Simple Summary
This study employed integrated metagenomic and environmental analyses to elucidate the ecological roles of microbial communities (viruses, archaea, fungi) in lotus–fish co-culture (LFC) systems versus intensive pond culture (IPC), focusing on their impacts on water quality, sediment health, fish gut immunity, and ecosystem sustainability. The LFC system efficiently decreased the concentration of total nitrogen, total phosphorus, chemical oxygen demand in water, and organic matter in sediment via plant–microbe symbiosis. Simultaneously, LFC mediated the structure of microbial communities: archaea enhanced ammonia oxidation and organic matter degradation, which helped counterbalance methanogenesis, whereas the richness of fungi in sediment and fish gut promoted ecological self-purification. The suppression of viral diversity in water helped to reduce pathogen risks. LFC advances sustainable aquaculture by strengthening ecological resilience and health in large-scale operations, achieving dual goals of high productivity and environmental stewardship via closed-loop resource cycling.
Abstract
The lotus–fish co-culture (LFC) system leverages plant–fish symbiosis to optimize aqua-culture environments, enhancing both economic and ecological yields. However, the eco-logical mechanisms of microbial communities in LFC systems remain poorly understood, particularly regarding the functional roles of fungi, archaea, and viruses. This study compared microbiota (viruses, archaea, fungi) in water, sediment, and fish (crucian carp) gut of LFC and intensive pond culture (IPC) systems using integrated metagenomic and environmental analyses. Results demonstrated that LFC significantly reduced concentrations of total nitrogen, total phosphorus, and nitrite nitrogen and chemical oxygen demand in water, and organic matter and total nitrogen in sediment compared to IPC. Community diversity analysis, LefSe, and KEGG annotation revealed suppressed viral diversity in LFC, yet increased complexity and stability of intestinal virus communities compared to IPC. Archaeal and functional analyses revealed significantly enhanced ammonia oxidation and OM decomposition in LFC versus IPC, promoting methane metabolism equilibrium and sediment organic matter decomposition. Moreover, crucian carp intestines in LFC harbored abundant Methanobacteria, which contributed to maintaining a low hydrogen partial pressure, suppressing facultative anaerobes and reducing intestinal infection risk. The abundance of fungi in sediment and crucian carp intestine in LFC was significantly higher than that in IPC, showing higher ecological self-purification ability and sustainability potential in LFC. Collectively, LFC's optimized archaeal–fungal networks strengthened host immunity and environmental resilience, while viral community suppression reduced pathogen risks. These findings elucidate microbiome-driven mechanisms underlying LFC’s ecological advantages, providing a framework for designing sustainable aquaculture systems through microbial community modulation.
1. Introduction
Decades-long depletion of wild fish resources, exemplified by the decline in breeding stocks of the “Four Domesticated Fish” in the Yangtze River to below 10% of the historical levels by the 1960s, has driven China to launch the Yangtze River Protection Initiative with its Ten-Year Fishing Ban, spurring aquaculture growth [1,2,3,4]. According to the China Fishery Statistical Yearbook (2020–2024), aquaculture area expanded by 7.56% and total production rose by 16.04%. Rising demand for quality protein and heightened food safety awareness have further accelerated the adoption of eco-friendly and sustainable models in the sector [4,5,6]. Integrated agro-aquaculture systems (e.g., lotus–fish and rice–fish systems) can enhance resource efficiency via dual-use water/land systems [6,7]. It forms a composite system that synergizes ecological restoration, economic valorization, and cultural heritage preservation [8,9]. Lotus–fish co-culture (LFC), practiced in China for millennia, is depicted in the Han Dynasty Jiangnan folk song (Yuefu tradition) as a natural agricultural synergy where fish swim among lotus leaves. Lotus plants absorb excess nutrients from water and soil, providing a natural food source and habitat for fish [10,11,12]. On the other hand, cultured species (e.g., crucian carp and common carp) contribute to pest control, soil decompaction, and waste recycling into organic fertilizers through their excretion [6,10,11,13]. Therefore, the bidirectional synergy in LFC boosts fish health (e.g., growth metrics, immune markers) and optimizes the rearing environment (e.g., water quality, microbiome dynamics), outperforming intensive pond culture (IPC). Unofficial statistics show that China’s lotus pond coverage surpasses 100,000 hectares (ha). Therefore, full-scale implementation of LFC could not only elevate aquatic product quality but also diversify aquatic product portfolios. How can the superiority of the LFC system be quantitatively validated? Microbiome research provides a robust framework to dissect this, leveraging multi-omics integration to uncover mechanistic links.
The microbiome serves as a revolutionary bioindicator for fish health and the aquatic ecosystem, surpassing traditional morphological, physiological, and immunological markers via three strengths: dynamic responsiveness, functional integration, and environmental responsiveness [14,15,16,17]. Acting as an ecological sentinel, it enables early pathogen detection and reveals environmental stress adaptation, making it indispensable for sustainable aquaculture and ecosystem conservation [18,19,20]. Therefore, the microbiome transcends conventional health metrics by integrating host–microbe–environment interactions, offering a paradigm shift in aquatic health monitoring and ecosystem stewardship.
Furthermore, the gut microbiota critically shapes cultured fish health by regulating immune responses, stress response, reproduction, endocrine function, nutrient metabolism, and development [21,22]. Understanding microbial diversity and functional roles has always been pivotal for aquaculture advancement [23,24,25,26]. While current research on aquaculture microbiota predominantly focuses on bacterial communities (with metagenomic analyses providing critical insights into host–pathogen dynamics, disease progression, and ecological interactions) [23,27,28,29,30], bacterial communities engage in complex cross-kingdom interactions with archaea, fungi, and host organisms, collectively modulating host physiology and microbial functionality [28,31]. However, other key microbial taxa—particularly fungi, archaea, and viruses—remain systematically understudied. Major knowledge gaps persist regarding their ecological roles (e.g., nutrient cycling) and pathogenic impacts (e.g., viral outbreaks) in aquaculture systems [25,28].
Mammalian and fish gut microbiotas exhibit conserved functional profiles in nutrient metabolism and immune regulation [32,33]. In addition to bacteria, evolutionarily conserved mechanisms indicate that fungi, archaea, and viruses play critical roles in digestion, endocrine signaling, and stress adaptation [28]. Although archaea typically constitute <2% of gut microbiota (e.g., dominated by Methanomicrobia in freshwater fish), their methanogenesis enhances polysaccharide fermentation and detoxifies bacterial metabolites [34,35,36,37]. Fungi, while similarly low in abundance, degrade nutrients and modulate microbial communities via chitin-rich cell walls, with immunomodulatory functions conserved from teleosts to mammals [38,39,40]. Gut viruses, notably including Herpesviridae and Retroviridae, predominantly maintain immune and metabolic homeostasis through symbiotic interactions, as shown by aquatic virome studies [41,42,43,44].
Current knowledge of archaea, viruses, and fungi in aquaculture remains limited, particularly between IPC and LFC systems. This study bridges the gap via a metagenomic analysis of crucian carp gut and its environment, revealing microbial diversity patterns and ecological impacts across farming systems. By elucidating functional interactions and metabolic contributions, it provides insights on how to optimize microbial-driven nutrient cycling, host resilience, and precision aquaculture.
2. Materials and Methods
2.1. Experimental Setup
The field experiment was conducted in two adjacent sites of Large-scale Pond Aquaculture (an area of approximately 3.33 ha for both P1 and P2) in Shuangfeng County, Hunan Province, China, with P1 configured as LFC and P2 as IPC. In P1, lotus seedlings were planted directly in the bottom mud, and the recommended planting density for lotus rhizomes is 3.00 m row spacing and 2.00 m plant-to-plant spacing. The P2 implemented traditional pond aquaculture for crucian carp via mud sediment without aquatic vegetation. The fingerlings of crucian carp (95.5 ± 8.3 g, mean ± SD) were introduced into the pond once lotus seedlings reached 0.20–0.30 m in height, with the water level gradually elevated to 0.50 m through controlled hydrological adjustments [11]. Healthy crucian carp fingerlings (20,000 ind/ha) from the National Education Ministry Breeding Center of Polyploidy Fish, Hunan Normal University were stocked in P1 and P2 during May 2022. Both P1 and P2 implemented standardized artificial feeding protocols for crucian carp [45], but P1 received a 50% lower daily feed ration than P2 despite identical feeding frequency and pellet specifications. This reduction was feasible due to the stratified architecture of lotus root systems enhancing benthic–pelagic coupling, which sustains diverse food webs replacing a large part of formulated feeds [10,11]. The two ponds were continuously supplied by rainfall and the nearby Juan River (a tributary of the Xiangjiang River), ensuring stable hydrological conditions for P1 and P2.
2.2. Sampling and DNA Extraction
One week after stocking, water samples (four spatial repetitions, Figure 1), sediment samples (four spatial repetitions), and fish gut samples (five to six biological repetitions) were methodically collected for comprehensive monitoring. And the first sampling served as early-stage aquaculture diagnostic samples: E_W1 (water in P1), E_S1 (sediment in P1), E_F1 (fish intestines in P1), E_W2 (water in P2), E_S2 (sediment in P2), and E_F2 (fish intestines in P2). Subsequent to a 7-month aquaculture period, comprehensive sample collection (water, sediment, and fish intestinal contents) was conducted in November as the post-cultivation stage (late-stage) samples, maintaining technical repetitions (4 per pond) and biological repetitions (5–6 individuals) for comparative analysis. And the samples were correspondingly named as L_W1, L_S1, L_F1, L_W2, L_S2, and L_F2, respectively. Furthermore, thirty individual C. auratus were randomly sampled from each of P1 and P2, weighed to the nearest 0.1 g, and their mean body weight (±SD) was calculated. All sampling tools were sterilized with 75% ethanol and UV-irradiated. Samples were stored in sterile cryovials pre-treated with RNase/DNase inhibitors. The collected gut contents were rapidly snap-frozen in liquid nitrogen and stored at −80 °C until DNA extraction. Water samples (200 mL) were prefiltered (5 μm) to remove zooplankton and large particles, followed by 0.2 μm Millipore Isopore filtration (Merck KgaA, Burlington, MA, USA) for bacterial collection. Surface sediments (500 g) were harvested using a Peterson mud harvester (TC-600BD-1/40, Institute of Hydrobiology, Wuhan, China) and homogenized, cleaned of visible plant residues, and stored in sterile bags. Both filters and soil samples (2 g) were preserved at −80 °C prior to DNA extraction. Total DNA from intestinal, water, and soil samples were extracted using the E.Z.N.A.® Soil DNA Kit (Omega Bio-tek, Norcross, GA, USA) per the manufacturer’s protocol, quantified using a NanoDrop2000 UV-vis spectrophotometer (Thermo Scientific, Waltham, MA, USA), and stored at −80 °C prior to sequencing.

Figure 1.
Sampling sites in (P1) and (P2) (four areas per pond) labeled with red inverted triangles (1–4).
2.3. Physicochemical Parameters in Water and Sediment
In situ measurements of temperature (T, °C), pH, and dissolved oxygen (DO, mg/L) were conducted using an AP-2000 portable multi-parameter analyzer (Shanghai P-NAV Scientific Instruments Co., Ltd., Shanghai, China). Additionally, water and sediment samples were collected for laboratory quantification of physicochemical parameters: total nitrogen (TN), total phosphorus (TP), nitrate nitrogen (NO3−–N), ammoniacal nitrogen (NH4+–N), phosphate (PO43−–P), nitrite nitrogen (NO2−–N), and chemical oxygen demand (COD). Standardized methods were employed, including (1) alkaline persulfate digestion UV spectrophotometry (TN, GB 11894-89) [46]; (2) molybdenum–antimony–scandium spectrophotometry (TP, PO43−–P, GB 11893-89) [47]; (3) UV spectrophotometry (NO3−–N, HJ/T 346-2007) [48]; (4) Nessler’s reagent colorimetry (NH4+–N, GB 7479-87) [49]; (5) UV spectrophotometry (NO2−–N, GB 7493-1987) [50]; and (6) potassium dichromate digestion (COD, GB/T 32208-2015) [51].
Sediment physicochemical indicators (pH, organic matter, TN, TP, NH4+–N, NO3−–N) were analyzed using standardized methods: (1) pH (NY/T 1377-2007) [52]; (2) organic matter (OM) (NY/T 1121.6-2006) [53]; (3) TN (Automatic Kjeldahl, NY/T 1121.24-2012) [54]; (4) TP (NY/T 88-1988) [55]; (5) NH4+–N (KCl extraction-spectrophotometry, HJ 634-2012) [56]; and (6) NO3−–N (ultraviolet spectrophotometry, GB/T 32737-2016) [57].
Physicochemical indicator analyses and visualizations were performed using R (v4.2.3) with standardized methods. Statistical comparisons between experimental modes were performed using parametric independent t-tests. Welch’s correction was applied when variances were unequal, as determined by Levene’s tests. All statistical procedures aligned with assumptions’ validation. Data were reported as mean ± SD to quantify variability and ensure reproducibility.
2.4. Metagenomic Sequencing and Annotation
The metagenomic sequencing and preliminary sequence analysis were conducted by Ma-jorbio Bio-Pharm Technology Co., Ltd. (Shanghai, China). Metagenomic analysis was performed by indexed multiplexed sequencing. Post-sequencing, samples were demultiplexed, and raw FASTQ data underwent quality control using Fastp to remove (1) bases with Phred quality <20; (2) adapter contaminants; (3) reads <50 bp; and (4) host DNA (via BWA alignment) [58]. The high-quality reads were assembled into contigs using Megahit. Open reading frames (ORFs) were predicted from all contigs >300 bp using Prodigal v2.6.3 [59]. Non-redundant gene catalogs were generated by clustering genes at 95% similarity (CD-HIT). The high-quality reads were aligned to the non-redundant gene set using SOAPaligner v2.21 at 95% similarity [60]. Functional annotation was conducted by BLASTP v2.14.0 against the NCBI nonredundant (NR) protein sequences and KEGG database. Gene abundance of each taxonomy or KEGG metabolic potential was calculated as reads per kilobase per million mapped reads (RPKM) to normalize sequencing depth biases [61].
Independent gene catalogues were built for viral, archaeal, and fungal taxa. Non-redundant sequences were annotated using DIAMOND (v2.1.8.162) in BLASTP against NCBI NR (E-value ≤ 1 × 10−5) [62], and taxonomic classifications were retrieved from the NCBI taxonomy hierarchy. Species abundances were quantified by summing the gene counts per species. Subsequently, phylogenetic profiling was performed at the eight ranks to generate abundance matrices. Alpha diversity was calculated (mothur, https://mothur.org/ (accessed on 25 December 2024)) and compared using an ANOVA (Tukey’s HSD). Community similarity/dissimilarity was visualized using a PCoA based on Bray–Curtis dissimilarity, and group differences were tested using Adonis, ANOSIM, and PERMANOVA (999 permutations). LEfSe was used to identify ecologically divergent taxa between LFC and IPC. Their discriminatory power was quantified by linear discriminant analysis (LDA) with habitat-specific thresholds: water: LDA > 3.5 (reduce noise); sediment: LDA > 2.5 (balance sensitivity and specificity); and fish gut: LDA > 4.0 (reduce false positives). Top five discriminatory taxa per habitat were visualized.
