Biochemical Profile of the Soybean Seed Embryonic Axis and Its Changes during Accelerated Aging
Abstract
:1. Introduction
2. Materials and Methods
2.1. Plant Material, Accelerated Aging, Germination, and Vigor Tests
2.2. Isolation of the Soybean Embryonic Axis
2.3. Biochemical Analyses
2.3.1. Antioxidant Enzymes
2.3.2. Lipid Peroxidation, Hydrogen Peroxide, and Maillard Reaction
2.3.3. Protein, Glucose, Cholesterol, Triglycerides, Starch, and Sugars
2.4. Macronutrients Analysis
2.5. Extraction and Estimation Analysis, DNA Oxidation
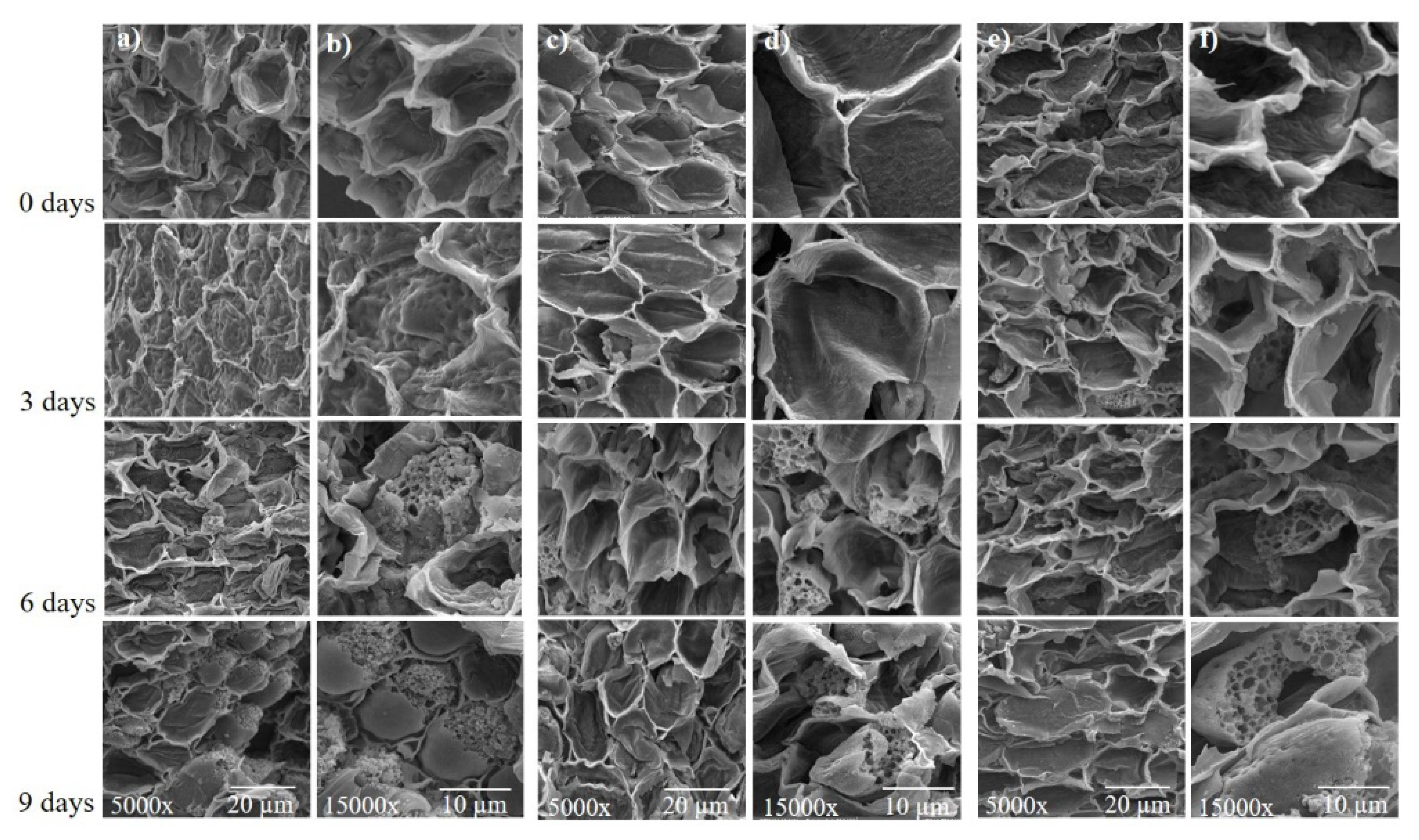
2.6. Scanning Electron Microscopy (SEM)
2.7. Statistical Analysis
3. Results
