Vitamin D3-Coated Surfaces and Their Role in Bone Repair and Peri-Implant Biomechanics
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Titanium Implants and Discs
2.2. Vitamin D3 Surface—Coating Technique
2.3. Physicochemical Tests
2.4. In Vivo Tests
2.5. Mesenchymal Cell Culture and Cell Differentiation Analysis
2.6. Statistical Analysis
3. Results
3.1. Scanning Electron Microscopy (SEM)
3.2. Microtomography Analysis (Micro-CT)
3.3. Peri-Implant Biomechanical Analysis
3.4. Mesenchymal Cell Culture and Cell Differentiation Analysis
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| TLA | Three letter acronym |
| LD | Linear dichroism |
| Ti | Titanium and titanium surface |
| vD | Vitamin D3 |
| vD40µl | Vitamin D40µl surface |
| vD400µl | Vitamin D400µl surface |
| SEM | Scanning electron microscopy |
| DD | Drug delivery systems |
| MTT | 3-(4,5-dimethylthiazol-2-yl)2,5-diphenyltetrazolium bromide |
| ALP | Alkaline phosphatase |
References
- Kitagawa, I.L.; Miyazaki, C.M.; Pitol-Palin, L.; Okamoto, R.; de Vasconcellos, L.M.R.; Constantino, C.J.L.; Lisboa-Filho, P.N. Titanium-Based Alloy Surface Modification with TiO2 and Poly(Sodium 4-Styrenesulfonate) Multilayers for Dental Implants. ACS Appl. Bio Mater. 2021, 4, 3055–3066. [Google Scholar] [CrossRef] [PubMed]
- Sivaswamy, V.; Bahl, V. Surface Modifications of Commercial Dental Implant Systems: An Overview. J. Long-Term Eff. Med. Implants 2023, 33, 71–77. [Google Scholar] [CrossRef] [PubMed]
- Suh, M.S.; Kastellorizios, M.; Tipnis, N.; Zou, Y.; Wang, Y.; Choi, S.; Burgess, D.J. Effect of Implant Formation on Drug Release Kinetics of In Situ Forming Implants. Int. J. Pharm. 2021, 592, 120105. [Google Scholar] [CrossRef] [PubMed]
- Shirazi, S.; Huang, C.-C.; Kang, M.; Lu, Y.; Leung, K.S.; Pitol-Palin, L.; Gomes-Ferreira, P.H.S.; Okamoto, R.; Ravindran, S.; Cooper, L.F. Evaluation of Nanoscale versus Hybrid Micro/Nano Surface Topographies for Endosseous Implants. Acta Biomater. 2024, 173, 199–216. [Google Scholar] [CrossRef]
- Sinjab, K.; Sawant, S.; Ou, A.; Fenno, J.C.; Wang, H.-L.; Kumar, P. Impact of Surface Characteristics on the Peri-implant Microbiome in Health and Disease. J. Periodontol. 2024, 95, 244–255. [Google Scholar] [CrossRef]
- Khalil, M.I.; Sakr, H. Implant Surface Topography Following Different Laser Treatments: An In Vitro Study. Cureus 2023, 15, e38731. [Google Scholar] [CrossRef]
- Kunrath, M.F.; Garaicoa-Pazmino, C.; Giraldo-Osorno, P.M.; Haj Mustafa, A.; Dahlin, C.; Larsson, L.; Asa’ad, F. Implant Surface Modifications and Their Impact on Osseointegration and Peri-implant Diseases through Epigenetic Changes: A Scoping Review. J. Periodontal Res. 2024, 59, 1095–1114. [Google Scholar] [CrossRef]
- Heng, P.W.S. Controlled Release Drug Delivery Systems. Pharm. Dev. Technol. 2018, 23, 833. [Google Scholar] [CrossRef]
- De Jong, W.H.; Borm, P.J. Drug Delivery and Nanoparticles: Applications and Hazards. Int. J. Nanomed. 2008, 133, 133–149. [Google Scholar] [CrossRef]
- Mitchell, M.J.; Billingsley, M.M.; Haley, R.M.; Wechsler, M.E.; Peppas, N.A.; Langer, R. Engineering Precision Nanoparticles for Drug Delivery. Nat. Rev. Drug Discov. 2021, 20, 101–124. [Google Scholar] [CrossRef]
- Farnoud, A.M. Nano-Bio Interactions in Drug Delivery. Phys. Biol. 2020, 17, 050201. [Google Scholar] [CrossRef] [PubMed]
