Lessons from SARS-CoV-2 Pandemic: Evolution, Disease Dynamics and Future
Abstract
1. SARS-CoV-2, Structure, Epidemiology and Symptoms of COVID-19
1.1. SARS-CoV-2: Structure and Similarity to Other Coronaviruses
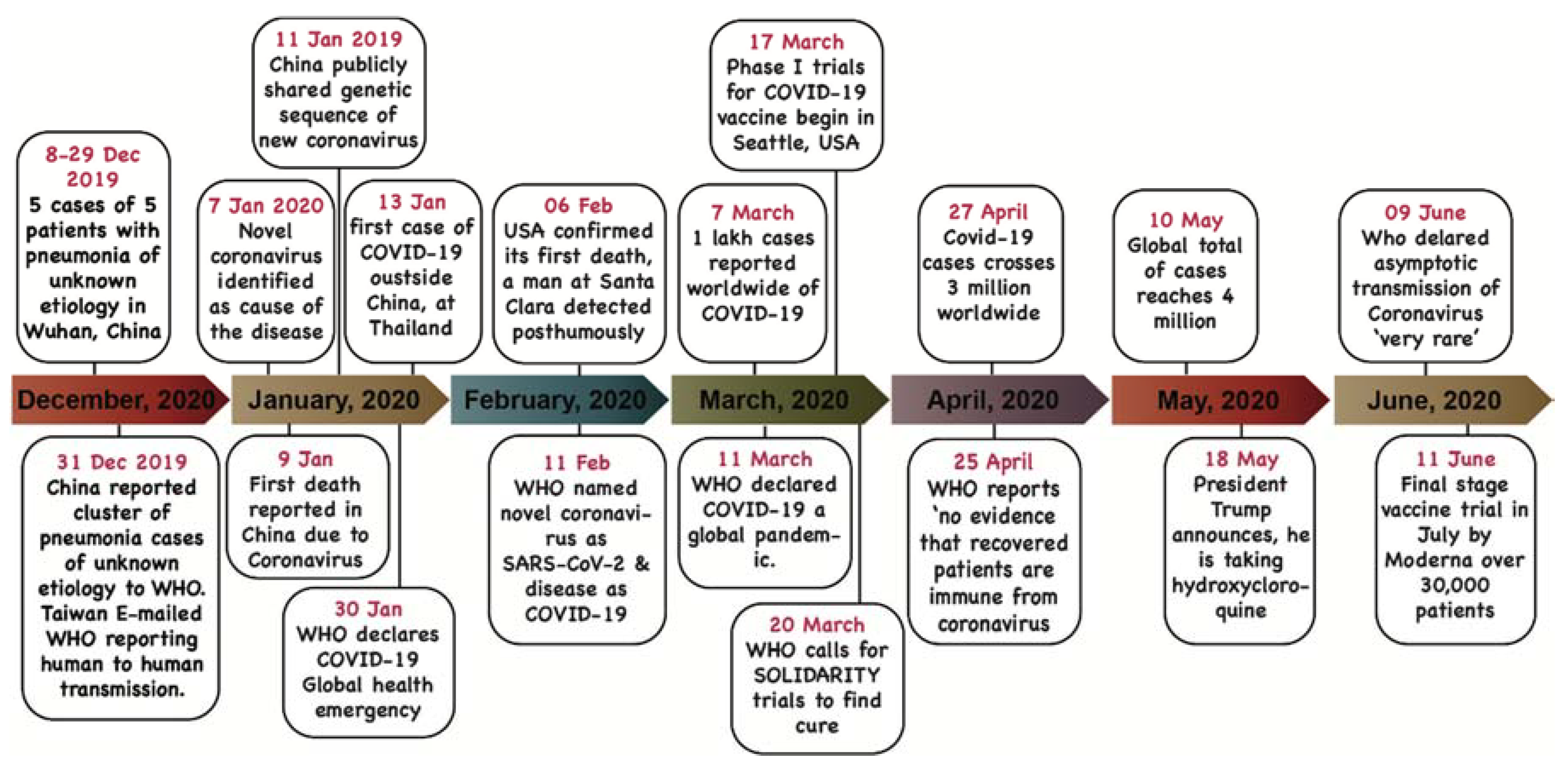
1.2. Epidemiology
1.3. Symptoms
2. Status of Human Interventions
2.1. Drugs and Treatment
2.2. Vaccine Trials and Challenges
3. Lessons from SARS-CoV-2 Pandemic
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Zhu, N.; Zhang, D.; Wang, W.; Li, X.; Yang, B.; Song, J.; Zhao, X.; Huang, B.; Shi, W.; Lu, R.; et al. A novel coronavirus from patients with pneumonia in China, 2019. N. Engl. J. Med. 2020, 382, 727–733. [Google Scholar] [CrossRef] [PubMed]
- Velavan, T.P.; Meyer, C.G. The COVID-19 epidemic. Trop. Med. Int. Heal. 2020, 25, 278–280. [Google Scholar] [CrossRef] [PubMed]
- Lu, R.; Zhao, X.; Li, J.; Niu, P.; Yang, B.; Wu, H.; Wang, W.; Song, H.; Huang, B.; Zhu, N.; et al. Genomic characterisation and epidemiology of 2019 novel coronavirus: Implications for virus origins and receptor binding. Lancet 2020, 395, 565–574. [Google Scholar] [CrossRef]
- Wu, F.; Zhao, S.; Yu, B.; Chen, Y.-M.; Wang, W.; Song, Z.-G.; Hu, Y.; Tao, Z.-W.; Tian, J.-H.; Pei, Y.-Y.; et al. A new coronavirus associated with human respiratory disease in China. Nature 2020, 579, 265–269. [Google Scholar] [CrossRef] [PubMed]
