysis of polymorphic variants of UCP genes with changes in thyroid homeostasis (SPINA) the sample (n = 279) was divided into three groups according to body mass index categories [53]: underweight (≤18.49 kg/m2), normal weight (18.5–24.99 kg/m2), and overweight/obese (≥25 kg/m2) (Supplementary Materials, Chapter S4). BSA (n = 279) was calculated according to Haycock’s formula [54].
2.3. Hormonal Measurement
Venous blood for the study was collected in the morning after an 8 h fast from 94 participants. Levels of TSH (µU/mL), FT3 (pmol/L), and FT4 (pmol/L) in serum were detected by time-resolved immunofluorescence analysis using DELFIA hTSH Ultra, DELFIA Free Thyroxine, and DELFIA Free Triiodothyronine kits (PerkinElmer Inc., Waltham, MA, USA), respectively. The concentrations of the three hormones in the samples were measured at a wavelength of 450 nm on a VICTOR X5 Multilabel Plate Reader (PerkinElmer Inc., USA). The reference values according to the kit recommendations were TSH 0.63–4.2 µU/mL, FT3 4.6–7.8 pmol/L, and FT4 9.8–16.8 pmol/L. TSH levels in all study participants (n = 94) were within normal limits (2.20 ± 0.83 μU/mL); one individual was found to have elevated levels of FT3 (7.96 pmol/L) and FT4 (17.2 pmol/L), and five individuals showed elevated levels of FT4 (17 pmol/L to 18.8 pmol/L). For further analysis, the sample was normalized, and the males with elevated levels of FT3 and FT4 were excluded (n = 6).
2.4. PCR-RFLP Analysis
Genomic DNA was isolated from blood using phenol-chloroform extraction. Genotyping of 9 polymorphisms of UCP1-6 genes was performed for all participants (n = 279) using the polymerase chain reaction-restriction fragment length polymorphism (PCR-RFLP) assay. Data, including primer sequence, annealing temperature, PCR product size, and restriction enzymes, are presented in Table 1. PCR was performed on a Bio Rad T100 thermocycler (Bio-Rad Laboratories, Inc., Berkeley, CA, USA). Genotype and allele frequencies of 9 polymorphic variants of UCP1-6 uncoupling protein genes are presented in Supplementary Materials, Table S1.

Table 1.
List of primer sequences, annealing temperature, and allelic profiles of the studied SNPs.
2.5. SPINA Parameters
Thyroid homeostasis assessment parameters were calculated for 94 individuals using the SPINA Thyr software (version 4.1.0, SPINA Thyr, RRID:SCR_014352, doi 10.5281/zenodo.3596049). The software is based on a mathematical model of thyroid hormone homeostasis. The results of the evaluation studies and the algorithms underlying the mathematical theory were published in several papers [55,56,57]. Reference values according to SPINA Thyr program recommendations were SPINA-GT—1.4–8.7 pmol/s, and SPINA-GD—20–40 nmol/s.
2.6. Search for Natural Selection Signals for Polymorphic Variants of UCP Genes
Data on the frequencies of the studied alleles were obtained from the “1000 Genomes Project” open database [58] for the following 31 populations: Esan (Nigeria), Gambians (Gambia), Luhya (Webuye, Kenya), Mende (Sierra Leone), Yoruba (Ibadan, Nigeria), African Caribbean (Barbados), Finns (Finland), Britons (England and Scotland), Iberians (Spain), Tuscans (Italy), Bengalis (Bangladesh), Gujarati Indians (USA), Indian Telugu (UK), Punjabis (Lahore, Pakistan), Sri Lankan Tamils (UK), Chinese Dai (Xishuangbanna, China), Han Chinese (Beijing, China), Han Chinese South (China), Japanese (Tokyo, Japan), Vietnamese (Ho Chi Minh City, Vietnam), Mexicans (United States, Mexico), Puerto Ricans (Puerto Rico), Colombians (Medellin, Colombia), Peruvians (Lima, Peru), Estonians (Estonia), Dutch (Netherlands), indigenous Oceanians (Oceania), Koreans (Republic of Korea), Swedes (Sweden), Qataris (Qatar), Danes (Denmark). Additionally, from the published studies [49,59,60], the data on the allele frequencies of rs1800849 of the UCP3 gene were obtained for 8 populations: Nivkhi (Russia), Koryak (Russia), Chukchi (Russia), Buryats (Russia), Khanty (Russia), Kets (Russia), Brazilians (Brazil), and Turks (Turkey). The map illustrating allele frequency distribution in populations across North and South America, Eurasia, and Africa was created using Surfer 12.0 software (Golden Software, Golden, CO, USA).
2.7. Evidence of Involvement of Polymorphic Variants of UCP Genes in Adaptation to Cold Climate
For the first time, a method utilizing four criteria has been developed to assess the role of polymorphic variants in UCP genes in human adaptation to cold environments. The following criteria were applied: (1) the presence of associations of polymorphic variants of UCP genes with the levels of hormones of the pituitary–thyroid axis (TSH, FT3 and FT4) (n = 94), (2) the presence of associations of polymorphic variants of UCP genes with changes in thyroid homeostasis (SPINA) (n = 61), (3) the presence of associations of polymorphic variants of UCP genes with BSA (n = 279), (4) the presence of directional selection natural signals for polymorphic variants of UCP genes (n = 279). Each criterion was evaluated based on the strength of its influence, expressed in points (ranging from 0 to 3), according to the significance level of the p-value determined by the results of the association analyses (Table 2). The maximum total score for all four criteria was 21 points. To determine the minimum threshold, we used a cut-score percentage method. In this method, we fixed the 25th percentage as the minimum threshold. The study design is presented in the Supplementary Materials (Chapter S1, Figure S1).

Table 2.
Strengths of influence (in points) of four parameters on human adaptation to the cold.
2.8. Statistical Analysis
The results were analyzed using the computer software for statistical data editing, Statistica 13.5 (TIBCO Software Inc., Santa Clara, CA, USA). Values of p ≤ 0.05 were considered statistically significant. Quantitative results are reported as the mean ± standard deviation. Comparative analysis between the three genotypes was performed using the Kruskal–Wallis H test. Comparative analysis between two genotypes and alleles was carried out using the Mann–Whitney U test.
3. Results
3.1. Assessment of the Contribution of Polymorphic Variants of UCP Genes in Human Adaptation to Cold
For the first time, the combined approach for analyzing the contribution of polymorphic variants of UCP genes in human adaptation to cold was developed. This approach was based on four criteria that can show signs of involvement of genes and their variants in cold adaptation. For each criterion, statistical analyses were performed for nine polymorphic variants of UCP1-6 genes (Supplementary Materials, Chapters S2–S6, Tables S1–S5, and Figure S2). According to the results of these analyses and the evaluation of the strength of the criteria influence on cold adaptation, certain scores (from 0 to 3) were assigned for the nine polymorphisms of UCP genes (Table 3). Based on the minimum threshold of six points, two gene variants, UCP1 (rs3811787) and UCP3 (rs1800849), were found to be most involved in the process of adaptive thermogenesis, as shown in Table 3. The results indicate the involvement of these variants in human adaptation to a cold climate. The rs3811787 (−412A > C) polymorphism of the UCP1 gene is located at the − 412 position upstream of the transcription start site, and it is in moderate linkage disequilibrium (r2 = 0.53) with the second polymorphism rs1800592 [61]. Perhaps for this reason, the frequencies of these two polymorphisms of the UCP1 gene (rs1800592 and rs3811787) are similar, and both have positive selection signals for cold climates. The rs1800849 (−55C/T) polymorphism of the UCP3 gene is situated in the promoter region, and the second polymorphism, rs2075577 (+2546T/C) of this gene is located in exon 3 [62]. We assume that these two polymorphisms are independent of each other, and therefore, for the rs2075577 polymorphism, no associations or natural selection signals similar to those observed for rs1800849 were found.

Table 3.
Assessment of the contribution of polymorphic variants of UCP genes to human adaptation to cold.
Some other polymorphic variants of the UCP genes scored less than six points, indicating their indirect involvement in human adaptation to cold climates (Table 3). The polymorphic variant rs1010978 of the UCP5 gene was associated with the first criterion (FT3) and was assigned two points. According to the other criteria, there were no associations found for this variant of the UCP5 gene. Additionally, the search for natural selection signals for the T rs1010978 allele of the UCP5 gene revealed that the high frequency of this allele is characteristic of warm climates (83–99%). Another polymorphic variant, rs9526067, of the UCP6 gene has been shown to be associated with two criteria, TSH and SPINA-GT, so it was awarded five points. The search for natural selection signals for the A rs9526067 allele showed that a high frequency of distribution of this allele is found in the Eastern regions of Eurasia (81–92%), as in the south (92%), and in the north (81%). However, the observed associations between uncoupling protein genes and levels of pituitary–thyroid hormones, as well as thyroid homeostasis (SPINA), indicate the existence of several biochemical processes regulated by thyroid hormones in which the uncoupling proteins UCP5 and UCP6 are involved, but this requires further research.