- Farzan, M.; Roth, R.; Schoelkopf, J.; Huwyler, J.; Puchkov, M. The Processes Behind Drug Loading and Release in Porous Drug Delivery Systems. Eur. J. Pharm. Biopharm. 2023, 189, 133–151. [Google Scholar] [CrossRef] [PubMed]
- Bouillon, R. Comparative Analysis of Nutritional Guidelines for Vitamin D. Nat. Rev. Endocrinol. 2017, 13, 466–479. [Google Scholar] [CrossRef] [PubMed]
- Sundar, R.; Bhagavandas Rai, A.; Naveen Kumar, J.; Devang Divakar, D. The Role of Vitamin D as an Adjunct for Bone Regeneration: A Systematic Review of Literature. Saudi Dent. J. 2023, 35, 220–232. [Google Scholar] [CrossRef]
- Werny, J.G.; Sagheb, K.; Diaz, L.; Kämmerer, P.W.; Al-Nawas, B.; Schiegnitz, E. Does Vitamin D Have an Effect on Osseointegration of Dental Implants? A Systematic Review. Int. J. Implant Dent. 2022, 8, 16. [Google Scholar] [CrossRef]
- Colotta, F.; Jansson, B.; Bonelli, F. Modulation of Inflammatory and Immune Responses by Vitamin D. J. Autoimmun. 2017, 85, 78–97. [Google Scholar] [CrossRef]
- Guillot, X.; Semerano, L.; Saidenberg-Kermanac’h, N.; Falgarone, G.; Boissier, M.-C. Vitamin D and Inflammation. Jt. Bone Spine 2010, 77, 552–557. [Google Scholar] [CrossRef]
- Garbossa, S.G.; Folli, F. Vitamin D, Sub-Inflammation and Insulin Resistance. A Window on a Potential Role for the Interaction Between Bone and Glucose Metabolism. Rev. Endocr. Metab. Disord. 2017, 18, 243–258. [Google Scholar] [CrossRef]
- Trino, L.D.; Bronze-Uhle, E.S.; Ramachandran, A.; Lisboa-Filho, P.N.; Mathew, M.T.; George, A. Titanium Surface Bio-Functionalization Using Osteogenic Peptides: Surface Chemistry, Biocompatibility, Corrosion and Tribocorrosion Aspects. J. Mech. Behav. Biomed. Mater. 2018, 81, 26–38. [Google Scholar] [CrossRef]
- Hernandez-Montelongo, J.; Corrales Ureña, Y.R.; Machado, D.; Lancelloti, M.; Pinheiro, M.P.; Rischka, K.; Lisboa-Filho, P.N.; Cotta, M.A. Electrostatic Immobilization of Antimicrobial Peptides on Polyethylenimine and Their Antibacterial Effect against Staphylococcus Epidermidis. Colloids Surf. B Biointerfaces 2018, 164, 370–378. [Google Scholar] [CrossRef]
- Percie du Sert, N.; Hurst, V.; Ahluwalia, A.; Alam, S.; Avey, M.T.; Baker, M.; Browne, W.J.; Clark, A.; Cuthill, I.C.; Dirnagl, U.; et al. The ARRIVE Guidelines 2.0: Updated Guidelines for Reporting Animal Research. PLoS Biol. 2020, 18, e3000410. [Google Scholar] [CrossRef]
- Wajima, C.S.; Pitol-Palin, L.; de Souza Batista, F.R.; dos Santos, P.H.; Matsushita, D.H.; Okamoto, R. Morphological and Biomechanical Characterization of Long Bones and Peri-Implant Bone Repair in Type 2 Diabetic Rats Treated with Resveratrol. Sci. Rep. 2024, 14, 2860. [Google Scholar] [CrossRef] [PubMed]
- Bouxsein, M.L.; Boyd, S.K.; Christiansen, B.A.; Guldberg, R.E.; Jepsen, K.J.; Müller, R. Guidelines for Assessment of Bone Microstructure in Rodents Using Micro–Computed Tomography. J. Bone Miner. Res. 2010, 25, 1468–1486. [Google Scholar] [CrossRef] [PubMed]
- Andrade, P.D.; Vasconcellos, L.M.; Carvalho, I.; Forte, L.; Santos, E.; Prado, R.F.; Santos, D.R.; Cairo, C.A.; Carvalho, Y.R. Titanium-35niobium Alloy as a Potential Material for Biomedical Implants: In Vitro Study. Mat. Sci. Eng. C 2015, 56, 538–544. [Google Scholar] [CrossRef]
- International Standard ISO 10993-5:2009; Biological Evaluation of Medical Devices—Part 5: Tests for In Vitro Cytotoxicity. ISO: Geneva, Switzerland, 2019.
