Extraction, Storage Duration, and Storage Temperature Affect the Activity of Ascorbate Peroxidase, Glutathione Reductase, and Superoxide Dismutase in Rice Tissue
Abstract
:1. Introduction
2. Materials and Methods
2.1. Plant Materials
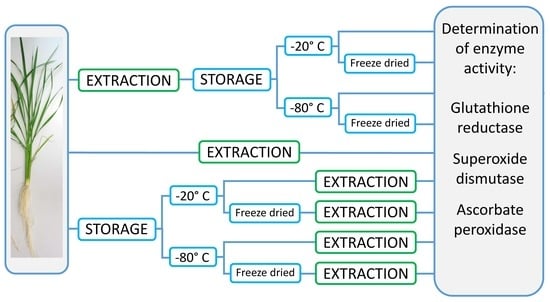
2.2. Extraction and Storage of Extracts and Samples
2.3. Determination of Enzyme Activities
3. Results
3.1. Varietal Differences
3.2. Storing as Intact Tissue or Extract?
3.3. Storage Methods and Duration of Storage
4. Discussion
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Alscher, R.G.; Erturk, N.; Heath, L.S. Role of superoxide dismutases (sods) in controlling oxidative stress in plants. J. Exp. Bot. 2002, 53, 1331–1341. [Google Scholar] [CrossRef] [PubMed]
- Mittler, R. Oxidative stress, antioxidants and stress tolerance. Trends Plant Sci. 2002, 7, 405–410. [Google Scholar] [CrossRef]
- Engel, K.; Asch, F.; Becker, M. Classification of rice genotypes based on their mechanisms of adaptation to iron toxicity. J. Plant Nutr. Soil Sci. 2012, 175, 871–881. [Google Scholar] [CrossRef]
- Moura, B.B.; Silveira, N.M.; Machado, E.C.; Ribeiro, R.V. Effects of storage time and freeze-drying on the activity of antioxidant enzymes in sugarcane leaves. Rev. Bras. Bot. 2016, 39, 373–376. [Google Scholar] [CrossRef]
- Höller, S.; Ueda, Y.; Wu, L.; Wang, Y.; Hajirezaei, M.R.; Ghaffari, M.R.; von Wirén, N.; Frei, M. Ascorbate biosynthesis and its involvement in stress tolerance and plant development in rice (Oryza sativa L.). Plant Mol. Biol. 2015, 88, 545–560. [Google Scholar] [CrossRef] [PubMed]
- Kabir, A.H.; Begum, M.C.; Haque, A.; Amin, R.; Swaraz, A.M.; Haider, S.A.; Paul, N.K.; Hossain, M.M. Genetic variation in Fe toxicity tolerance is associated with the regulation of translocation and chelation of iron along with antioxidant defence in shoots of rice. Func. Plant Biol. 2016, 43, 1070–1081. [Google Scholar] [CrossRef] [Green Version]
- Lacan, D.; Baccou, J.C. High levels of antioxidant enzymes correlate with delayed senescence in nonnetted muskmelon fruits. Planta 1998, 204, 377–382. [Google Scholar] [CrossRef]
- Stein, R.J.; Duarte, G.L.; Spohr, M.G.; Lopes, S.I.G.; Fett, J.P. Distinct physiological responses of two rice cultivars subjected to iron toxicity under field conditions. Ann. Appl. Biol. 2009, 154, 269–277. [Google Scholar] [CrossRef]
- Stein, R.J.; Lopes, S.I.G.; Fett, J.P. Iron toxicity in field-cultivated rice: Contrasting tolerance mechanisms in distinct cultivars. Theor. Exp. Plant Phys. 2014, 26, 135–146. [Google Scholar] [CrossRef]
- Frei, M.; Tanaka, J.P.; Chen, C.P.; Wissuwa, M. Mechanisms of ozone tolerance in rice: Characterization of two QTLs affecting leaf bronzing by gene expression profiling and biochemical analyses. J. Exp. Bot. 2010, 61, 1405–1417. [Google Scholar] [CrossRef]
- Cakmak, I.; Strbac, D.; Marschner, H. Activities of hydrogen peroxide-scavenging enzymes in germinating wheat seeds. J. Exp. Bot. 1993, 44, 127–132. [Google Scholar] [CrossRef]
- Bode, K.; Döring, O.; Lüthje, S.; Neue, H.U.; Böttger, M. The role of active oxygen in iron tolerance of rice (Oryza sativa L.). Protoplasma 1995, 184, 249–255. [Google Scholar] [CrossRef]
- Cao, E.; Chen, Y.; Cui, Z.; Foster, P.R. Effect of freezing and thawing rates on denaturation of proteins in aqueous solutions. Biotech. Bioeng. 2003, 82, 684–690. [Google Scholar] [CrossRef] [PubMed]
- Roy, I.; Gupta, M.N. Freeze-drying of proteins: Some emerging concerns. Biotech. Appl. Biochem. 2004, 39, 165–177. [Google Scholar] [CrossRef] [PubMed]
- Lester, G.E.; Hodges, D.M.; Meyer, R.D.; Munro, K.D. Pre-extraction preparation (fresh, frozen, freeze-dried, or acetone powdered) and long-term storage of fruit and vegetable tissues: Effects on antioxidant enzyme activity. J. Agri. Food Chem. 2004, 52, 2167–2173. [Google Scholar] [CrossRef] [PubMed]
- Arakawa, T.; Prestrelski, S.J.; Kenney, W.C.; Carpenter, J.F. Factors affecting short-term and long-term stabilities of proteins. Adv. Drug Deliv. Rev. 2001, 46, 307–326. [Google Scholar] [CrossRef]
