Abstract
Allorecognition, the ability to distinguish self or kin from unrelated conspecifics, plays several important biological roles in invertebrate animals. Two of these roles include negotiating limited benthic space for colonial invertebrates, and inbreeding avoidance through self-incompatibility systems. Subphylum Tunicata (Phylum Chordata), the sister group to the vertebrates, is a promising group in which to study allorecognition. Coloniality has evolved many times independently in the tunicates, and the best known invertebrate self-incompatibility systems are in tunicates. Recent phylogenomic studies have coalesced around a phylogeny of the Tunicata as well as the Order Stolidobranchia within the Tunicata, providing a path forward for the study of allorecognition in this group.
1. Allorecognition in Invertebrate Animals
Allorecognition, broadly defined as the ability to recognize self from non-self, is widespread across the tree of life [1]. Allorecognition may have initially evolved as a way to alert the immune system to a pathogen or parasite, as innate immunity is evolutionarily conserved [2,3]. Interspecific immune-related systems seem to be the foundation upon which intraspecific recognition systems evolved [4]. Intraspecific recognition systems tied to disease resistance include MHC-mediated mate choice in many vertebrates (reviewed in [5]), gametic interactions in plants [6,7] and invertebrates [8,9], and fusion in invertebrates [10,11].
This review focuses on intraspecific recognition systems and will therefore use a narrower definition of allorecognition: the ability to distinguish self or kin from unrelated conspecifics. In invertebrate animals, allorecognition plays two important biological roles: negotiating space and regulating mating.
In space-limited benthic habitats, colonial growth forms are common, and colonies often encounter conspecifics [12,13]. There are at least two explanations for why allorecognition systems evolved in the context of coloniality: (1) to re-connect sections of colonies that were separated by predation, disease, or storms [14] (2) as an adaptation to stem cell parasitism [1]. Stem cell parasitism is a potential outcome of fusion between two colonies, where the stem cells of a winning colony invade the losing colony [15]. Stem cell parasitism often results in a precipitous decline in fitness for the losing colony, as usually only the winner’s genotype is represented in the offspring of the fused colony [15]. If fusions are restricted to kin, then the fitness declines experienced by losing colonies are mitigated [1].
Allorecognition is also linked to self-incompatibility mating systems in marine invertebrates. Self-incompatibility systems prevent inbreeding depression, and may be selected for in marine invertebrates that broadcast spawn (release sperm and eggs, fertilization occurs in the water) or spermcast (release sperm only, fertilization occurs inside the maternal animal) [16]. In these species, there is no copulatory behavior through which selection can favor individuals who choose unrelated mates [17].
Inbreeding prevalence in marine invertebrates is more common than previously thought [17]. The percentage of FIS values greater than 0.1 in broadcast or spermcast spawning species is 62% (raw data obtained from [17]). In these species, lowering the fitness cost of inbreeding through the purging of recessive deleterious alleles [18] may have evolved as an alternative to self-incompatibility systems. However, 38% of broadcasting and spermcasting species have FIS values less than 0.1 (raw data obtained from [17]). In these outcrossing species, selection for inbreeding avoidance, including self-incompatibility systems, should be strong.
In this review, allorecognition will be explored in the Tunicata. Subphylum Tunicata (Phylum Chordata), the sister group to the vertebrates, is a promising group in which to study allorecognition. One of the best-known systems of allorecognition, and furthermore one of the only invertebrate allorecognition systems for which the genetic underpinnings are known, occurs in the botryllid ascidians (family Styelidae). The evolution of allorecognition can be examined in the context of coloniality and mating systems. The tunicates contain both colonial and solitary species, and coloniality has evolved many times independently [19]. The best known invertebrate self-incompatibility systems are in tunicates: in the phlebobranch ascidian Ciona robusta [20] and the stolidobranch ascidians Halocynthia aurantium and Halocynthia roretzi [21,22].
2. Relationships within the Subphylum Tunicata
Tunicate biologists have long debated the evolution of coloniality, and therefore allorecognition, owing to uncertainties in the phylogenetic relationships within this group. Recent phylogenomic studies have coalesced around a phylogeny of the Tunicata, providing a path forward for the study of allorecognition in this group.
Subphylum Tunicata comprises Class Appendicularia (pelagic larvaceans), Class Ascidiacea (benthic ascidians), and Class Thaliacea (pelagic doliolids, pyrosomes, and salps) [23]. For the last 20 years, phylogenies based on 18S rRNA [24,25,26,27] and mitochondrial genomes [28,29,30] have consistently agreed that Class Ascidiacea is paraphyletic with respect to Class Thaliacea: thaliaceans belong in a monophyletic group with the ascidian orders Phlebobranchia and Aplousobranchia (Figure 1). This thaliacean/phlebobranch/aplousobranch clade is sister to a clade comprising the ascidian order Stolidobranchia (Figure 1). But the relationships within the thaliacean/phlebobranch/aplousobranch clade, and the position of Class Appendicularia, were unresolved.
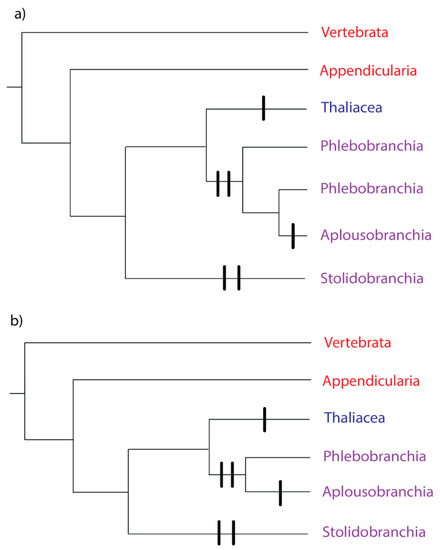
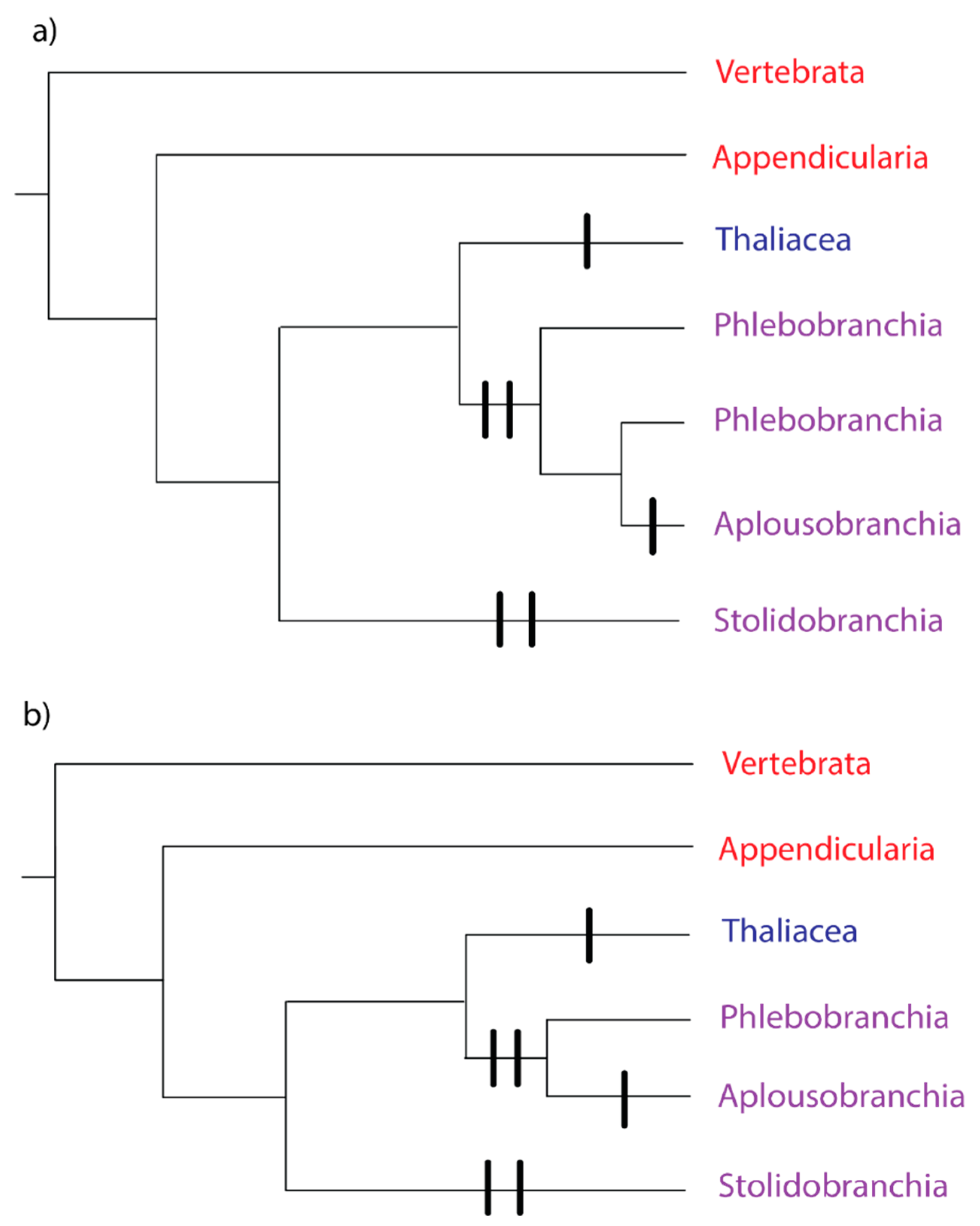
Figure 1.
