Oral Microbiota and Immune System Crosstalk: A Translational Research
Abstract
:1. Introduction
2. Experimental Section
2.1. Patient Inclusion and Criteria
2.2. Human Peripheral Blood Monocyte-Derived Macrophages
2.3. May–Grünwald Giemsa (MGG) Staining
2.4. Macrophages Immunofluorescence
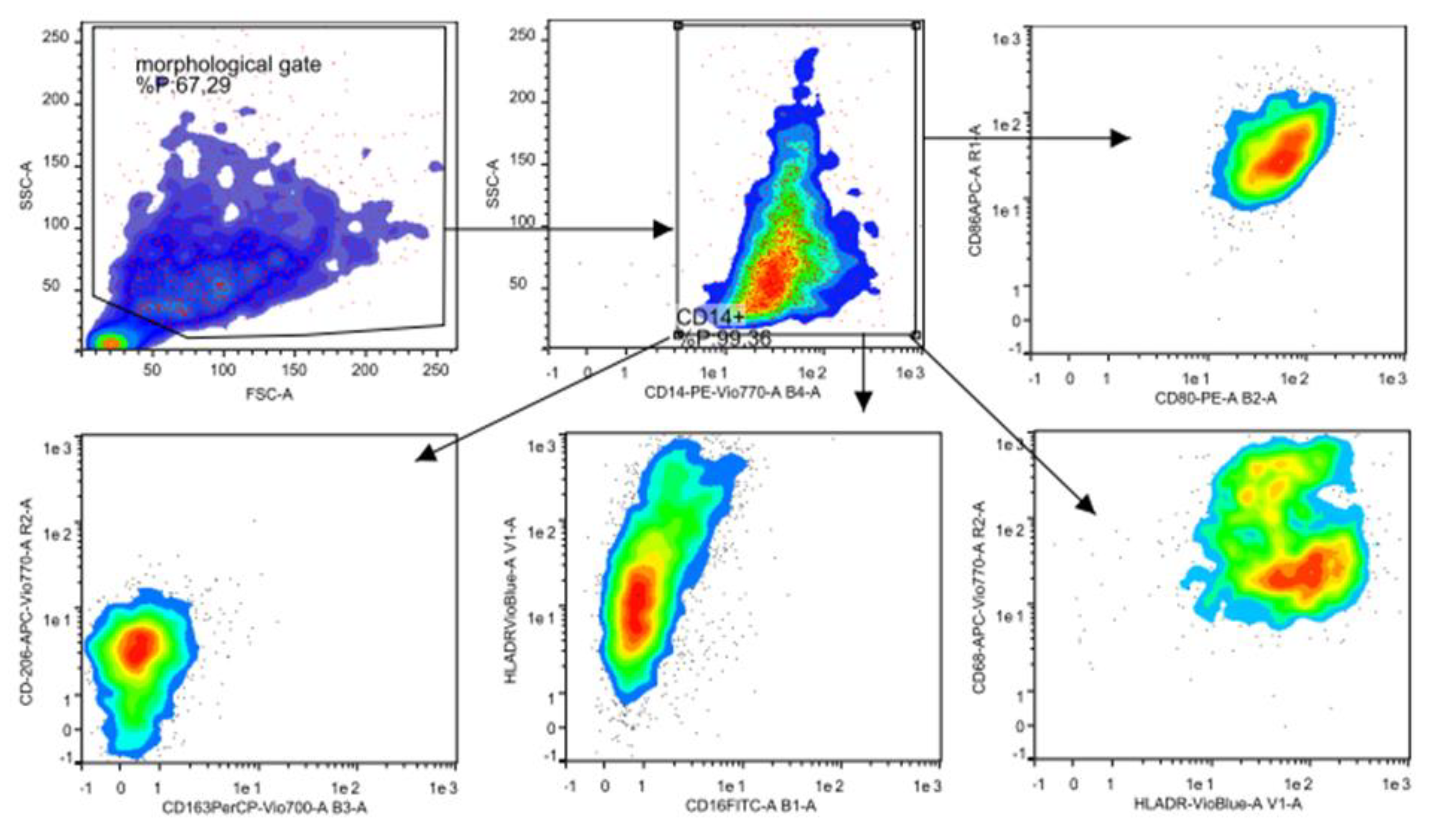
2.5. Flow Cytometry Analysis
2.6. Statistical Analysis
3. Results
3.1. Heterogeneity in Macrophages Morphology
3.2. Flow Cytometry Characterization
4. Discussion
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Cantore, S.; Mirgaldi, R.; Ballini, A.; Coscia, M.F.; Scacco, S.; Papa, F.; Inchingolo, F.; Dipalma, G.; De Vito, D. Cytokine gene polymorphisms associate with microbiogical agents in periodontal disease: Our experience. Int. J. Med. Sci. 2014, 11, 674–679. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ballini, A.; Santacroce, L.; Cantore, S.; Bottalico, L.; Dipalma, G.; Topi, S.; Saini, R.; De Vito, D.; Inchingolo, F. Probiotics efficacy on oxidative stress values in inflammatory bowel disease: A randomized double-blinded placebo-controlled pilot study. Endocr. Metab. Immune. Disord. Drug Targets 2019, 19, 373–381. [Google Scholar] [CrossRef] [PubMed]
- Inchingolo, F.; Martelli, F.S.; Gargiulo Isacco, C.; Borsani, E.; Cantore, S.; Corcioli, F.; Boddi, A.; Nguyễn, K.C.D.; De Vito, D.; Aityan, S.K.; et al. Chronic Periodontitis and Immunity, Towards the Implementation of a Personalized Medicine: A Translational Research on Gene Single Nucleotide Polymorphisms (SNPs) Linked to Chronic Oral Dysbiosis in 96 Caucasian Patients. Biomedicines 2020, 8, 115. [Google Scholar] [CrossRef]
- Mori, G.; Brunetti, G.; Colucci, S.; Oranger, A.; Ciccolella, F.; Sardone, F.; Pignataro, P.; Mori, C.; Karapanou, V.; Ballini, A.; et al. Osteoblast apoptosis in periodontal disease: Role of TNF-related apoptosis-inducing ligand. Int. J. Immunopathol. Pharmacol. 2009, 22, 95–103. [Google Scholar] [CrossRef]
- Caton, J.G.; Armitage, G.; Berglundh, T.; Chapple, I.L.C.; Jepsen, S.; Kornman, K.S.; Mealey, B.L.; Papapanou, P.N.; Sanz, M.; Tonetti, M.S. A new classification scheme for periodontal and peri-implant diseases and conditions-Introduction and key changes from the 1999 classification. J. Clin. Periodontol. 2018, 89, S1–S8. [Google Scholar] [CrossRef] [PubMed]
- Mantovani, A.; Sica, A.; Sozzani, S.; Allavena, P.; Vecchi, A.; Locati, M. The Chemokine System in Diverse Forms of Macrophage Activation and Polarization. Trends Immunol. 2004, 25, 677–686. [Google Scholar] [CrossRef]
- Eligini, S.; Crisci, M.; Bono, E.; Songia, P.; Tremoli, E.; Colombo, G.I.; Colli, S. Human monocyte-derived macrophages spontaneously differentiated in vitro show distinct phenotypes. J. Cell. Physiol. 2013, 228, 1464–1472. [Google Scholar] [CrossRef]
- Ballini, A.; Cantore, S.; Farronato, D.; Cirulli, N.; Inchingolo, F.; Papa, F.; Malcangi, G.; Inchingolo, A.D.; Dipalma, G.; Sardaro, N.; et al. Periodontal disease and bone pathogenesis: The crosstalk between cytokines and porphyromonas gingivalis. J. Biol. Regul. Homeost. Agents 2015, 29, 273–281. [Google Scholar]