- Helmy, Y.A.; Fawzy, M.; Elaswad, A.; Sobieh, A.; Kenney, S.P.; Shehata, A.A. The COVID-19 pandemic: A comprehensive review of taxonomy, genetics, epidemiology, diagnosis, treatment, and control. J. Clin. Med. 2020, 9, 1225. [Google Scholar] [CrossRef]
- Walls, A.C.; Tortorici, M.A.; Xiong, X.; Snijder, J.; Frenz, B.; Bosch, B.-J.; DiMaio, F.; Corti, D.; Rey, F.A.; Veesler, D. Structural studies of coronavirus fusion proteins. Microsc. Microanal. 2019, 25, 1300–1301. [Google Scholar] [CrossRef]
- Hindson, J. COVID-19: Faecal–oral transmission? Nat. Rev. Gastroenterol. Hepatol. 2020, 17, 259. [Google Scholar] [CrossRef]
- Goh, G.K.M.; Keith Dunker, A.; Foster, J.A.; Uversky, V.N. Rigidity of the outer shell predicted by a protein intrinsic disorder model sheds light on the COVID-19 (Wuhan-2019-nCoV) infectivity. Biomolecules 2020, 10, 331. [Google Scholar] [CrossRef]
- Li, L.Q.; Huang, T.; Wang, Y.Q.; Wang, Z.P.; Liang, Y.; Huang, T.B.; Zhang, H.Y.; Sun, W.-M.; Wang, Y.-P. 2019 novel coronavirus patients’ clinical characteristics, discharge rate, and fatality rate of meta-analysis. J. Med. Virol. 2020, 92, 577–583. [Google Scholar] [CrossRef]
- Wu, Z.; McGoogan, J.M. Characteristics of and important lessons from the coronavirus disease 2019 (COVID-19) outbreak in China. J. Am. Med. Assoc. 2020, 323, 1239–1242. [Google Scholar] [CrossRef]
- WHO Coronavirus Disease 2019 (COVID-19) Situation Report–85; WHO: Geneva, Switzerland, April 2020.