3.1. Germination and Vigor under Accelerated Aging
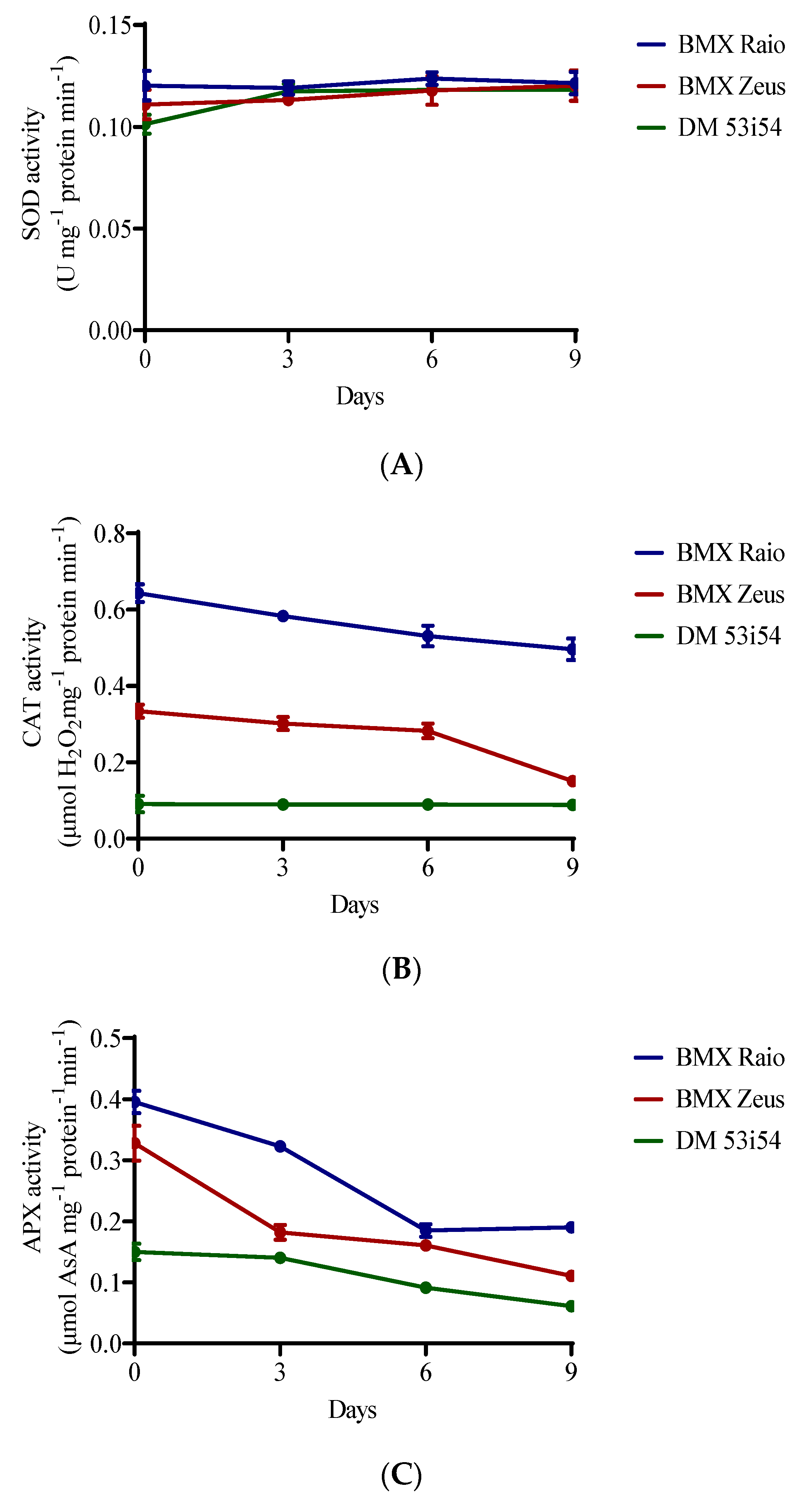
3.2. Antioxidant Enzyme Activity
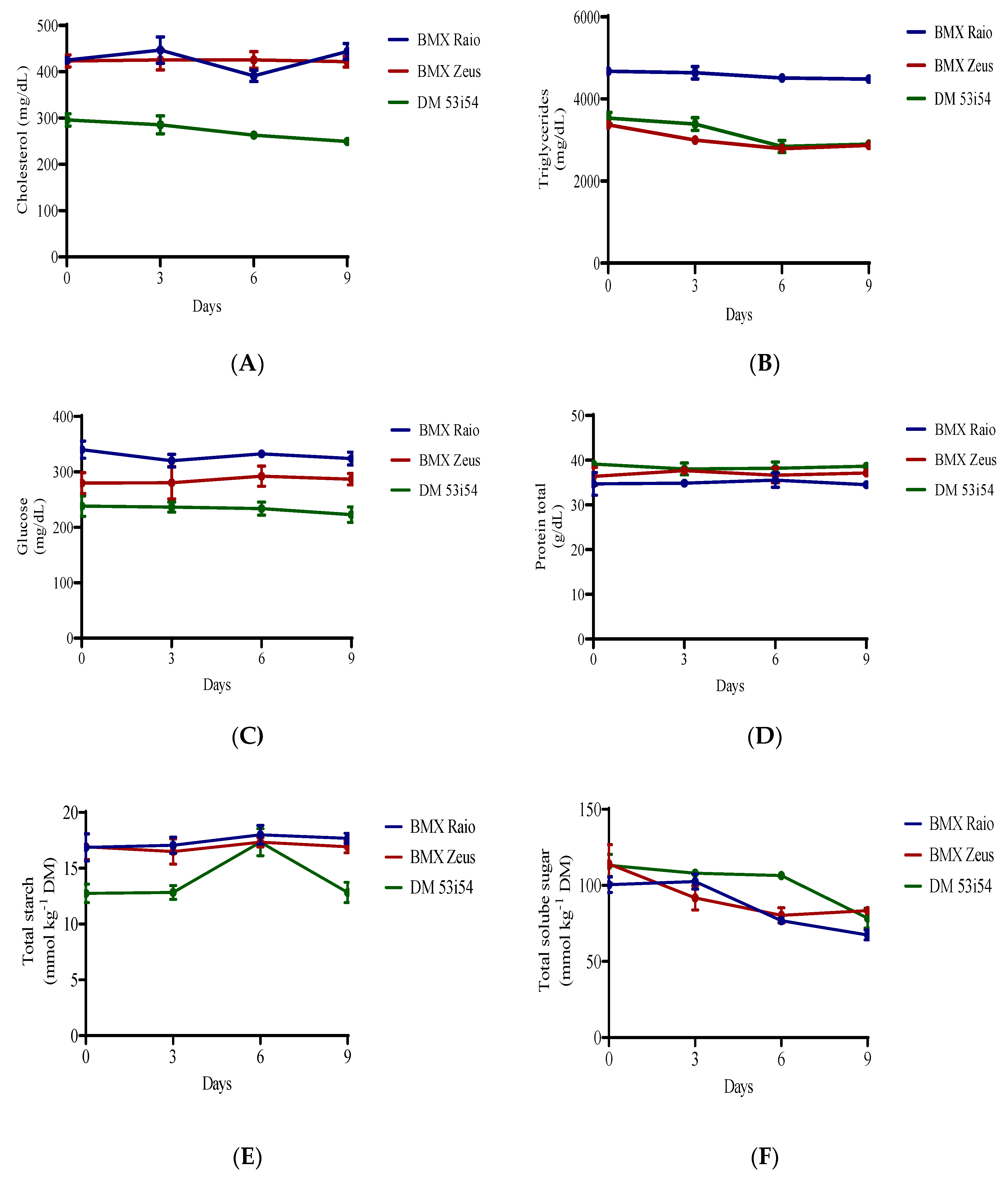
3.3. Hydrogen Peroxide, Lipid Peroxidation, and Maillard Reaction
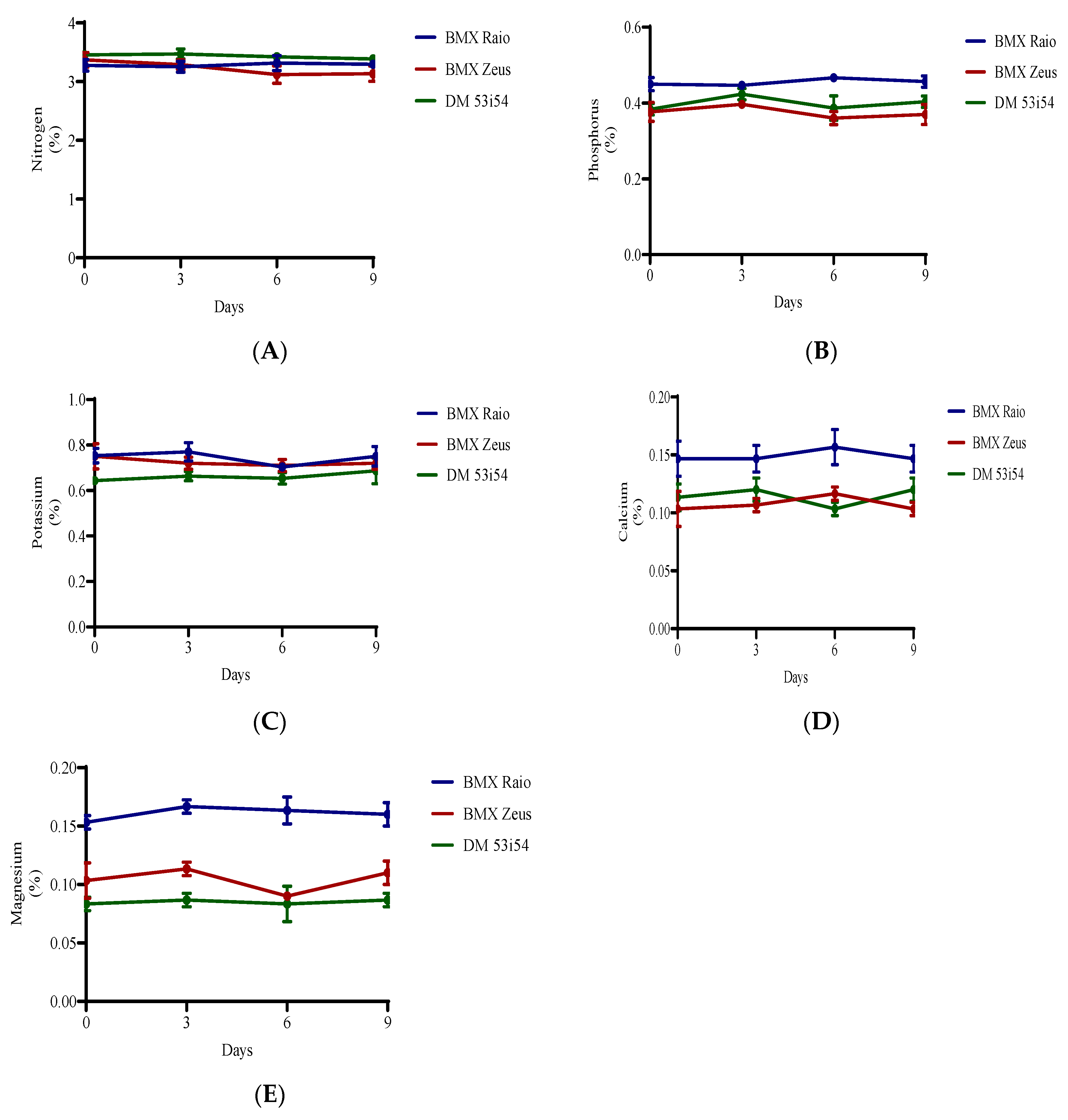
3.4. Macronutrients Analysis
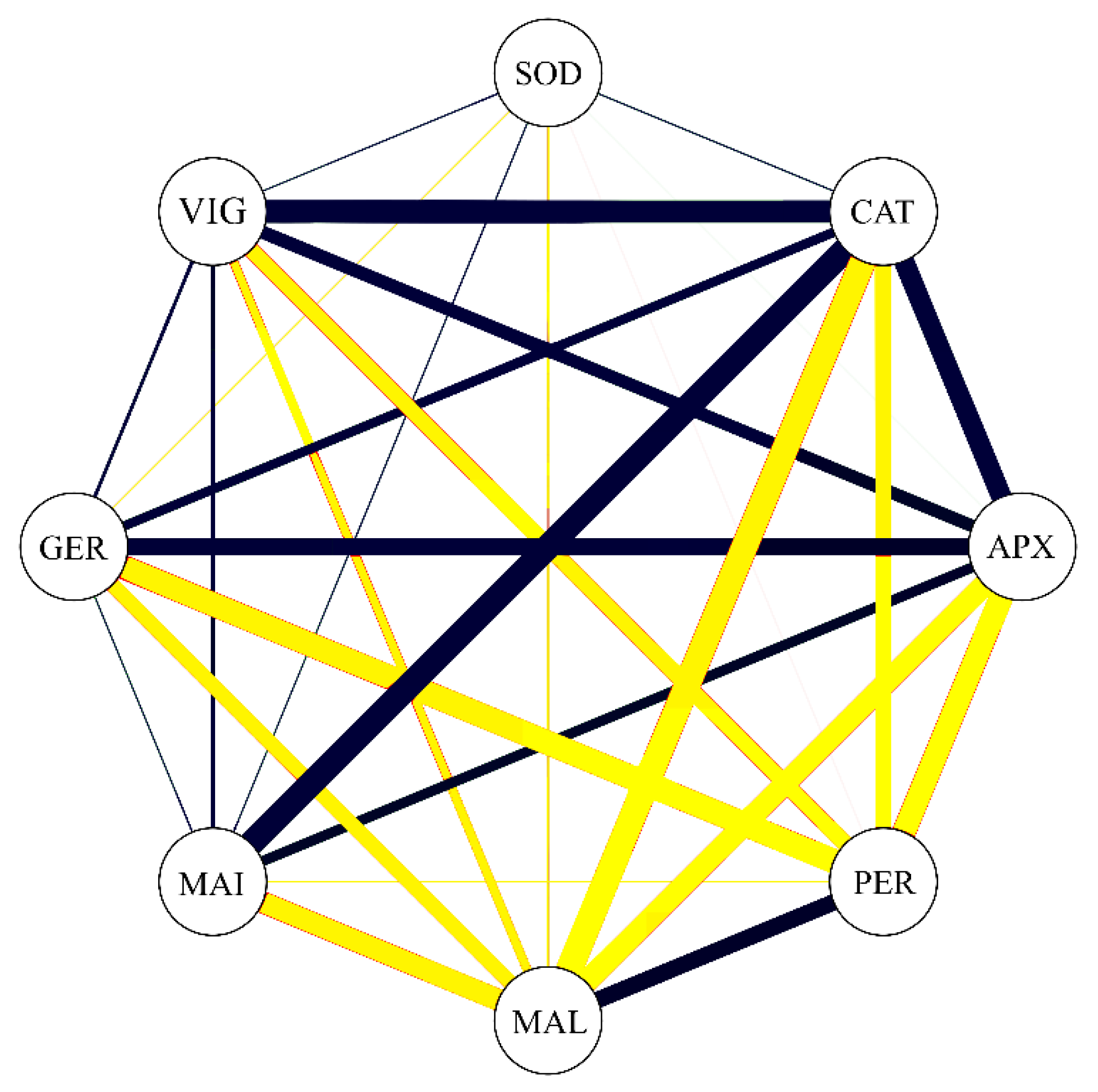
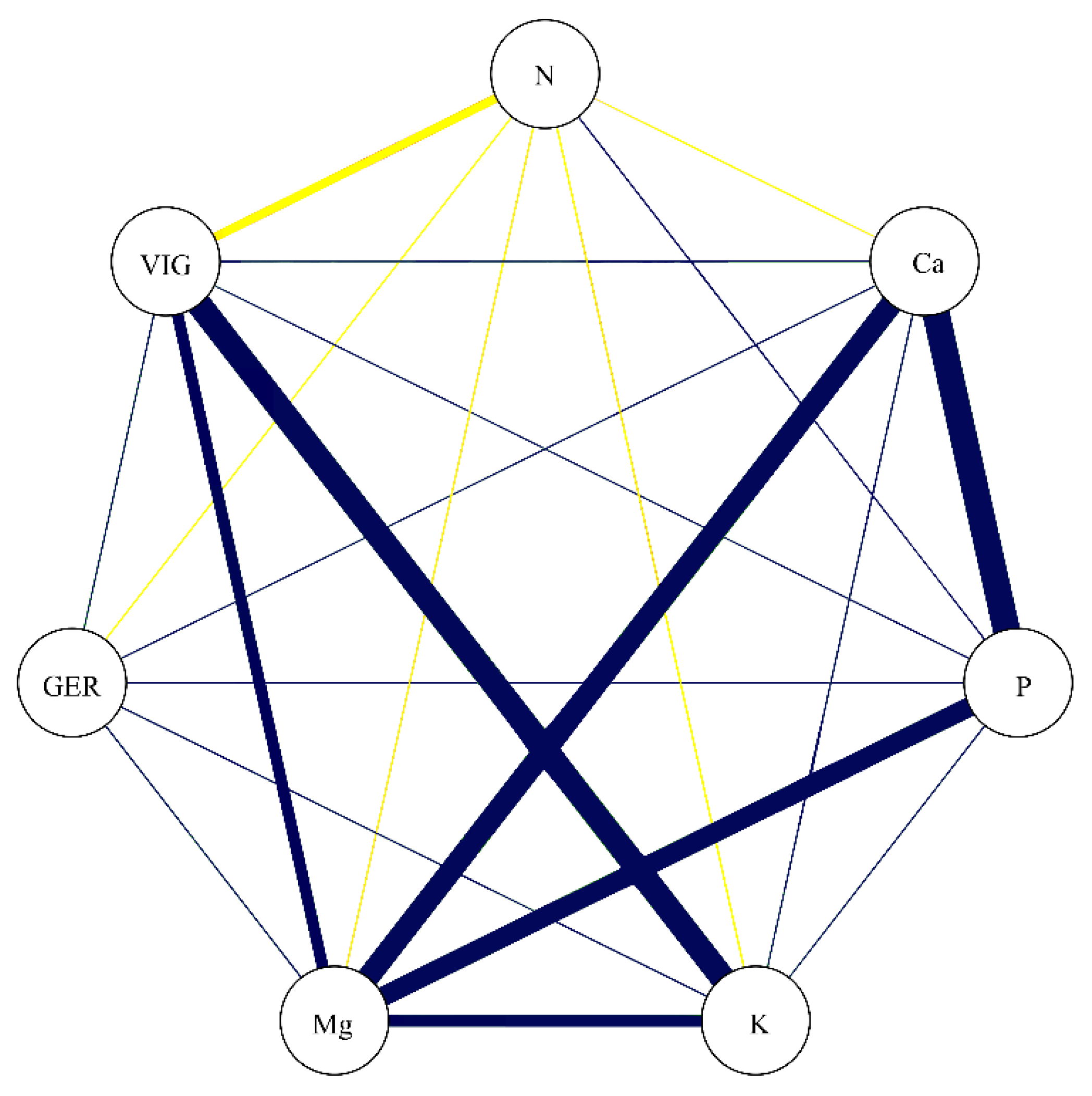
3.5. Correlations
3.6. Dissimilarity
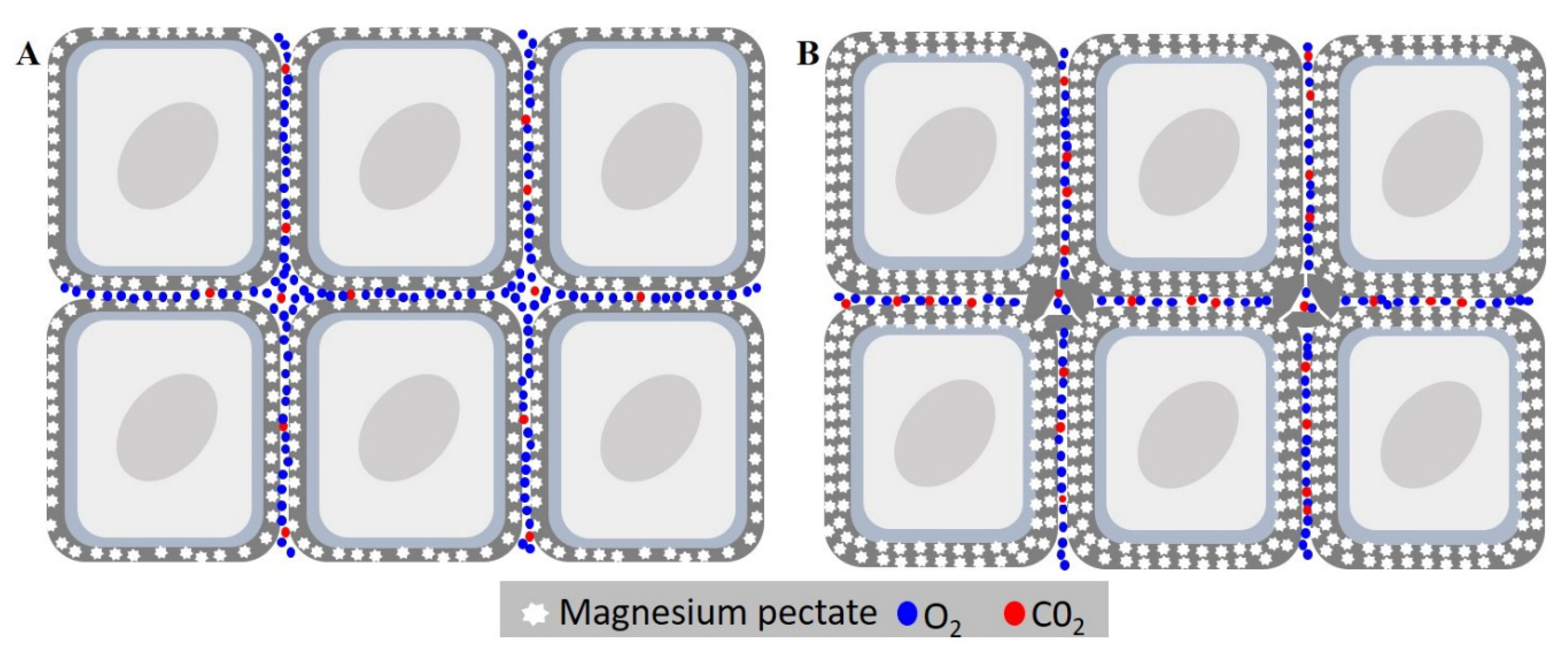
3.7. Scanning Electron Microscopy Analysis
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Finch-Savage, W.E.; Bassel, G.W. Seed vigour and crop establishment: Extending performance beyond adaptation. J. Exp. Bot. 2016, 67, 567–591. