Polyamine-Mediated Transcriptional Regulation of Enzymatic Antioxidative Response to Excess Soil Moisture during Early Seedling Growth in Soybean
Abstract
1. Introduction
2. Materials and Methods
2.1. Plant Materials and Seed Treatment Assays
2.2. Excess Moisture-Stress Treatment and Tissue Collection
2.3. RNA Extraction and cDNA Synthesis
2.4. Primers
2.5. Real-Time qPCR Assays
2.6. Protein Extraction
2.7. Enzyme Activity Assays
2.8. Statistical Analysis
3. Results
3.1. Germination
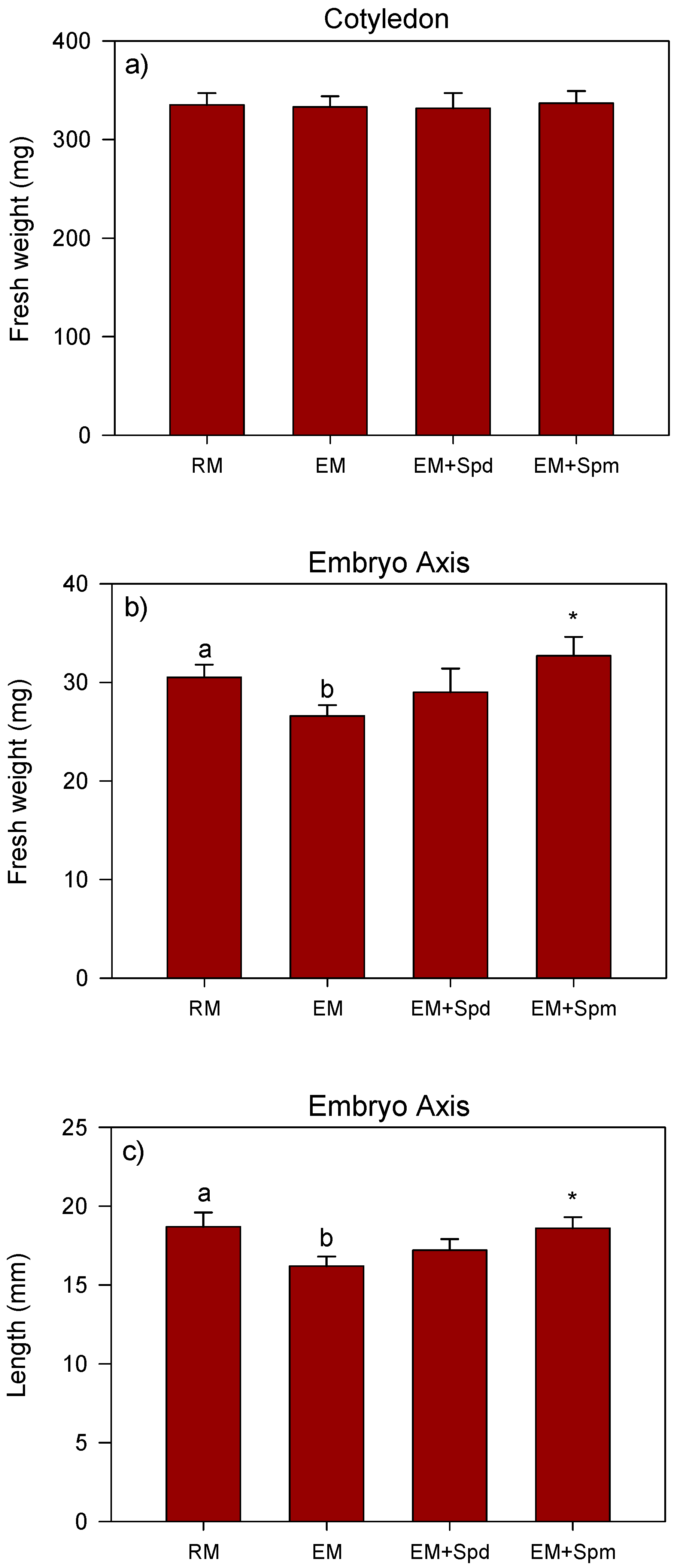
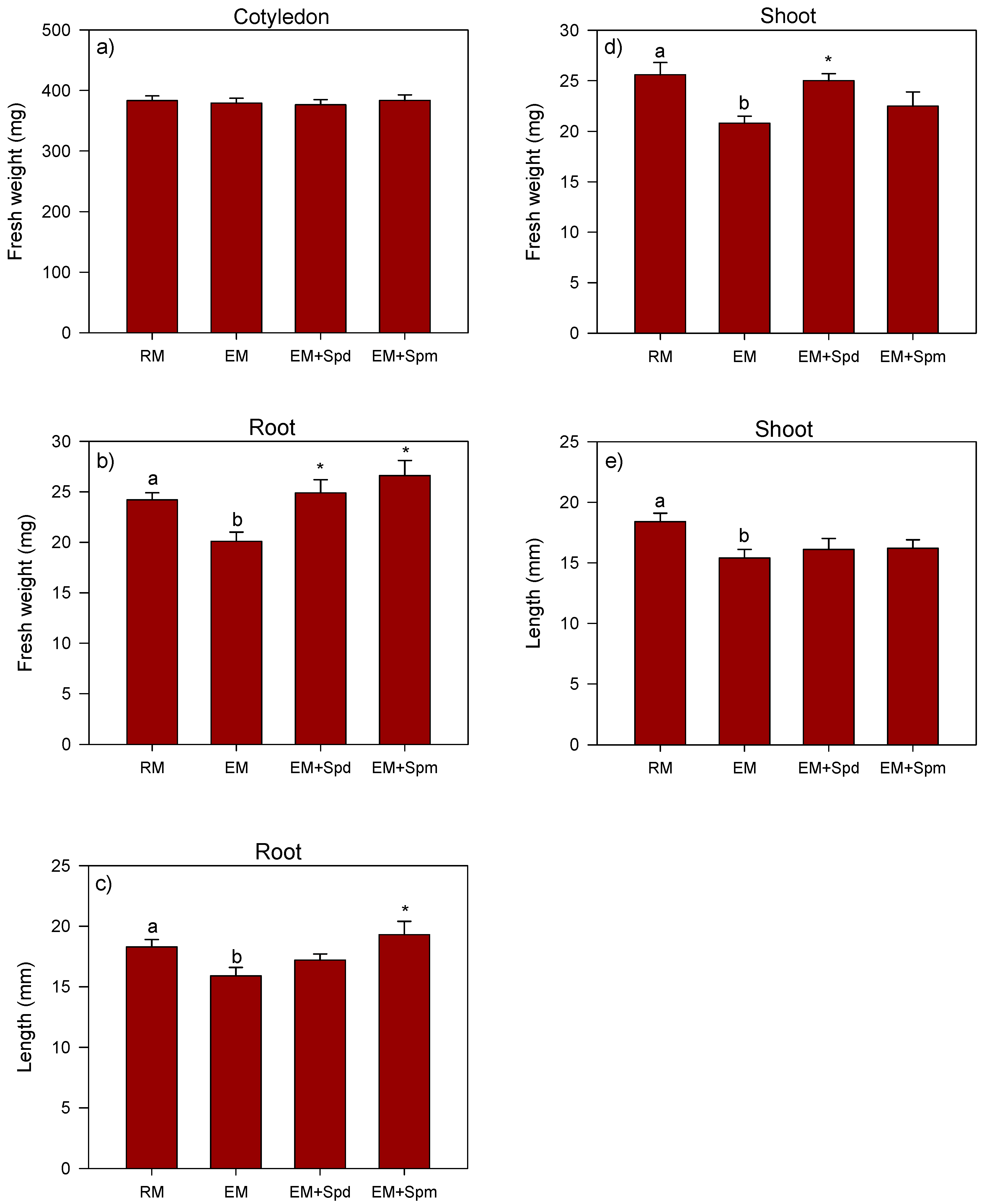
3.2. Growth Response of Polyamine-Treated Seedlings to Excess Moisture
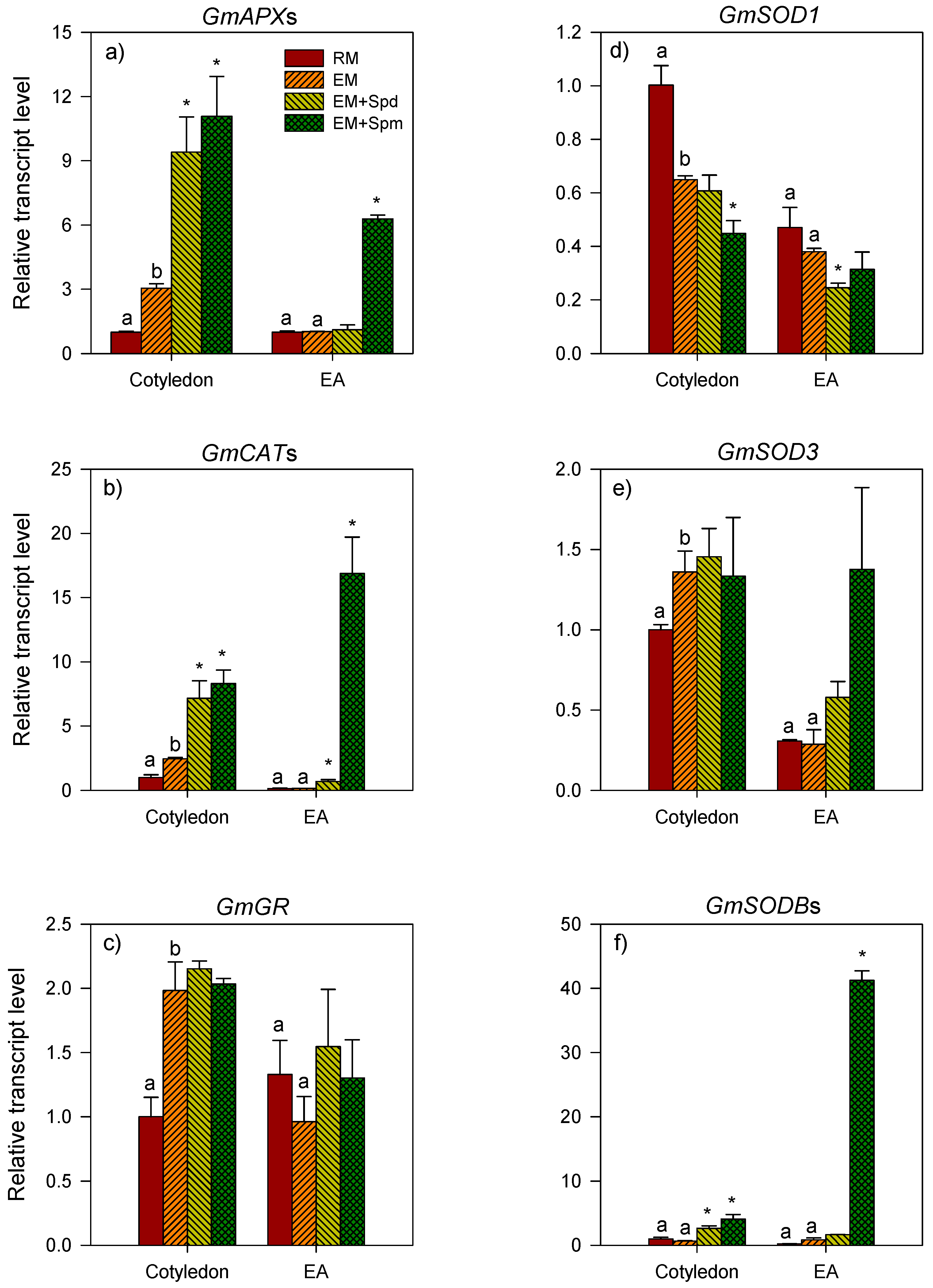
3.3. Response of the Expression of Antioxidative Genes to Excess Moisture
3.3.1. Ascorbate Peroxidase Genes
3.3.2. Catalase Genes
3.3.3. Glutathione Reductase Genes
3.3.4. Superoxide Dismutase Genes
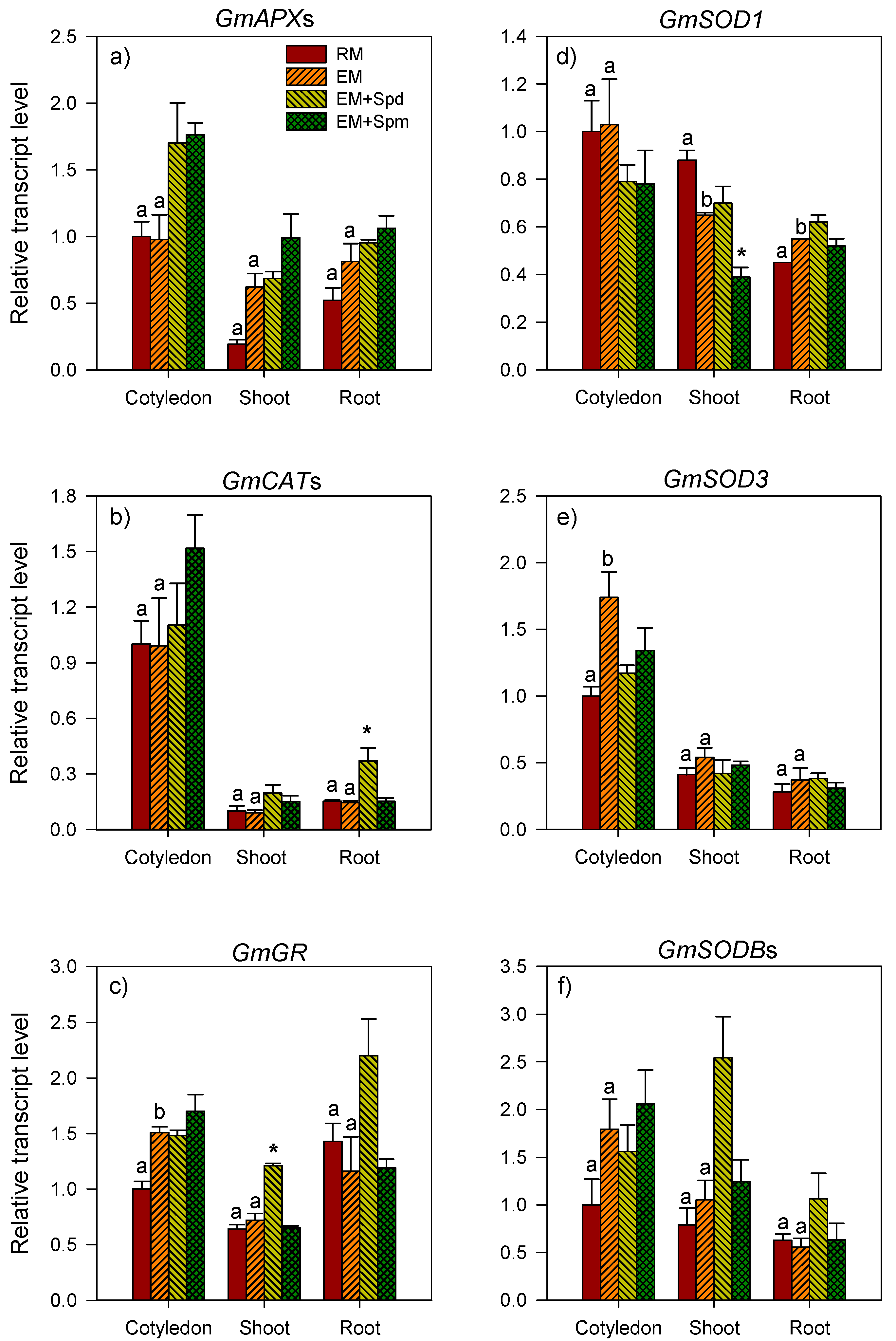
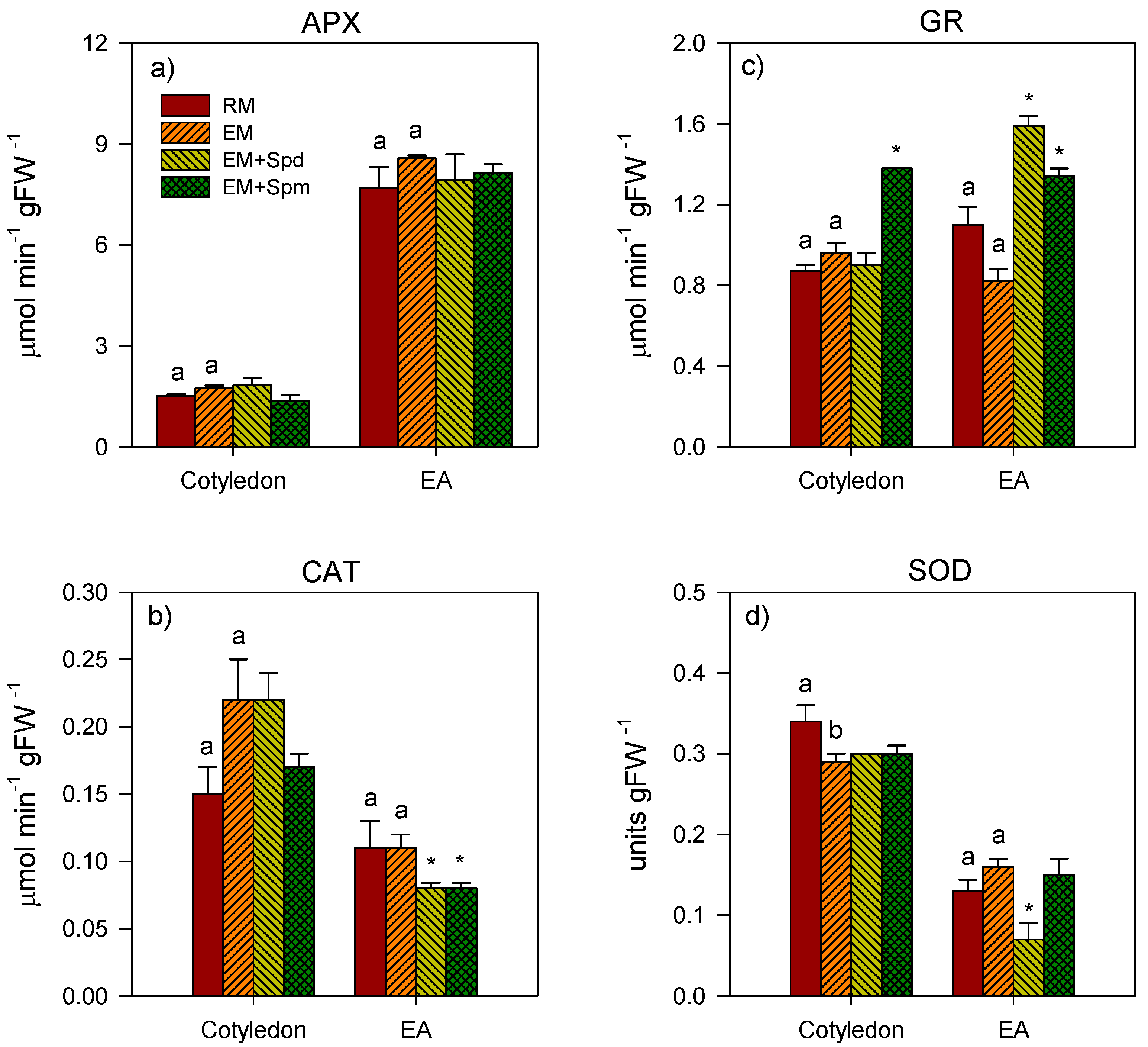
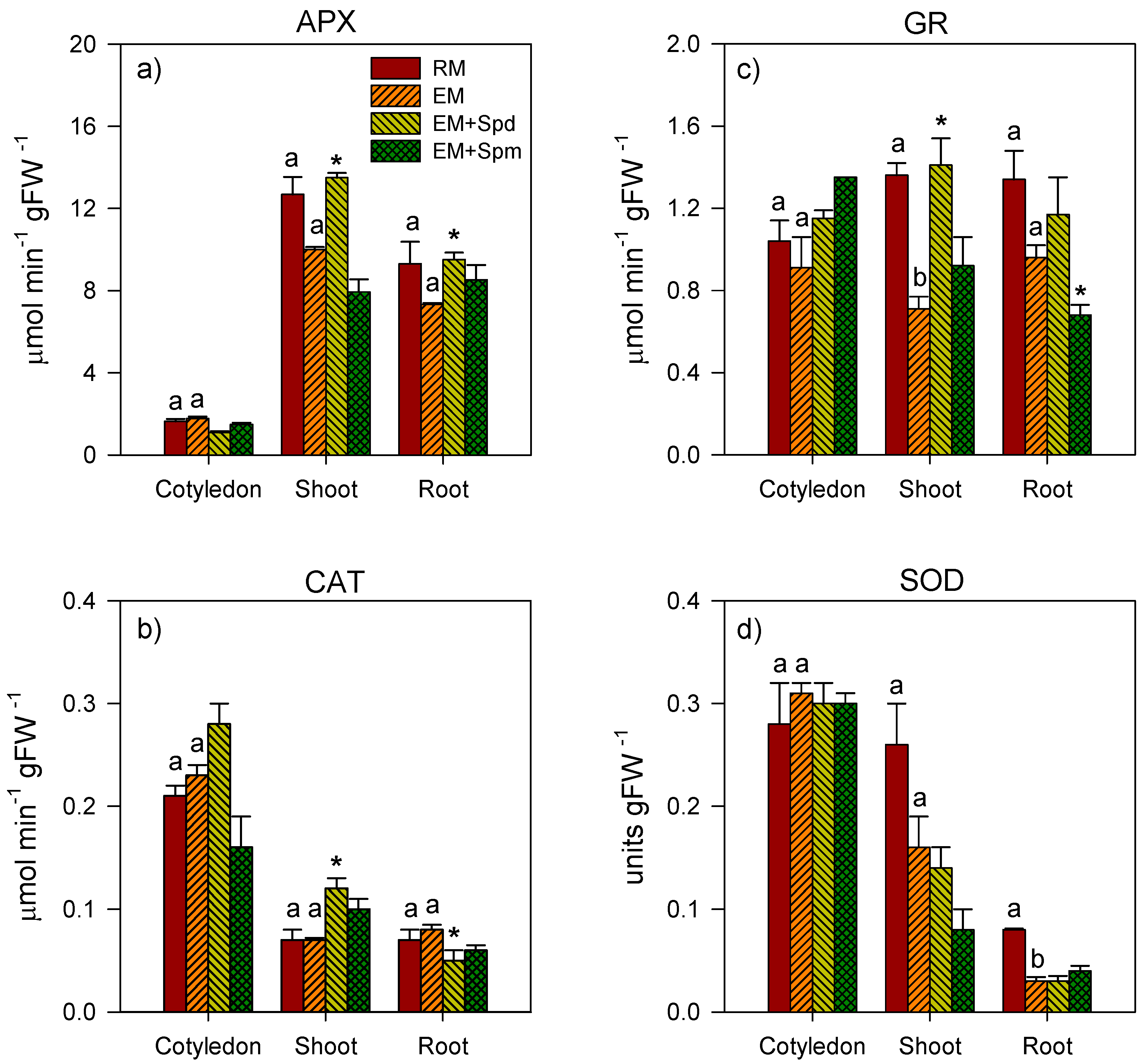
3.4. Changes in the Activities of Antioxidative Enzymes in Seedling Tissues under Excess Moisture
3.5. Polyamine-Induced Modulation of Antioxidative Enzyme Activities under Excess Moisture
4. Discussion
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
| APX | ascorbate peroxidase |
| CAT | catalase |
| DAI | days after imbibition |
| DAP | days after planting |
| EA | embryo axis |
| EM | excess moisture |
| GR | glutathione reductase |
| RM | regular watering |
| ROS | reactive oxygen species |
| SOD | superoxide dismutase |
| Spd | spermidine |
| Spm | spermine |
References
- Bailey-Serres, J.; Colmer, T.D. Plant tolerance of flooding stress—Recent advances. Plant Cell Environ. 2014, 37, 2211–2215. [Google Scholar] [CrossRef] [PubMed]
- Bailey-Serres, J.; Voesenek, L.A.C.J. Flooding stress: Acclimations and genetic diversity. Annu. Rev. Plant Biol. 2008, 59, 313–339. [Google Scholar] [CrossRef] [PubMed]