- Duffy, S. Why are RNA virus mutation rates so damn high? PLoS Biol. 2018, 16, e3000003. [Google Scholar] [CrossRef] [PubMed]
- Vankadari, N. Overwhelming mutations or SNPs of SARS-CoV-2: A point of caution. Gene 2020, 752, 144792. [Google Scholar] [CrossRef] [PubMed]
- Peck, K.M.; Lauring, A.S. Complexities of viral mutation rates. J. Virol. 2018, 92. [Google Scholar] [CrossRef] [PubMed]
- Sanjuán, R.; Nebot, M.R.; Chirico, N.; Mansky, L.M.; Belshaw, R. Viral mutation rates. J. Virol. 2010, 84, 9733–9748. [Google Scholar] [CrossRef] [PubMed]
- Cui, J.; Li, F.; Shi, Z.L. Origin and evolution of pathogenic coronaviruses. Nat. Rev. Microbiol. 2019, 17, 181–192. [Google Scholar] [CrossRef]
- Tang, X.; Wu, C.; Li, X.; Song, Y.; Yao, X.; Wu, X.; Duan, Y.; Zhang, H.; Wang, Y.; Qian, Z.; et al. On the origin and continuing evolution of SARS-CoV-2. Natl. Sci. Rev. 2020, nwaa036. [Google Scholar] [CrossRef]
- Yang, X.-L.; Hu, B.; Wang, B.; Wang, M.-N.; Zhang, Q.; Zhang, W.; Wu, L.-J.; Ge, X.-Y.; Zhang, Y.-Z.; Daszak, P.; et al. Isolation and characterization of a novel bat coronavirus closely related to the direct progenitor of severe acute respiratory syndrome coronavirus. J. Virol. 2016, 90, 3253–3256. [Google Scholar] [CrossRef]
- Hu, B.; Zeng, L.P.; Yang, X.L.; Ge, X.Y.; Zhang, W.; Li, B.; Xie, J.Z.; Shen, X.R.; Zhang, Y.Z.; Wang, N.; et al. Discovery of a rich gene pool of bat SARS-related coronaviruses provides new insights into the origin of SARS coronavirus. PLoS Pathog. 2017, 13, e1006698. [Google Scholar] [CrossRef]
- Cyranoski, D. SARS outbreak linked to Chinese bat cave. Nature 2017, 552, 15–16. [Google Scholar] [CrossRef]
- Guan, W.J.; Liang, W.H.; Zhao, Y.; Liang, H.R.; Chen, Z.S.; Li, Y.M.; Liu, X.Q.; Chen, R.C.; Tang, C.L.; Wang, T.; et al. Comorbidity and its impact on 1590 patients with COVID-19 in China: A nationwide analysis. Eur. Respir. J. 2020, 55. [Google Scholar] [CrossRef]
- Singh, A.K.; Gupta, R.; Misra, A. Comorbidities in COVID-19: Outcomes in hypertensive cohort and controversies with renin angiotensin system blockers. Diabetes Metab. Syndr. Clin. Res. Rev. 2020, 14, 283–287. [Google Scholar] [CrossRef] [PubMed]
- Graham, F. Coronavirus Latest: First Vaccine Clinical Trials Begin in United States. Available online: https://www.nature.com/articles/d41586-020-00802-1 (accessed on 18 March 2020).