3. Results
3.1. The Physicochemical Properties in Water and Sediment
Contrary to expectations, the crucian carp in P1 achieved a comparable growth performance (0.39 ± 0.18 kg/ind) to those in P2 (0.45 ± 0.09 kg/ind). The physicochemical indicators in water and sediment of P1 and P2 were determined using Chinese national standard (GB) methods. In the early-stage aquaculture, all physicochemical indicators showed statistically significant differences between P1 and P2 (Figures S1 and S2), while the absolute disparities in measured values remained relatively small (e.g., TN: 2.74 ± 0.22 vs. 2.51 ± 0.13 mg/L, p = 0.011; TP: 0.13 ± 0.03 vs. 0.15 ± 0.01 mg/L, p = 0.038, Table 1). However, during the later-stage aquaculture, nearly all values of physicochemical indicators demonstrated marked variations between P1 and P2 (e.g., TN: 1.45 ± 25 vs. 2.77 ± 0.14 mg/L, p = 3.6 × 10−5, Figure S1), with the exception of pH values in both water and sediment remaining similar. In P1, except for NO3−-N, T, DO, and TP, the other six water quality parameters were all significantly lower in the late stage than early (p < 0.01, Figure S1). In contrast, most water quality parameters in P2 exhibited a pronounced upward trend in the late stage, with pH and NO2−–N being the exception. Although the trends of these physicochemical indicators in the sediment generally paralleled those in the water, the magnitude of their variations was relatively smaller. Notably, there were no significant differences between early and late stages in P1 sediment indicators. However, TN and NH4+-N in P2 sediment demonstrated a substantial increase in the late stage (p < 0.01, Figure S2), with their levels significantly surpassing those in the early stage.

Table 1.
Concentrations of physicochemical indicators in water (mg/L) and sediment (mg/kg), mean ± SD.
3.2. Quality Assessment in Metagenomic Sequencing
Post-QC high-quality sequence counts exhibited a range of 4.6 × 107 to 5.8 × 107 across samples, with a mean of 4.6 × 107 (±0.4 × 107) sequences per sample (Table S1). The proportion of high-quality sequences to raw sequences averaged 98.1% (range: 95.8–99.0%). Q20 values averaged 97.1% (range: 94.8–98.5%) across samples, while Q30 values averaged 93.8% (range: 92.1–95.5%), and the GC content averaged 53.8% (range: 41.5–61.5%). The metagenomic sequencing data demonstrated high reliability with Q30 > 90%, GC content stability (53.8% ± 4.7%), and validated reproducibility across technical replicates, meeting the quality thresholds for the following genomic analysis.
3.3. Alpha Diversity of Fungal–Viral–Archaeal Communities
Microbial α-diversity (Chao, Pielou, Shannon indices) was assessed for viral, archaeal, and fungal communities in water, sediment, and crucian carp gut samples (P1/P2). All samples reached full coverage (index = 1), ensuring complete taxon detection (Table 2, Table 3 and Table 4).

Table 2.
α diversity indices of viruses in different ponds in different periods (E represents the early stage, L represents the late stage; 1 represents P1, 2 represents P2; W represents water, S represents sediment; F represents fish gut), the same as below.

Table 3.
α diversity indices of archaea in different ponds in different periods.

Table 4.
α diversity indices of fungi in different ponds in different periods.
Water viral richness remained persistently lower in P1 than P2, with early-stage evenness (0.16 vs. 0.38) and diversity (1.02 vs. 2.60, Shannon index) significantly reduced in P1 (p < 0.05), but late-stage values comparable. Sediment viral diversity showed transient reduction in early-stage P1 (p < 0.05), while richness and evenness showed no inter-pond differences at either stage. Gut viral richness was suppressed in late-stage P1 (p < 0.05), accompanied by early-stage reductions in evenness and diversity (p < 0.05), with no late-stage inter-pond differences (Table 2).
Archaeal richness in water exhibited sustained suppression in P1 compared to P2 across both stages (p < 0.05), while evenness and diversity showed no inter-pond differences (Table 3). Archaeal richness in sediment was significantly higher in P1 in the early stage, but late-stage values comparable with P2. Archaeal richness and evenness in the gut were similar between P1 and P2 in the early stage. However, in the late stage, P1 exhibited higher richness (p < 0.05) but lower evenness (p < 0.05).
Sediment fungal richness was significantly lower in P1 vs. P2 (p < 0.05), but it became marginally higher in P1 by the late stage (Table 4). For gut fungi, P1 consistently exceeded P2 (p < 0.05), with no temporal shifts within ponds. Early-stage gut fungal evenness and diversity showed no inter-pond differences, whereas both indices were significantly elevated in P1 in the late stage (p < 0.05).
3.4. Structure of Fungal–Viral–Archaeal Communities
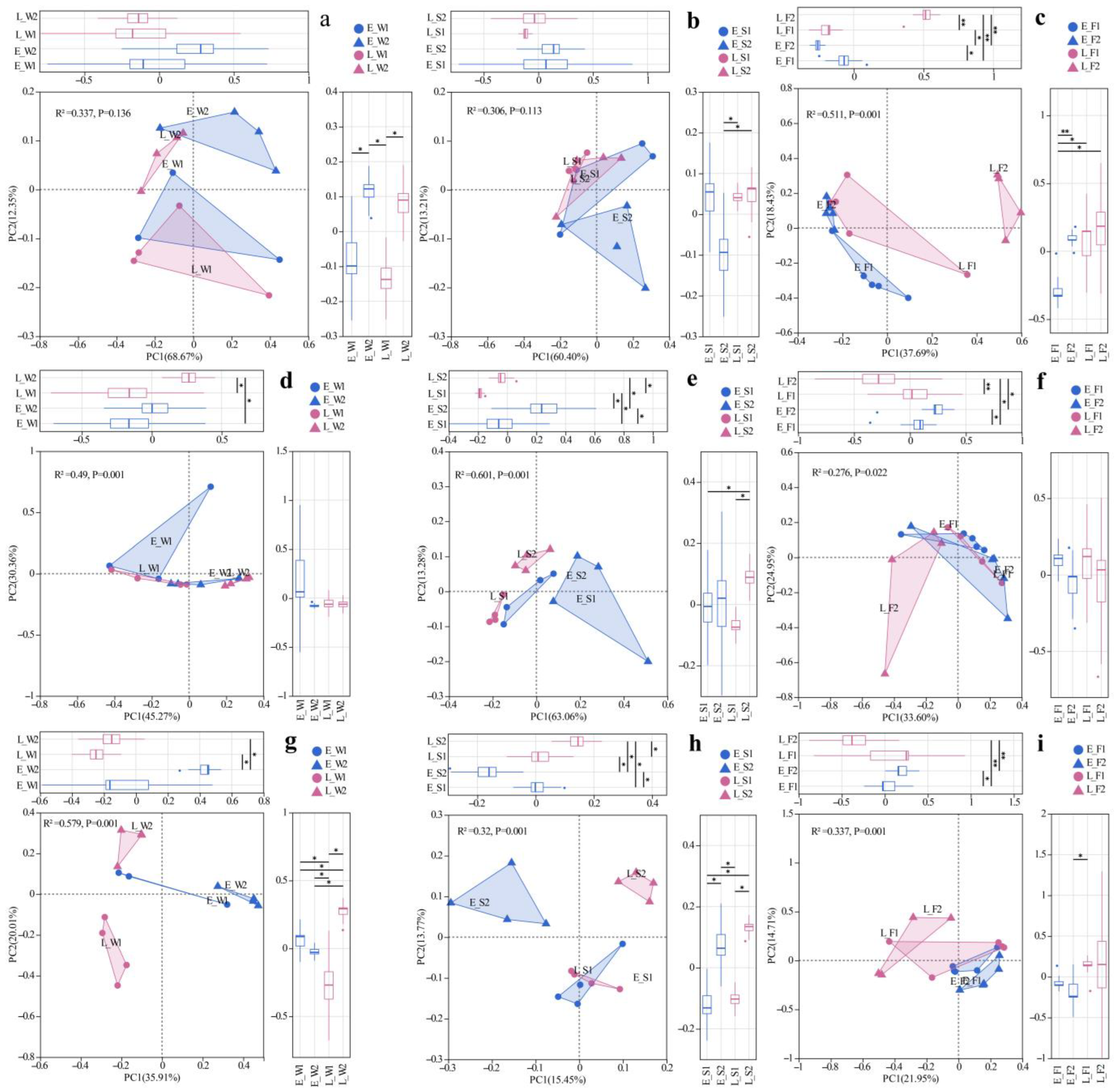
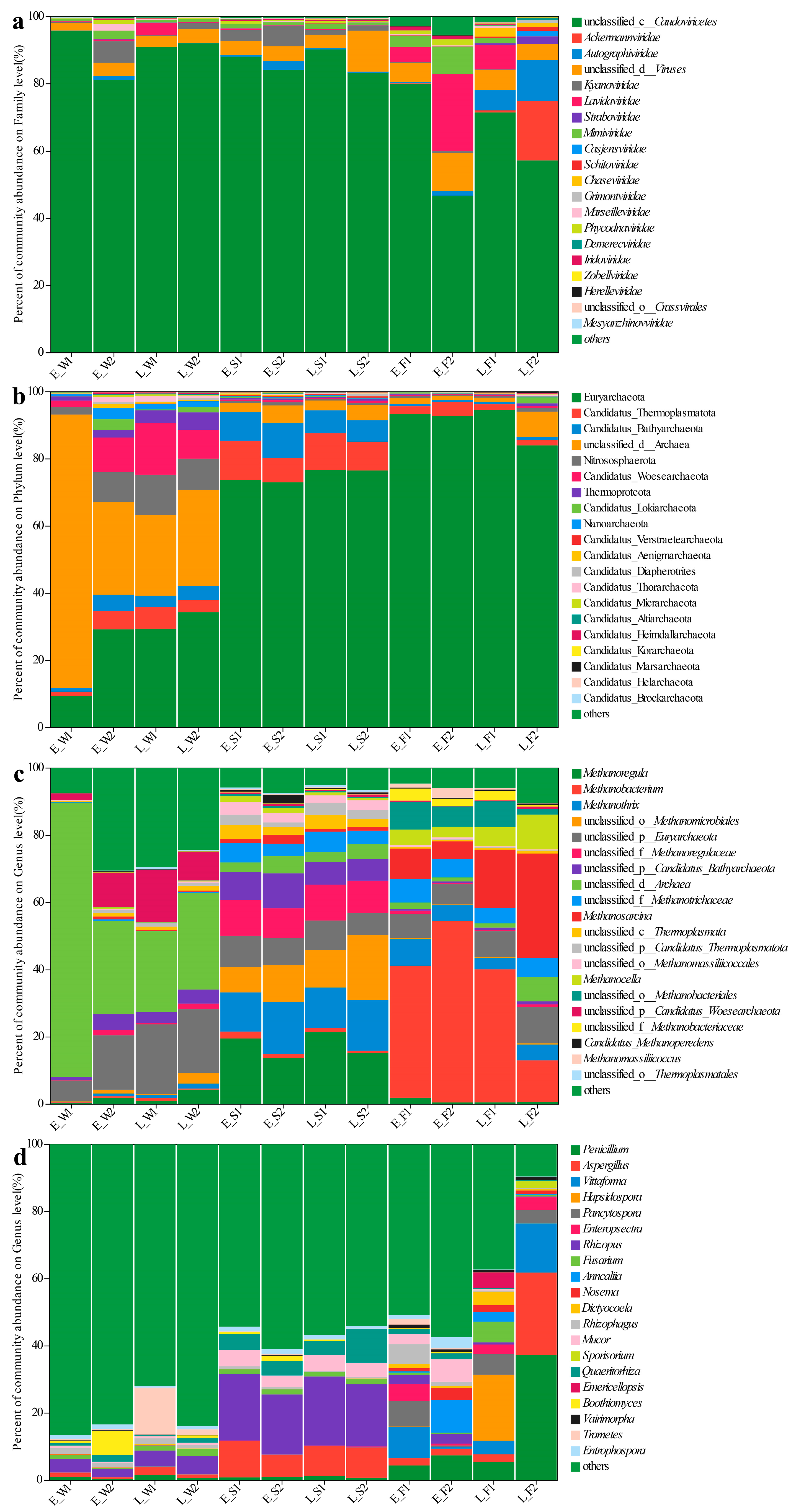
Principal coordinate analysis (PCoA) based on the Bray–Curtis distance for viral species at the taxonomic level in water, sediment, and the gut were conducted, respectively. Bray–Curtis-based PCoA revealed the viral community structure, while PERMANOVA indicated no significant spatial/temporal variations either in water or sediment (Figure 2a,b). However, gut viromes displayed significant pond/temporal variations (PC1/2: 37.69%/18.43%; p < 0.05, Figure 2c). Unclassified Caudoviricetes dominated the water (80.94–95.64%) and sediment (83.10–90.09%) viromes (Figure 3a). Late-stage differentials included Lavidaviridae (P1: 3.77%) vs. Cyanoviridae (P2: 1.93%) in water; P2 sediment indicated a succession to unclassified virus dominance (12.17%); and gut Caudoviricetes varied substantially (46.52–79.93%), with dynamic subdominant taxa (Figure 3a).
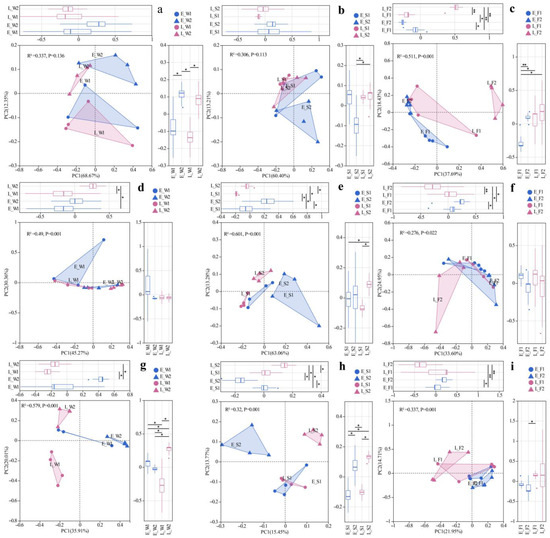
Figure 2.
PCoA of viral (a–c), archaeal (d–f), and fungal (g–i) communities across habitats (W: water; S: sediment; F: fish gut) under two models (1: LFC; 2: IPC) and periods (E: early stage; L: late stage). Significance levels: *, p < 0.05; **, p < 0.01.