- Dat, J.F.; Capelli, N.; Folzer, H.; Bourgeade, P.; Badot, P.-M. Sensing and signalling during plant flooding. Plant Physiol. Biochem. 2004, 42, 273–282. [Google Scholar] [CrossRef]
- Wuebker, E.F.; Mullen, R.E.; Koehler, K. Flooding and temperature effects on soybean germination. Crop Sci. 2001, 41, 1857–1861. [Google Scholar] [CrossRef]
- Lee, K.-W.; Chen, P.W.; Yu, S.-M. Metabolic adaptation to sugar/O2 deficiency for anaerobic germination and seedling growth in rice. Plant Cell Environ. 2014, 37, 2234–2244. [Google Scholar]
- Bewley, J.D. Seed germination and dormancy. Plant Cell 1997, 9, 1055–1066. [Google Scholar] [CrossRef]
- Liu, M.Y.; Sun, J.; Wang, K.Y.; Liu, D.; Li, Z.Y.; Zhang, J. Spermidine enhances waterlogging tolerance via regulation of antioxidant defence, heat shock protein expression and plasma membrane H+-ATPase activity in Zea mays. J. Agron. Crop Sci. 2014, 200, 199–211. [Google Scholar] [CrossRef]
- Bailey-Serres, J.; Lee, S.C.; Brinton, E. Waterproofing crops: Effective flooding survival strategies. Plant Physiol. 2012, 160, 1698–1709. [Google Scholar] [CrossRef]
- Hashiguchi, A.; Sakata, K.; Komatsu, S. Proteome analysis of early-stage soybean seedlings under flooding stress. J. Proteome Res. 2009, 8, 2058–2069. [Google Scholar] [CrossRef]
- Apel, K.; Hirt, H. Reactive oxygen species: Metabolism, oxidative stress, and signal transduction. Annu. Rev. Plant Biol. 2004, 55, 373–399. [Google Scholar] [CrossRef]
- Gill, S.S.; Tuteja, N. Reactive oxygen species and antioxidant machinery in abiotic stress tolerance in crop plants. Plant Physiol. Biochem. 2010, 48, 909–930. [Google Scholar] [CrossRef] [PubMed]
- Bailly, C.; El-Maarouf-Bouteau, H.; Corbineau, F. From intracellular signaling networks to cell death: The dual role of reactive oxygen species in seed physiology. C. R. Biol. 2008, 331, 806–814. [Google Scholar] [CrossRef] [PubMed]
- Alscher, R.G.; Erturk, N.; Heath, L.S. Role of superoxide dismutases (SODs) in controlling oxidative stress in plants. J. Exp. Bot. 2002, 53, 1331–1341. [Google Scholar] [CrossRef] [PubMed]