Phylogeny of the Subphylum Tunicata, based on [31,32]. The tree includes Subphylum Vertebrata as the outgroup. Red text denotes a group where all species have solitary growth forms, blue text denotes a group where all species have colonial growth forms, and purple text denotes a group with both solitary and colonial growth forms. Vertical bars represent independent evolutions of the colonial growth form. (a) Phlebobranchia paraphyletic, (b) Phlebobranchia monophyletic.
Recent nuclear phylogenomic studies [31,32] have made progress on the unresolved nodes. In both studies, the thaliaceans are the outgroup to the phlebobranch/aplousobranch clade, with Appendicularia as the outgroup to the rest of the Tunicata (Figure 1). The remaining unanswered question is whether Phlebobranchia is paraphyletic with respect to Aplousobranchia (Figure 1a), or whether Phlebobranchia and Aplousobranchia are monophyletic groups (Figure 1b). The majority of the analyses in [32] recovered phlebobranch paraphyly, but with variable support. A subset of the 50 genes with the most stable amino acid frequencies across the tree produced a monophyletic and well-supported Phlebobranchia [32]. The analyses in [31] resulted in a paraphyletic Phlebobranchia, but with no statistical support.
3. Evolution of Allorecognition within the Subphylum Tunicata: Negotiating Space
As discussed above, allorecognition in many colonial invertebrates likely evolved as a response to competitive interactions in crowded benthic environments [13]. Therefore, the evolution of allorecognition systems governing fusion is closely linked to the evolution of coloniality. Within the Subphylum Tunicata, only Class Ascidiacea has adopted a benthic lifestyle, so ascidians will be the focus of this section. For each ascidian order (Aplousobranchia, Phlebobranchia, Stolidobranchia), the evolution of coloniality will be reviewed, followed by the evolution of allorecognition.
Tunicata and Vertebrata together form the clade Olfactores [33], and the ancestor of this clade most likely had a solitary growth form and a benthic lifestyle [34]. The tunicate ancestor was also likely to be solitary (Figure 1). Coloniality likely evolved independently on the lineage, leading to Class Thaliacea (Figure 1), because the asexual buds develop from an endostyle outgrowth, a mechanism distinct from other tunicates [35].
3.1. Order Stolidobranchia
3.1.1. Coloniality in Order Stoliobranchia
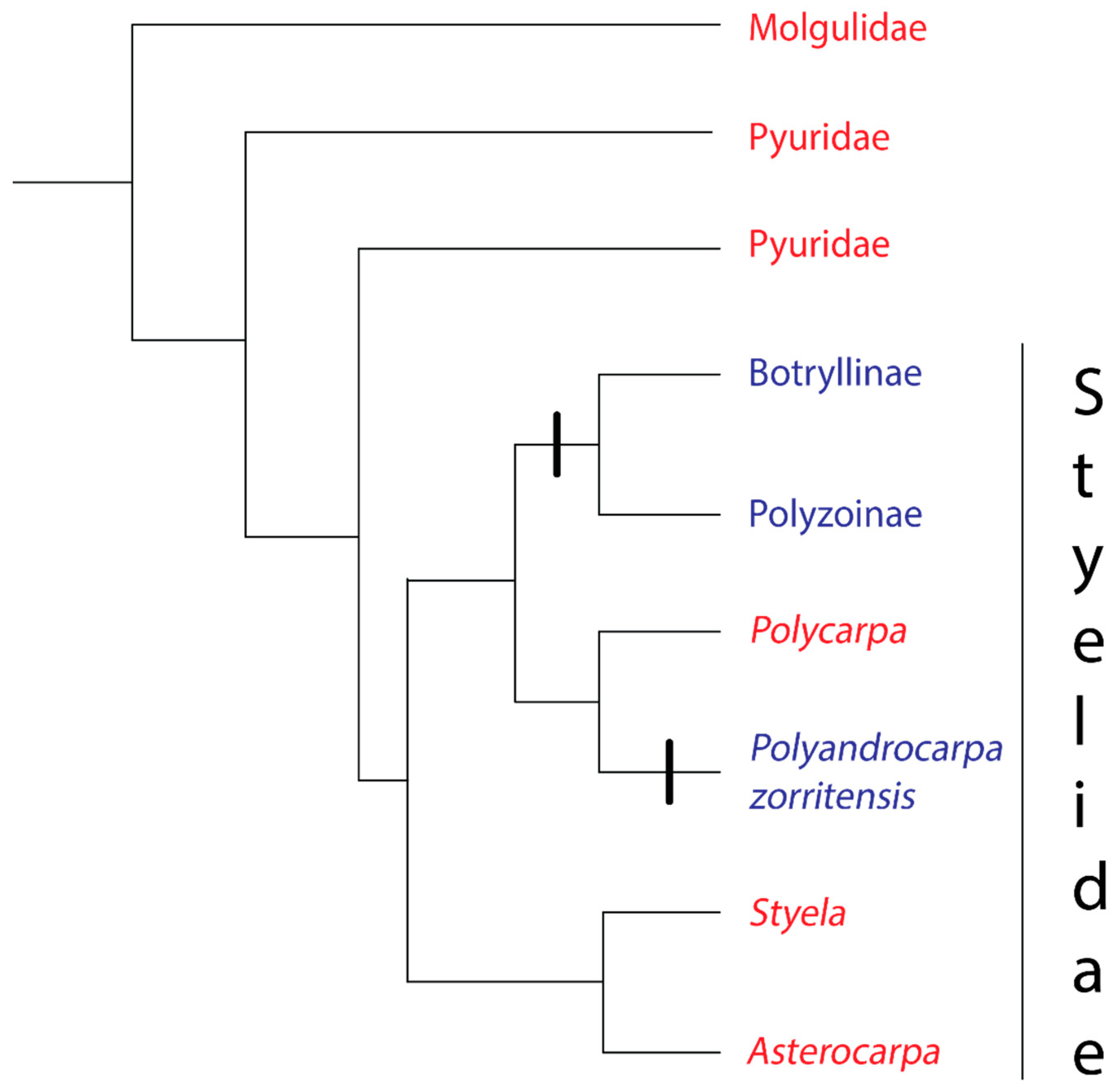
The families at the base of the stolidobranch tree (Molgulidae and Pyuridae) are solitary (Figure 2). The third family in the Order Stolidobranchia, Styelidae, contains both solitary and colonial species [23]. Within the Styelidae, coloniality evolved twice, once in the ancestor of the Botryllinae + Polyzoinae clade [25,36] and again in Polyandrocarpa zorritensis [36] (Figure 2). Convergent rather than homologous evolution of coloniality in the family Styelidae is indicated by two strong lines of evidence. First, Botryllinae + Polyzoinae create colonies through peribranchial budding [36,37], with Botryllinae + Symplegma in the Polyzoinae also capable of vascular budding [38,39], whereas P.zorritensis is unique among the tunicates in its use of vasal budding [40]. Second, an evolution of coloniality in the most recent common ancestor of Botryllinae, Polyzoinae, and P.zorritensis would contradict the principle of parsimony, requiring four to six character state changes [36].