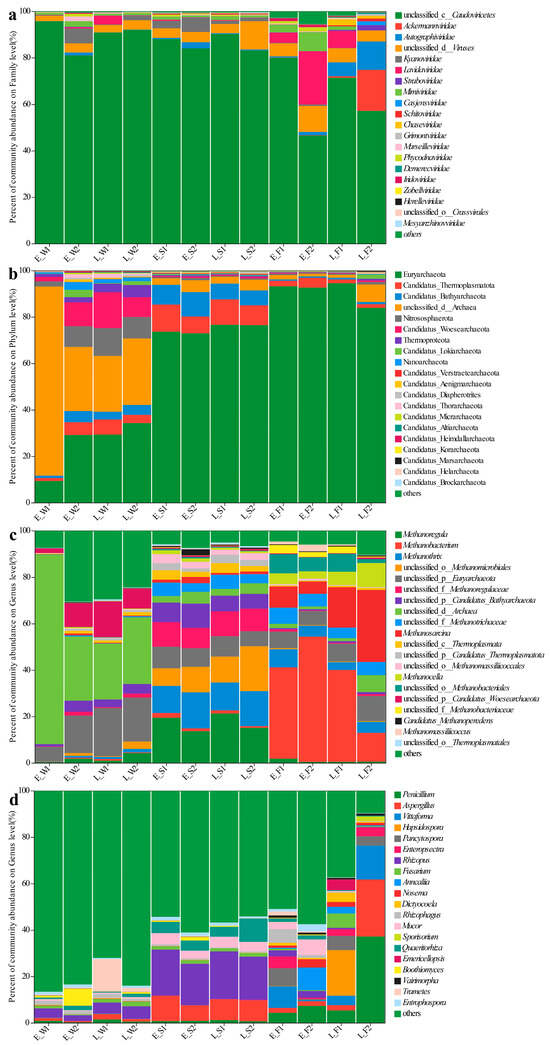
Figure 3.
Species composition of viral families (a), archaeal phyla (b) and archaeal genera (c), and fungal genera (d) across water (W), sediment (S), and fish gut (F) samples under two culture models (1: LFC and 2: IPC) during early (E) and late (L) culture stages. Different colors represent different taxonomic groups.
Archaeal communities, visualized via Bray–Curtis PCoA, exhibited no significant spatial or temporal variation in water (PERMANOVA, p > 0.05), but it did indicate significant inter-pond differences and temporal variations in sediment and gut (PERMANOVA, p < 0.05, Figure 2d–f). The dominant archaeal phyla, including Euryarchaeota and Candidatus_Thermoplasmatota, demonstrated significant spatiotemporal variations in abundance (Figure 3b). In the late stages, the water exhibited a notable increase in the prevalence of Euryarchaeota and Nitrososphaerota. The composition of sediment archaea remained stable throughout. The gut archaea were consistently dominated by Euryarchaeota (Figure 3b). At the genus level, the water communities displayed divergence in their relative abundances, with P1 indicating major shifts in the late stages (e.g., unclassified archaea decreased from 81.5% to 24%). The sediment maintained a consistent taxonomic composition, albeit with variable abundances. The dominance in the gut shifted from Methanobacterium and Methanosarcina in the early stages to pond-specific assemblages in the late stages (Figure 3c).
For fungal communities, PERMANOVA detected substantial differences among ponds, as well as notable temporal variations in the water, sediment, and gut samples (p < 0.05, Figure 2g–i). Fungal communities exhibited divergence between ponds, with P1′s water shifting to Trametes dominance (13.97% compared to P2′s 1.74%) while sediments maintained compositional similarity (Figure 3d). In the gut, fungi underwent a temporal restructuring—in P1, there was a transition from Vittaforma to Hapsidospora dominance, whereas in P2, there was a shift from Anncaliia to Penicillium/Aspergillus dominance—likely influenced by host–microbe interactions (Figure 3d).
3.5. LEfSe Differential Discriminant Analysis of Fungal–Viral–Archaeal Abundance
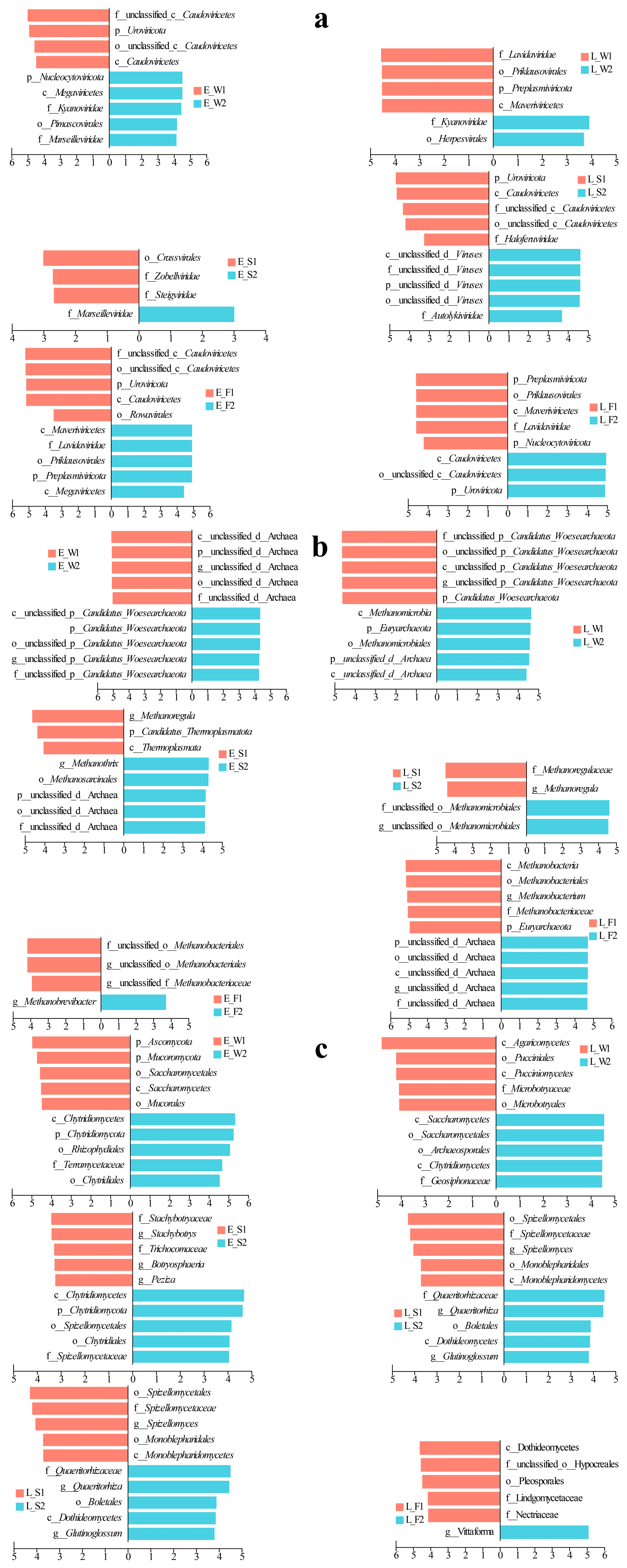
LEfSe applies linear discriminant analysis (LDA) to identify taxonomic biomarkers exhibiting significant differential abundance across experimental groups. In terms of viral community dynamics in water, P1 demonstrated early dominance of Caudovirales, transitioning to Lavidaviridae in its late stages (Figure 4a). Conversely, P2 exhibited early enrichment of Kyanoviridae/Marseilleviridae, shifting to Kyanoviridae/Herpesvirales by its late stages. In sediments, P1 was initially enriched with Crassvirales/Zobelliridae/Steigviridae, later succeeded by Caudoviricetes/Haloferviridae (Figure 4a). P2, on the other hand, shifted from early Marseilleviridae dominance to unclassified viruses/Autolykiviridae. In the fish guts, P1 displayed early enrichment of Uroviricota and Caudoviricetes, evolving into p__Preplasmiviricota, o__Priklausovirales, and c__Maveriviricetes. Meanwhile, P2 transitioned from early Marseilleviridae and Megaviricetes to Uroviricota and Caudoviricetes dominance (Figure 4a).
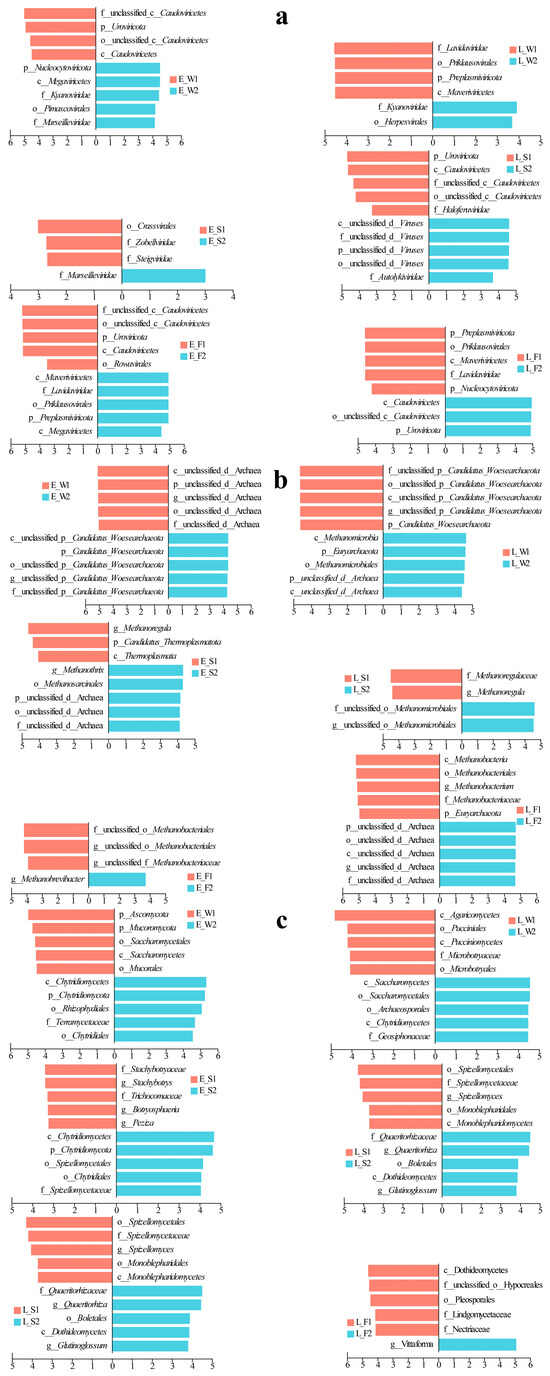
Figure 4.
LEfSe analysis of hierarchical viral (a), archaeal (b), and fungal (c) taxa across water, sediment, and fish gut samples under two culture models (LFC and IPC) during early and late culture stages.
In the water, the archaeal community experienced changes throughout the different stages of P1 and P2 (Figure 4b). During the early stage of P1, there was an enrichment of unclassified archaea, which later gave way to an enrichment of Woesearchaeota in P2. It is important to note that the late stage of P2 significantly increased the ratio of Woesearchaeota to Euryarchaeota as well as methane-producing archaea (Methanomicrobia/Methanomicrobiales). In the sediments, the early P1 stage was characterized by the presence of g__Methanoregula/p__Thermoplasmatota. As it progressed, a shift occurred towards f__Methanoregulaea/g__Methanoregula. The P2 stage began with a dominance of unclassified archaea/g__Methanothrix, which eventually advanced to a dominance of Methanomicrobiales (Figure 4b). Within the guts, the P1 stage saw a transition from early Methanobacteriales to late-stage Euryarchaeota and taxa related to Methanobacteriales. In contrast, the P2 stage switched from Methanobrevibacter to a dominance of unclassified archaea (Figure 4b).
In the fungal community, P1 in water transitioned from an early dominance of Ascomycota to a late-stage supremacy of Agaricomycetes, while P2 shifted from Chytridiomycota-related taxa to a dominance of Saccharomycetes (Figure 4c). In sediments, P1 evolved from an enrichment of Stachybotrys to taxa associated with Spizellomycetales; and P2 transitioned from early Chytridiomycota to Boletales/Dothideomycetes (Figure 4c). In fish guts, P1 restructured from early Vittaforma to late Dothideomycetes dominance; and P2 displayed a late-stage enrichment of Vittaforma following an early prevalence of Tubulinosema (Figure 4c).
3.6. Environmental Driver Associations with Fungal–Viral–Archaeal Abundance
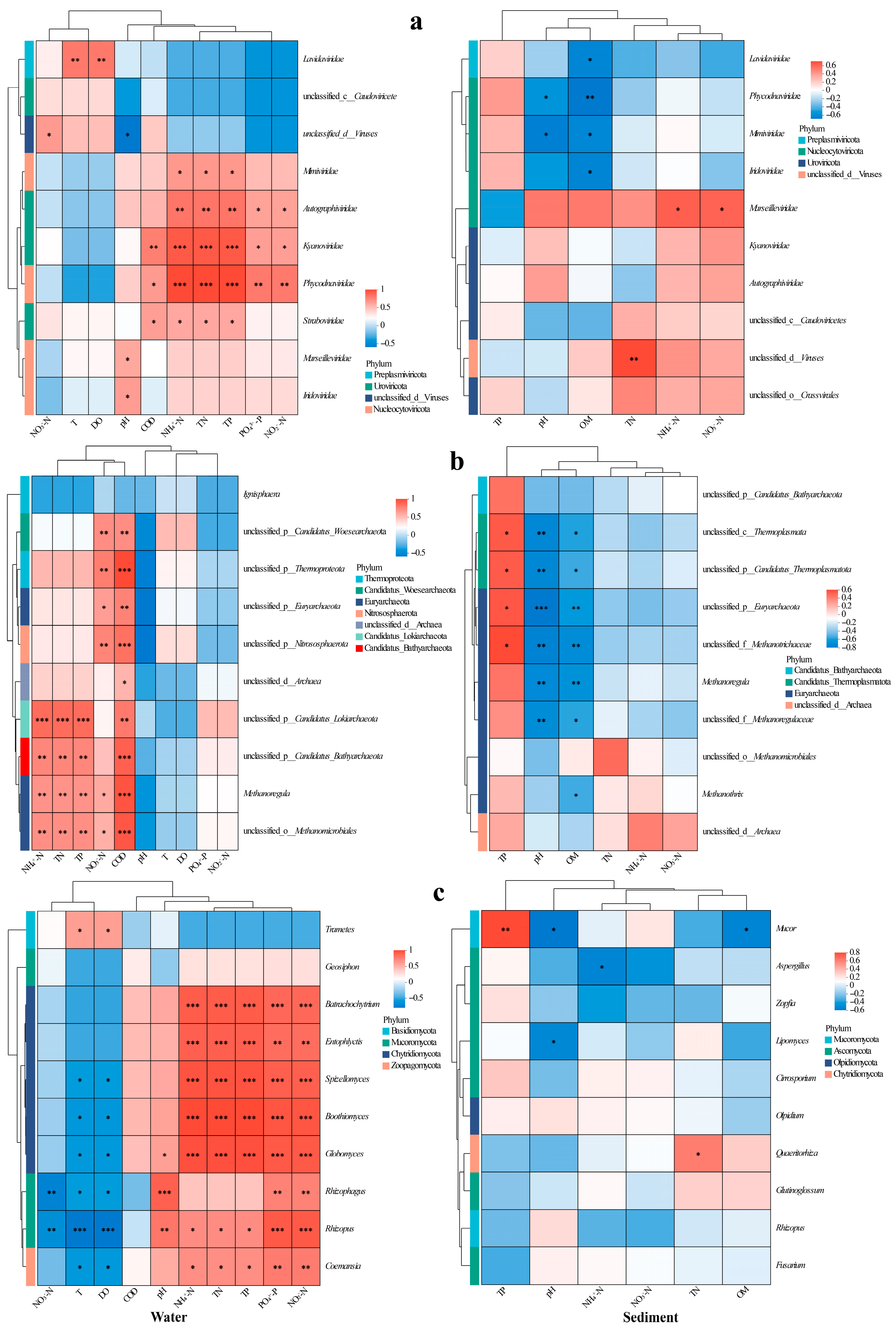
Type I error control in multiple correlation analyses was achieved via the Benjamini–Hochberg false discovery rate (FDR) procedure, with the significance threshold set at an FDR < 0.05. We analyzed viral taxa–environment correlations using heatmaps (Figure 5), revealing distinct preferences: Phycodnaviridae and algal DNA virus correlated positively with COD, TN, NH4+-N, NO2−-N, TP, and PO43−-P in water (Figure 5a); Mimiviridae, Autolykiviridae, and Guttaviridae were associated with TN/TP/NH4+-N (Autolykiviridae additionally with NO2−-N/PO43−-P, Guttaviridae with COD); Megaviricetes was linked with T/DO; while Marseilleviridae and Iridoviridae showed pH-dependence. In sediment, Megaviricetes, Phycodnaviridae, Mimiviridae, and Iridoviridae inversely correlated with OM; Phycodnaviridae and Mimiviridae decreased with pH; and Marseilleviridae covaried positively with NH4+-N/NO3−-N (Figure 5a).