- Ahmad, P.; Jaleel, C.A.; Salem, M.A.; Nabi, G.; Sharma, S. Roles of enzymatic and nonenzymatic antioxidants in plants during abiotic stress. Crit. Rev. Biotechnol. 2010, 30, 161–175. [Google Scholar] [CrossRef]
- Yiu, J.-C.; Liu, C.-W.; Yi-Tan Fang, D.; Lai, Y.-S. Waterlogging tolerance of Welsh onion (Allium fistulosum L.) enhanced by exogenous spermidine and spermine. Plant Physiol. Biochem. 2009, 47, 710–716. [Google Scholar] [CrossRef]
- Kusano, T.; Berberich, T.; Tateda, C.; Takahashi, Y. Polyamines: Essential factors for growth and survival. Planta 2008, 228, 367–381. [Google Scholar] [CrossRef]
- Alcázar, R.; Altabella, T.; Marco, F.; Bortolotti, C.; Reymond, M.; Koncz, C.; Carrasco, P.; Tiburcio, A.F. Polyamines: Molecules with regulatory functions in plant abiotic stress tolerance. Planta 2010, 231, 1237–1249. [Google Scholar] [CrossRef]
- Tiburcio, A.F.; Altabella, T.; Bitrián, M.; Alcázar, R. The roles of polyamines during the lifespan of plants: From development to stress. Planta 2014, 240, 1–18. [Google Scholar] [CrossRef]
- Hussain, S.S.; Ali, M.; Ahmad, M.; Siddique, K.H.M. Polyamines: Natural and engineered abiotic and biotic stress tolerance in plants. Biotechnol. Adv. 2011, 29, 300–311. [Google Scholar] [CrossRef]
- Scoccianti, V.; Torrigiani, P.; Bagni, N. Distribution of diamine oxidase activity and polyamine pattern in bean and soybean seedlings at different stages of germination. Physiol. Plant. 1990, 80, 515–519. [Google Scholar] [CrossRef]
- Jiménez-Bremont, J.F.; Marina, M.; Guerrero-González, M.d.l.L.; Rossi, F.R.; Sánchez-Rangel, D.; Rodríguez-Kessler, M.; Ruiz, O.A.; Gárriz, A. Physiological and molecular implications of plant polyamine metabolism during biotic interactions. Front. Plant Sci. 2014, 5, 95. [Google Scholar] [PubMed]
- Gupta, K.; Dey, A.; Gupta, B. Plant polyamines in abiotic stress responses. Acta Physiol. Plant. 2013, 35, 2015–2036. [Google Scholar] [CrossRef]