3.2. Effect of Polymorphic Variant rs3811787 of the UCP1 Gene on Human Adaptation to Cold
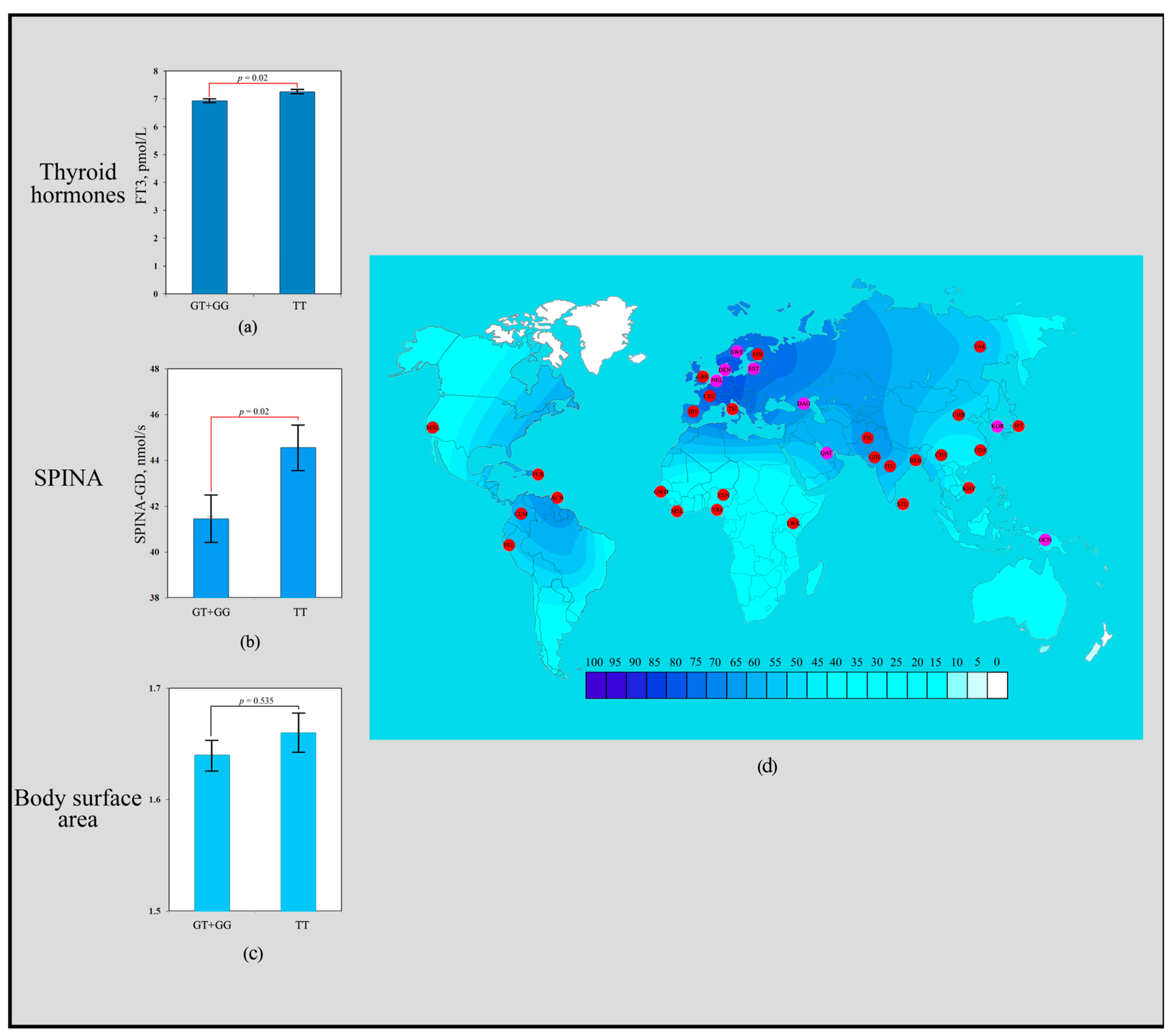
The scoring results revealed that the rs3811787 polymorphism of the UCP1 gene received a total of seven points. Carriers of the homozygous TT genotype exhibited significantly higher levels of FT3 (7.26 ± 0.30 pmol/L; p = 0.02) compared to carriers of the heterozygous GT genotype (6.98 ± 0.35 pmol/L) and the homozygous GG genotype (6.85 ± 0.33 pmol/L) (Figure 1a). Individuals with the TT genotype showed significantly higher SPINA-GD values (44.56 ± 5.12 nmol/s; p = 0.02) than carriers of the GT + GG genotype (41.44 ± 6.08 nmol/s) (Figure 1b). No associations of the rs3811787 polymorphism with BSA were observed (Figure 1c). The T allele of rs3811787 demonstrated a signal of directional selection for cold climate adaptation. Low frequencies of the T allele (rs3811787) are common throughout Africa (20–37%), where warm and hot climates prevail. High frequency distribution is characteristic of the northwestern regions of Europe (86–78%), where the climate is considered temperate, transitioning to the subarctic type of climate (Figure 1d).
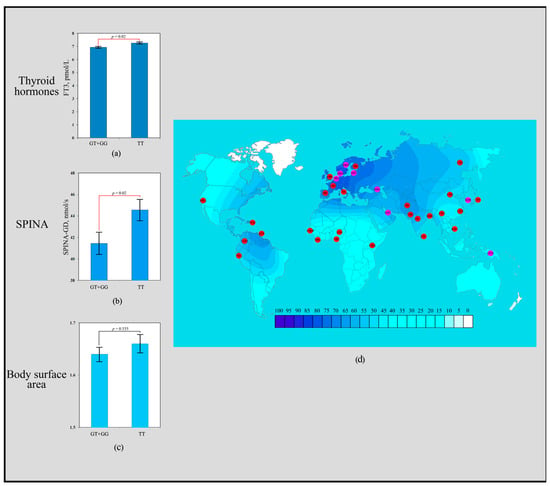
Figure 1.
Effect of the rs3811787 of UCP1 gene in human adaptation to cold: (a) The levels of FT3 as a function of rs3811787 genotypes of the UCP1 gene; (b) Association analysis of the TT rs3811787 genotype between SPINA-GD; (c) Association analysis of the TT rs3811787 genotype between BSA; (d) The geographical distribution of the frequencies of the T rs3811787 (UCP1) allele in global human populations to identify signals of natural selection for cold climate adaptation.