- International Standard ISO 10993-1; Biological Evaluation of Medical Devices—Part 1: Evaluation and Testing Within a Risk Management Process. ISO: Geneva, Switzerland, 2018.
- Rosa, M.L.; Beloti, M.M.; Prando, N.; Queiroz, R.H.C.; de Oliveira, P.T.; Rosa, A.L. Chronic Ethanol Intake Inhibits in Vitro Osteogenesis Induced by Osteoblasts Differentiated from Stem Cells. J. Appl. Toxicol. 2008, 28, 205–211. [Google Scholar] [CrossRef]
- Prado, R.F.; Rabêlo, S.B.; De Andrade, D.P.; Nascimento, R.D.; Henriques, V.A.; Carvalho, Y.R.; Cairo, C.A.; De Vasconcellos, L.M. Porous Titanium and Ti-35Nb Alloy: Effects on Gene Expression of Osteoblastic Cells Derived from Human Alveolar Bone. J. Mater. Sci. Mater. Med. 2015, 26, 259. [Google Scholar] [CrossRef]
- Trino, L.D.; Dias, L.F.G.; Albano, L.G.S.; Bronze-Uhle, E.S.; Rangel, E.C.; Graeff, C.F.O.; Lisboa-Filho, P.N. Zinc Oxide Surface Functionalization and Related Effects on Corrosion Resistance of Titanium Implants. Ceram. Int. 2018, 44, 4000–4008. [Google Scholar] [CrossRef]
- Pujari, S.P.; Scheres, L.; Marcelis, A.T.M.; Zuilhof, H. Covalent Surface Modification of Oxide Surfaces. Angew. Chem. Int. Ed. Engl. 2014, 53, 6322–6356. [Google Scholar] [CrossRef]
- Hu, H.; Wang, L.; Dou, J.; Shang, Y.; Liu, X.; Shen, J.; Yuan, J. Nitric Oxide-Releasing Porous Coating with Antibacterial Activity and Blood Compatibility. Langmuir 2024, 40, 1286–1294. [Google Scholar] [CrossRef]
- Yano, H.; Sakai, N.; Ebina, Y.; Ma, R.; Osada, M.; Fujimoto, K.; Sasaki, T. Construction of Multilayer Films and Superlattice- and Mosaic-like Heterostructures of 2D Metal Oxide Nanosheets via a Facile Spin-Coating Process. ACS Appl. Mater. Interfaces 2021, 13, 43258–43265. [Google Scholar] [CrossRef]
- Gigante, A.; Brugè, F.; Cecconi, S.; Manzotti, S.; Littarru, G.P.; Tiano, L. Vitamin MK-7 Enhances Vitamin D3-Induced Osteogenesis in hMSCs: Modulation of Key Effectors in Mineralization and Vascularization: Vitamin MK-7 Enhances Vitamin D3 Effects on hMSCs. J. Tissue Eng. Regen. Med. 2015, 9, 691–701. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Bao, Q.; Suh, M.S.; Kastellorizios, M.; Wang, R.; Burgess, D.J. Novel Adapter Method for in Vitro Release Testing of in Situ Forming Implants. Int. J. Pharm. 2022, 621, 121777. [Google Scholar] [CrossRef] [PubMed]