- Inchingolo, F.; Dipalma, G.; Cirulli, N.; Cantore, S.; Saini, R.S.; Altini, V.; Santacroce, L.; Ballini, A.; Saini, R. Microbiological results of improvement in periodontal condition by administration of oral Probiotics. J. Biol. Regul. Homeost. Agents 2018, 32, 1323–1328. [Google Scholar]
- Zhou, Q.; Desta, T.; Fenton, M.; Graves, D.T.; Amar, S. Cytokine profiling of macrophages exposed to Porphyromonas gingivalis, its lipopolysaccharide, or its FimA protein. Infect. Immun. 2005, 73, 935–943. [Google Scholar] [CrossRef] [Green Version]
- Cicinelli, E.; Ballini, A.; Marinaccio, M.; Poliseno, A.; Coscia, M.F.; Monno, R.; De Vito, D. Microbiological findings in endometrial specimen: Our experience. Arch. Gynecol. Obstet. 2012, 285, 1325–1329. [Google Scholar] [CrossRef] [PubMed]
- Xu, W.; Roos, A.; Schlagwein, N.; Woltman, A.M.; Daha, M.R.; van Kooten, C. IL-10 producing macrophages preferentially clear apoptotic cells. Blood 2006, 107, 4930–4937. [Google Scholar] [CrossRef] [Green Version]
- Gow, D.J.; Garceau, V.; Kapetanovic, R.; Sester, D.P.; Fici, G.J.; Shelly, J.A.; Wilson, T.L.; Hume, D.A. Cloning and expression of porcine Colony Stimulating Factor-1 (CSF-1) and Colony Stimulating Factor-1 Receptor (CSF-1R) and analysis of the species specificity of stimulation by CSF-1 and Interleukin 34. Cytokine 2012, 60, 793–805. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Waldo, S.W.; Li, Y.; Buono, C.; Zhao, B.; Billings, E.M.; Chang, J.; Kruth, H.S. Heterogeneity of human macrophages in culture and in atherosclerotic plaques. Am. J. Pathol. 2008, 172, 1112–1126. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Martinez, F.O.; Gordon, S. The M1 and M2 paradigm of macrophage activation: Time for reassessment. F1000 Prime Rep. 2014, 6, 13. [Google Scholar] [CrossRef] [Green Version]
- Ballini, A.; Scacco, S.; Coletti, D.; Pluchino, S.; Tatullo, M. Mesenchymal stem cells as promoters, enhancers, and playmakers of the translational regenerative medicine. Stem Cells Int. 2017, 2017, 3292810. [Google Scholar] [CrossRef] [Green Version]
- Yang, J.; Zhu, Y.; Duan, D.; Wang, P.; Xin, Y.; Bai, L.; Liu, Y.; Xu, Y. Enhanced activity of macrophage M1/M2 phenotypes in periodontitis. Arch. Oral. Biol. 2018, 96, 234–242. [Google Scholar] [CrossRef]
- Hanania, R.; Sun, H.S.; Xu, K.; Pustylnik, S.; Jeganathan, S.; Harrison, R.E. Classically activated macrophages use stable microtubules for matrix metalloproteinase-9 (MMP-9) secretion. J. Biol. Chem. 2012, 287, 8468–8483. [Google Scholar] [CrossRef] [Green Version]
- Ricci, S.; Pinto, F.; Auletta, A.; Giordano, A.; Giovane, A.; Settembre, G.; Boccellino, M.; Boffo, S.; Di Carlo, A.; Di Domenico, M. The enigmatic role of matrix metalloproteinases in epithelial-to-mesenchymal transition of oral squamous cell carcinoma: Implications and nutraceutical aspects. J. Cell. Biochem. 2019, 3. [Google Scholar] [CrossRef]