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fu, Y.; Ahmed, Z.; Diederichsen, A. Towards a better monitoring of seed ageing under ex situ seed conservation. Conserv. Physiol. 2015, 3. [Google Scholar] [CrossRef] [Green Version]
- Caverzan, A.; Giacomin, R.; Müller, M.; Biazus, C.; Lângaro, N.C.; Chavarria, G.L. How does seed vigor affect soybean yield componentes? Agron. J. 2018, 110, 1318–1327. [Google Scholar] [CrossRef]
- Ebone, L.A.; Caverzan, A.; Tagliari, A.; Chiomento, J.L.T.; Silveira, D.C.; Chavarria, G. Soybean seed vigor: Uniformity and growth as key factors to improve yield. Agronomy 2020, 10, 545. [Google Scholar] [CrossRef]
- Ebone, L.A.; Caverzan, A.; Chavarria, G. Physiologic alterations in orthodox seeds due to deterioration processes. Plant Physiol. Biochem. 2019, 145, 34–42. [Google Scholar] [CrossRef]
- Yin, G.; Xin, X.; Song, C.; Chen, X.; Zhang, J.; Wu, S.; Li, R.; Liu, X.; Lu, X. Activity levels and expression of antioxidant enzymes in the ascorbate-glutathione cycle in artificially aged rice seed. Plant Physiol. Biochem. 2014, 80, 1–9. [Google Scholar] [CrossRef]
- Gill, S.S.; Tuteja, N. Reactive oxygen species and antioxidant machinery in abiotic stress tolerance in crop plants. Plant Physiol. Biochem. 2010, 48, 909–930. [Google Scholar] [CrossRef]