- do Prado, R.F.; de Oliveira, F.S.; Nascimento, R.D.; de Vasconcellos, L.M.R.; Carvalho, Y.R.; Cairo, C.A.A. Osteoblast Response to Porous Titanium and Biomimetic Surface: In Vitro Analysis. Mater. Sci. Eng. C Mater. Biol. Appl. 2015, 52, 194–203. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Li, B.; Song, Y.; Ma, A.; Li, C.; Zhang, X.; Li, H.; Zhang, Q.; Zhang, K. Improved Osteoblast Adhesion and Osseointegration on TiO2 Nanotubes Surface with Hydroxyapatite Coating. Dent. Mater. J. 2019, 38, 278–286. [Google Scholar] [CrossRef]
- Baishya, K.; Bacova, J.; Al Chimali, B.; Capek, J.; Michalicka, J.; Gautier, G.; Le Borgne, B.; Rousar, T.; Macak, J.M. Ultrathin ALD Coatings of Zr and V Oxides on Anodic TiO2 Nanotube Layers: Comparison of the Osteoblast Cell Growth. ACS Appl. Mater. Interfaces 2024, 17, 739–749. [Google Scholar] [CrossRef]
- Tshephe, T.S.; Akinwamide, S.O.; Olevsky, E.; Olubambi, P.A. Additive Manufacturing of Titanium-Based Alloys- A Review of Methods, Properties, Challenges, and Prospects. Heliyon 2022, 8, e09041. [Google Scholar] [CrossRef]
- Dias Corpa Tardelli, J.; Duarte Firmino, A.C.; Ferreira, I.; Cândido dos Reis, A. Influence of the Roughness of Dental Implants Obtained by Additive Manufacturing on Osteoblastic Adhesion and Proliferation: A Systematic Review. Heliyon 2022, 8, e12505. [Google Scholar] [CrossRef]
- Obata, Y.; Bale, H.A.; Barnard, H.S.; Parkinson, D.Y.; Alliston, T.; Acevedo, C. Quantitative and Qualitative Bone Imaging: A Review of Synchrotron Radiation Microtomography Analysis in Bone Research. J. Mech. Behav. Biomed. Mater. 2020, 110, 103887. [Google Scholar] [CrossRef]
- Takeda, S.; Saito, M.; Sakai, S.; Yogo, K.; Marumo, K.; Endo, K. Eldecalcitol, an Active Vitamin D3 Derivative, Prevents Trabecular Bone Loss and Bone Fragility in Type I Diabetic Model Rats. Calcif. Tissue Int. 2017, 101, 433–444. [Google Scholar] [CrossRef]