3.3. Effect of the rs1800849 Polymorphic Variant of the UCP3 Gene on Human Adaptation to Cold
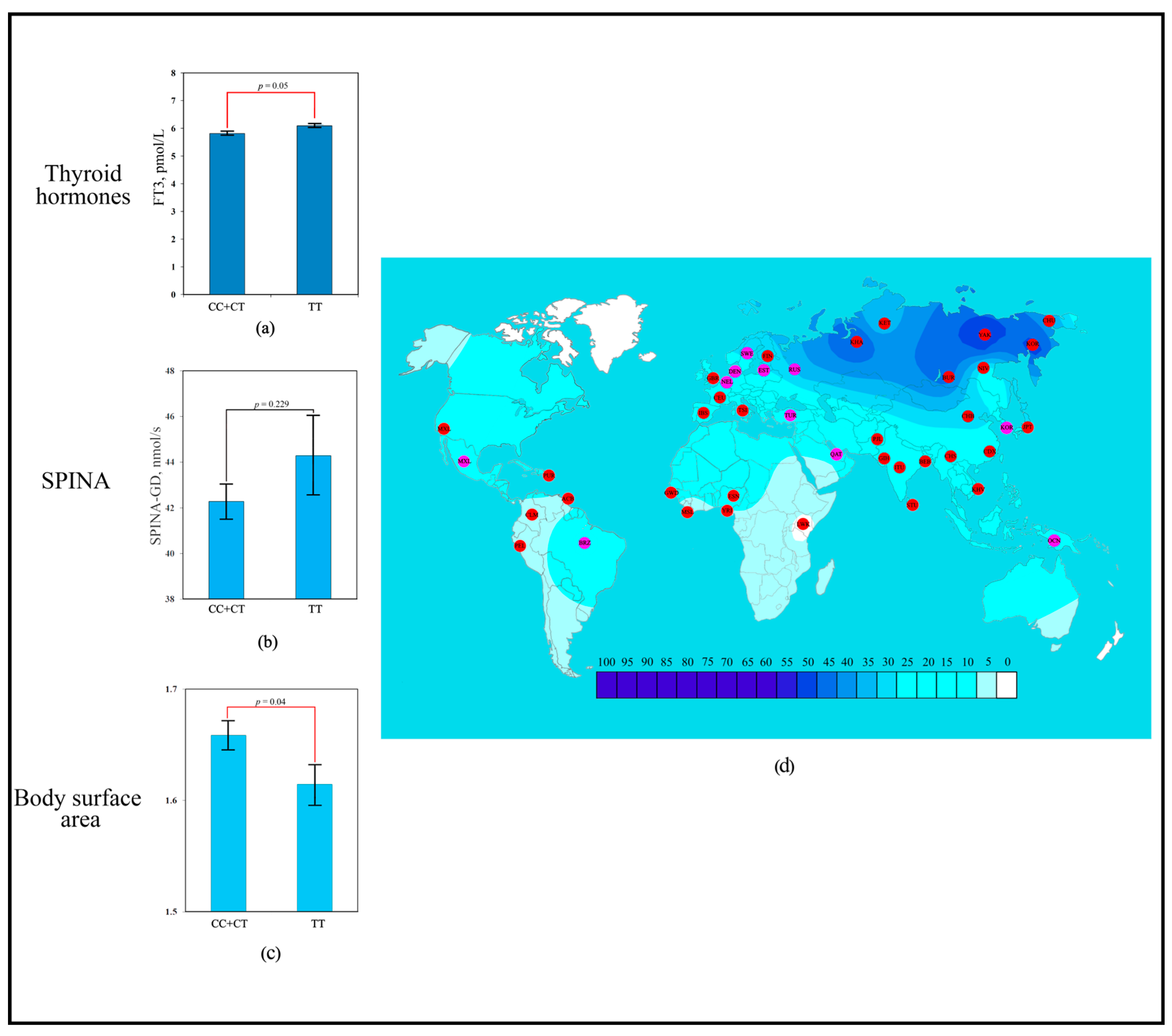
The scoring revealed that the strongest contribution to human adaptation to cold climate was made by the polymorphic variant rs1800849 of the UCP3 gene (six points). Association analysis showed that the TT genotype of rs1800849 was significantly associated with more elevated blood FT3 levels (6.1 ± 0.29 pmol/L; p = 0.05) compared to the opposite CC + CT genotype (5.83 ± 0.41 pmol/L) (Figure 2a). No association with SPINA-GD was detected for rs1800849 (UCP3) (Figure 2b). Association analysis showed that the TT genotype of rs1800849 was significantly associated with lower BSA (1.61 ± 0.17 m2; p = 0.04) compared to the opposite CC + CT genotype (1.66 ± 0.19 m2) (Figure 2c). For the T rs1800849 allele, signals of directional selection for cold climate adaptation were detected, where a high frequency of the T rs1800849 allele was detected in regions dominated by temperate and arctic climates (Figure 2d).
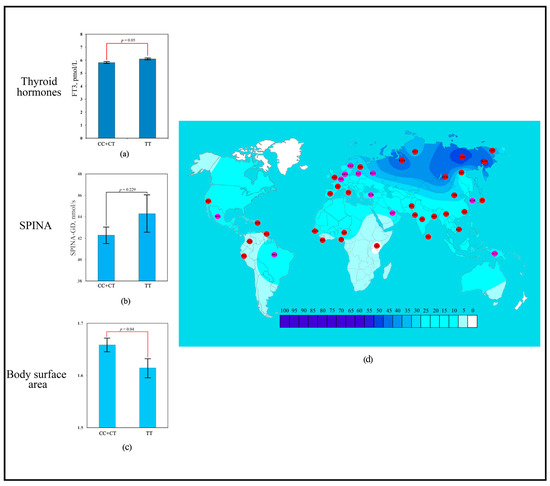
Figure 2.
Effect of the rs1800849 of UCP3 gene on human adaptation to cold: (a) The levels of FT3 as a function of rs1800849 genotypes of the UCP3 gene; (b) Association analysis of TT rs1800849 genotype between SPINA-GD; (c) Association analysis of TT rs1800849 genotype between BSA; (d) The geographical distribution of frequencies of the T rs1800849 (UCP3) allele in global human populations to identify signals of natural selection for cold climate adaptation.
4. Discussion
Based on the evaluation of the contribution of polymorphic variants of UCP genes to human adaptation to cold, it was revealed that two polymorphisms, rs3811787 of the UCP1 gene and rs1800849 of the UCP3 gene, showed the strongest influence. According to the functional role of UCP1 and UCP3 genes, it can be assumed that their role in cold adaptation may be related to heat generation processes during shivering and non-shivering thermogenesis [1,5,17,18,19,20,21,22,23,24,25,26].
4.1. The Role of the rs3811787 of the UCP1 Gene in Non-Shivering Thermogenesis
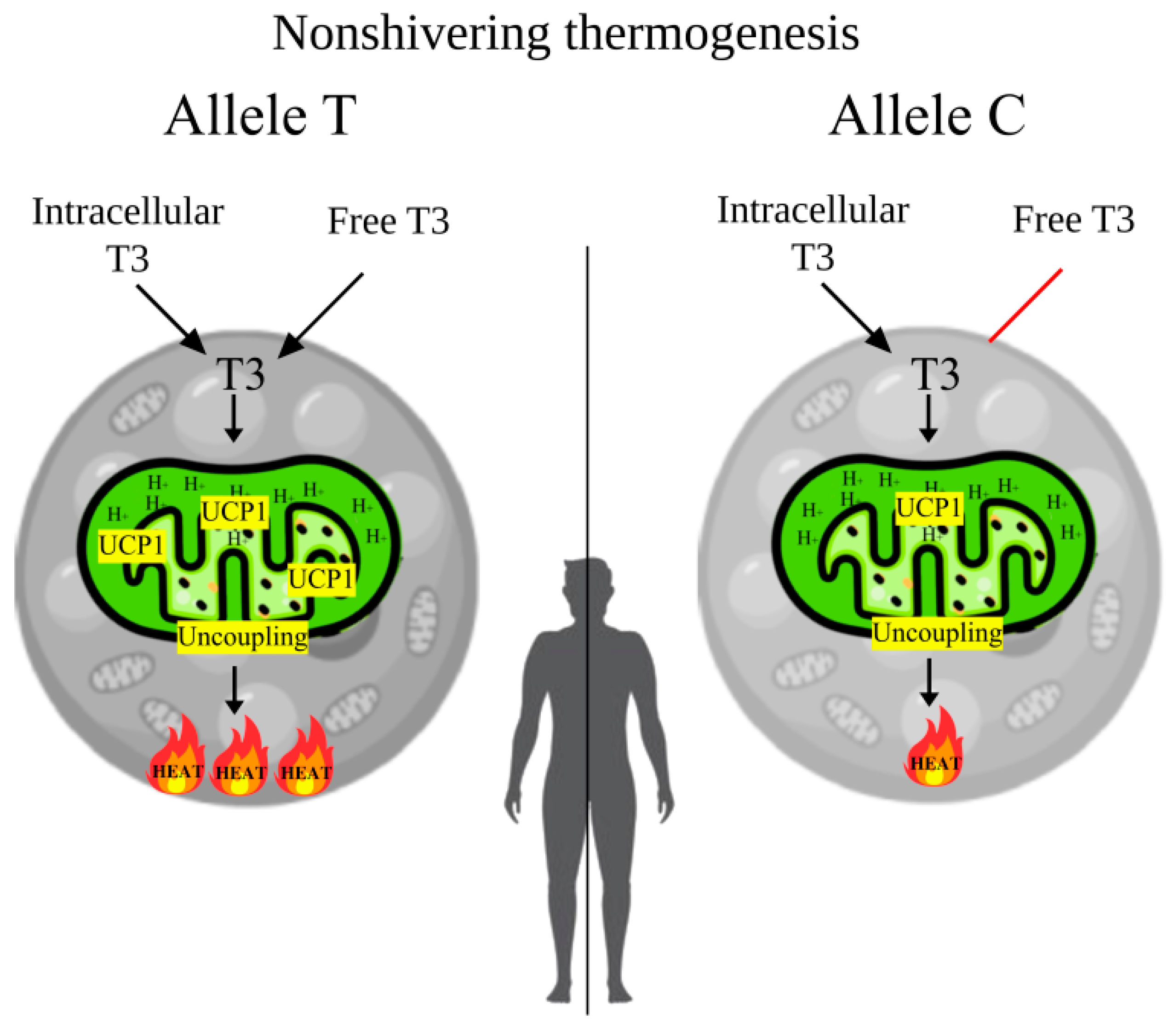
In the present study, a significant involvement of the rs3811787 polymorphic variant of the UCP1 gene in cold climate adaptation (seven points) was demonstrated, which may be associated with non-shivering thermogenesis in BAT. It was found that the TT genotype of rs3811787 is associated with higher T3 and SPINA-GD levels, as well as with cold climate. Therefore, it is possible that when exposed to cold, active BAT may shift thyroid hormone homeostasis toward type 2 allostasis, which leads to increased levels of FT3 (elevated values peripheral deiodination—SPINA-GD) and accelerated basal metabolism and increased energy expenditure. In addition, the BSA values tend to be higher in carriers of the TT genotype, which also supports the increased basal metabolism (Figure 1c). In turn, it has already been shown that in winter, people living in Eastern Siberia have increased basal metabolic rates by 5.8–6% [63]. However, the increase in thyroid hormone levels during type 2 allostasis can be mitigated by the polar T3 syndrome, which is characterized by decreased levels of FT3 in winter [64,65]. The detailed mechanism of BAT-mediated regulation of thyroid hormones and basal metabolic rate is presented in Figure 3.