- Bouchereau, A.; Aziz, A.; Larher, F.; Martin-Tanguy, J. Polyamines and environmental challenges: Recent development. Plant Sci. 1999, 140, 103–125. [Google Scholar] [CrossRef]
- Velikova, V.; Yordanov, I.; Edreva, A. Oxidative stress and some antioxidant systems in acid rain-treated bean plants: Protective role of exogenous polyamines. Plant Sci. 2000, 151, 59–66. [Google Scholar] [CrossRef]
- Kamiab, F.; Talaie, A.; Khezri, M.; Javanshah, A. Exogenous application of free polyamines enhance salt tolerance of pistachio (Pistacia vera L.) seedlings. Plant Growth Regul. 2014, 72, 257–268. [Google Scholar] [CrossRef]
- Parvin, S.; Lee, O.R.; Sathiyaraj, G.; Khorolragchaa, A.; Kim, Y.-J.; Yang, D.-C. Spermidine alleviates the growth of saline-stressed ginseng seedlings through antioxidative defense system. Gene 2014, 537, 70–78. [Google Scholar] [CrossRef]
- Nguyen, T.-N.; Tuan, P.A.; Mukherjee, S.; Son, S.; Ayele, B.T. Hormonal regulation in adventitious roots and during their emergence under waterlogged conditions in wheat. J. Exp. Bot. 2018, 69, 4065–4082. [Google Scholar] [CrossRef]
- Mukherjee, S.; Liu, A.; Deol, K.K.; Kulichikhin, K.; Stasolla, C.; Brûlé-Babel, A.; Ayele, B.T. Transcriptional coordination and abscisic acid mediated regulation of sucrose transport and sucrose-to-starch metabolism related genes during grain filling in wheat (Triticum aestivum L.). Plant Sci. 2015, 240, 143–160. [Google Scholar] [CrossRef]
- Yao, Z.; Liu, L.; Gao, F.; Rampitsch, C.; Reinecke, D.M.; Ozga, J.A.; Ayele, B.T. Developmental and seed aging mediated regulation of antioxidative genes and differential expression of proteins during pre- and post-germinative phases in pea. J. Plant Physiol. 2012, 169, 1477–1488. [Google Scholar] [CrossRef]
- Nguyen, T.-N.; Son, S.; Jordan, M.C.; Levin, D.B.; Ayele, B.T. Lignin biosynthesis in wheat (Triticum aestivum L.): Its response to waterlogging and association with hormonal levels. BMC Plant Biol. 2016, 16, 28. [Google Scholar] [CrossRef]