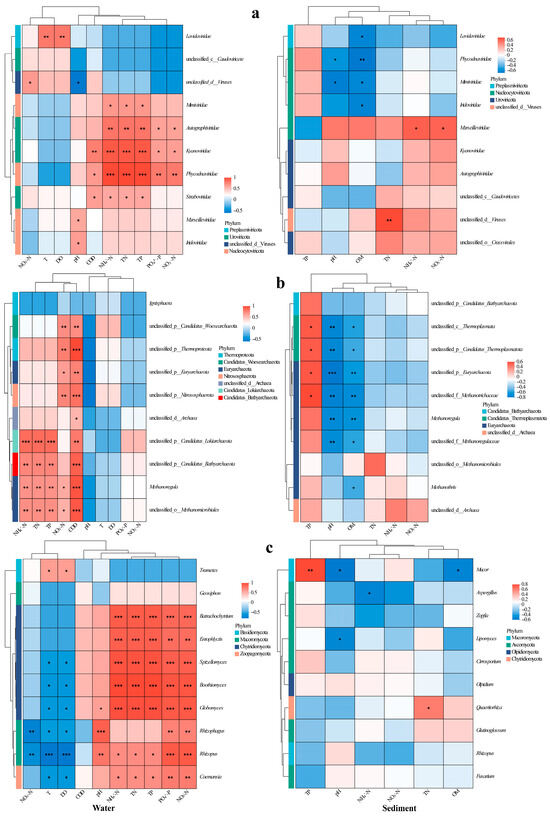
Figure 5.
Heatmaps of correlations between major viral families (a), archaeal genera (b), and fungal genera (c) and environmental indicators in water (left) and sediment (right). Significance levels: *, p < 0.05; **, p < 0.01; ***, p < 0.001.
In water, the archaea Euryarchaeota, Thermoproteota, Woesearchaeota, and Nitrososphaerota correlated positively with NO3−-N and COD (Figure 5b); Thermoproteota additionally showed a negative pH correlation. Bathyarchaeota, Lokiarchaeota, and Thermoplasmata was associated with elevated TN/NH4+-N/COD/TP, while Nanoarchaeota demonstrated broader nutrient linkages (TN/NH4+-N/COD/TP/PO43−-P/NO2−-N). Most archaeal genera exhibited similar TN/NH4+-N/TP/COD correlations. In sediment, Lokiarchaeota and Thermoplasmata increased with TP but decreased with pH/OM; Euryarchaeota and Thermoproteota similarly declined with pH/OM; and Candidatus Verstraetearchaeota was inversely correlated with pH. Genus-level analyses confirmed these patterns: unclassified taxa within Thermoproteota, Thermoplasmata, Euryarchaeota, and Methanosaetaceae thrived under high TP but diminished with increasing pH and OM, mirroring the sensitivity of Methanoregula and unclassified Methanoregulaceae to pH and OM gradients (Figure 5b).
In water, the abundance of the fungal Trametes increased in correlation with T and DO, whereas Coelomomyces exhibited an inverse relationship with these variables but showed a positive correlation with TN, NH4+-N, TP, PO43−-P, and NO2−-N (Figure 5c). Distinctively strong nutrient associations were observed for Rhizophydium, Entophlyctis, Hyphochytrium, Blastocladia, and Globomyces (all p < 0.01), with the latter three genera preferring habitats that were cooler and had lower oxygen levels. The populations of Rhizosporangium and Rhizopus were dependent on pH and sensitive to NO3−-N, thus exhibiting contrasting distribution patterns along the temperature–oxygen gradient. In sediment, the abundance of Mucor increased with TP but decreased with pH and OM. Conversely, Aspergillus was negatively correlated with NH4+-N, Lipomyces displayed a pH-dependent distribution, while Rhizophagus populations showed a positive correlation with TN (Figure 5c).
3.7. Functional Annotation and Statistical Analysis of Fungal–Viral–Archaeal Genes
The key findings from the comparative metagenomic analyses of viral, archaeal, and fungal gene functions across different aquaculture phases (early vs. late) and modes (LFC vs. IPC) are summarized below (Figure 6a–c).
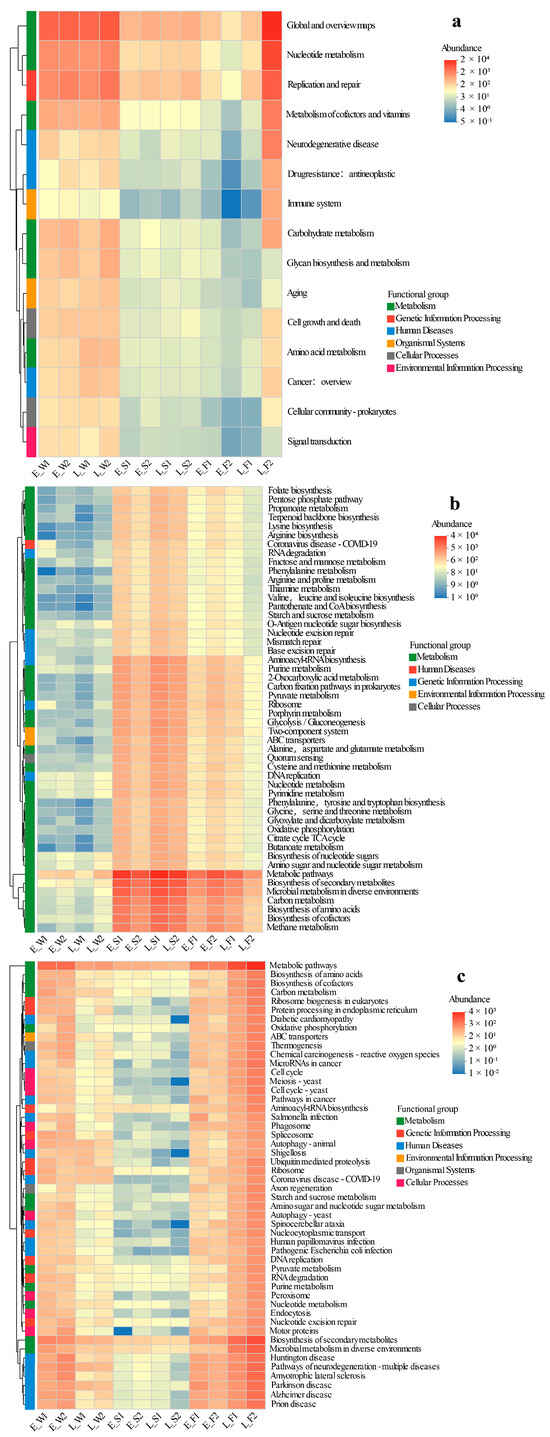
Figure 6.
Heatmap clustering of viral KEGG pathways (hierarchy level 2 (a)), archaeal KEGG functional profiles (level 3 (b)), and fungal KEGG functional profiles (level 3 (c)) across habitats under two models (LFC and IPC) during the early and late stages.
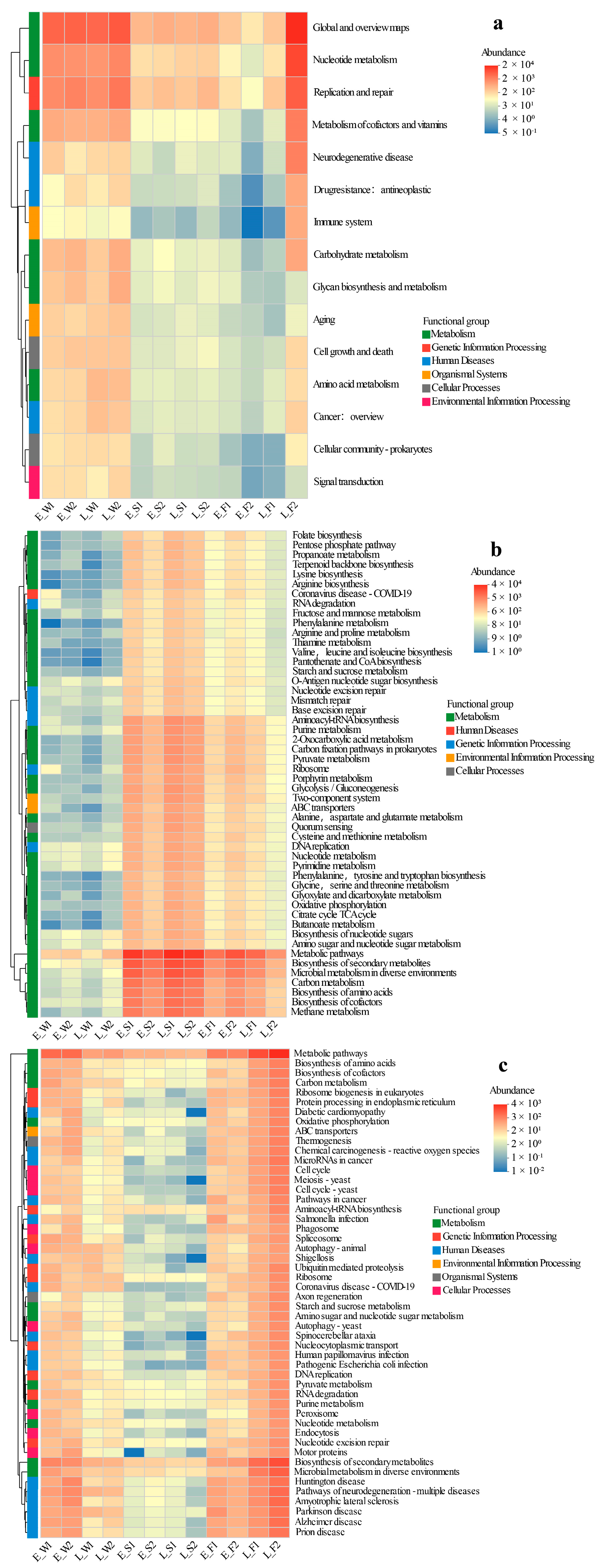
Viral functions displayed a significantly higher relative abundance in water compared to sediment or fish gut microbiomes, with no discernible temporal (early vs. late) or spatial (P1 vs. P2) differences noted in water and sediment, suggesting functional stability. The primary metabolic pathways for water viruses encompassed Global and Overview Maps, Replication and Repair, Metabolism of Cofactors and Vitamins, Nucleotide Metabolism, Carbohydrate Metabolism, and Glycan Biosynthesis and Metabolism. Gut viral communities exhibited minimal functional variation between P1 and P2 in the early stage, but most pathways (e.g., Global Maps, Nucleotide Metabolism, Replication/Repair) were significantly lower in P1 compared to P2 by the late stage (Figure 6a).
The abundances of functional genes in Archaea were notably lower in water compared to sediment and gut environments. Temporally and spatially, the profiles remained consistent between P1 and P2 stages. For early-stage sediment archaeal functions such as Methane Metabolism, Carbon Metabolism, and Propanoate Metabolism, P1 exhibited significantly higher values than P2. However, this disparity was not evident in the late stage. In the gut, while P1 demonstrated reduced core archaeal functions (e.g., Methane Metabolism, TCA Cycle) initially, it eventually surpassed P2 in the late stage (Figure 6b).
The functional profiles of fungi were substantially elevated in water and gut samples compared to sediment, with no discernible P1/P2 differences observed across stages in these compartments. In the late stage, sediment from P1 exhibited a significant upregulation of pathways related to human diseases (e.g., Diabetic Cardiomyopathy, Shigellosis) and cellular functions (e.g., Protein Processing in Endoplasmic Reticulum, Endocytosis). The fungal functions in the gut during P1 were marginally lower than those in P2′s late stage. Temporally, the abundance of water fungi peaked in the early stage and declined in the late stage, while the abundance in the gut increased by the late stage (Figure 6c).
4. Discussion
Compared to IPC, the LFC system demonstrated significantly improved water and sediment quality, characterized by lower levels of TN, TP, NO2−-N, and COD in water and reduced OM and TN in sediment. Furthermore, it exhibited reduced viral risks in both the environment (lower viral richness) and the fish gut (reduced viral functional abundance). Concurrently, the LFC system enhanced beneficial archaeal communities, showing increased diversity and metabolic function abundance in the fish gut. Finally, the LFC displayed an improved fungal community within the fish gut, marked by higher richness and a stable structure. In the following, we present a comparative analysis of both physicochemical parameters and microbial communities between LFC and IPC in detail.