- Kurek, K.; Plitta-Michalak, B.; Ratajczak, E. Reactive oxygen species as potential drivers of the seed aging process. Plants 2019, 8, 174. [Google Scholar] [CrossRef] [Green Version]
- Waterworth, W.M.; Bray, C.M.; West, C.E. The importance of safeguarding genome integrity in germination and seed longevity. J. Exp. Bot. 2015, 66, 3549–3558. [Google Scholar] [CrossRef] [Green Version]
- Min, C.W.; Lee, S.H.; Cheon, Y.E.; Han, W.Y.; Ko, J.M.; Kang, H.W.; Kim, Y.C.; Agrawal, G.K.; Rakwal, R.; Gupta, R.; et al. In-depth proteomic analysis of Glycine max seeds during controlled deterioration treatment reveals a shift in seed metabolism. J. Proteom. 2017, 169, 125–135. [Google Scholar] [CrossRef]
- Wettlaufer, S.H.; Leopold, A.C. Relevance of Amadori and Maillard Products to Seed Deterioration. Plant Physiol. 1991, 97, 165–169. [Google Scholar] [CrossRef]
- Fleming, M.B.; Richards, C.M.; Walters, C. Decline in RNA integrity of dry-stored soybean seeds correlates with loss of germination potential. J. Exp. Bot. 2017, 68, 2219–2230. [Google Scholar] [CrossRef] [Green Version]
- Ballesteros, D.; Walters, C. Solid-State Biology and Seed Longevity: A Mechanical Analysis of Glasses in Pea and Soybean Embryonic Axes. Front. Plant Sci. 2019, 10, 920. [Google Scholar] [CrossRef] [Green Version]
- Brasil—Ministério da Agricultura, Pecuária e Abastecimento. Regras Para Análise de Sementes, 1st ed.; Ministério da Agricultura, Pecuária e Abastecimento, Secretária de Defesa Agropecuária: Brasília, Brazil, 2009; pp. 147–220.