- Livak, K.J.; Schmittgen, T.D. Analysis of relative gene expression data using Real-time quantitative PCR and the 2−ΔΔCT method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef] [PubMed]
- Agarwal, S.; Sairam, R.K.; Srivastava, G.C.; Tyagi, A.; Meena, R.C. Role of ABA, salicylic acid, calcium and hydrogen peroxide on antioxidant enzymes induction in wheat seedlings. Plant Sci. 2005, 169, 559–570. [Google Scholar] [CrossRef]
- Dionisio-Sese, M.L.; Tobita, S. Antioxidant responses of rice seedlings to salinity stress. Plant Sci. 1998, 135, 1–9. [Google Scholar] [CrossRef]
- Nakano, Y.; Asada, K. Hydrogen peroxide is scavenged by ascorbate-specific peroxidase in spinach chloroplasts. Plant Cell Physiol. 1981, 22, 867–880. [Google Scholar]
- Aebi, H. Catalase in vitro. Methods Enzymol. 1984, 105, 121–126. [Google Scholar] [PubMed]
- Fryer, M.J.; Andrews, J.R.; Oxborough, K.; Blowers, D.A.; Baker, N.R. Relationship between CO2 assimilation, photosynthetic electron transport, and active O2 metabolism in leaves of maize in the field during periods of low temperature. Plant Physiol. 1998, 116, 571–580. [Google Scholar] [CrossRef]
- Huang, Y.; Lin, C.; He, F.; Li, Z.; Guan, Y.; Hu, Q.; Hu, J. Exogenous spermidine improves seed germination of sweet corn via involvement in phytohormone interactions, H2O2 and relevant gene expression. BMC Plant Biol. 2017, 17, 1. [Google Scholar] [CrossRef]
- Li, Z.; Peng, Y.; Zhang, X.-Q.; Ma, X.; Huang, L.-K.; Yan, Y.-H. Exogenous spermidine improves seed germination of white clover under water stress via involvement in starch metabolism, antioxidant defenses and relevant gene expression. Molecules 2014, 19, 18003–18024. [Google Scholar] [CrossRef]
- Bagni, N. Metabolic changes of polyamines during the germination of Phaseolus vulgaris. New Phytol. 1970, 69, 159–164. [Google Scholar] [CrossRef]