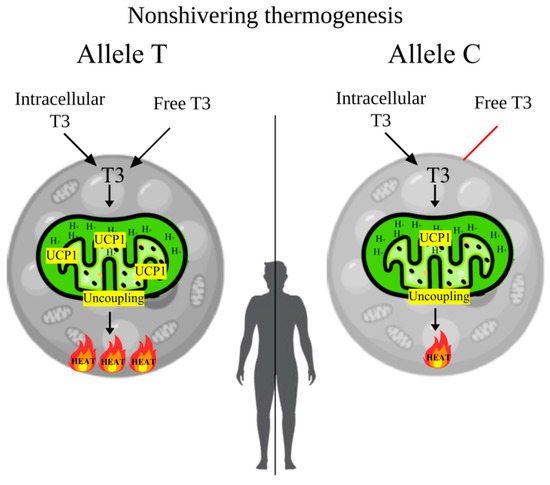
Figure 3.
The mechanism of BAT-mediated regulation of thyroid hormones and basal metabolic rate from the allelic variants rs3811787 of the UCP1 gene. Note. In the carriers of the T allele, with the active form of the UCP1 protein, brown adipocytes mitochondria use higher concentrations of T3 for increased heat generation during adaptive thermogenesis, which increases T3 clearance and in response increases peripheral deiodination (SPINA-GD), which raises blood levels of FT3 and basal metabolic rate. In carriers of the C allele, with a less active form of the protein, brown adipocytes will use less T3, which will not significantly increase SPINA-GD and blood levels of FT3, hence the metabolic rate will not significantly improve.
In turn, Fernández-Verdejo R. et al., based on the results of multiple studies on energy expenditure during active BAT, suggested that clinically meaningful weight loss would require maximally activated BAT throughout the day, which is unlikely [66]. The maximum capacity for non-shivering thermogenesis in BAT becomes inversely proportional to mass as we get older, and in mammals weighing more than 10,000 g, there is little or no predicted thermal contribution [67,68]. We assume that active BAT is maintained in adult humans not at all for direct heat generation, but rather to accelerate peripheral deiodination (conversion of T4 to T3), which in turn shifts homeostasis of the pituitary–thyroid axis toward type 2 allostasis, resulting in an increase in basal metabolism that is crucial for supporting the survival mechanisms for survival of an organism exposed to chronic cold stress.
4.2. The Role of the rs1800849 Polymorphism of the UCP3 Gene in Shivering Thermogenesis
The current study demonstrates that the rs1800849 polymorphic variant of the UCP3 gene significantly affects human adaptation to cold climates (six points), which may be associated with “uncoupling to survive” in skeletal muscles during shivering thermogenesis. It was previously assumed that in skeletal muscle mitochondria, UCP3 participates in “uncoupling to survive” by creating a proton leak in the respiratory chain, resulting in the conversion of electrical charge into thermal energy at the expense of ATP synthesis [69]. Studies on model animals and in vivo models have confirmed the uncoupling ability of UCP3 in skeletal muscle mitochondria [70,71,72,73,74]. Mice with a UCP3 gene knockout (UCP3 KO) exhibited enhanced mitochondrial respiration, indicating the protein’s proton transport activity [70,71]. In UCP1 knockout mice, the loss of thermogenesis in brown adipocytes was compensated by increased shivering thermogenesis in muscles, accompanied by elevated UCP3 expression [72]. Another study on UCP3 KO mice confirmed that UCP3 is a critical mediator of physiological thermogenesis in skeletal muscles [73]. An in vivo study demonstrated that UCP3 overexpression in skeletal muscles reduces the ATP synthesis-to-mitochondrial oxidation ratio, suggesting uncoupling via UCP3 [74].
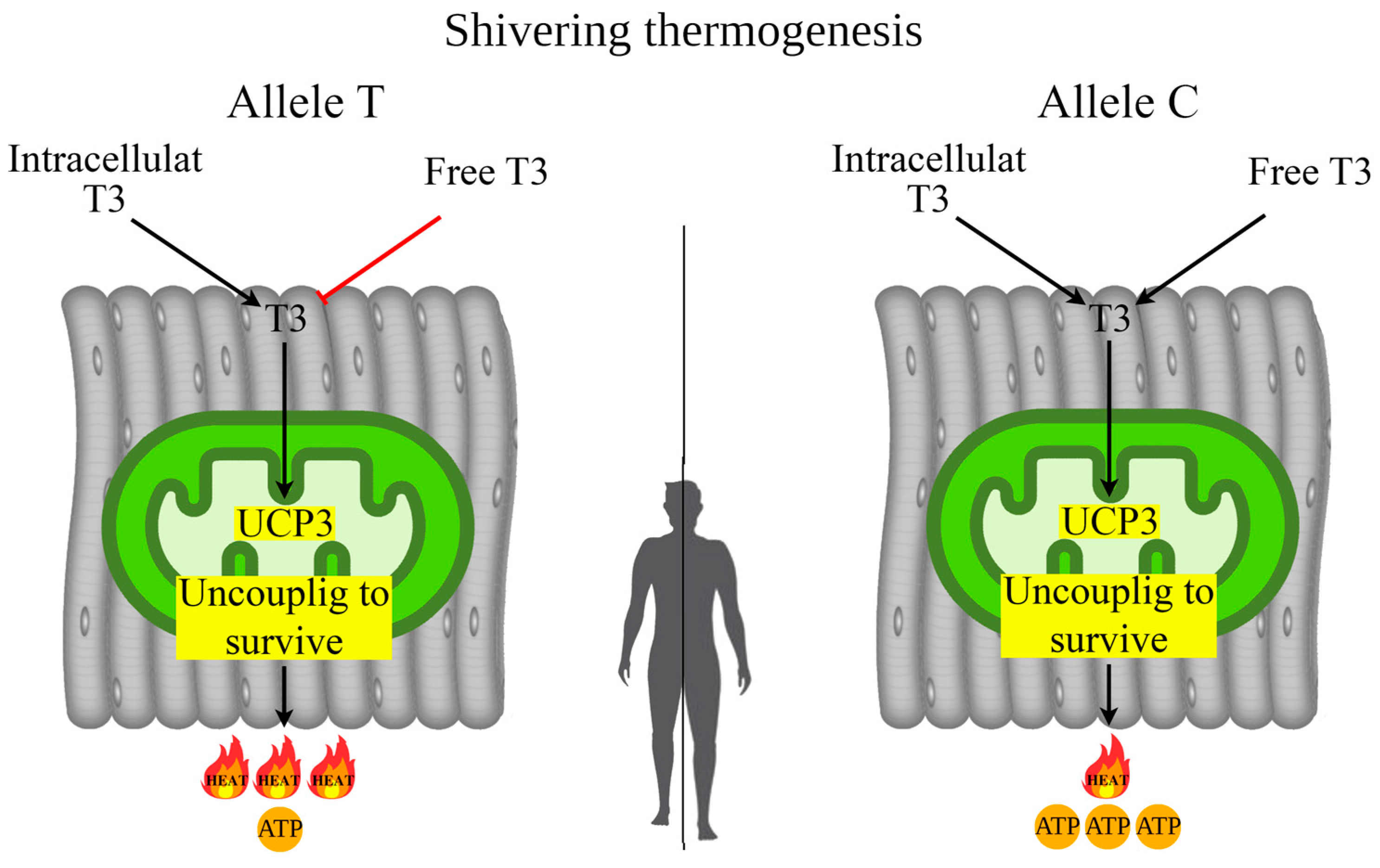
It is hypothesized that UCP3-dependent “uncoupling to survive” is associated with less prolonged shivering thermogenesis but with a greater thermal contribution [40,75]. Other studies have found that cold-adapted individuals exhibited a lower shivering threshold (short-term act of shivering) [76,77]. In turn, the TT genotype rs1800849 of the UCP3 gene showed a tendency with less efficient skeletal muscle contractions; such effects may be related to UCP-dependent differences in mitochondrial proton leak/uncoupling, and thus inefficiency of ATP genesis relative to oxygen consumption [78]. These data are consistent with our earlier results, where we found associations of the TT genotype rs1800849 with increased levels of irisin in female from Eastern Siberia, indicating that carriers of the TT genotype, due to increased heat production, use less irisin (less shivering) for UCP3-dependent “uncoupling to survive” processes [52]. The mechanism of UCP3-dependent “uncoupling to survive” during shivering thermogenesis is presented in Figure 4.