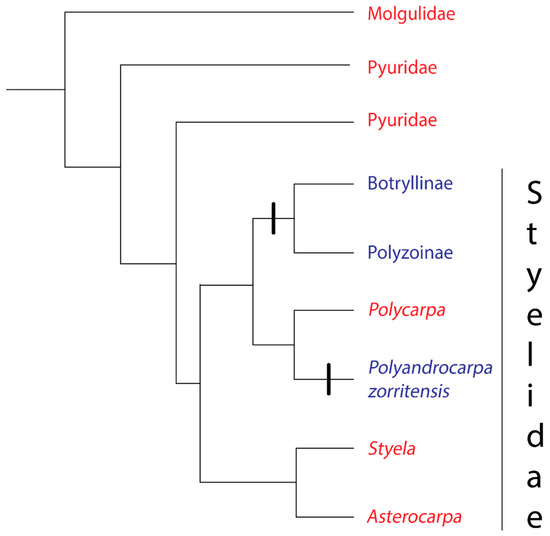
Figure 2.
Phylogeny of the Order Stolidobranchia, based on [36]. The order contains three families: Molgulidae, Pyuridae, and Styelidae. Red text denotes a group where all species have solitary growth forms, Blue text denotes a group where all species have colonial growth forms.
3.1.2. Allorecognition in Order Stolidobranchia
While there is evidence for tunic fusion in two species of solitary stolidobranchs (Molgula complanata and Styela plicata) it is unclear whether the fusion involves the creation of a common vascular system and whether the fusion is discriminatory [41,42]. Setting aside these uncertain cases, allorecognition evolved at least twice: at least once the base of the Polyzoinae/Botryllinae clade when coloniality evolved in this group, and again when coloniality evolved in P. zorritensis (Figure 2).
Allorecognition occurs in Symplegma reptans in the subfamily Polyzoinae [43] and in 10 species in the subfamily Botryllinae: Botrylloides diegensis, Botrylloides fuscus, Botrylloides lentus, Botrylloides simodensis, Botrylloides violaceus, Botryllus delicatus, Botryllus primigenus, Botryllus promiscuus, Botryllus puniceus, and Botryllus scalaris [44,45,46,47,48,49,50,51,52]. Symplegma and botryllids have similarities in their rejection reactions [43]. It would be interesting to determine if the genes involved in allorecognition in botryllids [53] are found in S. reptans. This information could help determine whether allorecognition evolved at the base of the Polyzoinae/Botryllinae clade (as is posited for coloniality in [36]), or separately in each subfamily.
The evolution of allorecognition within the subfamily Botryllinae is well understood, owing to 60 years of study by Oka, Saito, Watanabe and students. Each botryllid species studied thus far exhibits one of three types of allogeneic rejection responses, which vary in the amount of physical interaction between colonies before points of rejection are formed [10]. In Type I, the outer coverings (tunics) fuse in limited areas, whereas the complete fusion of the outer tunics occurs in Type II and III [10]. In Type III but not Type I or II responses, the vascular systems fuse prior to rejection [10]. Type III allorecognition evolved first, followed by Type II and Type I [10], with the amount of physical contact and tissue remodeling before rejection occurs decreasing over evolutionary time. B. scalaris, the only known species with Type III allorecognition, occupies the basal position in the botryllid phylogeny [10], followed by two species with Type II allorecognition: B. primigenus and Botryllus schlosseri [10,54]. Type I allorecognition is found in Botrylloides species, which are the most derived in the botryllid phylogeny [10,54]. This evolution from allorecognition mechanisms requiring extensive physical contact between colonies to those requiring limited contact suggests that external allorecognition in botryllids evolved from systems that already existed within individuals: defense against pathogens or stem cell parasitism, self-incompatibility or lineage sorting during development [10].
One botryllid species, Botryllus horridus, does not engage in fusion or rejection reactions under natural conditions [50]. While B. horridus is an early-branching lineage in the Botryllus/Botrylloides phylogeny [54], B. primigenus branches earlier and has allorecognition abilities [47]. Therefore, the B. horridus lineage must have lost the ability to engage in allorecognition reactions.
Returning to P.zorritensis, the second place in the stolidobranch phylogenetic tree where coloniality exists, no one has published on allorecognition in this species. A congener, Polyandrocarpa misakiensis, exhibits no allorecognition behaviors [55]. However, P. misakiensis and P. zorritensis are not closely related; Polyandrocarpa is a polyphyletic genus [36]. Because coloniality likely evolved separately in Polyzoinae + Botryllinae and P. zorritensis, it would be interesting to learn whether the allorecognition mechanisms in Symplegma, Botryllus/Botrylloides and P. zorritensis share any similarities at the genetic level, assuming P. zorritensis evolved allorecognition.
3.2. Order Phlebobranchia
3.2.1. Coloniality in Order Phlebobranchia
The Order Phlebobranchia contains both colonial and solitary species, but each family has a single growth form [23]. The ancestor of the Phlebobranchia was likely solitary, as the majority of the Phlebobranchia are solitary. There are three colonial genera and 58 colonial species within the Phlebobranchia: Ecteinascidia (29 species), Perophora (23 species), and Plurella (6 species), compared with 36 solitary genera and 278 solitary species [23]. In the genera Ecteinascidia and Perophora (family Perophoridae), the inner vesicle is derived from the stolonial mesenchymal septum [35,56]. There are no published phylogenetic trees that include Plurella, but Kott [57] did not find evidence for a close relationship between Plurella and Perophoridae because Plurella zooids are embedded in a test rather than being connected by stolons as in Perophoridae. Although the mechanism of budding is unknown in Plurella, it is very unlikely to be stolonic as in Perophoridae. Evolutionarily, Plurella should be close to the solitary phlebobranch family Ascidiidae [57]. For these reasons, coloniality likely evolved twice in the Phlebobranchia: once in the ancestor of the Perophoridae, and once in the ancestor of the genus Plurella.
3.2.2. Allorecognition in Order Phlebobranchia
Within the Order Phlebobranchia, there are two colonial groups: family Perophoridae and Genus Plurella. There are no studies of Plurella species in an allorecognition context. Within the family Perophoridae, Ecteinascidia tortugensis, Perophora bermudensis, Perophora japonica, Perophora sagamiensis and Perophora viridis show evidence of allorecognition abilities, whereas Perophora orientalis does not undergo natural fusion with either self or non-self-colonies [58,59,60]. Further study of the evolution of allorecognition in the colonial phlebobranchs awaits investigations of allorecognition in a larger number of species, as well as a phylogeny of the family Perophoridae.