- Murray, P.J.; Allen, J.E.; Biswas, S.K.; Fisher, E.A.; Gilroy, D.W.; Goerdt, S.; Gordon, S.; Hamilton, J.A.; Ivashkiv, L.B.; Lawrence, T.; et al. Macrophage activation and polarization: Nomenclature and experimental guidelines. Immunity 2014, 41, 14–20. [Google Scholar] [CrossRef] [Green Version]
- Menditti, D.; Laino, L.; Milano, M.; Caputo, C.; Boccellino, M.; D’Avino, A.; Baldi, A. Intraoral lymphoepithelial carcinoma of the minor salivary glands. In Vivo 2012, 26, 1087–1089. [Google Scholar] [PubMed]
- Ricciardiello, F.; Caraglia, M.; Iorio, B.; Abate, T.; Boccellino, M.; Colella, G.; Oliva, F.; Ferrise, P.; Zappavigna, S.; Faenza, M.; et al. Aggressiveness pattern and second primary tumor risk associated with basaloid squamous cell carcinoma of the larynx. Oncotarget 2017, 8, 5791–95798. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sinder, B.P.; Pettit, A.R.; McCauley, L.K. Macrophages: Their Emerging Roles in Bone. J. Bone Miner. Res. 2015, 30, 2140–2149. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Davies, L.C.; Jenkins, S.J.; Allen, J.E.; Taylor, P.R. Tissue-resident macrophages. Nat. Immunol. 2013, 14, 986–995. [Google Scholar] [CrossRef]
- Grassi, F.R.; Pappalettere, C.; Di Comite, M.; Corsalini, M.; Mori, G.; Ballini, A.; Crincoli, V.; Pettini, F.; Rapone, B.; Boccaccio, A. Effect of different irrigating solutions and endodontic sealers on bond strength of the dentin-post interface with and without defects. Int. J. Med. Sci. 2012, 9, 642–654. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Crincoli, V.; Ballini, A.; Fatone, L.; Di Bisceglie, M.B.; Nardi, G.M.; Grassi, F.R. Cytokine genotype distribution in patients with periodontal disease and rheumatoid arthritis or diabetes mellitus. J. Biol. Regul. Homeost. Agents 2016, 30, 863–866. [Google Scholar] [PubMed]
- Garceau, V.; Smith, J.; Paton, I.R.; Davey, M.; Sester, D.P.; Burt, D.W.; Hume, D.A. Pivotal advance: Avian colony-stimulating factor 1 (CSF-1), interleukin-34 (IL-34), and CSF-1 receptor genes and gene products. J. Leukoc. Biol. 2010, 87, 753–764. [Google Scholar] [CrossRef] [Green Version]
- Bashir, S.; Sharma, Y.; Elahi, A.; Khan, F. Macrophage polarization: The link between inflammation and related diseases. Inflamm. Res. 2016, 65, 1–11. [Google Scholar] [CrossRef]
- Ballini, A.; Santacroce, L.; Cantore, S.; Bottarico, L.; Dipalma, G.; De Vito, D.; Saini, R.; Inchingolo, F. Probiotics Improve Urogenital Health in Women. Open Access Maced. J. Med. Sci. 2018, 6, 1845–1850. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, L.; Messas, E.; Batista, E.L.; Levine, R.A.; Amar, S. Porphyromonas gingivalis infection accelerates the progression of atherosclerosis in a heterozygous apolipoprotein E-deficient murine model. Circulation 2002, 105, 861–867. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Seguier, S.; Gogly, B.; Bodineau, A.; Godeau, G.; Brousse, N. Is collagen breakdown during periodontitis linked to inflammatory cells and expression of matrix metalloproteinases and tissue inhibitors of metalloproteinases in human gingival tissue? J. Periodontol. 2001, 72, 1398–1406. [Google Scholar] [CrossRef] [PubMed]