In aquatic ecosystems, macrophytes play a crucial role in water purification by absorbing decomposed nutrients from both the water and sediment, thereby enhancing the remediation of aquaculture water quality [63,64]. Most physicochemical parameters of water and sediment between P1 (LFC) and P2 (IPC) were found to be significantly different in the early stage, but the differences were relatively small in magnitude. In the later stage, P1 showed significantly lower values than P2 for all parameters except pH and sediment TP (Figures S1 and S2), suggesting that the lotus played a significant role in reducing nitrogen and phosphorus loads in water, thereby decreasing the accumulation of organic pollutants and contributing to the water purification [65,66]. Additionally, ecological floating beds in rice–aquaculture systems effectively removed N and P; and aquaponics systems significantly reduced NH4+-N and NO2−-N concentrations in aquatic environments [19,67,68]. The OM content in P1 was significantly lower than that in P2 (p < 0.05). This suggested that LFC effectively enhanced the degradation and utilization of organic matter, thereby reducing its accumulation in sediments, as opposed to IPC [65]. LEfSe revealed enrichment of organic matter-degrading microorganisms in LFC sediments, with fungal (Trichoderma, Aspergillus) and archaeal (Methanosarcina) taxa as key contributors. Tao et al. (2012) quantified OM and TN reduction in lotus ponds, showing 0.59 g/kg OM and 0.74 mg/kg TN loss, respectively [69]. The LFC could significantly reduce the nitrogen content in sediment and even reduce the release of nitrogen and phosphorus [12,66]. Therefore, LFC exhibits substantial benefits over IPC in diminishing nitrogen and phosphorus loads and alleviating organic pollution in aquatic ecosystems.
Notably, viruses, regarded as the most diverse biological entities on our planet, display an unprecedented functional diversity in controlling nutrient cycles across various ecosystems, including freshwater, marine, and terrestrial systems [70,71]. Their ecological integration—from nutrient cycling to microbiome modulation—underscores their indispensable role in planetary biogeochemistry [71,72]. Viral diversity dynamics are influenced by both biotic and abiotic environmental factors [73,74]. This study revealed that the viral richness under the LFC demonstrated a significantly lower diversity compared to the IPC, with notable differences observed in both water and fish intestinal samples (p < 0.05, Table 2). The observed disparity likely stemmed from elevated nutrient inputs (e.g., aquafeed) in IPC, which elevated the OM content and created eutrophic conditions that enhance viral proliferation. Conversely, LFC systems mitigated nitrogen/phosphorus loads, reducing sediment OM and nitrogen accumulation (Figures S1 and S2). This environmental optimization suppressed viral transmission and replication, consequently decreasing infections in fish intestines [75,76]. The diversity of viruses in freshwater ecosystems is influenced by multiple environmental factors, particularly nutrient levels [77,78]. Higher concentrations of nutrients (e.g., nitrogen, phosphorus) in aquatic systems correlated with elevated viral diversity and species richness, driven by enhanced microbial productivity and host availability [79]. Our results revealed that the vast majority of viruses exhibited significant positive correlations with TN, NH4+-N, and TP (p < 0.05, Figure 5a), suggesting their tight coupling with nutrient cycling in aquatic ecosystems [80,81]. Furthermore, dominant viruses (e.g., Caudovirales) reduce viral community alpha diversity by competitively occupying key niches like host receptors or nutrients, driving winner-takes-all dynamics [82,83].
In contrast to IPC, LFC displayed no substantial differences in the dominant viral species and prevalent viral taxa in water and sediment (Figure 3a). However, distinct disparities were observed in the viral community structures within the fish’s intestine, especially in the late stage (Figure 3a). In contrast to LFC, IPC demonstrated a significant enrichment of Kyanoviridae and Herpesviruses in water (Figure 4a). Kyanoviridae, viruses that specifically infect cyanobacteria, have a broad distribution in various environments such as lakes, ponds, and sediments [84]. They showed considerable potential in controlling algal blooms, regulating cyanobacterial populations, and maintaining water quality through mechanisms like host lysis and nutrient cycling [85,86]. Herpesviruses are notable pathogens that extensively spread amongst mammals, birds, and fish. They pose a severe risk to the health of fish, particularly during aquaculture [87,88]. Recent research has determined that the gill-bleeding disease observed in farmed crucian carp across China is attributed to herpesvirus infection, which has brought heavy losses to some aquaculture farms in China [89,90]. High levels of two viruses in the IPC pond water indicated algal blooms and worsening water quality, threatening fish health and growth. Cyanobacteriophages (algae-targeting viruses) showed strong positive correlations with key pollutants, including COD, TN, NH4−-N, NO2−-N, TP, and PO43−-P (Figure 5a). This result further highlighted the high risk of water degradation in the IPC, while the LFC could maintain a relatively high-quality water environment by rhizosphere filtration and microbial self-purification, thus reducing viral pathogen transmission risks. The inhibition of herpesvirus mediated by LFC does not merely augment production by diminishing mortality rates; it also transforms disease management paradigms. This is achieved by decreasing reliance on chemical controls and enriching antagonistic microbiota, which competitively inhibit viral proliferation [91].
Furthermore, the fish intestinal viral communities exhibited significantly greater diversity in the LFC group compared to the IPC group (Table 2, Figure 3a and Figure 4a), with a more stable community structure [92]. Functional analysis indicated suppressed virus metabolic activity in the LFC, contrasting with the active metabolism observed in the IPC. This discrepancy may reflect unfavorable ecological conditions in the LFC that suppress viral replication [8], thereby bolstering host immunity and intestinal health.
Notably, ecological regulation of viral dynamics rarely operates in isolation; archaeal groups, often underappreciated, frequently act as critical intermediaries within microbial communities [93]. As crucial drivers of biogeochemical cycles, archaeal groups possess extraordinary genetic and metabolic diversity. Specific archaeal groups act as keystone species, shaping microbial community composition and function [93]. Their implications for host health and disease prevention remain significant yet largely unexplored [3,94,95]. Compared with the IPC, the sediment archaeal richness in LFC exhibited a significant increase in the early stage (p < 0.05, Table 3). Archaeal richness and diversity in sediments were strongly influenced by plant root exudates, as evidenced by previous studies [96,97]. Lotus roots act as pivotal ecosystem engineers within sediment systems, exerting their influence through three interconnected mechanisms: (1) physical restructuring that enhances sediment porosity and promotes particle aggregation; (2) chemical regulation that modulates redox potential and mediates nutrient fluxes; and (3) biological facilitation of microbial consortia, which drive organic matter degradation and pollutant transformation [98,99,100]. As a result, during the initial cultivation, lotus roots modified sediment, elevating archaeal richness. Later growth stagnation weakened this effect, leading to a gradual convergence of the archaeal community within the sediment between LFC and IPC (Table 3).
Nitrososphaerota are key drivers of nitrification in the nitrogen cycle and critical participants in the carbon cycle [101]. Our results revealed that Nitrososphaerota abundance was significantly positively correlated with NO3−-N levels in water (Figure 5b). By enhancing Nitrososphaerota-mediated ammonia oxidation and organic decomposition capacity, LFC promotes efficient nitrogen transformation, reducing toxic NH4+-N/NO2−-N accumulation and improving water quality (Figure S1). Substantial research has demonstrated the pivotal role of ammonia-oxidizing archaea (AOA) in wastewater treatment and nitrogen pollution remediation [29,102,103]. Euryarchaeota exhibited high relative abundance across diverse habitats under both LFC and IPC systems, representing a pivotal archaeal phylum with a ubiquitous distribution and significant ecological functions [104,105]. Furthermore, unclassified archaea showed a high abundance in sediments and fish gut samples, indicating unresolved ecological and host-adaptive functions that warrant deeper investigation.
Archaeal communities differed significantly between LFC and IPC sediments (Figure 4b). LFC enriched methane-regulating archaea (e.g., Methanoregulaceae, Methanoregula) that couple organic decomposition with methanogenesis to sustain methane equilibrium [106,107]. In contrast, IPC sediments were dominated by obligate acetoclastic (Methanothrix) and versatile methylotrophic methanogens (Methanosarcinales), indicating adaptation to high-organic-load methanogenesis [108,109]. The dominance of Methanothrix and Methanosarcina in acetoclastic methanogenesis—contributing ∼60% to biogenic methane emissions—highlights their ecological resilience under high-organic-load conditions typical of conventional aquaculture [110,111]. IPC fostered benthic methanogen proliferation through continuous organic deposition, ultimately exacerbating methane flux and sediment anoxia, with concomitant risks of eutrophication and microbial dysbiosis. In contrast, the LFC enriched archaeal consortia (e.g., Methanoregula spp.) that orchestrated a methane production–consumption equilibrium via coupled organic decomposition and methanotrophy, reflecting its environmentally benign nature and low-emission characteristics [112]. The Methanoregula-driven organic matter depletion in LFC sediments mechanistically explained the observed negative correlation (Figure 5b), coupled with reduced organic matter levels in sediments under the LFC (Figure S2). This aligns with the reported function of Methanoregula in accelerating organic matter mineralization while suppressing methanogenesis, thereby depleting sediment organic carbon pools [113].
Furthermore, our findings revealed that the fish gut microbiota in the LFC system exhibited continuous and significant enrichment of Methanobacteriales-like archaea, correlating with a more stable gut microbial community structure. The persistent dominance of Methanobacteriales archaea (e.g., Methanobrevibacter) in LFC fish guts likely stabilized microbial networks by consuming excess H2 from bacterial fermentation, thereby suppressing hydrogen accumulation-induced dysbiosis [114]. Methanobacteriales archaea utilize H2/CO2 for methanogenesis while oxidizing formate, sustaining low gut H2 pressure [115,116,117]. This dual activity boosts microbial activity, prevents formate buildup, and optimizes intestinal health and microbial balance.
Notably, the metabolic functional abundance of diverse archaeal groups—particularly those involved in methanogenesis, nitrogen fixation, and organic matter mineralization—was markedly elevated in LFC relative to IPC in the late stage (Figure 6b). This archaeal functional enhancement likely expedited sediment nutrient cycling, with methanogenic archaea converting organic waste into bioavailable methane/CO2 and nitrogen-fixing archaea increasing ammonium availability for primary producers. The collaborative impact of archaea-driven nutrient recycling and host metabolic optimization collectively enhanced the stability of the aquaculture environment. This resulted in a reduction in ammonia-N accumulation and COD in comparison to IPC (Figure S1), ultimately contributing to improved fish health [118]. The KEGG-annotated archaeal metabolic enhancement and carbon fixation align with LFC’s sustainable design: lotus roots secrete oxygen to create redox gradients, promoting archaeal syntropy with fermentative bacteria while suppressing sulfate-reducing competitors. This archaeal functional resilience underscores LFC’s potential in mitigating aquaculture-induced methane emissions [119].
These archaeal-driven biogeochemical shifts further create niches for fungi, which are relatively rare but play essential roles in substrate mineralization, micronutrient supply, and intestinal barrier function [38,120]. The LFC system demonstrated significantly (p < 0.05) higher sediment fungal richness and gut microbiota diversity than IPC (Table 4), with improved community stability in sediment and the fish gut (Figure 2h,i and Figure 3c). Our findings align with evidence that root exudates (e.g., sugars, organic acids, and secondary metabolites) selectively recruit specific fungal taxa such as arbuscular mycorrhizal fungi (AMF), thereby modulating rhizosphere fungal diversity [121,122]. Therefore, the LFC system showed a superior performance over IPC in optimizing aquaculture microenvironments (sediment/gut) and facilitating nutrient–energy transfer, offering practical benefits for sustainable production.
The IPC water was dominated by Geotrichum and Coemansia fungi, with significant enrichment of chytrids and yeast-like taxa. Notably, Coemansia (Zygomycota) represents a saprophytic fungal group associated with organic matter decomposition [123,124]. The dominance of saprophytic Coemansia and Geotrichum suggested active organic matter processing in IPC water [125]. Coemansia abundance positively correlated with nitrogen (TN, NH4+–N, NO2−–N) and phosphorus (TP, PO43−–P) levels (Figure 5c), indicating organic pollution from sources like fish feed. This pattern implies that Coemansia thrives under elevated nutrient conditions, making it a potential microbial sentinel for aquaculture-derived organic pollution. Yeasts are recognized organic matter degraders in eutrophic systems [126], aligning with the observed COD accumulation in IPC water—a hallmark of microbial carbon processing under nutrient enrichment.
In contrast, the LFC water exhibited significant enrichment of fungal taxa from Agaricomycetes and Aspergillus spp. This enrichment facilitated the enhancement of organic matter turnover by means of extracellular enzymes, thereby mitigating eutrophication through the acceleration of the degradation of complex organic compounds [127,128]. Hyphal exudates of Rhizophagus spp. chemoattract phosphate-solubilizing bacteria, forming synergistic partnerships that boost P uptake by plants and fungi [129,130]. Therefore, within the LFC, this interaction likely facilitated fungal-mediated phosphorus cycling, decreasing water phosphorus levels and reducing eutrophication risk. Our findings supported this view, with PO43−–P levels significantly decreased at the later vs. early stage in LFC (p < 0.001). Conversely, IPC showed a reversal in trend (Figure S1i). Notably, late-stage TP in LFC remained significantly lower than in IPC (p < 0.001; Figure S1e).
Furthermore, compared to IPC, the LFC was dominated by taxa specialized in organic degradation in sediment (e.g., Trichodermaceae, Peziza, Monoblepharidomycetes, Figure 3c). Notably, Trichoderma species (Trichodermaceae) exert dual advantageous functions: (1) promoting nitrogen/phosphorus transformation and reducing organic phosphorus pollution to improve plant nutrient absorption; and (2) inhibiting pathogens by sequestering Fe3+ for biological control [131,132]. Therefore, the LFC achieved (1) dynamic phosphorus regulation through microbial interventions (Figure S2); (2) self-purification through optimized organic degradation (Figure S2); and (3) sustainability by minimizing chemical inputs and reducing pathogen loads (Figure 6c).
The fish intestines in LFC were significantly enriched with the fungal taxa Dothideomycetes, Pleosporales, Lindneromyces, and Nectriaceae (Figure 4c). These fungi facilitated the decomposition of organic matter and nutrient cycling, significantly contributing to ecological interactions and host adaptation [133,134]. Notably, they played pivotal roles in pathogen suppression, immune modulation, and the enhancement of symbiotic networks and community stability [135]. In contrast, only the fungal genus Vittaforma was significantly enriched in the late-stage IPC fish intestines (Figure 4c), reflecting intestinal environmental homogenization and potentially increased pathogen pressure. Conversely, enriched fungal taxa in the LFC enhanced ecological functions and health benefits, underscoring its superiority for ecosystem stability and sustainability.