3.3. Order Aplousobranchia
3.3.1. Coloniality in Order Aplousobranchia
The ancestral aplousobranch was colonial [30]. The species in the Order Aplousobranchia are all colonial, with the interesting exception of the family Diazonidae; genera Pseudorhopalaea and Rhopalaea are solitary but the genera Diazona, Pseudodiazona and Tylobranchion are colonial [30,61,62]. Given that the rest of the Aplousobranchia are colonial, the solitary Diazonidae genera must be a reversion back to the ancestral growth form found at the base of the Phlebobranchia/Aplousobranchia clade [30].
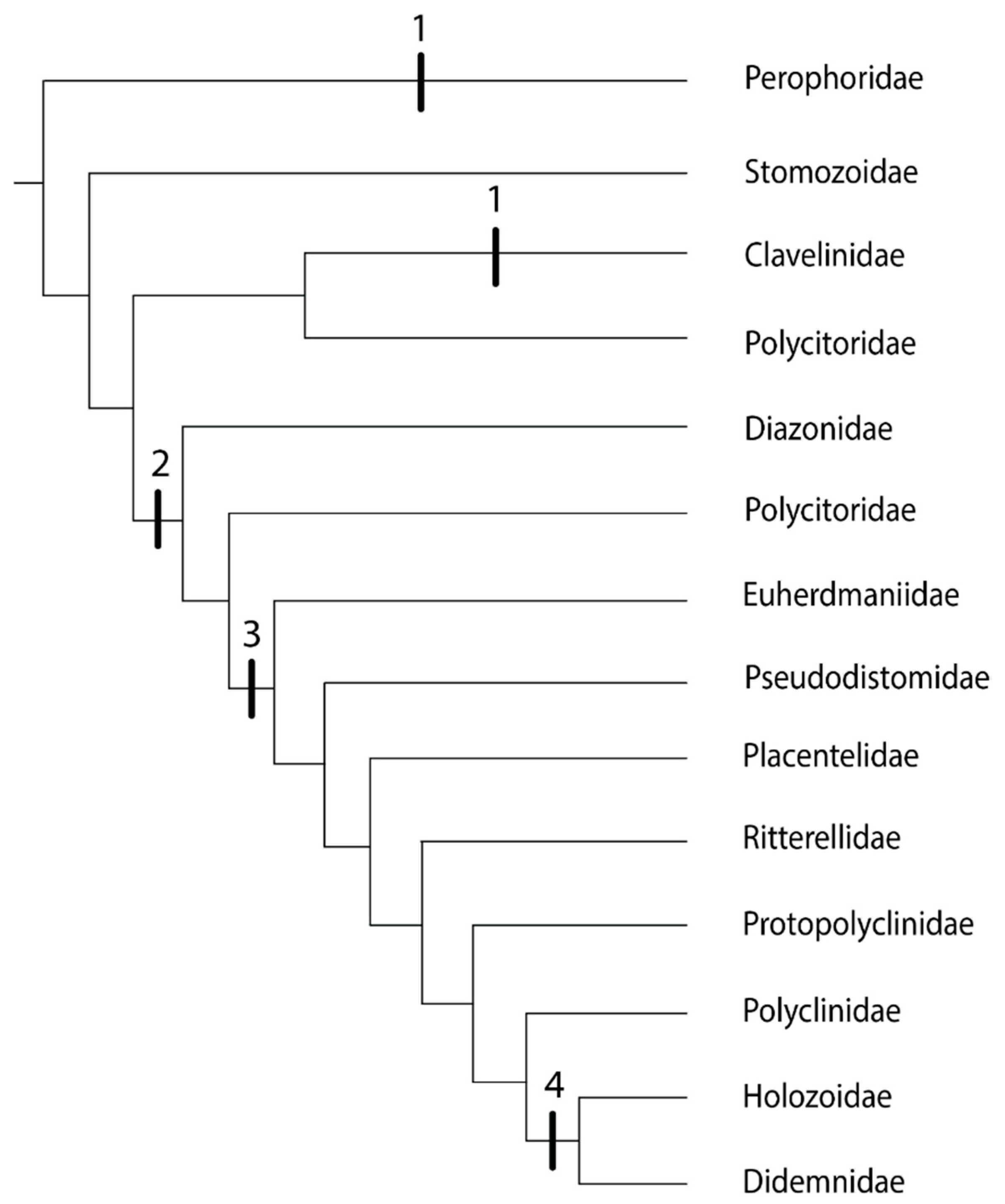
In the genera of the family Clavelinidae within the Aplousobranchia for which budding is known (Clavelina and Nephtheis), the inner vesicles of the buds form from the stolonial mesenchymal septum, the same mechanism as in the phlebobranch family Perophoridae [35]. Both of these families have zooids connected by stolons [63]. However, these two groups are separated by family Stomozoidae (Figure 3). Although budding in Stomozoidae is unknown, there is no evidence that species in this group reproduce by stolonic budding [61]. Polycitor circes, a member of the polyphyletic family Polycitoridae, groups with family Clavelinidae and also shows no evidence of stolonic budding [63]. An ancestral aplousobranch with stolonic budding would mean a loss of stolonic budding in Stomozoidae and P. circes (Figure 3), so the convergent evolution of this trait in the families Perophoridae and Clavelinidae is the more parsimonious explanation.
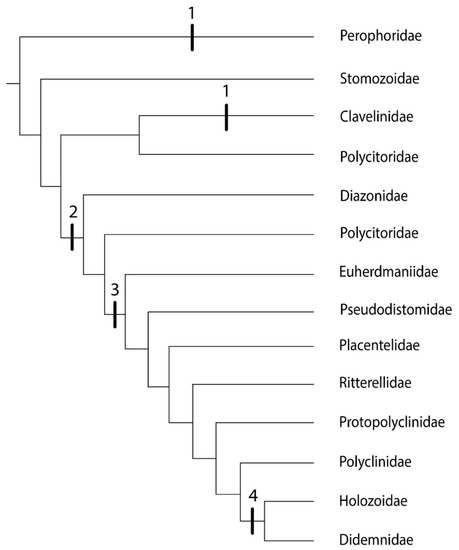
Figure 3.
Phylogeny of the Order Aplousobranchia, based on [30,63,65]. The tree includes family Perophoridae (Order Phlebobranchia) as the outgroup. Vertical bars represent budding types. 1: stolonic; 2: inner bud vesicles form by epidermal constriction of the abdomen; 3: inner bud vesicles formed by epidermal constriction of the post-abdomen; 4: epicardial budding with no constriction of the abdomen or post-abdomen.
Aside from the family Clavelinidae, there are three additional budding modes in the Aplousobranchia, and therefore three more possibilities for the independent evolution of coloniality in this group. The next clades after the family Clavelinidae are family Diazonidae and a clade including the following members of the family Polycitoridae: Brevicollus, Eudistoma, Exostoma, and Polycitor crystallinus (Figure 3). In these groups, inner bud vesicles form by epidermal constriction of the abdomen when budding type is known [63]. The final clade in the aplousobranch phylogeny experienced two evolutions of coloniality: inner bud vesicles formed by epidermal constriction of the post-abdomen (Figure 3: Euherdmaniidae through Polyclinidae), and then epicardial budding with no constriction of the abdomen or post-abdomen as the most derived budding mechanism (Figure 3: Holozoidae and Didemnidae) [63].