- Topoll, H.H.; Zwadlo, G.; Lange, D.E.; Sorg, C. Phenotypic dynamics of macrophage subpopulations during human experimental gingivitis. J. Periodontal Res. 1989, 24, 106–112. [Google Scholar] [CrossRef] [PubMed]
- Huang, N.; Gibson, F.C. Immuno-pathogenesis of Periodontal Disease: Current and Emerging Paradigms. Curr. Oral Health Rep. 2014, 1, 124–132. [Google Scholar] [CrossRef] [PubMed]
- Heidari, Z.; Mahmoudzadeh-Sagheb, H.; Hashemi, M.; Ansarimoghaddam, S.; Moudi, B.; Sheibak, N. Association of macrophage migration inhibitory factor gene polymorphisms with chronic periodontitis in a South Eastern Iranian population. Dent. Res. J. 2017, 14, 395–402. [Google Scholar]
- Letta, F.; Vieira Ferro, E.A.; Bevilacqua, E.; Benincasa, L.; Maioli, E.; Paulesu, L. Role of the Macrophage Migration Inhibitory Factor (MIF) in the survival of first trimester human placenta under induced stress conditions. Sci. Rep. 2018, 8, 1–10. [Google Scholar]
- Wang, Y.; Li, Y.; Li, H.; Song, H.; Zhai, N.; Lou, L.; Wang, F.; Zhang, K.; Bao, W.; Jin, X.; et al. Brucella Dysregulates Monocytes and Inhibits Macrophage Polarization through LC3-Dependent Autophagy. Front. Immunol. 2017, 8, 691. [Google Scholar] [CrossRef] [PubMed]
- Wagener, J.; MacCallum, D.M.; Brown, G.D.; Gow, N.A. Candida albicans Chitin Increases Arginase-1 Activity in Human Macrophages, with an Impact on Macrophage Antimicrobial Functions. mBio 2017, 8, e01820-16. [Google Scholar] [CrossRef] [Green Version]
- Teixeira, F.B.; Saito, M.T.; Matheus, F.C.; Prediger, R.D.; Yamada, E.S.; Maia, C.S.F.; Lima, R.R. Periodontitis and Alzheimer’s Disease: A Possible Comorbidity between Oral Chronic Inflammatory Condition and Neuroinflammation. Front. Aging Neurosci. 2017, 9, 327. [Google Scholar] [CrossRef]
- Boccellino, M.; Camussi, G.; Giovane, A.; Ferro, L.; Calderaro, V.; Balestrieri, C.; Quagliuolo, L. Platelet-activating factor regulates cadherin-catenin adhesion system expression and beta-catenin phosphorylation during Kaposi’s sarcoma cell motility. Am. J. Pathol. 2005, 166, 1515–1522. [Google Scholar] [CrossRef]
- Garaicoa-Pazmino, C.; Fretwurst, T.; Squarize, C.H.; Berglundh, T.; Giannobile, W.V.; Larsson, L.; Castilho, R.M. Characterization of macrophage polarization in periodontal disease. J. Clin. Periodontol. 2019, 46, 830–839. [Google Scholar] [CrossRef]
- Chapple, C.C.; Srivastava, M.; Hunter, N. Failure of macrophage activation in destructive periodontal disease. J. Pathol. 1998, 186, 281–286. [Google Scholar] [CrossRef]
- Lovreglio, P.; Bukvic, N.; Fustinoni, S.; Ballini, A.; Drago, I.; Foà, V.; Guanti, G.; Soleo, L. Lack of genotoxic effect in workers exposed to very low doses of 1,3-butadiene. Arch. Toxicol. 2006, 80, 378–381. [Google Scholar] [CrossRef] [PubMed]