- McCloskey, B.; Heymann, D.L. SARS to novel coronavirus-Old lessons and new lessons. Epidemiol. Infect. 2020, 1–4. [Google Scholar] [CrossRef] [PubMed]
- WHO Coronavirus Disease 2019 (COVID-19) Situation Report–57; WHO: Geneva, Switzerland, 2020; Volume 49, pp. e99–e100. [CrossRef]
- Uddin, M.; Mustafa, F.; Rizvi, T.A.; Loney, T.; Suwaidi, H.A.; Marzouqi, A.A.; Eldin, A.K.; Alsabeeha, N.; Adrian, T.E. SARS-CoV-2 / COVID-19: Viral genomics, epidemiology, vaccines, and therapeutic interventions. Preprints 2020, 1–17. [Google Scholar] [CrossRef]
- Van Doremalen, N.; Bushmaker, T.; Morris, D.H.; Holbrook, M.G.; Gamble, A.; Williamson, B.N.; Tamin, A.; Harcourt, J.L.; Thornburg, N.J.; Gerber, S.I.; et al. Aerosol and surface stability of SARS-CoV-2 as compared with SARS-CoV-1. N. Engl. J. Med. 2020, 382, 1564–1567. [Google Scholar] [CrossRef] [PubMed]
- Drosten, C.; Meyer, B.; Müller, M.A.; Corman, V.M.; Al-Masri, M.; Hossain, R.; Madani, H.; Sieberg, A.; Bosch, B.J.; Lattwein, E.; et al. Transmission of MERS-coronavirus in household contacts. N. Engl. J. Med. 2014, 371, 828–835. [Google Scholar] [CrossRef] [PubMed]
- Highway, B. The difference in the incubation period of 2019 novel coronavirus (SARS-CoV-2) infection between travelers to Hubei and non-travelers: The need of a longer quarantine period. Infect. Control. Hosp. Epidemiol. 2020. [Google Scholar] [CrossRef]
- Guan, W.-J.; Ni, Z.-Y.; Hu, Y.; Liang, W.-H.; Ou, C.-Q.; He, J.-X.; Liu, L.; Shan, H.; Lei, C.-L.; Hui, D.S.C.; et al. Clinical Characteristics of Coronavirus Disease 2019 in China. N. Engl. J. Med. 2020, 1–13. [Google Scholar] [CrossRef]
- Li, Q.; Guan, X.; Wu, P.; Wang, X.; Zhou, L.; Tong, Y.; Ren, R.; Leung, K.S.M.; Lau, E.H.Y.; Wong, J.Y.; et al. Early transmission dynamics in Wuhan, China, of novel coronavirus-infected pneumonia. N. Engl. J. Med. 2020, 382, 1199–1207. [Google Scholar] [CrossRef]
- Lau, H.; Khosrawipour, V.; Kocbach, P.; Mikolajczyk, A.; Schubert, J.; Bania, J.; Khosrawipour, T. The positive impact of lockdown in Wuhan on containing the COVID-19 outbreak in China. J. Travel Med. 2020, 27, taaa037. [Google Scholar] [CrossRef]
- Xie, Z. Pay attention to SARS-CoV-2 infection in children. Pediatr. Investig. 2020, 4, 1–4. [Google Scholar] [CrossRef]
- Yuan, B.; Chen, Y.; Zhang, K.; Wang, C. Recurrence of positive SARS-CoV-2 Viral RNA in recovered COVID-19 patients during medical isolation observation. Nat. Res. 2020, 1–12. [Google Scholar] [CrossRef]
- Leroy, E.M.; Ar Gouilh, M.; Brugère-Picoux, J. The risk of SARS-CoV-2 transmission to pets and other wild and domestic animals strongly mandates a one-health strategy to control the COVID-19 pandemic. One Heal. 2020. [Google Scholar] [CrossRef] [PubMed]
- Shi, J.; Wen, Z.; Zhong, G.; Yang, H.; Wang, C.; Huang, B.; Liu, R.; He, X.; Shuai, L.; Sun, Z.; et al. Susceptibility of ferrets, cats, dogs, and other domesticated animals to SARS-coronavirus 2. Science 2020, 368, 1016–1020. [Google Scholar] [CrossRef] [PubMed]
- OIE. World Organisation for Animal Health-Questions And Answers on COVID-19; World Organisation for Animal Health: Paris, France, 2020; Volume 36, pp. 1–6. [Google Scholar]
- Tang, Y.W.; Schmitz, J.E.; Persing, D.H.; Stratton, C.W. The Laboratory diagnosis of COVID-19 infection: Current issues and challenges. J. Clin. Microbiol. 2020, 58. [Google Scholar] [CrossRef] [PubMed]
- COVID-19 Pandemic Compiled Data based on WHO Situation Reports and Other Sources. Available online: https://www.worldometers.info/coronavirus/#countries (accessed on 28 May 2020).
- Sex, Gender and COVID-19: Overview and Resources. Available online: https://globalhealth5050.org/covid19/ (accessed on 28 May 2020).