3.3.2. Allorecognition in Order Aplousobranchia
Although studies on this topic are not common in the speciose Order Aplousobranchia, allorecognition occurs across the aplousobranch phylogenetic tree, in the Polycitoridae, Polyclinidae, and Didemnidae. Researchers have not studied the two families that occupy the basal positions in the phylogenetic tree of Aplousobranchia [63], Stomozoidae and Clavelinidae, in a fusion or allorecognition context, so it is hard to speculate whether the ancestral aplousobranch was capable of allorecognition.
In the family Polycitoridae, Cystodytes dellechiajei exhibits allorecognition [64]. However, Cystodytes, Eudistoma and Archidistoma, all polycitorids, fall in different places in the phylogenetic tree [65], so C. dellechiajei likely does not represent all members of this polyphyletic family. In the family Polyclinidae, Aplidium constellatum [58] and Aplidium yamazii [66] exhibit allorecognition. In the family Didemnidae, Didemnum moseleyi [47,67] and Didemnum vexillum [68,69] exhibit allorecognition. The family Didemnidae is exceptionally speciose, so the behaviors of two species should not be taken to represent the entire family. Four other species in the family Didemnidae: Didemnum fulgens, Didemnum rodriguesi, Trididemnum solidum, and Trididemnum tenerum, fuse with conspecifics, but colony specificity is an open question [70,71,72,73].
In Diplosoma listerianum [74], a lack of colony specificity leads to indiscriminate fusion. Indiscriminate fusion in this species may be explained by loss of alleles in the allorecognition genes if the benefits of fusion are greater than the costs [74]. A potential risk of fusion is that one partner may contribute more to the tunic production than the other partner, but the tunic in D. listerianum is thin and insubstantial [74], even compared with other members of the genus.
4. Evolution of Allorecognition within the Subphylum Tunicata: Regulating Mating
Researchers have studied the genes/proteins involved in self-incompatibility in three ascidian species: the phlebobranch C. robusta [20] and the stolidobranchs H. aurantium and H. roretzi [21,22]. Some Ciona intestinalis and C. robusta individuals can self-fertilize in vitro, likely because of the high concentration of sperm used in the experiments [75,76,77]. However, individuals in these species cannot self-fertilize in a laboratory flume and are therefore unlikely to do so in nature [78]. In C. robusta, the proposed mechanism of the self-incompatibility system operates by self-recognition [20] and is under genetic control [79,80]. There are two sets of proteins involved: s-Themis A and B (sperm), which act as ligands, and v-Themis A and B (egg), which act as receptors [20]. When s-Themis ligands bind to v-Themis receptors, s-Themis recognizes self v-Themis, s-Themis detaches from v-Themis, and the sperm do not penetrate the vitelline coat [20].
In contrast to Ciona, species in the stolidobranch genus Halocynthia are not self-fertile, even at high sperm concentrations [81]. While researchers have not confirmed genetic control of allorecognition in Halocynthia, they have identified candidate molecules [81]. A protein on the surface of the vitelline coat in H. roretzi, named HrVC70, binds more effectively to non-self-sperm than to self-sperm [21]. HrVC70 binds to a sperm protein called HrUrabin [82]. While HrUrabin is not highly polymorphic, it seems to be necessary for self-sperm to bind to HrVC70, and also mediates the higher affinity of HrVC70 to non-self-sperm [81]. HaVC80 is a homologous protein to HrVC70 in H.aurantium [22].
Experimental fertilizations provide evidence for the existence of self-incompatibility systems in other tunicate species, although the genes and proteins underlying such systems are unknown. In the colonial stolidobranch B. schlosseri, classic studies show that sperm from one colony is not released at the same time that eggs in the same colony are mature [83,84], which limits the possibility of self-fertilization. However, if colonies are large enough, systems within the same colony can develop asynchronous development and self-fertilization could occur [78]. Indeed, some colonies in Monterey, California, contained newly ovulated eggs and mature testes releasing sperm at the same time [85]. Through a series of crossing experiments, B. schlosseri was shown to limit fertilizations, where the gametes are from the same colony, or from sibling colonies [85]. The fusibility locus was thought to control self-sterility, or the fusibility locus and the self-sterility locus were thought to be tightly linked [85]. Similar results were obtained from B. primigenus [86]. However, there was no evidence for allorecognition genotypes influencing sperm–egg interactions in a subsequent study in B. schlosseri [4], and the fusibility gene (FuHC) is not expressed in germline tissue in B. schlosseri [87]. Researchers have not subsequently investigated self-sterility in B. schlosseri, nor have they studied linkages between fusibility and self-sterility in B. primigenus.
The phlebobranch Ciona savignyi is self-fertile, but self-fertilization takes longer than non-self-fertilization, and non-self-fertilization was 100% in a mixture of self and non-self-sperm [88]. The phlebobranch Corella willmeriana is capable of both self-fertilization and outcrossing, and success at self-fertilization varies widely between individuals [89,90]. While this could result from varying levels of self-compatibility, varying levels of inbreeding depression is more likely [89]. The phlebobranchs Ascidia ceratodes [85] and Ascidia mentula [91] possess a block to self-fertilization as does the stolidobranch Herdmania momus [92]. The aplousobranch ascidian D. listerianum shows incompatibilities between potential mates [93,94], although these incompatibilities cannot be predicted by genetic relatedness [94].
5. Conclusions
In invertebrate animals such as tunicates, allorecognition is linked to coloniality and self-incompatibility mating systems. Within the subphylum Tunicata, coloniality likely evolved independently at least six times: once in the Class Thaliacea, twice in the Phlebobranchia, once in the Aplousobranchia, and twice in the Stolidobranchia. There are two colonial groups within the Phlebobranchia; of these colonial species, five species exhibit allorecognition and one does not. Further study of the evolution of allorecognition in the colonial phlebobranchs awaits investigations of allorecognition in a larger number of species. In the Aplousobranchia, allorecognition seems to be found across the phylogenetic tree, in the families Polycitoridae, Polyclinidae, and Didemnidae. Within the Stolidobranchia, allorecognition evolved either at the base of the Polyzoinae/Botryllinae clade, or separately in each subfamily.
Researchers have studied the genes/proteins involved in self-incompatibility in three ascidian species: the phlebobranch C.robusta and the stolidobranchs H.aurantium and H.roretzi. In C.robusta, the proposed mechanism of the self-incompatibility system operates by self-recognition and is under genetic control. There are two sets of proteins involved: s-Themis A and B (sperm), which act as ligands, and v-Themis A and B (egg), which act as receptors. When s-Themis ligands bind to v-Themis receptors, s-Themis recognizes self v-Themis, s-Themis detaches from v-Themis, and the sperm do not penetrate the vitelline coat. In H. roretzi, a protein on the surface of the vitelline coat, HrVC70, binds more effectively to non-self-sperm than to self-sperm. HrVC70 binds to a sperm protein called HrUrabin, which seems to be necessary for self-sperm to bind to HrVC70 and also mediates the higher affinity of HrVC70 to non-self-sperm.
Funding
This research received no external funding.
Conflicts of Interest
The author declares no conflicts of interest.