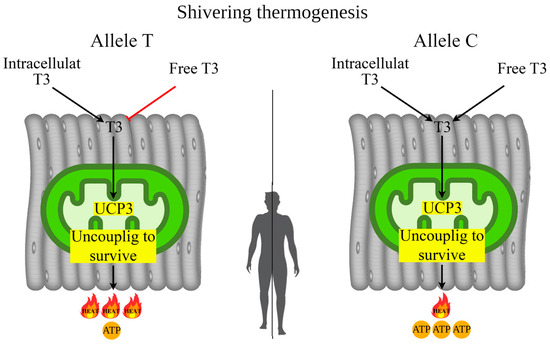
Figure 4.
Possible mechanism of UCP3-dependent “uncoupling to survive” during shivering thermogenesis depending on allelic variants of rs1800849 of the UCP3 gene in skeletal muscle. Note. In the carriers of the T allele (rs1800849) with increased UCP3 expression in skeletal muscle, heat generation during adaptive thermogenesis will occur mainly through “uncoupling to survive” without utilizing serum T3. In the carriers of the C allele (rs1800849) for enhanced UCP3 expression, skeletal muscle mitochondria will utilize T3 to enhance heat generation from “uncoupling to survive”, so serum T3 levels will be low compared to T allele carriers who do not utilize T3.
In the current study, the TT genotype of rs1800849 was found to be associated with increased levels of FT3 in the blood. These results confirm the involvement of T3 in the regulation of expression of the UCP3 gene [23,26]. However, UCP3-dependent “uncoupling to survive” has side effects that potentially affect BSA (growth and body weight). In the present study, rs1800849 of the UCP3 gene was found to be associated with BSA, where carriers of the TT genotype had a lower BSA compared to other genotypes. It is possible that a person with a smaller BSA will keep warm for a longer time. Moreover, this allele rs1800849 (UCP3) was associated with reduced weight and height in females from Eastern Siberia [64]. It is hypothesized that in carriers of the rs1800849 T allele, the lower BSA (reduced weight and height) may be due to competition of UCP3-dependent “uncoupling to survive” with hormones such as testosterone, growth hormones, and estrogens that utilize ATP to support anabolic pathways [34,79,80].
5. Conclusions
- Two polymorphic variants of UCP1 (rs3811787) and UCP3 (rs1800849) genes, out of nine analyzed polymorphic variants of uncoupling protein genes (UCP1, UCP2, UCP3, UCP4, UCP5, and UCP6), demonstrated a direct involvement in human adaptation to cold climates. The other seven polymorphic variants of UCP genes have scored fewer points, so it is assumed that their contribution to human adaptation to cold is less significant;
- The results we obtained on the association of the TT genotype of rs3811787 in the UCP1 gene with increased FT3 levels, and elevated SPINA-GD value, in the absence of association with BSA, indicate that the active form of UCP1 in brown adipocytes may utilize more T3, additionally extracting T3 from serum. This increases T3 clearance and rate of peripheral deiodination (conversion of T4 to T3), which shifts the homeostasis of the pituitary–thyroid axis toward type 2 allostasis and ultimately leads to a higher basal metabolic rate;
- The findings on the association of the TT genotype of rs1800849 in the UCP3 gene with increased FT3 levels in blood and with body weight deficiency demonstrate that the uncoupling protein UCP3 in skeletal muscle mitochondria actively participates in the processes of “uncoupling to survive”. This involves creating a proton leak in the respiratory chain, resulting in the conversion of electrical charge into thermal energy at the expense of ATP synthesis. A secondary effect of UCP3-dependent “uncoupling to survive” is likely to be competition with anabolic pathways for ATP, which may affect BSA, growth, and weight.
Supplementary Materials
The following supporting information can be downloaded at https://www.mdpi.com/article/10.3390/biology14050454/s1, Figure S1: Study design; Table S1: Genotype and allele frequencies of 9 polymorphic variants of 6 genes of UCP1-6 uncoupling proteins in the Yakut population and their conformity to the Hardy-Weinberg equilibrium (HWE); Table S2: Association analysis of 9 polymorphic variants of UCP1-6 genes with the levels of pituitary–thyroid hormones TSH, FT3 and FT4 in subjects from the group I; Table S3: Association analysis of 9 polymorphic variants of UCP1-6 genes with levels of pituitary–thyroid hormones TSH, FT3 and FT4 in subjects from the group II; Table S4: Associative analysis of genotypes of 9 polymorphic variants of UCP1-6 genes with SPINA parameters; Table S5: Associative analysis of 9 polymorphic variants of UCP1-6 genes with BSA; Figure S2: Distribution of allele frequencies of 9 polymorphic variants of UCP1-6 genes.
Author Contributions
Conceptualization, A.A.N. and N.A.B.; methodology, validation and formal analysis, V.G.P., G.P.R. and A.V.S.; investigation, resources and data curation, S.S.N. and A.A.N.; writing—original draft preparation, A.A.N. and N.A.B.; supervision, N.A.B. and S.A.F.; project administration, N.A.B. and S.A.F.; funding acquisition, N.A.B. and S.A.F. All authors have read and agreed to the published version of the manuscript.
Funding
This study was supported by the YSC CMP project “Study of the genetic structure and burden of hereditary pathology of the populations of the Republic of Sakha (Yakutia)” (to A.A.N., V.G.P. and N.A.B.), by the Ministry of Science and Higher Education of the Russian Federation (FSRG-2023-0003) (S.S.N., G.P.R., A.V.S. and S.A.F.).
Institutional Review Board Statement
The study was conducted in accordance with the Declaration of Helsinki, and approved by the local Biomedical Ethics Committee at the Yakut Scientific Center of Complex Medical Problems, Siberian Branch of the Russian Academy Scientific of Medical Sciences, Yakutsk, Russia (Yakutsk, Protocol No. 45, 12 October 2017).
Informed Consent Statement
Informed consent was obtained from all subjects involved in the study.