- Villanueva, V.R.; Adlakha, R.C.; Cantera-Soler, A.M. Changes in polyamine concentration during seed germination. Phytochemistry 1978, 17, 1245–1249. [Google Scholar] [CrossRef]
- Liu, Y.-Z.; Tang, B.; Zheng, Y.-L.; Ma, K.-J.; Xu, S.-Z.; Qiu, F.-Z. Screening methods for waterlogging tolerance at maize (Zea mays L.) seedling stage. Agr. Sci. China 2010, 9, 362–369. [Google Scholar] [CrossRef]
- Gill, S.S.; Tuteja, N. Polyamines and abiotic stress tolerance in plants. Plant Signal. Behav. 2010, 5, 26–33. [Google Scholar] [CrossRef] [PubMed]
- Nahar, K.; Hasanuzzaman, M.; Alam, M.M.; Rahman, A.; Mahmud, J.-A.; Suzuki, T.; Fujita, M. Insights into spermine-induced combined high temperature and drought tolerance in mung bean: Osmoregulation and roles of antioxidant and glyoxalase system. Protoplasma 2017, 254, 445–460. [Google Scholar] [CrossRef] [PubMed]
- Blokhina, O.; Virolainen, E.; Fagerstedt, K.V. Antioxidants, oxidative damage and oxygen deprivation stress: A review. Ann. Bot. 2003, 91, 179–194. [Google Scholar] [CrossRef]
- Ozga, J.A.; Reinecke, D.M.; Knowles, N.R.; Blenis, P. Characterization of the loss of seedling vigor in pea (Pisum sativum L.). Can. J. Plant Sci. 2004, 84, 443–451. [Google Scholar] [CrossRef]
- Kranner, I.; Roach, T.; Beckett, R.P.; Whitaker, C.; Minibayeva, F.V. Extracellular production of reactive oxygen species during seed germination and early seedling growth in Pisum sativum. J. Plant Physiol. 2010, 167, 805–811. [Google Scholar] [CrossRef]
- Verma, G.; Mishra, S.; Sangwan, N.; Sharma, S. Reactive oxygen species mediate axis-cotyledon signaling to induce reserve mobilization during germination and seedling establishment in Vigna radiata. J. Plant Physiol. 2015, 184, 79–88. [Google Scholar] [CrossRef]
- Tamang, B.G.; Magliozzi, J.O.; Maroof, M.A.S.; Fukao, T. Physiological and transcriptomic characterization of submergence and reoxygenation responses in soybean seedlings. Plant Cell Environ. 2014, 37, 2350–2365. [Google Scholar] [CrossRef]