References
- Buss, L.W. Somatic cell parasitism and the evolution of somatic tissue compatibility. PNAS 1982, 79, 5337–5341. [Google Scholar] [CrossRef] [PubMed]
- Medzhitov, R.; Preston-Hurlburt, P.; Janeway, C.A. A human homologue of the Drosophila Toll protein signals activation of adaptive immunity. Nature 1997, 388, 394–397. [Google Scholar] [CrossRef] [PubMed]
- Rauta, P.R.; Samanta, M.; Dash, H.R.; Nayak, B.; Das, S. Toll-like receptors (TLRs) in aquatic animals: Signaling pathways, expressions and immune responses. Immunol. Lett. 2014, 158, 14–24. [Google Scholar] [CrossRef] [PubMed]
- Grosberg, R.K.; Hart, M.W. Mate selection and the evolution of highly polymorphic self/nonself recognition genes. Science 2000, 289, 2111–2114. [Google Scholar] [CrossRef]
- Milinski, M. Mate choice optimizes offspring MHC genetics and drives sexual reproduction. Immunogenetics 2016, 1. [Google Scholar] [CrossRef]
- Hiscock, S.J.; Kües, U.; Dickinson, H.G. Molecular mechanisms of self-incompatibility in flowering plants and fungi—Different means to the same end. Trends Cell Biol. 1996, 6, 421–428. [Google Scholar] [CrossRef]
- Nasrallah, J.B. Recognition and rejection of self in plant self-incompatibility: Comparisons to animal histocompatibility. Trends Immunol. 2005, 26, 412–418. [Google Scholar] [CrossRef]
- Vacquier, V.D.; Lee, Y.-H. Abalone sperm lysin: Unusual mode of evolution of a gamete recognition protein. Zygote 1993, 1, 181–196. [Google Scholar] [CrossRef]
- Vacquier, V.D.; Swanson, W.J.; Lee, Y.-H. Positive Darwinian selection on two homologous fertilization proteins: What is the selective pressure driving their divergence? J. Mol. Evol. 1997, 44, S15–S22. [Google Scholar] [CrossRef]
- Cohen, C.S.; Saito, Y.; Weissman, I.L. Evolution of allorecognition in botryllid ascidians inferred from a molecular phylogeny. Evolution 1998, 52, 746–756. [Google Scholar] [CrossRef]
- Khalturin, K.; Bosch, T.C. Self/nonself discrimination at the basis of chordate evolution: Limits on molecular conservation. Curr. Opin. Immunol. 2007, 19, 4–9. [Google Scholar] [CrossRef] [PubMed]
- Bancroft, F.W. Variation and fusion in colonies of compound ascidians. Proc. Calif. Acad. Sci. (3rd Ser.) 1903, 3, 137–186. [Google Scholar]
- Grosberg, R.K. The evolution of allorecognition specificity in clonal invertebrates. Q. Rev. Biol. 1988, 63, 377–412. [Google Scholar] [CrossRef]
- Feldgarden, M.; Yund, P.O. Allorecognition in colonial marine invertebrates: Does selection favor fusion with kin of fusion with self? Biol. Bull. 1992, 182, 155–158. [Google Scholar] [CrossRef]
- Stoner, D.S.; Weissman, I.L. Somatic and germ cell parasitism in a colonial ascidian: Possible role for a highly polymorphic allorecognition system. PNAS 1996, 93, 15254–15259. [Google Scholar] [CrossRef]
- Bishop, J.D.D.; Pemberton, A.J. The third way: Spermcast mating in sessile marine invertebrates. Integr. Comp. Biol. 2006, 46, 398–406. [Google Scholar] [CrossRef]
- Olsen, K.C.; Ryan, W.H.; Winn, A.A.; Kosman, E.T.; Moscoso, J.A.; Krueger-Hadfield, S.A.; Burgess, S.C.; Carlon, D.B.; Grosberg, R.K.; Kalisz, S.; et al. Inbreeding shapes the evolution of marine invertebrates. Evolution 2020. [Google Scholar] [CrossRef]
- Charlesworth, D.; Charlesworth, B. Inbreeding depression and its evolutionary consequences. Annu. Rev. Ecol. Evol. Syst. 1987, 18, 237–268. [Google Scholar] [CrossRef]
- Perez-Portela, R.; Bishop, J.D.D.; Davis, A.R.; Turon, X. Phylogeny of the families Pyuridae and Styelidae (Stolidobranchiata, Ascidiacea) inferred from mitochondrial and nuclear DNA sequences. Mol. Phylogenet. Evol. 2009, 50, 560–570. [Google Scholar] [CrossRef]
- Harada, Y.; Takagaki, Y.; Sunagawa, M.; Saito, T.; Yamada, L.; Taniguchi, H.; Shoguchi, E.; Sawada, H. Mechanism of self-sterility in a hermaphroditic chordate. Science 2008, 320, 548–550. [Google Scholar] [CrossRef][Green Version]
- Sawada, H.; Tanaka, E.; Ban, S.; Yamasaki, C.; Fujino, J.; Ooura, K.; Abe, Y.; Matsumoto, K.I.; Yokosawa, H. Self/nonself recognition in ascidian fertilization: Vitelline coat protein HrVC70 is a candidate allorecognition molecule. PNAS 2004, 101, 15615–15620. [Google Scholar] [CrossRef] [PubMed]
- Ban, S.; Harada, Y.; Yokosawa, H.; Sawada, H. Highly polymorphic vitelline-coat protein HaVC80 from the ascidian, Halocynthia aurantium: Structural analysis and involvement in self/nonself recognition during fertilization. Dev. Biol. 2005, 286, 440–451. [Google Scholar] [CrossRef] [PubMed][Green Version]
- World Register of Marine Species. Available online: http://www.marinespecies.org (accessed on 18 April 2020).
- Swalla, B.J.; Cameron, C.B.; Corley, L.S.; Garey, J.R. Urochordates are monophyletic within the deuterostomes. Syst. Biol. 2000, 49, 52–64. [Google Scholar] [CrossRef] [PubMed]
- Zeng, L.; Swalla, B.J. Molecular phylogeny of the protochordates: Chordate evolution. Can. J. Zool. 2005, 83, 24–33. [Google Scholar] [CrossRef]
- Tsagkogeorga, G.; Turon, X.; Hopcroft, R.R.; Tilak, M.K.; Feldstein, T.; Shenkar, N.; Loya, Y.; Huchon, D.; Douzery, E.J.; Delsuc, F. An updated 18S rRNA phylogeny of tunicates based on mixture and secondary structure models. BMC Evol. Biol. 2009, 9, 187. [Google Scholar] [CrossRef]
- Govindarajan, A.F.; Bucklin, A.; Madin, L.P. A molecular phylogeny of the Thaliacea. J. Plank. Res. 2011, 33, 843–853. [Google Scholar] [CrossRef]
- Singh, T.R.; Tsagkogeorga, G.; Delsuc, F.; Blanquart, S.; Shenkar, N.; Loya, Y.; Douzery, E.J.; Huchon, D. Tunicate mitogenomics and phylogenetics: Peculiarities of the Herdmania momus mitochondrial genome and support for the new chordate phylogeny. BMC Genom. 2009, 10, 534. [Google Scholar] [CrossRef] [PubMed]
- Rubinstein, N.D.; Feldstein, T.; Shenkar, N.; Botero-Castro, F.; Griggio, F.; Mastrototaro, F.; Delsuc, F.; Douzery, E.J.; Gissi, C.; Huchon, D. Deep sequencing of mixed total DNA without barcodes allows efficient assembly of highly plastic ascidian mitochondrial genomes. Genome Biol. Evol. 2013, 5, 1185–1199. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Shenkar, N.; Koplovitz, G.; Dray, L.; Gissi, C.; Huchon, D. Back to solitude: Solving the phylogenetic position of the Diazonidae using molecular and developmental characters. Mol. Phylogenet. Evol. 2016, 100, 51–56. [Google Scholar] [CrossRef]