In conclusion, the significantly higher fungal abundance observed in LFC sediments and crucian carp intestines (vs. IPC; p < 0.05) revealed a heightened ecological self-purification capability. This is likely attributed to the fungal-mediated decomposition of organic matter and suppression of pathogens. Such functional augmentation suggested a superior sustainability potential for the LFC system, potentially due to enhanced nutrient cycling efficiency and resilience against environmental disturbances. These findings on LFC enable scalable aquaculture integration, resolving water pollution, disease risk, and sustainability challenges through microbiome-driven mechanisms. Firstly, the efficacy of LFC stems from its promotion of beneficial archaeal and fungal networks, which suppress viral pathogens while mitigating pollutants (total nitrogen, phosphorus, COD). To scale this self-purification capacity, modular systems could integrate layered sediment zones to enhance archaeal ammonia oxidation and fungal organic matter decomposition, alongside controlled aeration to sustain a low hydrogen partial pressure—a condition stabilized by Methanobacteria. Biofilters enriched with these functional microbes may further optimize pollutant removal in industrial aquaculture. Secondly, these findings highlight the role of specific microbes (e.g., Methanobacteria in intestines, ammonia-oxidizing archaea, and fungi in sediment) in improving host health and environmental resilience. Probiotics co-encapsulating Methanobacteria and antifungal strains (e.g., Saccharomyces) can be integrated into aquafeeds to deplete intestinal H2, suppress facultative anaerobes (e.g., pathogenic E. coli or Aeromonas), and reduce infection-driven mortality in high-density aquaculture [136]. Thirdly, LFC’s higher ecological self-purification ability and sustainability potential align with growing consumer and regulatory demands for eco-certified aquaculture.
5. Conclusions
The present metagenomic analysis revealed a comparison of microbial communities (viruses, archaea, fungi) between LFC and IPC. The LFC increased sustainability through three primary mechanisms: (1) by reducing viral diversity and stabilizing host–virus interactions, thereby creating a healthier environment; (2) by modifying the structure of archaeal communities to enhance environmental remediation, energy efficiency, and host health; and (3) by optimizing fungal communities, thereby improving phosphorus cycling, organic degradation, and pathogen resistance. Taken together, these microbial-driven effects contribute to a more stable and low-risk aquaculture ecosystem. Future studies must resolve archaeal–fungal syntropy in LFC by quantifying metabolite exchange between ammonia-oxidizing archaea and ligninolytic fungi, and its role in regulating methanogenic pathways. Single-cell transcriptomics of Methanobacteria should define H2-depletion-mediated suppression of facultative anaerobes, while delineating hydrogenase-dependent immunomodulatory networks that mitigate infection.
Supplementary Materials
The following supporting information can be downloaded at https://www.mdpi.com/article/10.3390/biology14081092/s1. Figure S1: Variations in water physicochemical parameters under LFC and IPC in different culture periods. a, temperature (T, °C); b, dissolved oxygen (DO, mg/L); c, pH; d, total nitrogen (TN, mg/L); e, total phosphorus (TP, mg/L); f, ammonia nitrogen (NH4+–N, mg/L); g, nitrate nitrogen (NO3−–N, mg/L); h, nitrite nitrogen (NO2−–N, mg/L); i, phosphate (PO43−–P, mg/L); j, chemical oxygen demand (COD, mg/L). E represents the early stage, L represents the late stage; 1 represents P1 (LFC), 2 represents P2 (IPC). Figure S2: Variations in sediment physicochemical parameters under LFC and IPC in different culture periods. a, pH; b, total nitrogen (TN, mg/kg); c, total phosphorus (TP, mg/kg); d, ammonia nitrogen (NH4+–N, mg/kg); e, nitrate nitrogen (NO3−–N, mg/kg); f, organic matter (OM, g/kg). E represents the early stage, L represents the late stage. Table S1: Quality assessment in metagenomic sequencing including accession number, read number, and GC content of clean data.
Author Contributions
Q.Z.: investigation, methodology, visualization, and writing—original draft. Z.W.: investigation, methodology, visualization, and editing. Z.S.: investigation. W.L.: investigation. K.L.: project administration and funding acquisition. Q.Q.: funding acquisition. S.L.: investigation, methodology, validation, and writing—review and editing. Q.G.: conceptualization, formal analysis, investigation, methodology, and writing—review and editing. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the National Key Research and Development Program of China, grant number 2023YFD2400902; the earmarked fund for HARS, grant number HARS-07; the National Key Research and Development Plan Program, grant number 2023YFD2401605; the Scientific Research Foundation of Hunan Provincial Education Department, grant number 23B0073; and the aid program of the Science and Technology Innovative Research Team in Higher Educational Institutions of Hunan Province.
Institutional Review Board Statement
This study received approval from Hunan Normal University’s College of Life Sciences Animal Ethics Committee (No. 2024-825) for all piscine experimental protocols.
Informed Consent Statement
Not applicable.
Data Availability Statement
The data are contained within the article.
Conflicts of Interest
Authors Wuhui Li and Qinbo Qin were employed by the company Hunan Yuelu Mountain Science and Technology Co. Ltd. Amid at Aquatic Breeding. The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
References
- Wang, H.J.; Wang, P.Z.; Xu, C.; Sun, Y.F.; Shi, L.; Zhou, L.; Jeppesen, E.; Chen, J.; Xie, P. Can the “10-year fishing ban” rescue biodiversity of the Yangtze River? Innovation 2022, 3, 100235. [Google Scholar] [CrossRef]
- Jin, B.S.; Winemiller, K.O.; Ren, W.W.; Tickner, D.; Wei, X.H.; Guo, L.; Li, Q.; Zhang, H.; Pompeu, P.S.; Goichot, M.; et al. Basin-scale approach needed for Yangtze River fisheries restoration. Fish Fish. 2022, 23, 1009–1015. [Google Scholar] [CrossRef]
- Dong, F.; Fang, D.D.; Zhang, H.; Wei, Q.W. Protection and development after the ten-year fishing ban in the Yangtze River. J. Fish. China 2023, 47, 245–259. [Google Scholar]
- Gui, J.F. Chinese wisdom and modern innovation of aquaculture. Water Biol. Secur. 2024, 3, 100271. [Google Scholar] [CrossRef]
- Xie, B.; Qin, J.; Yang, H.; Wang, X.; Wang, Y.H.; Li, T.Y. Organic aquaculture in China: A review from a global perspective. Aquaculture 2013, 414, 243–253. [Google Scholar] [CrossRef]
- Chen, R.Z.; Wong, M.H. Integrated wetlands for food production. Environ. Res. 2016, 148, 429–442. [Google Scholar] [CrossRef]
- Gu, Q.H.; Zeng, Q.Q.; Li, Z.X.; Wang, X.; Peng, B.; Rao, K.; Shen, Z.Y.; Wu, C.; Qin, Q.B.; Luo, K.K.; et al. Effects on rice grains cadmium reduction and the characteristics of rice and fish in rice-fish coculture system. J. Fish. China 2023, 47, 142–152. [Google Scholar]
- Chen, X.; Fan, L.M.; Qiu, L.P.; Dong, X.X.; Wang, Q.; Hu, G.D.; Meng, S.L.; Li, D.D.; Chen, J.Z. Metagenomics Analysis Reveals Compositional and Functional Differences in the Gut Microbiota of Red Swamp Crayfish, Procambarus clarkii, Grown on Two Different Culture Environments. Front. Microbiol. 2021, 12, 735190. [Google Scholar] [CrossRef]
- Ren, W.Z.; Hu, L.L.; Guo, L.; Zhang, J.; Tang, L.; Zhang, E.T.; Zhang, J.E.; Luo, S.M.; Tang, J.J.; Chen, X. Preservation of the genetic diversity of a local common carp in the agricultural heritage rice-fish system. Proc. Natl. Acad. Sci. USA 2018, 115, E546–E554. [Google Scholar] [CrossRef]
- Ignowski, L.; Belton, B.; Ali, H.; Thilsted, S.H. Integrated aquatic and terrestrial food production enhances micronutrient and economic productivity for nutrition-sensitive food systems. Nat. Food 2023, 4, 866–873. [Google Scholar] [CrossRef] [PubMed]
- Yang, Z.; Sun, M.; Peng, L.; Dai, L.L.; Zhu, J.Q.; Li, G.; Tao, L.; Zhang, H. Reduction of nutrient fluxes across the sediment-water interface and nutrient accumulation in lotus-fish co-culture aquaculture ponds. Aquacult. Int. 2024, 32, 7683–7694. [Google Scholar] [CrossRef]
- Yang, Z.; Yao, Y.H.; Sun, M.; Li, G.; Zhu, J.Q. Metagenomics Reveal Microbial Effects of Lotus Root-Fish Co-Culture on Nitrogen Cycling in Aquaculture Pond Sediments. Microorganisms 2022, 10, 1740. [Google Scholar] [CrossRef] [PubMed]
- Ma, L.; Zhu, J.; Chen, Q.; Li, W.; Huang, G.H. Beneficial effects of fish stocking on performance and pest control in the lotus field system. Aquac. Environ. Interact. 2017, 9, 321–329. [Google Scholar] [CrossRef]
- Chen, C.Z.; Li, P.; Liu, L.; Li, Z.H. Exploring the interactions between the gut microbiome and the shifting surrounding aquatic environment in fisheries and aquaculture: A review. Environ. Res. 2022, 214, 114202. [Google Scholar] [CrossRef]
- Hamilton, E.F.; Element, G.; de Groot, P.V.; Engel, K.; Neufeld, J.D.; Shah, V.; Walker, V.K. Anadromous Arctic Char Microbiomes: Bioprospecting in the High Arctic. Front. Bioeng. Biotechnol. 2019, 7, 32. [Google Scholar] [CrossRef]
- Sehnal, L.; Brammer-Robbins, E.; Wormington, A.M.; Blaha, L.; Bisesi, J.; Larkin, I.; Martyniuk, C.J.; Simonin, M.; Adamovsky, O. Microbiome Composition and Function in Aquatic Vertebrates: Small Organisms Making Big Impacts on Aquatic Animal Health. Front. Microbiol. 2021, 12, 567408. [Google Scholar] [CrossRef] [PubMed]
- Singh, B.K.; Thakur, K.; Kumari, H.; Mahajan, D.; Sharma, D.; Sharma, A.K.; Kumar, S.; Singh, B.; Pankaj, P.P.; Kumar, R. A review on comparative analysis of marine and freshwater fish gut microbiomes: Insights into environmental impact on gut microbiota. FEMS Microbiol. Ecol. 2025, 101, fiae169. [Google Scholar] [CrossRef] [PubMed]
- Kiat, E.O.J.; Nair, T.; Sobana, M.; Hann, N.T.; Domingos, J.A.; Gomes, G.B. Identification of scale drop disease virus based on environment DNA in an aquaculture facility of Singapore. Aquaculture 2023, 563, 738993. [Google Scholar] [CrossRef]
- Li, F.B.; Feng, J.F.; Zhou, X.Y.; Xu, C.C.; Jijakli, M.H.; Zhang, W.J.; Fang, F.P. Impact of rice-fish/shrimp co-culture on the N2O emission and NH3 volatilization in intensive aquaculture ponds. Sci. Total Environ. 2019, 655, 284–291. [Google Scholar] [CrossRef]
- Yajima, D.; Fujita, H.; Hayashi, I.; Shima, G.; Suzuki, K.; Toju, H. Core species and interactions prominent in fish-associated microbiome dynamics. Microbiome 2023, 11, 53. [Google Scholar] [CrossRef]
- El-Son, M.A.M.; Elbahnaswy, S.; Khormi, M.A.; Aborasain, A.M.; Abdelhaffez, H.H.; Zahran, E. Harnessing the fish gut microbiome and immune system to enhance disease resistance in aquaculture. Fish Shellfish. Immunol. 2025, 163, 110394. [Google Scholar] [CrossRef]
- Jia, P.P.; Junaid, M.; Wen, P.P.; Yang, Y.F.; Li, W.G.; Yang, X.G.; Pei, D.S. Role of germ-free animal models in understanding interactions of gut microbiota to host and environmental health: A special reference to zebrafish. Environ. Pollut. 2021, 279, 116925. [Google Scholar] [CrossRef] [PubMed]
- Ghosh, A.; Rathore, A.; Gaba, S.; Kamli, M.R.; Maigoro, A.Y.; Kwon, H.W.; Mahajan, N.; Kim, C.B.; Malik, A. The Chinese mitten crab (Eriocheir sinensis) and its microbiome: A review. Aquaculture 2025, 595, 741518. [Google Scholar] [CrossRef]
- Martínez-Porchas, M.; Vargas-Albores, F. Microbial metagenomics in aquaculture: A potential tool for a deeper insight into the activity. Rev. Aquac. 2017, 9, 42–56. [Google Scholar] [CrossRef]
- Moschos, S.; Kormas, K.A.; Karayanni, H. Prokaryotic diversity in marine and freshwater recirculating aquaculture systems. Rev. Aquac. 2022, 14, 1861–1886. [Google Scholar] [CrossRef]
- Zhang, Z.M.; Yang, Q.S.; Liu, H.K.; Jin, J.Y.; Yang, Y.X.; Zhu, X.M.; Han, D.; Zhou, Z.G.; Xie, S.Q. Potential Functions of the Gut Microbiome and Modulation Strategies for Improving Aquatic Animal Growth. Rev. Aquac. 2025, 17, e12959. [Google Scholar] [CrossRef]
- Borges, N.; Keller-Costa, T.; Sanches-Fernandes, G.M.M.; Louvado, A.; Gomes, N.C.M.; Costa, R. Bacteriome Structure, Function, and Probiotics in Fish Larviculture: The Good, the Bad, and the Gaps. Annu. Rev. Anim. Biosci. 2021, 9, 423–452. [Google Scholar] [CrossRef] [PubMed]
- Medina-Félix, D.; Garibay-Valdez, E.; Vargas-Albores, F.; Martínez-Porchas, M. Fish disease and intestinal microbiota: A close and indivisible relationship. Rev. Aquac. 2023, 15, 820–839. [Google Scholar] [CrossRef]
- Wang, Q.F.; Ruan, Z.C.; Wang, Y.B.; Zhang, Y.; Chai, X.J. Comparison of growth performance, oxidative stress, and intestinal microbiota of rockfish (Sebastiscus marmoratus) in different culture modes. Fish Physiol. Biochem. 2025, 51, 54. [Google Scholar] [CrossRef]
- Xavier, R.; Severino, R.; Silva, S.M. Signatures of dysbiosis in fish microbiomes in the context of aquaculture. Rev. Aquac. 2024, 16, 706–731. [Google Scholar] [CrossRef]
- Vargas-Albores, F.; Martínez-Córdova, L.R.; Hernández-Mendoza, A.; Cicala, F.; Lago-Lestón, A.; Martínez-Porchas, M. Therapeutic modulation of fish gut microbiota, a feasible strategy for aquaculture? Aquaculture 2021, 544, 737050. [Google Scholar] [CrossRef]
- Lee, J.G.; Cho, H.J.; Jeong, Y.M.; Lee, J.S. Genetic Approaches Using Zebrafish to Study the Microbiota-Gut-Brain Axis in Neurological Disorders. Cells 2021, 10, 566. [Google Scholar] [CrossRef]
- Zhuang, M.M.; Zhang, X.; Cai, J. Microbiota-gut-brain axis: Interplay between microbiota, barrier function and lymphatic system. Gut Microbes 2024, 16, 2387800. [Google Scholar] [CrossRef] [PubMed]
- Chuphal, N.; Singha, K.P.; Sardar, P.; Sahu, N.P.; Shamna, N.; Kumar, V. Scope of Archaea in Fish Feed: A New Chapter in Aquafeed Probiotics? Probiotics Antimicrob. 2021, 13, 1668–1695. [Google Scholar] [CrossRef] [PubMed]
- Deb, S.; Das, L.; Das, S.K. Composition and functional characterization of the gut microbiome of freshwater pufferfish (Tetraodon cutcutia). Arch. Microbiol. 2020, 202, 2761–2770. [Google Scholar] [CrossRef]
- Gaci, N.; Borrel, G.; Tottey, W.; O'Toole, P.W.; Brugère, J.F. Archaea and the human gut: New beginning of an old story. World J. Gastroenterol. 2014, 20, 16062–16078. [Google Scholar] [CrossRef]
- Pimentel, M.; Gunsalus, R.P.; Rao, S.S.; Zhang, H. Methanogens in human health and disease. Am. J. Gastroenterol. Suppl. 2012, 1, 28–33. [Google Scholar] [CrossRef]
- Richard, M.L.; Sokol, H. The gut mycobiota: Insights into analysis, environmental interactions and role in gastrointestinal diseases. Nat. Rev. Gastro. Hepat. 2019, 16, 331–345. [Google Scholar] [CrossRef]
- Nyman, A.; Huyben, D.; Lundh, T.; Dicksved, J. Effects of microbe- and mussel-based diets on the gut microbiota in Arctic charr (Salvelinus alpinus). Aquac. Rep. 2017, 5, 34–40. [Google Scholar] [CrossRef]
- Egerton, S.; Culloty, S.; Whooley, J.; Stanton, C.; Ross, R.P. The gut microbiota of marine fish. Front. Microbiol. 2018, 9, 873. [Google Scholar] [CrossRef]
- Chen, Y.Q.; Yang, C.; Deng, Z.H.; Xiang, T.W.; Tan, J.L.; Xu, J.Z.; Sun, D.; Luo, F. Alterations of gut virome with close interaction in the progression of estrogen deficiency-induced osteoporosis. Gut Microbes 2024, 16, 2437250. [Google Scholar] [CrossRef]
- Pfeiffer, J.K.; Virgin, H.W. Transkingdom control of viral infection and immunity in the mammalian intestine. Science 2016, 351, aad5872. [Google Scholar] [CrossRef]
- Tiamani, K.; Luo, S.Q.; Schulz, S.; Xue, J.L.; Costa, R.; Mirzaei, M.K.; Deng, L. The role of virome in the gastrointestinal tract and beyond. FEMS Microbiol. Rev. 2022, 46, fuac027. [Google Scholar] [CrossRef]
- Filipa-Silva, A.; Parreira, R.; Martínez-Puchol, S.; Bofill-Mas, S.; Crespo, M.T.B.; Nunes, M. The Unexplored Virome of Two Atlantic Coast Fish: Contribution of Next-Generation Sequencing to Fish Virology. Foods 2020, 9, 1634. [Google Scholar] [CrossRef]
- Noureldin, S.M.; Diab, A.M.; Salah, A.S.; Mohamed, R.A. Effect of different monochromatic LED light colors on growth performance, behavior, immune-physiological responses of gold fish, Carassius auratus. Aquaculture 2021, 538, 736532. [Google Scholar] [CrossRef]
- GB 11894-89. Available online: https://codeofchina.com/standard/GB11894-1989.html (accessed on 12 August 2025).