- Rai, D. Young Indians Comprise More Than Half of Confirmed COVID-19 Cases. Available online: https://www.indiatoday.in/diu/story/coronavirus-india-young-patients-age-groups-covid19-1662698-2020-04-03 (accessed on 28 May 2020).
- Wang, S.; Guo, L.; Chen, L.; Liu, W.; Cao, Y.; Zhang, J.; Feng, L. A case report of neonatal COVID-19 infection in China. Clin. Infect. Dis. 2020, ciaa225. [Google Scholar] [CrossRef]
- Rothan, H.A.; Byrareddy, S.N. The epidemiology and pathogenesis of coronavirus disease (COVID-19) outbreak. J. Autoimmun. 2020, 109, 102433. [Google Scholar] [CrossRef]
- Wang, L.S.; Wang, Y.R.; Ye, D.W.; Liu, Q.Q. A review of the 2019 Novel Coronavirus (COVID-19) based on current evidence. Int. J. Antimicrob. Agents 2020, 105948. [Google Scholar] [CrossRef]
- Zhao, D.; Yao, F.; Wang, L.; Zheng, L.; Gao, Y.; Ye, J.; Guo, F.; Zhao, H.; Gao, R. A comparative study on the clinical features of COVID-19 pneumonia to other pneumonias. Clin. Infect. Dis. 2020. [Google Scholar] [CrossRef]
- Nishiura, H.; Kobayashi, T.; Suzuki, A.; Jung, S.-M.; Hayashi, K.; Kinoshita, R.; Yang, Y.; Yuan, B.; Akhmetzhanov, A.R.; Linton, N.M.; et al. Estimation of the asymptomatic ratio of novel coronavirus infections (COVID-19). Int. J. Infect. Dis. 2020. [Google Scholar] [CrossRef]
- Peeri, N.C.; Shrestha, N.; Rahman, S.; Tan, Z.; Bibi, S.; Baghbanzadeh, M. The SARS, MERS and novel coronavirus (COVID-19) epidemics, the newest and biggest global health threats: What lessons have we learned? Int. J. Epidemiol. 2020, 1–10. [Google Scholar] [CrossRef]
- Gautret, P.; Lagier, J.-C.; Parola, P.; Hoang, V.T.; Meddeb, L.; Sevestre, J.; Mailhe, M.; Doudier, B.; Aubry, C.; Amrane, S.; et al. Clinical and microbiological effect of a combination of hydroxychloroquine and azithromycin in 80 COVID-19 patients with at least a six-day follow up: A pilot observational study. Travel Med. Infect. Dis. 2020, 8, 101663. [Google Scholar] [CrossRef] [PubMed]
- McAuliffe, J.; Vogel, L.; Roberts, A.; Fahle, G.; Fischer, S.; Shieh, W.J.; Butler, E.; Zaki, S.; St. Claire, M.; Murphy, B.; et al. Replication of SARS coronavirus administered into the respiratory tract of African Green, rhesus and cynomolgus monkeys. Virology 2004, 330, 8–15. [Google Scholar] [CrossRef] [PubMed]
- Woolsey, C.B.; Borisevich, V.; Prasad, A.N.; Agans, K.N.; Deer, D.J.; Dobias, N.S.; Heymann, J.C.; Foster, S.L.; Levine, C.B.; Medina, L.; et al. Establishment of an African green monkey model for COVID-19. bioRxiv 2020. bioRxiv: 2020.05.17.100289. [Google Scholar] [CrossRef]
- Chen, W.-H.; Strych, U.; Hotez, P.J.; Bottazzi, M.E. The SARS-CoV-2 Vaccine Pipeline: An Overview. Curr. Trop. Med. Rep. 2020, 1–4. [Google Scholar] [CrossRef]
- WHO Draft Landscape of Covid-19 Candidate Vaccines–27; WHO: Geneva, Switzerland, May 2020.