- Anchordoquy, T.J.; Carpenter, J.F. Polymers protect lactate dehydrogenase during freeze-drying by inhibiting dissociation in the frozen state. Arch. Biochem. Biophys. 1996, 332, 231–238. [Google Scholar] [CrossRef] [PubMed]
- Çelik, Ö.; Çakır, B.C.; Atak, Ç. Identification of the antioxidant defense genes which may provide enhanced salt tolerance in Oryza sativa L. Phys. Mol. Biol. Plants 2019, 25, 85–99. [Google Scholar] [CrossRef]
- Dey, S.K.; Kar, M. Antioxidant efficiency during callus initiation from mature rice embryo. Plant Cell Phys. 1995, 36, 543–549. [Google Scholar]
- Wu, L.-B.; Ueda, Y.; Lai, S.-K.; Frei, M. Shoot tolerance mechanisms to iron toxicity in rice (Oryza sativa L.). Plant Cell Env. 2017, 40, 570–584. [Google Scholar] [CrossRef]
- Nguyen, H.T.T.; Ie, S.S.; Kobayashi, K.; Usui, K. Effects of salt stress on ion accumulation and antioxidative enzyme activities of Oryza sativa L. and Echinochloa oryzicola Vasing. Weed Biol. Manag. 2005, 5, 1–7. [Google Scholar] [CrossRef]
- Qin, J.; Wang, X.; Hu, F.; Li, H. Growth and physiological performance responses to drought stress under non-flooded rice cultivation with straw mulching. Plant Soil Env. 2010, 56, 51–59. [Google Scholar] [CrossRef] [Green Version]
- Yoshida, S.; Forno, D.A.; Cock, J.H.; Gomez, K.A. Routine procedure for growing rice plants in culture solution. In Laboratory Manual for Physiological Studies of Rice; The International Rice Research Institute: Manila, Philippines, 1976; Chapter 17; pp. 61–63. Available online: http://books.irri.org/9711040352_content.pdf (accessed on 23 September 2019).
- Hartmann, J.; Asch, F. Micro-method to determine iron concentrations in plant tissues using 2,2′ bipyridine. J. Plant Nutr. Soil Sci. 2018, 181, 357–363. [Google Scholar] [CrossRef]
- Nakano, Y.; Asada, K. Hydrogen peroxide is scavenged by ascorbate-specific peroxidase in spinach chloroplasts. Plant Cell Phys. 1981, 22, 867–880. [Google Scholar]
- Maksimović, J.J.D.; Živanović, B.D. Quantification of the antioxidant activity in salt-stressed tissues. In Plant Salt Tolerance: Methods and Protocols, 1st ed.; Shabala, S., Cuin, T.A., Eds.; Humana Press: New York, NY, USA, 2012; Volume 913, pp. 237–250. [Google Scholar]
- Foyer, C.H.; Halliwell, B. The presence of glutathione and glutathione reductase in chloroplasts: A proposed role in ascorbic acid metabolism. Planta 1976, 133, 21–25. [Google Scholar] [CrossRef] [PubMed]
- Giannopolitis, C.N.; Ries, S.K. Superoxide dismutases: I. Occurrence in higher plants. Plant Phys. 1977, 59, 309–314. [Google Scholar] [CrossRef]
- Asch, F.; Becker, M.; Kpongor, D.S. A quick and efficient screen for resistance to iron toxicity in lowland rice. J. Plant Nutr. Soil Sci. 2005, 168, 764–773. [Google Scholar] [CrossRef]






© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hartmann, J.; Asch, F. Extraction, Storage Duration, and Storage Temperature Affect the Activity of Ascorbate Peroxidase, Glutathione Reductase, and Superoxide Dismutase in Rice Tissue. Biology 2019, 8, 70. https://doi.org/10.3390/biology8040070
Hartmann J, Asch F. Extraction, Storage Duration, and Storage Temperature Affect the Activity of Ascorbate Peroxidase, Glutathione Reductase, and Superoxide Dismutase in Rice Tissue. Biology. 2019; 8(4):70. https://doi.org/10.3390/biology8040070
Chicago/Turabian StyleHartmann, Julia, and Folkard Asch. 2019. "Extraction, Storage Duration, and Storage Temperature Affect the Activity of Ascorbate Peroxidase, Glutathione Reductase, and Superoxide Dismutase in Rice Tissue" Biology 8, no. 4: 70. https://doi.org/10.3390/biology8040070
APA StyleHartmann, J., & Asch, F. (2019). Extraction, Storage Duration, and Storage Temperature Affect the Activity of Ascorbate Peroxidase, Glutathione Reductase, and Superoxide Dismutase in Rice Tissue. Biology, 8(4), 70. https://doi.org/10.3390/biology8040070