- Delsuc, F.; Philippe, H.; Tsagkogeorga, G.; Simion, P.; Tilak, M.K.; Turon, X.; López-Legentil, S.; Piette, J.; Lemaire, P.; Douzery, E.J. A phylogenomic framework and timescale for comparative studies of tunicates. BMC Biol. 2018, 16, 39. [Google Scholar] [CrossRef]
- Kocot, K.M.; Tassia, M.G.; Halanych, K.M.; Swalla, B.J. Phylogenomics offers resolution of major tunicate relationships. Mol. Phylogenet. Evol. 2018, 121, 166–173. [Google Scholar] [CrossRef]
- Delsuc, F.; Brinkmann, H.; Chourrout, D.; Philippe, H. Tunicates and not cephalochordates are the closest living relatives of vertebrates. Nature 2006, 439, 965–968. [Google Scholar] [CrossRef]
- Stolfi, A.; Brown, F.D. Tunicata. In Evolutionary Developmental Biology of Invertebrates, 1st ed.; Wanninger, A., Ed.; Springer: Vienna, Austria, 2015; Volume 6, pp. 135–204. [Google Scholar]
- Berrill, N.J. Regeneration and budding in tunicates. Biol. Rev. 1951, 26, 456–475. [Google Scholar] [CrossRef]
- Alié, A.; Hiebert, L.S.; Simion, P.; Scelzo, M.; Prünster, M.M.; Lotito, S.; Delsuc, F.; Douzery, E.J.; Dantec, C.; Lemaire, P.; et al. Convergent acquisition of nonembryonic development in styelid ascidians. Mol. Biol. Evol. 2018, 35, 1728–1743. [Google Scholar] [CrossRef]
- Brown, F.D.; Swalla, B.J. Evolution and development of budding by stem cells: Ascidian coloniality as a case study. Dev. Biol. 2012, 369, 151–162. [Google Scholar] [CrossRef]
- Oka, H.; Watanabe, H. Vascular budding, a new type of budding in Botryllus. Biol. Bull. 1957, 112, 225–240. [Google Scholar] [CrossRef]
- Gutierrez, S.; Brown, F.D. Vascular budding in Symplegma brakenhielmi and the evolution of coloniality in styelid ascidians. Dev. Biol. 2017, 423, 152–169. [Google Scholar] [CrossRef]
- Scelzo, M.; Alié, A.; Pagnotta, S.; Lejeune, C.; Henry, P.; Gilletta, L.; Hiebert, L.S.; Mastrototaro, F.; Tiozzo, S. Novel budding mode in Polyandrocarpa zorritensis: A model for comparative studies on asexual development and whole body regeneration. EvoDevo 2019, 10, 7. [Google Scholar] [CrossRef]
- Schmidt, G.H. Aggregation and fusion between conspecifics of a solitary ascidian. Biol. Bull. 1982, 162, 195–201. [Google Scholar] [CrossRef]
- Kingsley, E.A.; Briscoe, D.A.; Raftos, D.A. Correlation of histocompatibility reactions with fusion between conspecifics in the solitary urochordate Styela plicata. Biol. Bull. 1989, 176, 282–289. [Google Scholar] [CrossRef]
- Shirae, M.; Hirose, E.; Saito, Y. Behavior of hemocytes in the allorejection reaction in two compound ascidians, Botryllus scalaris and Symplegma reptans. Biol. Bull. 1999, 197, 188–197. [Google Scholar] [CrossRef]
- Yund, P.O.; Feldgarden, M. Rapid proliferation of historecognition alleles in populations of a colonial ascidian. J. Exp. Zool. 1992, 263, 442–452. [Google Scholar] [CrossRef]
- Hirose, E.; Shirae, M.; Saito, Y. Colony specificity in the xenogeneic combinations among four Botrylloides species (Urochordata, Ascidiacea). Zool. Sci. 2002, 19, 747–753. [Google Scholar] [CrossRef][Green Version]
- Okuyama, M.; Saito, Y. Studies on Japanese botryllid ascidians. II. A new species of the genus Botryllus from the vicinity of Shimoda. Zool. Sci. 2002, 19, 809–815. [Google Scholar] [CrossRef][Green Version]
- Mukai, H.; Watanabe, H. On the occurrence of colony specificity in some compound ascidians. Biol. Bull. 1974, 147, 411–421. [Google Scholar] [CrossRef]
- Hirose, E.; Saito, Y.; Watanabe, H. A new type of the manifestation of colony specificity in the compound ascidian, Botrylloides violaceus Oka. Biol. Bull. 1988, 175, 240–245. [Google Scholar] [CrossRef][Green Version]
- Okuyama, M.; Saito, Y. Studies on Japanese botryllid ascidians. I. A new species of the genus Botryllus from the Izu Islands. Zool. Sci. 2001, 18, 261–267. [Google Scholar] [CrossRef]
- Saito, Y.; Okuyama, M. Studies on Japanese botryllid ascidians. IV. A new species of the genus Botryllus with a unique colony shape, from the vicinity of Shimoda. Zool. Sci. 2003, 20, 1153–1161. [Google Scholar] [CrossRef][Green Version]
- Saito, Y.; Nagasawa, N. Studies on Japanese botryllid ascidians. III. A new species of the genus Botryllus with a vivid colony color from the vicinity of Shimoda. Zool. Sci. 2003, 20, 765–771. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Saito, Y.; Watanabe, H. Colony specificity in the compound ascidian, Botryllus scalaris. Proc. Jpn. Acad. 1982, 58, 105–108. [Google Scholar] [CrossRef][Green Version]
- Taketa, D.A.; De Tomaso, A.W. Botryllus schlosseri allorecognition: Tackling the enigma. Dev. Comp. Immunol. 2015, 48, 254–265. [Google Scholar] [CrossRef] [PubMed]
- Nydam, M.L.; Lemmon, A.R.; Cherry, J.R.; Kortyna, M.L.; Hernandez, C.; Cohen, C.S. Phylogenetic relationships among botryllid ascidians. 2020; Unpublished. [Google Scholar]
- Ballarin, L.; Kawamura, K. The hemocytes of Polyandrocarpa mysakiensis: Morphology and immune-related activities. Invertebr. Surviv. J. 2009, 6, 154–161. [Google Scholar]
- Berrill, N.J.; Giese, A.C.; Pearse, J.S. Chordata: Tunicata. Reprod. Mar. Invertebr. 1975, 2, 241–282. [Google Scholar]
- Kott, P. The Australian Ascidiacea part 1, phlebobranchia and stolidobranchia. Mem. Qld. Mus. 1985, 23, 1–440. [Google Scholar]
- Freeman, G. Transplantation specificity in echinoderms and lower chordates. Transplant. Proc. 1970, 2, 236–239. [Google Scholar]
- Koyama, H.; Watanabe, H. Colony specificity in the colonial ascidian, Perophora japonica. Annot Zool Jpn. 1981, 54, 30–41. [Google Scholar]
- Koyama, H.; Watanabe, H. Colony specificity in the ascidian, Perophora sagamiensis. Biol. Bull. 1982, 162, 171–181. [Google Scholar] [CrossRef]
- Kott, P. The Australian Ascidiacea part 2, Aplousobranchia (1) and Supplement: Phlebobranchia and stolidobranchia. Mem. Qld. Mus. 1990, 29, 1–266. [Google Scholar]
- Monniot, C. Ascidies de Nouvelle-Calédonie. VIII. Phlébobranches (suite). Bull. Mus. Nat. d’Histoire Nat. Sect. A 1990, 12, 491–515. [Google Scholar]
- Moreno, T.R.; Rocha, R.M. Filogenia de Aplousobranchia (Tunicata: Ascidiacea). Rev. Bras. Zool. 2008, 25, 269–298. [Google Scholar] [CrossRef]