- Boccellino, M.; Quagliuolo, L.; Verde, A.; La Porta, R.; Crispi, S.; Piccolo, M.T.; Vitiello, A.; Baldi, A.; Signorile, P.G. In vitro model of stromal and epithelial immortalized endometriotic cells. J. Cell. Biochem. 2012, 113, 1292–1301. [Google Scholar] [CrossRef] [PubMed]
- Ballini, A.; Tetè, S.; Scattarella, A.; Cantore, S.; Mastrangelo, F.; Papa, F.; Nardi, G.M.; Perillo, L.; Crincoli, V.; Gherlone, E.; et al. The role of anti-cyclic citrullinated peptide antibody in periodontal disease. Int. J. Immunopathol. Pharmacol. 2010, 23, 677–681. [Google Scholar] [CrossRef]
- Ballini, A.; Cantore, S.; Dedola, A.; Santacroce, L.; Laino, L.; Cicciù, M.; Mastrangelo, F. IL-1 haplotype analysis in periodontal disease. J. Biol. Regul. Homeost. Agents 2018, 32, 433–437. [Google Scholar]
- Cantore, S.; Ballini, A.; Mori, G.; Dibello, V.; Marrelli, M.; Mirgaldi, R.; De Vito, D.; Tatullo, M. Anti-plaque and antimicrobial efficiency of different oral rinses in a 3-day plaque accumulation model. J. Biol. Regul. Homeost. Agents 2016, 30, 1173–1178. [Google Scholar]
- Foti, C.; Romita, P.; Rigano, L.; Zimerson, E.; Sicilia, M.; Ballini, A.; Ghizzoni, O.; Antelmi, A.; Angelini, G.; Bonamonte, D.; et al. Isobornyl acrylate: An impurity in alkyl glucosides. Cutan. Ocul. Toxicol. 2016, 35, 115–119. [Google Scholar] [CrossRef]
- Magrone, T.; Magrone, M.; Russo, M.A.; Jirillo, E. Peripheral Immunosenescence and Central Neuroinflammation: A Dangerous Liaison. A Dietary Approach. Endocr. Metab. Immune. Disord. Drug Targets 2020. [Google Scholar] [CrossRef]






© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ballini, A.; Dipalma, G.; Isacco, C.G.; Boccellino, M.; Di Domenico, M.; Santacroce, L.; Nguyễn, K.C.D.; Scacco, S.; Calvani, M.; Boddi, A.; et al. Oral Microbiota and Immune System Crosstalk: A Translational Research. Biology 2020, 9, 131. https://doi.org/10.3390/biology9060131
Ballini A, Dipalma G, Isacco CG, Boccellino M, Di Domenico M, Santacroce L, Nguyễn KCD, Scacco S, Calvani M, Boddi A, et al. Oral Microbiota and Immune System Crosstalk: A Translational Research. Biology. 2020; 9(6):131. https://doi.org/10.3390/biology9060131
Chicago/Turabian StyleBallini, Andrea, Gianna Dipalma, Ciro Gargiulo Isacco, Mariarosaria Boccellino, Marina Di Domenico, Luigi Santacroce, Kieu C.D. Nguyễn, Salvatore Scacco, Maura Calvani, Anna Boddi, and et al. 2020. "Oral Microbiota and Immune System Crosstalk: A Translational Research" Biology 9, no. 6: 131. https://doi.org/10.3390/biology9060131
APA StyleBallini, A., Dipalma, G., Isacco, C. G., Boccellino, M., Di Domenico, M., Santacroce, L., Nguyễn, K. C. D., Scacco, S., Calvani, M., Boddi, A., Corcioli, F., Quagliuolo, L., Cantore, S., Martelli, F. S., & Inchingolo, F. (2020). Oral Microbiota and Immune System Crosstalk: A Translational Research. Biology, 9(6), 131. https://doi.org/10.3390/biology9060131