- Oftadeh, R.; Perez-Viloria, M.; Villa-Camacho, J.C.; Vaziri, A.; Nazarian, A. Biomechanics and Mechanobiology of Trabecular Bone: A Review. J. Biomech. Eng. 2015, 137, 010802. [Google Scholar] [CrossRef]
- Stoppie, N.; Wevers, M.; Naert, I. Feasibility of Detecting Trabecular Bone around Percutaneous Titanium Implants in Rabbits by In Vivo Microfocus Computed Tomography. J. Microsc. 2007, 228, 55–61. [Google Scholar] [CrossRef] [PubMed]
- Wu, Y.-Y.; Yu, T.; Yang, X.-Y.; Li, F.; Ma, L.; Yang, Y.; Liu, X.-G.; Wang, Y.-Y.; Gong, P. Vitamin D3 and Insulin Combined Treatment Promotes Titanium Implant Osseointegration in Diabetes Mellitus Rats. Bone 2013, 52, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Javed, F.; Malmstrom, H.; Kellesarian, S.V.; Al-Kheraif, A.A.; Vohra, F.; Romanos, G.E. Efficacy of Vitamin D3 Supplementation on Osseointegration of Implants. Implant. Dent. 2016, 25, 281–287. [Google Scholar] [CrossRef] [PubMed]
- Zhou, C.; Li, Y.; Wang, X.; Shui, X.; Hu, J. 1,25Dihydroxy Vitamin D3 Improves Titanium Implant Osseointegration in Osteoporotic Rats. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. 2012, 114, S174–S178. [Google Scholar] [CrossRef]
- Maamar el Asri, M.; Pariente Rodrigo, E.; Díaz-Salazar de la Flor, S.; Pini Valdivieso, S.; Ramos Barrón, M.C.; Olmos Martínez, J.M.; Hernández Hernández, J.L. Trabecular bone score and 25-hydroxyvitamin D levels in microvascular complications of type 2 diabetes mellitus. Med. Clin. 2022, 158, 308–314. [Google Scholar] [CrossRef]
- Gomes-Ferreira, P.H.S.; Frigério, P.B.; de Moura, J.; Duarte, N.D.; de Oliveira, D.; Deering, J.; Grandfield, K.; Okamoto, R. Evaluation of Vitamin D Isolated or Associated with Teriparatide in Peri-Implant Bone Repair in Tibia of Orchiectomized Rats. Biology 2023, 12, 228. [Google Scholar] [CrossRef]
- Ma, X.; Gao, Y.; Zhao, D.; Zhang, W.; Zhao, W.; Wu, M.; Cui, Y.; Li, Q.; Zhang, Z.; Ma, C. Titanium Implants and Local Drug Delivery Systems Become Mutual Promoters in Orthopedic Clinics. Nanomaterials 2021, 12, 47. [Google Scholar] [CrossRef]
- Zhu, Y.-S.; Wu, J.; Zhi, F. Advances in Conjugate Drug Delivery System: Opportunities and Challenges. Int. J. Pharm. 2024, 667, 124867. [Google Scholar] [CrossRef]