- Rauch, S.; Jasny, E.; Schmidt, K.E.; Petsch, B. New vaccine technologies to combat outbreak situations. Front. Immunol. 2018, 9, 1963. [Google Scholar] [CrossRef]
- Decaro, N.; Martella, V.; Saif, L.J.; Buonavoglia, C. COVID-19 from veterinary medicine and one health perspectives: What animal coronaviruses have taught us. Res. Vet. Sci. 2020, 131, 21–23. [Google Scholar] [CrossRef]
- Decaro, N.; Lorusso, A. Novel human coronavirus (SARS-CoV-2): A lesson from animal coronaviruses. Vet. Microbiol. 2020, 244, 108693. [Google Scholar] [CrossRef]
- Chang, T.W. Recurrent viral infection (reinfection). N. Engl. J. Med. 1971, 284, 765–773. [Google Scholar] [CrossRef]

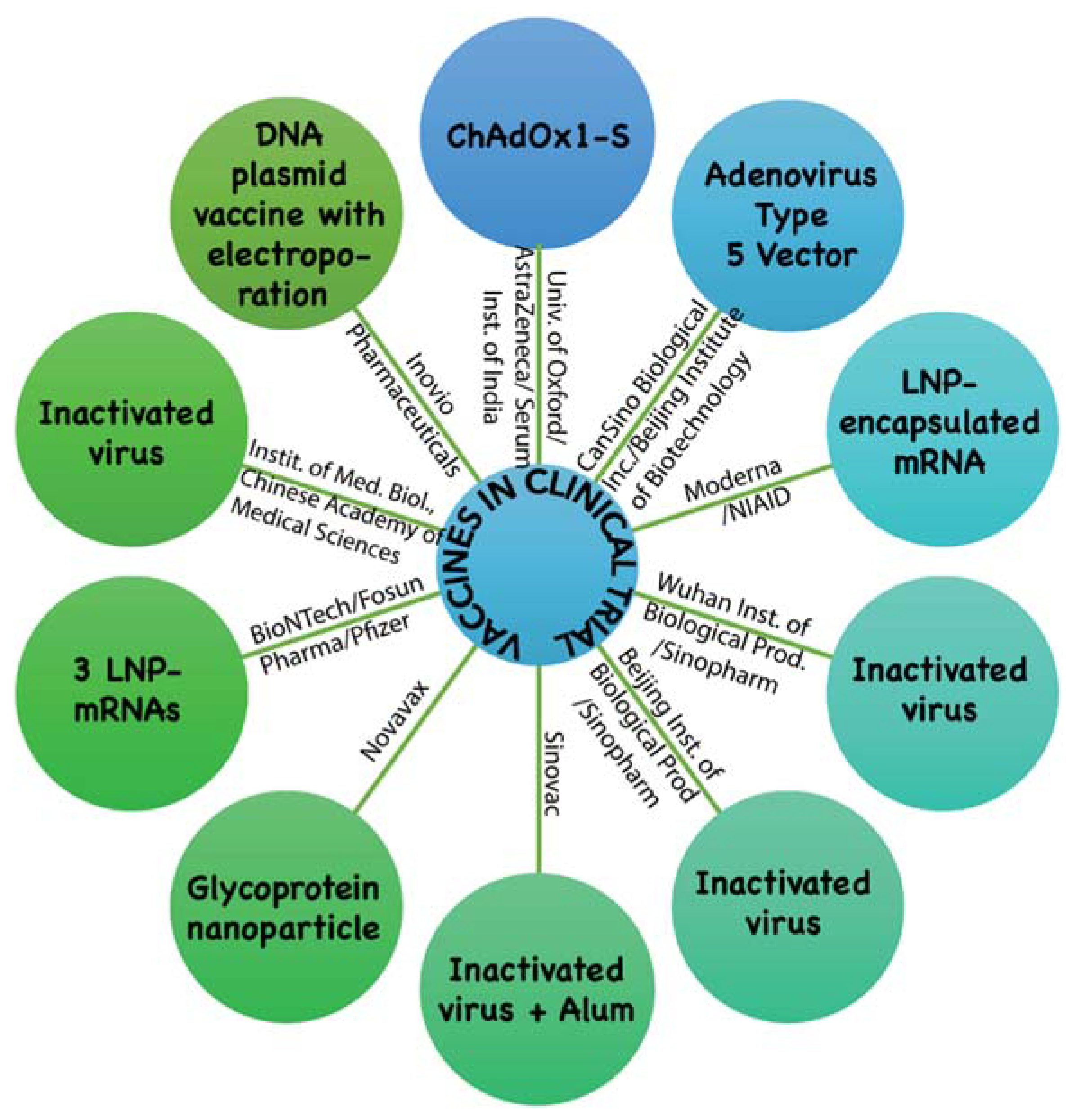
| Technical Platform | Candidate Vaccine in Clinical Trial | Developer | Current Stage |
|---|---|---|---|
| Non Replicating Viral Vector | ChAdOx1-S | University of Oxford/AstraZeneca, Cambridge, U.K. / Serum Institute of India | Phase2b/3 2020-001228-32 Phase 1/2 2020-001072-15 |
| Non Replicating Viral Vector | Adenovirus Type 5 Vector | CanSino Biological Inc., China/Beijing Institute of Biotechnology, China | Phase 2 ChiCTR2000031781 Phase 1 ChiCTR2000030906 |
| RNA | Lipid nanoparticles (LNP) encapsulated mRNA | Moderna Inc., USA/ National Institute of Allergy and Infectious Diseases (NIAID), USA | Phase 2 (IND submitted) Phase 1 NCT04283461 |
| Inactivated | Inactivated | Wuhan Institute of Biological Products, China/Sinopharm Group Co., Ltd., China | Phase 1/2 ChiCTR2000031809 |
| Inactivated | Inactivated | Beijing Institute of Biological Products/ Sinopharm Group Co., Ltd., China | Phase 1/2 ChiCTR2000032459 |