Data Availability Statement
The data presented in this study are available on request from the corresponding author.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Klingenberg, M.; Echtay, K.; Bienengraeber, M.; Winkler, E.; Huang, S. Structure–Function Relationship in UCP1. Int. J. Obes. 1999, 23, S24–S29. [Google Scholar] [CrossRef]
- Ricquier, D.; Bouillaud, F. The Uncoupling Protein Homologues: UCP1, UCP2, UCP3, StUCP and AtUCP. Biochem. J. 2000, 345, 161–179. [Google Scholar] [CrossRef] [PubMed]
- Echtay, K.S. Mitochondrial Uncoupling Proteins—What Is Their Physiological Role? Free Radic. Biol. Med. 2007, 43, 1351–1371. [Google Scholar] [CrossRef]
- Monteiro, B.S.; Freire-Brito, L.; Carrageta, D.F.; Oliveira, P.F.; Alves, M.G. Mitochondrial Uncoupling Proteins (UCP) as Key Modulators of ROS Homeostasis: A Crosstalk between Diabesity and Male Infertility? Antioxidants 2021, 10, 1746. [Google Scholar] [CrossRef] [PubMed]
- Virtanen, K.A.; Lidell, M.E.; Orava, J.; Heglind, M.; Westergren, R.; Niemi, T.; Taittonen, M.; Laine, J.; Savisto, N.-J.; Enerbäck, S.; et al. Functional Brown Adipose Tissue in Healthy Adults. N. Engl. J. Med. 2009, 360, 1518–1525. [Google Scholar] [CrossRef] [PubMed]
- Fleury, C.; Neverova, M.; Collins, S.; Raimbault, S.; Champigny, O.; Levi-Meyrueis, C.; Bouillaud, F.; Seldin, M.F.; Surwit, R.S.; Ricquier, D.; et al. Uncoupling Protein-2: A Novel Gene Linked to Obesity and Hyperinsulinemia. Nat. Genet. 1997, 15, 269–272. [Google Scholar] [CrossRef]
- Fisler, J.S.; Warden, C.H. Uncoupling Proteins, Dietary Fat and the Metabolic Syndrome. Nutr. Metab. 2006, 3, 38. [Google Scholar] [CrossRef]
- Stark, M.J.; Hodyl, N.A.; Butler, M.; Clifton, V.L. Localisation and Characterisation of Uncoupling Protein-2 (UCP2) in the Human Preterm Placenta. Placenta 2012, 33, 1020–1025. [Google Scholar] [CrossRef]
- Pierelli, G.; Stanzione, R.; Forte, M.; Migliarino, S.; Perelli, M.; Volpe, M.; Rubattu, S. Uncoupling Protein 2: A Key Player and a Potential Therapeutic Target in Vascular Diseases. Oxid. Med. Cell. Longev. 2017, 2017, 7348372. [Google Scholar] [CrossRef]
- Nigro, M.; De Sanctis, C.; Formisano, P.; Stanzione, R.; Forte, M.; Capasso, G.; Gigliotti, G.; Rubattu, S.; Viggiano, D. Cellular and Subcellular Localization of Uncoupling Protein 2 in the Human Kidney. J. Mol. Histol. 2018, 49, 437–445. [Google Scholar] [CrossRef]
- Wang, X.; Qian, H.; Huang, X.; Li, J.; Zhang, J.; Zhu, N.; Chen, H.; Zhu, C.; Wang, J.; Zhang, P.; et al. UCP2 Mitigates the Loss of Human Spermatozoa Motility by Promoting mROS Elimination. Cell Physiol. Biochem. 2018, 50, 952–962. [Google Scholar] [CrossRef] [PubMed]
- Boss, O.; Samec, S.; Paoloni-Giacobino, A.; Rossier, C.; Dulloo, A.; Seydoux, J.; Muzzin, P.; Giacobino, J.P. Uncoupling Protein-3: A New Member of the Mitochondrial Carrier Family with Tissue-Specific Expression. FEBS Lett. 1997, 408, 39–42. [Google Scholar] [CrossRef] [PubMed]
- Mao, W.; Yu, X.X.; Zhong, A.; Li, W.; Brush, J.; Sherwood, S.W.; Adams, S.H.; Pan, G. UCP4, a Novel Brain-Specific Mitochondrial Protein That Reduces Membrane Potential in Mammalian Cells. FEBS Lett. 1999, 443, 326–330. [Google Scholar] [CrossRef] [PubMed]
- Sanchis, D.; Fleury, C.; Chomiki, N.; Goubern, M.; Huang, Q.; Neverova, M.; Grégoire, F.; Easlick, J.; Raimbault, S.; Lévi-Meyrueis, C.; et al. BMCP1, a Novel Mitochondrial Carrier with High Expression in the Central Nervous System of Humans and Rodents, and Respiration Uncoupling Activity in Recombinant Yeast. J. Biol. Chem. 1998, 273, 34611–34615. [Google Scholar] [CrossRef]
- Haguenauer, A.; Raimbault, S.; Masscheleyn, S.; Gonzalez-Barroso, M.D.M.; Criscuolo, F.; Plamondon, J.; Miroux, B.; Ricquier, D.; Richard, D.; Bouillaud, F.; et al. A New Renal Mitochondrial Carrier, KMCP1, Is up-Regulated during Tubular Cell Regeneration and Induction of Antioxidant Enzymes. J. Biol. Chem. 2005, 280, 22036–22043. [Google Scholar] [CrossRef]
- Lizio, M.; Harshbarger, J.; Shimoji, H.; Severin, J.; Kasukawa, T.; Sahin, S.; Abugessaisa, I.; Fukuda, S.; Hori, F.; Ishikawa-Kato, S.; et al. Gateways to the FANTOM5 Promoter Level Mammalian Expression Atlas. Genome. Biol. 2015, 16, 22. [Google Scholar] [CrossRef]
- Garlid, K.D.; Orosz, D.E.; Modrianský, M.; Vassanelli, S.; Jezek, P. On the Mechanism of Fatty Acid-Induced Proton Transport by Mitochondrial Uncoupling Protein. J. Biol. Chem. 1996, 271, 2615–2620. [Google Scholar] [CrossRef]
- Cannon, B.; Nedergaard, J. Brown Adipose Tissue: Function and Physiological Significance. Physiol. Rev. 2004, 84, 277–359. [Google Scholar] [CrossRef]
- Bianco, A.C.; Silva, J.E. Cold Exposure Rapidly Induces Virtual Saturation of Brown Adipose Tissue Nuclear T3 Receptors. Am. J. Physiol. 1988, 255, E496–E503. [Google Scholar] [CrossRef]
- Carvalho, S.D.; Kimura, E.T.; Bianco, A.C.; Silva, J.E. Central Role of Brown Adipose Tissue Thyroxine 5′-Deiodinase on Thyroid Hormone-Dependent Thermogenic Response to Cold. Endocrinology 1991, 128, 2149–2159. [Google Scholar] [CrossRef]
- Rabelo, R.; Schifman, A.; Rubio, A.; Sheng, X.; Silva, J.E. Delineation of Thyroid Hormone-Responsive Sequences within a Critical Enhancer in the Rat Uncoupling Protein Gene. Endocrinology 1995, 136, 1003–1013. [Google Scholar] [CrossRef] [PubMed]
- Sentis, S.C.; Oelkrug, R.; Mittag, J. Thyroid Hormones in the Regulation of Brown Adipose Tissue Thermogenesis. Endocr. Connect 2021, 10, R106–R115. [Google Scholar] [CrossRef] [PubMed]
- Gong, D.W.; He, Y.; Karas, M.; Reitman, M. Uncoupling Protein-3 Is a Mediator of Thermogenesis Regulated by Thyroid Hormone, Beta3-Adrenergic Agonists, and Leptin. J. Biol. Chem. 1997, 272, 24129–24132. [Google Scholar] [CrossRef] [PubMed]
- Masaki, T.; Yoshimatsu, H.; Kakuma, T.; Hidaka, S.; Kurokawa, M.; Sakata, T. Enhanced Expression of Uncoupling Protein 2 Gene in Rat White Adipose Tissue and Skeletal Muscle Following Chronic Treatment with Thyroid Hormone. FEBS Lett. 1997, 418, 323–326. [Google Scholar] [CrossRef]
- Barbe, P.; Larrouy, D.; Boulanger, C.; Chevillotte, E.; Viguerie, N.; Thalamas, C.; Oliva Trastoy, M.; Roques, M.; Vidal, H.; Langin, D. Triiodothyronine-Mediated up-Regulation of UCP2 and UCP3 mRNA Expression in Human Skeletal Muscle without Coordinated Induction of Mitochondrial Respiratory Chain Genes. FASEB J. 2001, 15, 13–15. [Google Scholar] [CrossRef]
- de Lange, P.; Lanni, A.; Beneduce, L.; Moreno, M.; Lombardi, A.; Silvestri, E.; Goglia, F. Uncoupling Protein-3 Is a Molecular Determinant for the Regulation of Resting Metabolic Rate by Thyroid Hormone. Endocrinology 2001, 142, 3414–3420. [Google Scholar] [CrossRef]