- López-Legentil, S.; Ruchty, M.; Domenech, A.; Turon, X. Life cycles and growth rates of two morphotypes of Cystodytes (Ascidiacea) in the western Mediterranean. Mar. Ecol. Prog. Ser. 2005, 296, 219–228. [Google Scholar] [CrossRef][Green Version]
- Turon, X.; López-Legentil, S. Ascidian molecular phylogeny inferred from mtDNA data with emphasis on the Aplousobranchiata. Mol. Phylogenet. Evol. 2004, 33, 309–320. [Google Scholar] [CrossRef]
- Watanabe, H.; Taneda, Y. Self or non-self recognition in compound ascidians. Am. Zool. 1982, 22, 775–782. [Google Scholar] [CrossRef]
- Stocker, L.J. Effects of size and shape of colony on rates of fission, fusion, growth and mortality in a subtidal invertebrate. J. Exp. Mar. Biol. Ecol. 1991, 149, 161–175. [Google Scholar] [CrossRef]
- Smith, K.F.; Stefaniak, L.; Saito, Y.; Gemmill, C.E.; Cary, S.C.; Fidler, A.E. Increased inter-colony fusion rates are associated with reduced COI haplotype diversity in an invasive colonial ascidian Didemnum vexillum. PLoS ONE 2012, 7, e30473. [Google Scholar] [CrossRef]
- Weinberg, R.B.; Clancy, D.L.; Cohen, C.S. Genotypic variability following fusion in the invasive colonial tunicate Didemnum vexillum. Invertebr. Biol. 2019, 138, e12263. [Google Scholar] [CrossRef]
- Bak, R.P.M.; Sybesma, J.; Van Duyl, F.C. The ecology of the tropical compound ascidian Trididemnum solidum. 11. Abundance, growth and survival. Mar. Ecol. Prog. Ser. 1981, 6, 43–52. [Google Scholar] [CrossRef]
- Carlisle, D.B. Locomotory powers of adult ascidians. J. Zool. 1961, 136, 141–146. [Google Scholar] [CrossRef]
- López-Legentil, S.; Erwin, P.M.; Velasco, M.; Turon, X. Growing or reproducing in a temperate sea: Optimization of resource allocation in a colonial ascidian. Invertebr. Biol. 2013, 132, 69–80. [Google Scholar] [CrossRef]
- Ritzmann, N.F.; Rocha, R.M.D.; Roper, J.J. Sexual and asexual reproduction in Didemnum rodriguesi (Ascidiacea, Didemnidae). Iheringia Sér. Zool. 2009, 99, 106–110. [Google Scholar] [CrossRef]
- Bishop, J.D.; Sommerfeldt, A.D. Not like Botryllus: Indiscriminate post–metamorphic fusion in a compound ascidian. Proc. R. Soc. B 1999, 266, 241–248. [Google Scholar] [CrossRef]
- Rosati, F.; DeSantis, R. Studies on fertilization in ascidians. I. Self-sterility and specific recognition between gametes of Ciona intestinalis. Exp. Cell. Res. 1978, 121, 111–119. [Google Scholar] [CrossRef]
- Kawamura, K.; Fujita, H.; Nakauchi, M. Cytological characterization of self incompatibility in gametes of the ascidian, Ciona intestinalis. Dev. Growth Differ. 1987, 29, 627–642. [Google Scholar] [CrossRef]
- Phillippi, A. A Comparative Study of Self-Fertilization in the Life Histories of Three Ascidian Species with Contrasting Dispersal Patterns. Ph.D. Thesis, University of Maine, Orono, ME, USA, 2005. [Google Scholar]
- Phillippi, A.; Yund, P. Self-fertilization and inbreeding depression in three ascidian species that differ in genetic dispersal potential. Mar. Biol. 2017, 164, 179. [Google Scholar] [CrossRef]
- Morgan, T.H. The genetic and the physiological problems of self-sterility in Ciona. V. The genetic problem. J. Exp. Zool. 1942, 90, 199–228. [Google Scholar] [CrossRef]
- Morgan, T.H. The genetic and the physiological problems of self-sterility in Ciona. VI. Theoretical discussion of genetic data. J. Exp. Zool. 1944, 95, 37–59. [Google Scholar] [CrossRef]
- Harada, Y.; Sawada, H. Allorecognition mechanisms during ascidian fertilization. Int. J. Dev. Biol. 2008, 52, 637–645. [Google Scholar] [CrossRef]
- Urayama, S.; Harada, Y.; Nakagawa, Y.; Ban, S.; Akasaka, M.; Kawasaki, N.; Sawada, H. Ascidian sperm glycosylphosphatidylinositol-anchored CRISP-like protein as a binding partner for an allorecognizable sperm receptor on the vitelline coat. J. Biol. Chem. 2008, 283, 21725–21733. [Google Scholar] [CrossRef]
- Milkman, R. Genetic and developmental studies on Botryllus schlosseri. Biol. Bull. 1967, 132, 229–243. [Google Scholar] [CrossRef]
- Sabbadin, A. Self and cross-fertilization in the compound ascidian Botryllus schlosseri. Dev. Biol. 1971, 24, 379–391. [Google Scholar] [CrossRef]
- Scofield, V.L.; Schlumpberger, J.M.; Weissman, I.L. Colony specificity in the colonial tunicate Botryllus and the origins of vertebrate immunity. Am. Zool. 1982, 22, 783–794. [Google Scholar] [CrossRef]
- Oka, H. Colony specificity in compound ascidians. The genetic control of fusibility. In Profiles of Japanese Science and Scientists, 1st ed.; Yukawa, H., Ed.; Kodansha: Tokyo, Japan, 1970; pp. 195–206. [Google Scholar]
- De Tomaso, A.W.; Nyholm, S.V.; Palmeri, K.J.; Ishizuka, K.J.; Ludington, W.B.; Mitchel, K.; Weissman, I.L. Isolation and characterization of a protochordate histocompatibility locus. Nature 2005, 438, 454–459. [Google Scholar] [CrossRef] [PubMed]
- Jiang, D.; Smith, W.C. Self- and cross-fertilization in the solitary ascidian Ciona savignyi. Biol. Bull. 2005, 209, 107–112. [Google Scholar] [CrossRef] [PubMed]
- Cohen, C.S. The effects of contrasting modes of fertilization on levels of inbreeding in the marine invertebrate genus Corella. Evolution 1996, 50, 1896–1907. [Google Scholar] [CrossRef] [PubMed]
- Lambert, G.; Lambert, C.; Abbott, D. Corella species in the American Pacific Northwest: Distinction of C. inflata Huntsman, 1912 from C. willmeriana Herdman, 1898 (Ascidiacea, Phlebobranchia). Can. J. Zool. 1981, 59, 1493–1504. [Google Scholar] [CrossRef]
- Svane, I.; Young, C.M. The ecology and behaviour of ascidian larvae. Oceanogr. Mar. Biol. 1989, 27, 45–90. [Google Scholar]
- Morris, J.E. A ‘fertilization membrane’ in the ascidian Herdmania momus and its relation to self- and cross-fertilization. Experientia 1962, 18, 567–568. [Google Scholar] [CrossRef]
- Bishop, J.D.D.; Jones, C.S.; Noble, L.R. Female control of paternity in the internally-fertilizing compound ascidian Diplosoma listerianum. II. Investigation of male mating success using RAPD markers. Proc. R. Soc. B 1996, 263, 401–407. [Google Scholar]
- Pemberton, A.J.; Sommerfeldt, A.D.; Wood, C.A.; Flint, H.C.; Noble, L.R.; Clarke, K.R.; Bishop, J.D.D. Plant-like mating in an animal: Sexual compatibility and allocation trade-offs in a simultaneous hermaphrodite with remote transfer of sperm. J. Evol. Biol. 2004, 17, 506–518. [Google Scholar] [CrossRef]
© 2020 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).