- Li, Z.; Zhou, H.; Peng, Y.; Zhang, X.; Ma, X.; Huang, L.; Yan, Y. Exogenously applied spermidine improves drought tolerance in creeping bentgrass associated with changes in antioxidant defense, endogenous polyamines and phytohormones. Plant Growth Regul. 2015, 76, 71–82. [Google Scholar] [CrossRef]
- Wang, X.; Shi, G.; Xu, Q.; Hu, J. Exogenous polyamines enhance copper tolerance of Nymphoides peltatum. J. Plant Physiol. 2007, 164, 1062–1070. [Google Scholar] [CrossRef]
- Nayyar, H.; Chander, S. Protective effects of polyamines against oxidative stress induced by water and cold stress in chickpea. J. Agron. Crop Sci. 2004, 190, 355–365. [Google Scholar] [CrossRef]
- Guerra, D.; Crosatti, C.; Khoshro, H.H.; Mastrangelo, A.M.; Mica, E.; Mazzucotelli, E. Post-transcriptional and post-translational regulations of drought and heat response in plants: A spider’s web of mechanisms. Front. Plant Sci. 2015, 6, 57. [Google Scholar] [CrossRef] [PubMed]
- Handa, A.K.; Mattoo, A.K. Differential and functional interactions emphasize the multiple roles of polyamines in plants. Plant Physiol. Biochem. 2010, 48, 540–546. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.; Fan, L.; Gao, H.; Wu, X.; Li, J.; Lv, G.; Gong, B. Polyamine biosynthesis and degradation are modulated by exogenous gamma-aminobutyric acid in root-zone hypoxia-stressed melon roots. Plant Physiol. Biochem. 2014, 82, 17–26. [Google Scholar] [CrossRef] [PubMed]
- Wimalasekera, R.; Tebartz, F.; Scherer, G.F.E. Polyamines, polyamine oxidases and nitric oxide in development, abiotic and biotic stresses. Plant Sci. 2011, 181, 593–603. [Google Scholar] [CrossRef]
- Moschou, P.N.; Wu, J.; Cona, A.; Tavladoraki, P.; Angelini, R.; Roubelakis-Angelakis, K.A. The polyamines and their catabolic products are significant players in the turnover of nitrogenous molecules in plants. J. Exp. Bot. 2012, 63, 5003–5015. [Google Scholar] [CrossRef]

| Gene | Type | Sequence (5′ to 3′) | Amplicon Size | Accession Number |