- GB 11893-89. Available online: https://codeofchina.com/standard/GB11893-1989.html (accessed on 12 August 2025).
- HJ/T 346-2007. Available online: https://www.chinesestandard.net/PDF/English.aspx/HJT346-2007 (accessed on 12 August 2025).
- GB 7479-87. Available online: https://english.mee.gov.cn/standards_reports/standards/water_environment/method_standard2/201010/t20101027_196755.htm (accessed on 12 August 2025).
- GB 7493-1987. Available online: https://www.gbstandards.org/GB_standard_english.asp?code=GB/T%207493-1987 (accessed on 12 August 2025).
- GB/T 32208-2015. Available online: https://www.transcustoms.com/GB_Standards/GB_standards_english.asp?code=GB/T%2032208-2015 (accessed on 12 August 2025).
- NY/T 1377-2007. Available online: https://www.chinesestandard.net/PDF/English.aspx/NYT1377-2007 (accessed on 12 August 2025).
- NY/T 1121.6-2006. Available online: https://www.chinesestandard.net/PDF/English.aspx/NYT1121.6-2006 (accessed on 12 August 2025).
- NY/T 1121.24-2012. Available online: https://www.chinesestandard.net/PDF/English.aspx/NYT1121.24-2012 (accessed on 12 August 2025).
- NY/T 88-1988. Available online: https://www.chinesestandard.net/PDF/English.aspx/NYT88-1988 (accessed on 12 August 2025).
- HJ 634-2012. Available online: https://www.chinesestandard.net/PDF/English.aspx/HJ634-2012 (accessed on 12 August 2025).
- GB/T 32737-2016. Available online: https://www.codeofchina.com/standard/GBT32737-2016.html (accessed on 12 August 2025).
- Li, H.; Durbin, R. Fast and accurate short read alignment with Burrows-Wheeler transform. Bioinformatics 2009, 25, 1754–1760. [Google Scholar] [CrossRef]
- Hyatt, D.; Chen, G.L.; LoCascio, P.F.; Land, M.L.; Larimer, F.W.; Hauser, L.J. Prodigal: Prokaryotic gene recognition and translation initiation site identification. BMC Bioinform. 2010, 11, 119. [Google Scholar] [CrossRef] [PubMed]
- Gu, S.; Fang, L.; Xu, X. Using SOAPaligner for Short Reads Alignment. Curr. Protoc. Bioinform. 2013, 44, 11.11.1–11.11.17. [Google Scholar] [CrossRef] [PubMed]
- Ren, L.J.; Lu, Z.; Xia, X.M.; Peng, Y.Y.; Gong, S.Q.; Song, X.Y.; Jeppesen, E.; Han, B.P.; Wu, Q.L. Metagenomics reveals bacterioplankton community adaptation to long-term thermal pollution through the strategy of functional regulation in a subtropical bay. Water Res. 2022, 216, 118298. [Google Scholar] [CrossRef] [PubMed]
- Buchfink, B.; Reuter, K.; Drost, H.G. Sensitive protein alignments at tree-of-life scale using DIAMOND. Nat. Methods 2021, 18, 366. [Google Scholar] [CrossRef]
- Scheffer, M.; Hosper, S.H.; Meijer, M.L.; Moss, B.; Jeppesen, E. Alternative equilibria in shallow lakes. Trends Ecol. Evol. 1993, 8, 275–279. [Google Scholar] [CrossRef]
- Shen, C.Q.; Wu, D.; Chen, B.S.; Khazaei, B.; Han, C.H. Phosphorus removal potential of aquatic macrophytes in a shallow eutrophic system. Hydrobiologia 2023, 850, 3935–3948. [Google Scholar] [CrossRef]
- Seo, D.C.; DeLaune, R.D.; Han, M.J.; Lee, Y.C.; Bang, S.B.; Oh, E.J.; Chae, J.H.; Kim, K.S.; Park, J.H.; Cho, J.S. Nutrient uptake and release in ponds under long-term and short-term lotus (Nelumbo nucifera) cultivation: Influence of compost application. Ecol. Eng. 2010, 36, 1373–1382. [Google Scholar] [CrossRef]
- Wang, Y.K.; Ji, Z.H.; Li, X.Q.; Long, Z.W.; Pei, Y.S. Comprehensive analysis of the migration and transformation of nutrients between sediment and overlying water in complex habitat systems. Sci. Total Environ. 2022, 852, 158433. [Google Scholar] [CrossRef]
- Li, G.; Tao, L.; Li, X.L.; Peng, L.; Song, C.F.; Dai, L.L.; Wu, Y.Z.; Xie, L. Design and performance of a novel rice hydroponic biofilter in a pond-scale aquaponic recirculating system. Ecol. Eng. 2018, 125, 1–10. [Google Scholar] [CrossRef]
- Wongkiew, S.; Park, M.R.; Chandran, K.; Khanal, S.K. Aquaponic Systems for Sustainable Resource Recovery: Linking Nitrogen Transformations to Microbial Communities. Environ. Sci. Technol. 2018, 52, 12728–12739. [Google Scholar] [CrossRef]
- Tao, L.; Zhang, C.-X.; Zhu, J.-Q.; Zhang, S.-Y.; Li, X.-L.; Li, G. Improving fishpond sediment by aquatic vegetable rotation. Adv. J. Food Sci. Technol. 2012, 4, 327–331. [Google Scholar]
- Koonin, E.V.; Dolja, V.V. A virocentric perspective on the evolution of life. Curr. Opin. Virol. 2013, 3, 546–557. [Google Scholar] [CrossRef]
- Sime-Ngando, T. Environmental bacteriophages: Viruses of microbes in aquatic ecosystems. Front. Microbiol. 2014, 5, 355. [Google Scholar] [CrossRef]
- Jansson, J.K.; Wu, R.N. Soil viral diversity, ecology and climate change. Nat. Rev. Microbiol. 2023, 21, 296–311. [Google Scholar] [CrossRef]
- Jackson, E.F.; Jackson, C.R. Viruses in wetland ecosystems. Freshw. Biol. 2008, 53, 1214–1227. [Google Scholar] [CrossRef]
- Jiang, S.; Fu, W.; Chu, W.; Fuhrman, J.A. The vertical distribution and diversity of marine bacteriophage at a station off Southern California. Microb. Ecol. 2003, 45, 399–410. [Google Scholar] [CrossRef]
- Geoghegan, J.L.; Di Giallonardo, F.; Cousins, K.; Shi, M.; Williamson, J.E.; Holmes, E.C. Hidden diversity and evolution of viruses in market fish. Virus Evol. 2018, 4, vey031. [Google Scholar] [CrossRef]
- Moreno, P.; Olveira, J.G.; Labella, A.; Cutrín, J.M.; Baro, J.C.; Borrego, J.J.; Dopazo, C.P. Surveillance of viruses in wild fish populations in areas around the Gulf of Cadiz (South Atlantic Iberian Peninsula). Appl. Environ. Microb. 2014, 80, 6560–6571. [Google Scholar] [CrossRef]
- Bonachela, J.A. Viral plasticity facilitates host diversity in challenging environments. Nat. Commun. 2024, 15, 7473. [Google Scholar] [CrossRef]
- Che, R.Q.; Bai, M.; Xiao, W.; Zhang, S.Y.; Wang, Y.X.; Cui, X.L. Nutrient levels and prokaryotes affect viral communities in plateau lakes. Sci. Total Environ. 2022, 839, 156033. [Google Scholar] [CrossRef]
- Zhou, Z.C.; Tran, P.Q.; Martin, C.; Rohwer, R.R.; Baker, B.J.; McMahon, K.D.; Anantharaman, K. Unravelling viral ecology and evolution over 20 years in a freshwater lake. Nat. Microbiol. 2025, 10, 231–245. [Google Scholar] [CrossRef] [PubMed]
- Yu, Q.L.; Hu, X.Q.; Qian, Y.; Wang, Y.; Shi, C.W.; Qi, R.; Hedenec, P.; Nan, Z.B.; Li, H. Virus communities rather than bacterial communities contribute more on nutrient pool in polluted aquatic environment. J. Environ. Sci. 2025, 154, 550–562. [Google Scholar] [CrossRef]
- Yuan, L.; Yu, P.F.; Huang, X.Y.; Zhao, Z.; Chen, L.X.; Ju, F. Seasonal succession, host associations, and biochemical roles of aquatic viruses in a eutrophic lake plagued by cyanobacterial blooms. Environ. Int. 2024, 193, 109125. [Google Scholar] [CrossRef] [PubMed]
- Ojosnegros, S.; Delgado-Eckert, E.; Beerenwinkel, N. Competition-colonization trade-off promotes coexistence of low-virulence viral strains. J. R. Soc. Interface 2012, 9, 2244–2254. [Google Scholar] [CrossRef]
- Van Goethem, M.W.; Swenson, T.L.; Trubl, G.; Roux, S.; Northen, T.R. Characteristics of Wetting-Induced Bacteriophage Blooms in Biological Soil Crust. MBIO 2019, 10, e02287-19. [Google Scholar] [CrossRef]
- Yu, M.S.; Zhang, M.H.; Zeng, R.Y.; Cheng, R.L.; Zhang, R.; Hou, Y.P.; Kuang, F.F.; Feng, X.J.; Dong, X.Y.; Li, Y.F.; et al. Diversity and potential host-interactions of viruses inhabiting deep-sea seamount sediments. Nat. Commun. 2024, 15, 3228. [Google Scholar] [CrossRef]
- Safferman, R.S.; Morris, M.E. Algal virus: Isolation. Science 1963, 140, 679–680. [Google Scholar] [CrossRef]
- Zhang, Y.Y.; Huang, L.L.; Wang, X.Y.; Ding, S.M.; LIU, Z.H.; Tong, Y.D. Regulation of cyanobacteria population density by cyanophage and its effect on material circulation in water. J. Lake Sci. 2022, 34, 376–390. [Google Scholar] [CrossRef]
- Hanson, L.; Dishon, A.; Kotler, M. Herpesviruses that Infect Fish. Viruses 2011, 3, 2160–2191. [Google Scholar] [CrossRef]
- Tang, R.Z.; Lu, L.Q.; Wang, B.Y.; Yu, J.; Wang, H. Identification of the Immediate-Early Genes of Cyprinid Herpesvirus 2. Viruses 2020, 12, 994. [Google Scholar] [CrossRef] [PubMed]
- Su, M.Z.; Tang, R.Z.; Wang, H.; Lu, L.Q. Suppression effect of plant-derived berberine on cyprinid herpesvirus 2 proliferation and its pharmacokinetics in Crucian carp (Carassius auratus gibelio). Antivir. Res. 2021, 186, 105000. [Google Scholar] [CrossRef] [PubMed]
- Zhu, M.; Liu, B.; Cao, G.L.; Hu, X.L.; Wei, Y.H.; Yi, J.T.; Zhou, Y.; Pan, G.; Wang, J.H.; Xue, R.Y.; et al. Identification and rapid diagnosis of the pathogen responsible for haemorrhagic disease of the gill of Allogynogenetic crucian carp. J. Virol. Methods 2015, 219, 67–74. [Google Scholar] [CrossRef] [PubMed]
- Li, S.N.; Gu, Q.H.; Li, Z.X.; Zeng, Q.Q.; Zhong, H.; Liu, M.Q.; Chen, J.Y.; Zhou, Y.; Liu, S.J.; Hu, S.B. The effects of lotus-fish co-culture on the gut microbiome of Hefang crucian carp (Carassis auratus). Reprod. Breed. 2023, 3, 143–151. [Google Scholar] [CrossRef]
- Liang, Y.Y.; Wang, Z.J.; Gao, N.; Qi, X.X.; Zeng, J.T.; Cui, K.; Lu, W.X.; Bai, S.J. Variations and Interseasonal Changes in the Gut Microbial Communities of Seven Wild Fish Species in a Natural Lake with Limited Water Exchange during the Closed Fishing Season. Microorganisms 2024, 12, 800. [Google Scholar] [CrossRef]
- Baker, B.J.; De Anda, V.; Seitz, K.W.; Dombrowski, N.; Santoro, A.E.; Lloyd, K.G. Diversity, ecology and evolution of Archaea. Nat. Microbiol. 2020, 5, 887–900. [Google Scholar] [CrossRef]
- Kanika, N.H.; Liaqat, N.; Chen, H.F.; Ke, J.; Lu, G.Q.; Wang, J.; Wang, C.H. Fish gut microbiome and its application in aquaculture and biological conservation. Front. Microbiol. 2025, 15, 1521048. [Google Scholar] [CrossRef]
- Moissl-Eichinger, C.; Pausan, M.; Taffner, J.; Berg, G.; Bang, C.; Schmitz, R.A. Archaea Are Interactive Components of Complex Microbiomes. Trends Microbiol. 2018, 26, 70–85. [Google Scholar] [CrossRef] [PubMed]
- Simon, H.M.; Dodsworth, J.A.; Goodman, R.M. Crenarchaeota colonize terrestrial plant roots. Environ. Microbiol. 2000, 2, 495–505. [Google Scholar] [CrossRef]