- Kim, B. Thyroid hormone as a determinant of energy expenditure and the basal metabolic rate. Thyroid 2008, 18, 141–144. [Google Scholar] [CrossRef]
- Leonard, W.R.; Sorensen, M.V.; Galloway, V.A.; Spencer, G.J.; Mosher, M.J.; Osipova, L.; Spitsyn, V.A. Climatic Influences on Basal Metabolic Rates among Circumpolar Populations. Am. J. Hum. Biol. 2002, 14, 609–620. [Google Scholar] [CrossRef]
- Galloway, V.A.; Leonard, W.R.; Ivakine, E. Basal Metabolic Adaptation of the Evenki Reindeer Herders of Central Siberia. Am. J. Hum. Biol. 2000, 12, 75–87. [Google Scholar] [CrossRef]
- Snodgrass, J.J.; Leonard, W.R.; Sorensen, M.V.; Tarskaia, L.A.; Mosher, M.J. The Influence of Basal Metabolic Rate on Blood Pressure among Indigenous Siberians. Am. J. Phys. Anthr. 2008, 137, 145–155. [Google Scholar] [CrossRef]
- Snodgrass, J.J.; Leonard, W.R.; Tarskaia, L.A.; Schoeller, D.A. Total Energy Expenditure in the Yakut (Sakha) of Siberia as Measured by the Doubly Labeled Water Method2. Am. J. Clin. Nutr. 2006, 84, 798–806. [Google Scholar] [CrossRef] [PubMed]
- Levy, S.B.; Leonard, W.R.; Tarskaia, L.A.; Klimova, T.M.; Fedorova, V.I.; Baltakhinova, M.E.; Krivoshapkin, V.G.; Snodgrass, J.J. Seasonal and Socioeconomic Influences on Thyroid Function among the Yakut (Sakha) of Eastern Siberia: Seasonal Thyroid Changes in the Yakut. Am. J. Hum. Biol. 2013, 25, 814–820. [Google Scholar] [CrossRef]
- Chatzitomaris, A.; Hoermann, R.; Midgley, J.E.; Hering, S.; Urban, A.; Dietrich, B.; Abood, A.; Klein, H.H.; Dietrich, J.W. Thyroid Allostasis-Adaptive Responses of Thyrotropic Feedback Control to Conditions of Strain, Stress, and Developmental Programming. Front. Endocrinol. 2017, 8, 163. [Google Scholar] [CrossRef] [PubMed]
- Levy, S.B.; Bribiescas, R.G. Hierarchies in the Energy Budget: Thyroid Hormones and the Evolution of Human Life History Patterns. Evol. Anthr. 2023, 32, 275–292. [Google Scholar] [CrossRef]
- Dietrich, J.W.; Landgrafe-Mende, G.; Wiora, E.; Chatzitomaris, A.; Klein, H.H.; Midgley, J.E.M.; Hoermann, R. Calculated Parameters of Thyroid Homeostasis: Emerging Tools for Differential Diagnosis and Clinical Research. Front. Endocrinol. 2016, 7, 57. [Google Scholar] [CrossRef] [PubMed]
- Dietrich, J.W.; Tesche, A.; Pickardt, C.R.; Mitzdorf, U. Thyrotropic Feedback Control: Evidence for an Additional Ultrashort Feedback Loop from Fractal Analysis. Cybern. Syst. 2004, 35, 315–331. [Google Scholar] [CrossRef]
- Nikanorova, A.A.; Borisova, T.V.; Pshennikova, V.G.; Nakhodkin, S.S.; Fedorova, S.A.; Barashkov, N.A. Type 2 Thyroid Allostasis in the residents of Yakutia. Yakut. Med. J. 2024, 86, 80–84. [Google Scholar] [CrossRef]
- Hoffecker, J.F.; Elias, S.A. Environment and Archeology in Beringia. Evol. Anthropol. Issues News Rev. 2003, 12, 34–49. [Google Scholar] [CrossRef]
- Hoffecker, J.F. A Prehistory of the North: Human Settlement of the Higher Latitudes; Rutgers University Press: New Brunswick, NJ, USA, 2005; ISBN 978-0-8135-3469-5. [Google Scholar]
- Holliday, T.W. Body Proportions in Late Pleistocene Europe and Modern Human Origins. J. Hum. Evol. 1997, 32, 423–448. [Google Scholar] [CrossRef]
- Pearson, O.M. Activity, Climate, and Postcranial Robusticity: Implications for Modern Human Origins and Scenarios of Adaptive Change. Curr. Anthr. 2000, 41, 569–607. [Google Scholar] [CrossRef]
- Brown, G.M.; Bird, G.S.; Boag, L.M.; Delahaye, D.J.; Green, J.E.; Hatcher, J.D.; Page, J. Blood Volume and Basal Metabolic Rate of Eskimos. Metabolism 1954, 3, 247–254. [Google Scholar] [PubMed]
- Gagge, A.P.; Gonzalez, R.R. Mechanisms of Heat Exchange: Biophysics and Physiology. Compr. Physiol. 2010, 45–84. [Google Scholar]
- Frisancho, A.R. Human Adaptation and Accommodation; University of Michigan Press: Ann Arbor, MI, USA, 1993; ISBN 978-0-472-09511-7. [Google Scholar]
- Makinen, T.M. Different Types of Cold Adaptation in Humans. Front. Biosci. 2010, 2, 1047–1067. [Google Scholar] [CrossRef] [PubMed]
- Saltykova, M.M. The Main Physiological Mechanisms of Cold Adaptation in Humans. Neurosci. Behav. Physi. 2018, 48, 543–550. [Google Scholar] [CrossRef]
- Toner, M.M.; McArdle, W.D. Human thermoregulatory responses to acute cold stress with special reference to water immersion. Compr. Physiol. 1996, 2011, 379–397. [Google Scholar]
- Hancock, A.M.; Clark, V.J.; Qian, Y.; Di Rienzo, A. Population Genetic Analysis of the Uncoupling Proteins Supports a Role for UCP3 in Human Cold Resistance. Mol. Biol. Evol. 2011, 28, 601–614. [Google Scholar] [CrossRef]
- Stepanov, V.A.; Kharkov, V.N.; Vagaitseva, K.V.; Bocharova, A.V.; Popovich, A.A.; Khitrinskaya, I.Y.; Kazantsev, A.Y. Search for Genetic Markers of Climatic Adaptation in Populations of North Eurasia. Russ. J. Genet. 2017, 53, 1172–1183. [Google Scholar] [CrossRef]
- Watanabe, Y.; Wakiyama, Y.; Waku, D.; Valverde, G.; Tanino, A.; Nakamura, Y.; Oota, H. Cold adaptation in Upper Paleolithic hunter-gatherers of eastern Eurasia. bioRxiv 2024. [Google Scholar] [CrossRef]
- Nikanorova, A.A.; Barashkov, N.A.; Pshennikova, V.G.; Nakhodkin, S.S.; Gotovtsev, N.N.; Romanov, G.P.; Solovyev, A.V.; Kuzmina, S.S.; Sazonov, N.N.; Fedorova, S.A. The Role of Non-shivering Thermogenesis Genes on Leptin Levels Regulation in Residents of the Coldest Region of Siberia. Int. J. Mol. Sci. 2021, 22, 4657. [Google Scholar] [CrossRef]
- Nikanorova, A.A.; Barashkov, N.A.; Pshennikova, V.G.; Gotovtsev, N.N.; Romanov, G.P.; Solovyev, A.V.; Kuzmina, S.S.; Sazonov, N.N.; Fedorova, S.A. Relationships between Uncoupling Protein Genes UCP1, UCP2 and UCP3 and Irisin Levels in Residents of the Coldest Region of Siberia. Genes 2022, 13, 1612. [Google Scholar] [CrossRef]
- International Obesity Task Force. Obesity: Managing the Global Epidemic: Report of the World Health Organization (WHO) Consultation; World Health Organization: Geneva, Switzerland, 1997. [Google Scholar]
- Haycock, G.B.; Schwartz, G.J.; Wisotsky, D.H. Geometric Method for Measuring Body Surface Area: A Height-Weight Formula Validated in Infants, Children, and Adults. J. Pediatr. 1978, 93, 62–66. [Google Scholar] [CrossRef]
- Dietrich, J.W. Der Hypophysen-Schilddrüsen-Regelkreis. Entwicklung Und Klinische Anwendung Eines Nichtlinearen Modells; Logos-Verlag: Berlin, Germany, 2002; ISBN 978-3-89722-850-4. [Google Scholar]