- Wu, Y.; Sun, B.; Tang, Y.; Shen, A.; Lin, Y.; Zhao, X.; Li, J.; Monteiro, M.J.; Gu, W. Bone Targeted Nano-Drug and Nano-Delivery. Bone Res. 2024, 12, 51. [Google Scholar] [CrossRef]
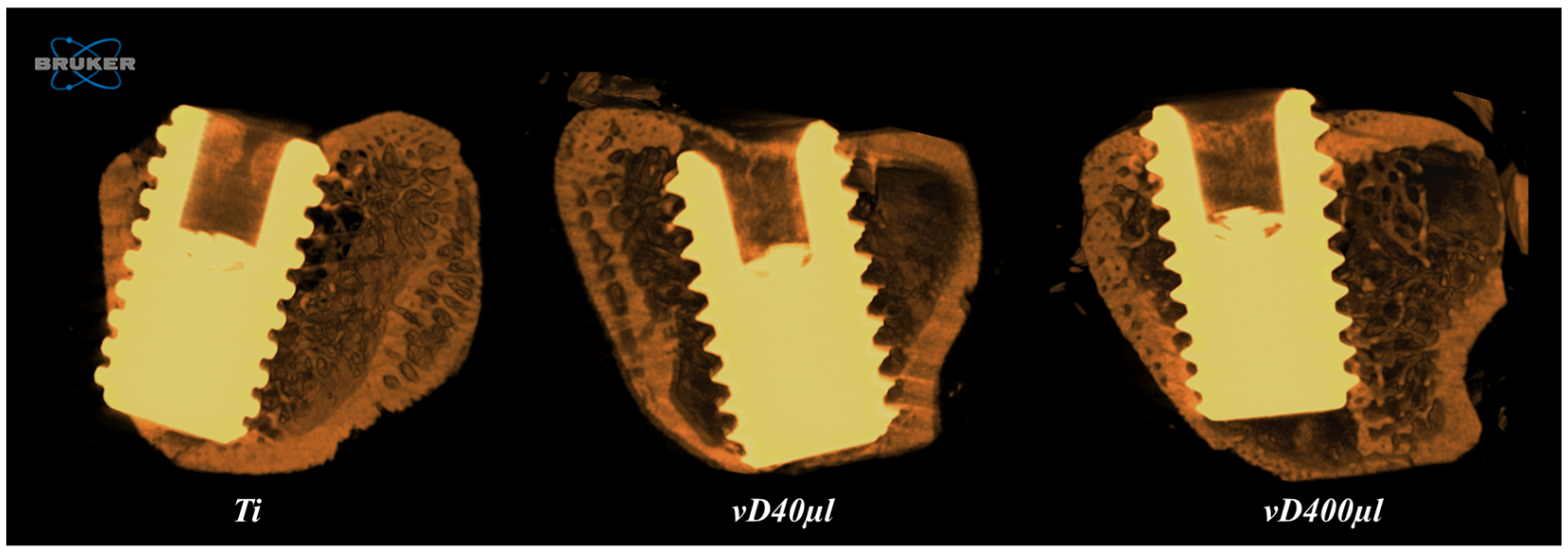



| Ti | vD40µl | vD400µl | |
|---|---|---|---|
| BV/TV (%) | 40.1 ± 13.28 | 46.8 ± 8.97 | 54.7 ± 2.87 |
| Po.Tot (%) † | 61.43 ± 12.01 | 59.26 ± 3.61 | 45.32 ± 2.87 |
| IS (mm2) † | 6.71 ± 1.95 | 8.62 ± 0.61 | 12.54 ± 1.56 |
| Tb.Th (mm) | 0.094 ± 0.008 | 0.092 ± 0.004 | 0.094 ± 0.003 |
| Tb.N (1/mm2) † | 3.82 ± 0.73 | 4.38 ± 0.23 | 5.75 ± 0.52 |
| Tb.Sp (mm) † | 0.108 ± 0.005 | 0.107 ± 0.004 | 0.092 ± 0.005 |
| Ti | vD40µl | vD400µl |
|---|---|---|
| 6.98 ± 1.181 A | 8.91 ± 2.091 AB | 9.65 ± 1.526 B |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pitol-Palin, L.; Sousa, I.C.; de Araújo, J.C.R.; de Souza Batista, F.R.; Inoue, B.K.N.; Botacin, P.R.; de Vasconcellos, L.M.R.; Lisboa-Filho, P.N.; Okamoto, R. Vitamin D3-Coated Surfaces and Their Role in Bone Repair and Peri-Implant Biomechanics. Biology 2025, 14, 476. https://doi.org/10.3390/biology14050476
Pitol-Palin L, Sousa IC, de Araújo JCR, de Souza Batista FR, Inoue BKN, Botacin PR, de Vasconcellos LMR, Lisboa-Filho PN, Okamoto R. Vitamin D3-Coated Surfaces and Their Role in Bone Repair and Peri-Implant Biomechanics. Biology. 2025; 14(5):476. https://doi.org/10.3390/biology14050476
Chicago/Turabian StylePitol-Palin, Letícia, Isadora Castaldi Sousa, Juliani Caroline Ribeiro de Araújo, Fábio Roberto de Souza Batista, Bruna Kaori Namba Inoue, Paulo Roberto Botacin, Luana Marotta Reis de Vasconcellos, Paulo Noronha Lisboa-Filho, and Roberta Okamoto. 2025. "Vitamin D3-Coated Surfaces and Their Role in Bone Repair and Peri-Implant Biomechanics" Biology 14, no. 5: 476. https://doi.org/10.3390/biology14050476
APA StylePitol-Palin, L., Sousa, I. C., de Araújo, J. C. R., de Souza Batista, F. R., Inoue, B. K. N., Botacin, P. R., de Vasconcellos, L. M. R., Lisboa-Filho, P. N., & Okamoto, R. (2025). Vitamin D3-Coated Surfaces and Their Role in Bone Repair and Peri-Implant Biomechanics. Biology, 14(5), 476. https://doi.org/10.3390/biology14050476