|---|---|---|---|---|
| Gmβ-actin1 | F a | CGGTGGTTCTATCTTGGCATC | 142 | NM_001289231 |
| R b | GTCTTTCGCTTCAATAACCCTA | |||
| GmAPXs | F | GATGCGCTCCTCTAATGCTC | 198 | NM_001250856, |
| R | AGAAATCGGCGTAGCTCAAA | NM_001248658 | ||
| GmCATs | F | CGCCTTCAATTCTCCCTTCT | 99 | NM_001250627, |
| R | TCCAGCAGAATTGGACCTCT | NM_001249045, NM_001250642, NM_001253092 | ||
| GmGR | F | GCGAGCTTCCTTTCTCCACT | 92 | NM_001251077 |
| R | CAGCAACTTCTTCGGCACAC | |||
| GmSOD1 | F | TGAAGGCTGTGGCAGTTCTT | 83 | NM_001248369 |
| R | GGTGGTTGGACCATTTCCCT | |||
| GmSOD3 | F | AATGGGACCACCCATGTGAC | 106 | NM_001255882 |
| R | CAGTGGAGTTGCAGCCATTG | |||
| GmSODBs | F | CTGCTGCTGCAACACAATTT | 141 | NM_001251557, |
| R | TCACAGCATTGGGACTCTTG | NM_001250972 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sidhu, G.K.; Tuan, P.A.; Renault, S.; Daayf, F.; Ayele, B.T. Polyamine-Mediated Transcriptional Regulation of Enzymatic Antioxidative Response to Excess Soil Moisture during Early Seedling Growth in Soybean. Biology 2020, 9, 185. https://doi.org/10.3390/biology9080185
Sidhu GK, Tuan PA, Renault S, Daayf F, Ayele BT. Polyamine-Mediated Transcriptional Regulation of Enzymatic Antioxidative Response to Excess Soil Moisture during Early Seedling Growth in Soybean. Biology. 2020; 9(8):185. https://doi.org/10.3390/biology9080185
Chicago/Turabian StyleSidhu, Gagandip K., Pham Anh Tuan, Sylvie Renault, Fouad Daayf, and Belay T. Ayele. 2020. "Polyamine-Mediated Transcriptional Regulation of Enzymatic Antioxidative Response to Excess Soil Moisture during Early Seedling Growth in Soybean" Biology 9, no. 8: 185. https://doi.org/10.3390/biology9080185
APA StyleSidhu, G. K., Tuan, P. A., Renault, S., Daayf, F., & Ayele, B. T. (2020). Polyamine-Mediated Transcriptional Regulation of Enzymatic Antioxidative Response to Excess Soil Moisture during Early Seedling Growth in Soybean. Biology, 9(8), 185. https://doi.org/10.3390/biology9080185