- Simon, H.M.; Jahn, C.E.; Bergerud, L.T.; Sliwinski, M.K.; Weimer, P.J.; Willis, D.K.; Goodman, R.M. Cultivation of mesophilic soil crenarchaeotes in enrichment cultures from plant roots. Appl. Environ. Microbiol. 2005, 71, 4751–4760. [Google Scholar] [CrossRef]
- Maranho, L.T.; Gomes, M.P. Morphophysiological Adaptations of Aquatic Macrophytes in Wetland-Based Sewage Treatment Systems: Strategies for Resilience and Efficiency under Environmental Stress. Plants 2024, 13, 2870. [Google Scholar] [CrossRef] [PubMed]
- Zeng, R.; Lin, Y.; Wan, Z.; Tu, M.; Jiao, J.; Zhang, G. An Investigation of Pull-Out Force of Semi-Buried Lotus Roots after Hydraulic Scouring. Agriculture 2021, 11, 706. [Google Scholar] [CrossRef]
- Yang, H.; He, S.; Feng, Q.; Liu, Z.; Xia, S.; Zhou, Q.; Wu, Z.; Zhang, Y. Lotus (Nelumbo nucifera): A multidisciplinary review of its cultural, ecological, and nutraceutical significance. Bioresour. Bioprocess. 2024, 11, 18. [Google Scholar] [CrossRef]
- Nakagawa, S.; Yagi, H.; Suyama, T.; Shimamura, S.; Yanaka, S.; Yagi-Utsumi, M.; Kato, S.; Ohkuma, M.; Kato, K.; Takai, K. Exploring protein N-glycosylation in ammonia-oxidizing Nitrososphaerota archaea through glycoproteomic analysis. MBIO 2025, 16, e03859-24. [Google Scholar] [CrossRef]
- Bai, Y.H.; Sun, Q.H.; Wen, D.H.; Tang, X.Y. Abundance of ammonia-oxidizing bacteria and archaea in industrial and domestic wastewater treatment systems. FEMS Microbiol. Ecol. 2012, 80, 323–330. [Google Scholar] [CrossRef]
- Zhao, W.H.; Bi, X.J.; Bai, M.; Wang, Y.Y. Research advances of ammonia oxidation microorganisms in wastewater: Metabolic characteristics, microbial community, influencing factors and process applications. Bioproc. Biosyst. Eng. 2023, 46, 621–633. [Google Scholar] [CrossRef] [PubMed]
- Barberán, A.; Fernández-Guerra, A.; Auguet, J.C.; Galand, P.E.; Casamayor, E.O. Phylogenetic ecology of widespread uncultured clades of the Kingdom Euryarchaeota. Mol. Ecol. 2011, 20, 1988–1996. [Google Scholar] [CrossRef]
- Yue, Y.H.; Yang, Z.H.; Cai, L.; Bai, C.Z.; Huang, Y.X.; Ma, J.; Yang, M. Effects of stratification and mixing on spatiotemporal dynamics and functional potential of microbial community in a subtropical large-deep reservoir driven by nutrients and ecological niche. Ecol. Indic. 2023, 156, 111128. [Google Scholar] [CrossRef]
- Juottonen, H. Disentangling the effects of methanogen community and environment on peatland greenhouse gas production by a reciprocal transplant experiment. Funct. Ecol. 2020, 34, 1268–1279. [Google Scholar] [CrossRef]
- Schulz, K.; Hunger, S.; Brown, G.G.; Tsai, S.M.; Cerri, C.C.; Conrad, R.; Drake, H.L. Methanogenic food web in the gut contents of methane-emitting earthworm Eudrilus eugeniae from Brazil. ISME J. 2015, 9, 1778–1792. [Google Scholar] [CrossRef]
- Halim, M.F.A.; Day, L.A.; Costa, K.C. Formate-Dependent Heterodisulfide Reduction in a Methanomicrobiales Archaeon. Appl. Environ. Microbiol. 2021, 87, e02698-20. [Google Scholar]
- Huang, Y.H.; Cai, B.Q.; Dong, H.; Li, H.Y.; Yuan, J.; Xu, H.Y.; Wu, H.B.; Xu, Z.Y.; Sun, D.Z.; Dang, Y.; et al. Enhancing anaerobic digestion of food waste with granular activated carbon immobilized with riboflavin. Sci. Total Environ. 2022, 851, 158172. [Google Scholar] [CrossRef] [PubMed]
- Smith, K.S.; Ingram-Smith, C. Methanosaeta, the forgotten methanogen? Trends Microbiol. 2007, 15, 150–155. [Google Scholar] [CrossRef] [PubMed]
- Xin, Y.; Gao, Q.; Zhang, S.B.; Zhang, Z.Z.; Wang, J.F.; Xia, X.H. Microbial regulatory mechanisms underlying methane emission in rivers with different land covers. Water Res. 2025, 281, 123680. [Google Scholar] [CrossRef]
- Bechtold, E.K.; Ellenbogen, J.B.; Villa, J.A.; Ferreira, D.K.D.; Oliverio, A.M.; Kostka, J.E.; Rich, V.I.; Varner, R.K.; Bansal, S.; Ward, E.J.; et al. Metabolic interactions underpinning high methane fluxes across terrestrial freshwater wetlands. Nat. Commun. 2025, 16, 944. [Google Scholar] [CrossRef]
- Shang, H.T. A generic hierarchical model of organic matter degradation and preservation in aquatic systems. Commun. Earth Environ. 2023, 4, 16. [Google Scholar] [CrossRef]
- Downing, B.E.; Nayak, D.D. Innovations in the electron transport chain fuel archaeal methane metabolism. Trends Biochem. Sci. 2025, 50, 425–437. [Google Scholar] [CrossRef]
- Enzmann, F.; Mayer, F.; Rother, M.; Holtmann, D. Methanogens: Biochemical background and biotechnological applications. AMB Express 2018, 8, 1. [Google Scholar] [CrossRef]
- Gill, S.R.; Pop, M.; DeBoy, R.T.; Eckburg, P.B.; Turnbaugh, P.J.; Samuel, B.S.; Gordon, J.I.; Relman, D.A.; Fraser-Liggett, C.M.; Nelson, K.E. Metagenomic analysis of the human distal gut microbiome. Science 2006, 312, 1355–1359. [Google Scholar] [CrossRef]
- Xu, J.B.; Jia, Z.J.; Lin, X.G.; Feng, Y.Z. DNA-based stable isotope probing identifies formate-metabolizing methanogenic archaea in paddy soil. Microbiol. Res. 2017, 202, 36–42. [Google Scholar] [CrossRef]
- Ding, X.; Jin, F.; Xu, J.W.; Zhang, S.L.; Chen, D.X.; Hu, B.J.; Hong, Y.J. The impact of aquaculture system on the microbiome and gut metabolome of juvenile Chinese softshell turtle (Pelodiscus sinensis). iMeta 2022, 1, e17. [Google Scholar] [CrossRef]
- Kato, H.; Mori, H.; Maruyama, F.; Toyoda, A.; Oshima, K.; Endo, R.; Fuchu, G.; Miyakoshi, M.; Dozono, A.; Ohtsubo, Y.; et al. Time-series metagenomic analysis reveals robustness of soil microbiome against chemical disturbance. DNA Res. 2015, 22, 413–424. [Google Scholar] [CrossRef] [PubMed]
- Findley, K.; Oh, J.; Yang, J.; Conlan, S.; Deming, C.; Meyer, J.A.; Schoenfeld, D.; Nomicos, E.; Park, M.; Kong, H.H.; et al. Topographic diversity of fungal and bacterial communities in human skin. Nature 2013, 498, 367. [Google Scholar] [CrossRef] [PubMed]
- Kumar, A.; Verma, J.P. Does plant-Microbe interaction confer stress tolerance in plants: A review? Microbiol. Res. 2018, 207, 41–52. [Google Scholar] [CrossRef] [PubMed]
- Lyu, D.; Smith, D.L. The root signals in rhizospheric inter-organismal communications. Front. Plant Sci. 2022, 13, 1064058. [Google Scholar] [CrossRef]
- Kwaśna, H.; Bateman, G.; Dawson, W. Coemansia species from the rhizospheres of wheat and barley in the United Kingdom. Mycol. Res. 1999, 103, 896–900. [Google Scholar] [CrossRef]
- Perkins, A.K.; Ganzert, L.; Rojas-Jimenez, K.; Fonvielle, J.; Hose, G.C.; Grossart, H.P. Highly diverse fungal communities in carbon-rich aquifers of two contrasting lakes in Northeast Germany. Fungal Ecol. 2019, 41, 116–125. [Google Scholar] [CrossRef]
- Bouhia, Y.; Hafidi, M.; Ouhdouch, Y.; Soulaimani, A.; Zeroual, Y.; Lyamlouli, K. Microbial intervention improves pollutant removal and semi-liquid organo-mineral fertilizer production from olive mill wastewater sludge and rock phosphate. J. Environ. Manag. 2024, 354, 120317. [Google Scholar] [CrossRef]
- Breyer, E.; Espada-Hinojosa, S.; Reitbauer, M.; Karunarathna, S.C.; Baltar, F. Physiological Properties of Three Pelagic Fungi Isolated from the Atlantic Ocean. J. Fungi 2023, 9, 439. [Google Scholar] [CrossRef]
- Zhou, Q.; Zhang, M.M.; Li, J.L.; Hu, K.D.; Li, Q.; Liu, S.L. Research Progress on the Degradation of Pyrethroids and 3-Phenoxybenzoic Acid by Filamentous Fungi. Food Sci. 2023, 44, 207–219. [Google Scholar]
- Zhao, Z.; Gao, H.; Yang, Y.Y.; Deng, Y.; Ju, F. Fungi as a Critical Component of Lake Microbiota in Response to Cyanobacterial Harmful Algal Blooms. Environ. Sci. Technol. 2025, 59, 11167–11180. [Google Scholar] [CrossRef]
- Richardson, A.; Hadobas, P.; Hayes, J. Acid phosphomonoesterase and phytase activities of wheat (Triticum aestivum L.) roots and utilization of organic phosphorus substrates by seedlings grown in sterile culture. Plant Cell Environ. 2000, 23, 397–405. [Google Scholar] [CrossRef]
- Sun, N.K.; Jiang, F.Y.; Zhang, L.; Feng, G. Hyphal exudates of an arbuscular mycorrhizal fungus Rhizophagus irregularis induce phosphate-solubilizing bacterium Rahnella aquatilis to swim towards its hyphae. Chin. Sci. Bull. 2021, 66, 4157–4168. [Google Scholar] [CrossRef]
- Macías-Rodríguez, L.; Contreras-Cornejo, H.A.; Adame-Garnica, S.G.; del-Val, E.; Larsen, J. The interactions of Trichoderma at multiple trophic levels: Inter-kingdom communication. Microbiol. Res. 2020, 240, 126552. [Google Scholar] [CrossRef] [PubMed]
- Tyskiewicz, R.; Nowak, A.; Ozimek, E.; Jaroszuk-Scisel, J. Trichoderma: The Current Status of Its Application in Agriculture for the Biocontrol of Fungal Phytopathogens and Stimulation of Plant Growth. Int. J. Mol. Sci. 2022, 23, 2329. [Google Scholar] [CrossRef]
- Littlejohn, P.T.; Metcalfe-Roach, A.; Poire, E.C.; Holani, R.; Bar-Yoseph, H.; Fan, Y.M.; Woodward, S.E.; Finlay, B.B. Multiple micronutrient deficiencies in early life cause multi-kingdom alterations in the gut microbiome and intrinsic antibiotic resistance genes in mice. Nat. Microbiol. 2023, 8, 2392. [Google Scholar] [CrossRef] [PubMed]
- Ohm, R.A.; Feau, N.; Henrissat, B.; Schoch, C.L.; Horwitz, B.A.; Barry, K.W.; Condon, B.J.; Copeland, A.C.; Dhillon, B.; Glaser, F.; et al. Diverse Lifestyles and Strategies of Plant Pathogenesis Encoded in the Genomes of Eighteen Dothideomycetes Fungi. PLoS Pathog. 2012, 8, e1003037. [Google Scholar] [CrossRef] [PubMed]
- Machouart, M.; Samerpitak, K.; de Hoog, G.S.; Gueidan, C. A multigene phylogeny reveals that Ochroconis belongs to the family Sympoventuriaceae (Venturiales, Dothideomycetes). Fungal Divers. 2014, 65, 77–88. [Google Scholar] [CrossRef]
- Latif, A.; Shehzad, A.; Niazi, S.; Zahid, A.; Ashraf, W.; Iqbal, M.W.; Rehman, A.; Riaz, T.; Aadil, R.M.; Khan, I.M.; et al. Probiotics: Mechanism of action, health benefits and their application in food industries. Front. Microbiol. 2023, 14, 1216674. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).