| Inactivated | Inactivated + Alum | Sinovac Biotech Ltd., China | Phase 1/2 NCT04383574 NCT04352608 |
| Protein Subunit | Full length recombinant SARS-CoV-2 glycoprotein nanoparticle vaccine adjuvanted with Matrix M | Novavax Inc., Maryland, USA | Phase 1/2 NCT04368988 |
| RNA | 3 LNP-mRNAs | BioNTech, Germany/Shanghai Fosun Pharmaceutical Co. Ltd., China /Pfizer, USA | Phase 1/2 2020-001038-36 NCT04368728 |
| Inactivated | Inactivated | Institute of Medical Biology, Chinese Academy of Medical Sciences | Phase 1 |
| DNA | DNA plasmid vaccine with electroporation | Inovio Pharmaceuticals Inc., USA | Phase 1 NCT04336410 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pandey, S.; Yadav, B.; Pandey, A.; Tripathi, T.; Khawary, M.; Kant, S.; Tripathi, D. Lessons from SARS-CoV-2 Pandemic: Evolution, Disease Dynamics and Future. Biology 2020, 9, 141. https://doi.org/10.3390/biology9060141
Pandey S, Yadav B, Pandey A, Tripathi T, Khawary M, Kant S, Tripathi D. Lessons from SARS-CoV-2 Pandemic: Evolution, Disease Dynamics and Future. Biology. 2020; 9(6):141. https://doi.org/10.3390/biology9060141
Chicago/Turabian StylePandey, Saurabh, Bharat Yadav, Arvind Pandey, Takshashila Tripathi, Masuma Khawary, Sashi Kant, and Deeksha Tripathi. 2020. "Lessons from SARS-CoV-2 Pandemic: Evolution, Disease Dynamics and Future" Biology 9, no. 6: 141. https://doi.org/10.3390/biology9060141
APA StylePandey, S., Yadav, B., Pandey, A., Tripathi, T., Khawary, M., Kant, S., & Tripathi, D. (2020). Lessons from SARS-CoV-2 Pandemic: Evolution, Disease Dynamics and Future. Biology, 9(6), 141. https://doi.org/10.3390/biology9060141