- Dietrich, J.W.; Stachon, A.; Antic, B.; Klein, H.H.; Hering, S. The AQUA-FONTIS Study: Protocol of a Multidisciplinary, Cross-Sectional and Prospective Longitudinal Study for Developing Standardized Diagnostics and Classification of Non-Thyroidal Illness Syndrome. BMC Endocr. Disord. 2008, 8, 13. [Google Scholar] [CrossRef] [PubMed]
- Dietrich, J.W.; Landgrafe, G.; Fotiadou, E.H. TSH and Thyrotropic Agonists: Key Actors in Thyroid Homeostasis. J. Thyroid Res. 2012, 2012, 351864. [Google Scholar] [CrossRef]
- The 1000 Genomes Project Consortium. A global reference for human genetic variation. Nature 2015, 526, 68–74. [Google Scholar] [CrossRef] [PubMed]
- Schnor, N.P.P.; Verlengia, R.; Novais, P.F.S.; Crisp, A.H.; Leite, C.V.D.S.; Rasera-Junior, I.; Oliveira, M.R.M. de Association of 5-HT2C (Rs3813929) and UCP3 (Rs1800849) Gene Polymorphisms with Type 2 Diabetes in Obese Women Candidates for Bariatric Surgery. Arch. Endocrinol. Metab. 2017, 61, 326–331. [Google Scholar] [CrossRef][Green Version]
- Verdi, H.; Kınık, S.T.; Baysan-Çebi, H.P.; Yalçın, Y.Y.; Yazıcı-Güvercin, A.C.; Aydın, B.; Tütüncü, N.B.; Ataç, F.B. Uncoupling Protein Gene UCP1-3826A/G, UCP2 Ins/Del and UCP3-55C/T Polymorphisms in Obese Turkish Children. Turk. J. Pediatr. 2020, 62, 921–929. [Google Scholar] [CrossRef] [PubMed]
- Jin, P.; Li, Z.; Xu, X.; He, J.; Chen, J.; Xu, X.; Du, X.; Bai, X.; Zhang, B.; He, X.; et al. Analysis of association between common variants of uncoupling proteins genes and diabetic retinopathy in a Chinese population. BMC Med. Genet. 2020, 21, 25. [Google Scholar] [CrossRef]
- Cha, M.H.; Shin, H.D.; Kim, K.S.; Lee, B.H.; Yoon, Y. The effects of uncoupling protein 3 haplotypes on obesity phenotypes and very low-energy diet–induced changes among overweight Korean female subjects. Metabolism 2006, 55, 578–586. [Google Scholar] [CrossRef]
- Leonard, W.R.; Levy, S.B.; Tarskaia, L.A.; Klimova, T.M.; Fedorova, V.I.; Baltakhinova, M.E.; Krivoshapkin, V.G.; Snodgrass, J.J. Seasonal Variation in Basal Metabolic Rates among the Yakut (Sakha) of Northeastern Siberia: Seasonality In BMR In The Yakut. Am. J. Hum. Biol. 2014, 26, 437–445. [Google Scholar] [CrossRef]
- Reed, H.L.; Silverman, E.D.; Shakir, K.M.M.; Dons, R.; Burman, K.D.; O’Brian, J.T. Changes in Serum Triiodothyronine (T3) Kinetics after Prolonged Antarctic Residence: The Polar T3 Syndrome. J. Clin. Endocrinol. Metab. 1990, 70, 965–974. [Google Scholar] [CrossRef]
- Nikanorova, A.A.; Barashkov, N.A.; Pshennikova, V.G.; Teryutin, F.M.; Nakhodkin, S.S.; Solovyev, A.V.; Romanov, G.P.; Burtseva, T.E.; Fedorova, S.A. A Systematic Review and Meta-Analysis of Free Triiodothyronine (FT3) Levels in Humans Depending on Seasonal Air Temperature Changes: Is the Variation in FT3 Levels Related to Non-shivering Thermogenesis? Int. J. Mol. Sci. 2023, 24, 14052. [Google Scholar] [CrossRef] [PubMed]
- Fernández-Verdejo, R.; Marlatt, K.L.; Ravussin, E.; Galgani, J.E. Contribution of Brown Adipose Tissue to Human Energy Metabolism. Mol. Asp. Med. 2019, 68, 82–89. [Google Scholar] [CrossRef]
- Heldmaier, G. Zitterfreie Wärmebildung und Körpergröße bei Säugetieren. Z Verg. Physiol. 1971, 73, 222–247. [Google Scholar] [CrossRef]
- Oelkrug, R.; Polymeropoulos, E.T.; Jastroch, M. Brown Adipose Tissue: Physiological Function and Evolutionary Significance. J. Comp. Physiol. B 2015, 185, 587–606. [Google Scholar] [CrossRef] [PubMed]
- Skulachev, V.P. Uncoupling: New Approaches to an Old Problem of Bioenergetics. Biochim. Biophys. Acta 1998, 1363, 100–124. [Google Scholar] [CrossRef]
- Vidal-Puig, A.J.; Grujic, D.; Zhang, C.Y.; Hagen, T.; Boss, O.; Ido, Y.; Szczepanik, A.; Wade, J.; Mootha, T.; Cortright, R.; et al. Energy metabolism in uncoupling protein 3 gene knockout mice. J. Biol. Chem. 2000, 275, 16258–16266. [Google Scholar] [CrossRef]
- Gong, D.W.; Monemdjou, S.; Gavrilova, O.; Leon, L.R.; Marcus-Samuels, B.; Chou, C.J.; Reitman, M.L. Lack of obesity and normal response to fasting and thyroid hormone in mice lacking uncoupling protein-3. J. Biol. Chem. 2000, 275, 16251–16257. [Google Scholar] [CrossRef]
- Shabalina, I.G.; Hoeks, J.; Kramarova, T.V.; Schrauwen, P.; Cannon, B.; Nedergaard, J. Cold Tolerance of UCP1-Ablated Mice: A Skeletal Muscle Mitochondria Switch toward Lipid Oxidation with Marked UCP3 up-Regulation Not Associated with Increased Basal, Fatty Acid- or ROS-Induced Uncoupling or Enhanced GDP Effects. Biochim. Biophys. Acta 2010, 1797, 968–980. [Google Scholar] [CrossRef] [PubMed]
- Riley, C.L.; Dao, C.; Kenaston, M.A.; Muto, L.; Kohno, S.; Nowinski, S.M.; Solmonson, A.D.; Pfeiffer, M.; Sack, M.N.; Lu, Z.; et al. The Complementary and Divergent Roles of Uncoupling Proteins 1 and 3 in Thermoregulation. J. Physiol. 2016, 594, 7455–7464. [Google Scholar] [CrossRef]
- Codella, R.; Alves, T.C.; Befroy, D.E.; Choi, C.S.; Luzi, L.; Rothman, D.L.; Kibbey, R.G.; Shulman, G.I. Overexpression of UCP3 Decreases Mitochondrial Efficiency in Mouse Skeletal Muscle in Vivo. FEBS Lett. 2023, 597, 309–319. [Google Scholar] [CrossRef]
- Wijers, S.L.J.; Schrauwen, P.; Saris, W.H.M.; van Marken Lichtenbelt, W.D. Human Skeletal Muscle Mitochondrial Uncoupling Is Associated with Cold Induced Adaptive Thermogenesis. PLoS ONE 2008, 3, e1777. [Google Scholar] [CrossRef]
- Park, S.; Cho, S.; Seo, H.J.; Lee, J.H.; Kim, M.Y.; Lee, S.D. Entire Mitochondrial DNA Sequencing on Massively Parallel Sequencing for the Korean Population. J. Korean Med. Sci. 2017, 32, 587–592. [Google Scholar] [CrossRef] [PubMed]
- Levy, S.B.; Klimova, T.M.; Zakharova, R.N.; Fedorov, A.I.; Fedorova, V.I.; Baltakhinova, M.E.; Bondy, M.; Atallah, D.; Thompson-Vasquez, J.; Dong, K.; et al. Brown Adipose Tissue Thermogenesis among Young Adults in Northeastern Siberia and Midwest United States and Its Relationship with Other Biological Adaptations to Cold Climates. Am. J. Hum. Biol. 2022, 34, e23723. [Google Scholar] [CrossRef] [PubMed]
- Dhamrait, S.S.; Williams, A.G.; Day, S.H.; Skipworth, J.; Payne, J.R.; World, M.; Humphries, S.E.; Montgomery, H.E. Variation in the Uncoupling Protein 2 and 3 Genes and Human Performance. J. Appl. Physiol. 2012, 112, 1122–1127. [Google Scholar] [CrossRef] [PubMed]
- Horber, F.F.; Haymond, M.W. Human Growth Hormone Prevents the Protein Catabolic Side Effects of Prednisone in Humans. J. Clin. Investig. 1990, 86, 265–272. [Google Scholar] [CrossRef]
- Das, S.; Morvan, F.; Jourde, B.; Meier, V.; Kahle, P.; Brebbia, P.; Toussaint, G.; Glass, D.J.; Fornaro, M. ATP Citrate Lyase Improves Mitochondrial Function in Skeletal Muscle. Cell Metab. 2015, 21, 868–876. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).