- Krzyzanowski, F.C.; Vieira, R.D.; França-Neto, J.B. Vigor de Sementes: Conceitos e Testes, 1st ed.; Associação Brasileira de Tecnologia de Sementes-ABRATES: Londrina, Brazil, 1999. [Google Scholar]
- Nakano, Y.; Asada, K. Hydrogen peroxide is scavenged by ascorbate-specific peroxidase in spinach chloroplasts. Plant Cell Physiol. 1981, 22, 1068–1072. [Google Scholar]
- Amako, K.; Chen, G.X.; Asada, K. Separate assays specific for ascorbate peroxidase and guaiacol peroxidase and for the chloroplastic and cytosolic isozymes of ascorbate peroxidase in plants. Plant Cell Physiol. 1994, 35, 497–504. [Google Scholar] [CrossRef]
- Havir, E.A.; McHale, N.A. Biochemical and developmental characterization of multiple forms of catalase in tobacco leaves. Plant Physiol. 1987, 84, 450–455. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Giannopolotis, C.N.; Ries, S.K. Superoxide dismutases: I. Occurrence in higher plants. Plant Physiol. 1977, 59, 309–314. [Google Scholar] [CrossRef] [PubMed]
- Rosa, S.B.; Caverzan, A.; Teixeira, F.K.; Lazzarotto, F.; Silveira, J.A.; Ferreira-Silva, S.L.; Abreu-Neto, J.; Margis, R.; Margis-Pinheiro, M. Cytosolic APx knockdown indicates an ambiguous redox responses in rice. Phytochemistry 2010, 71, 548–558. [Google Scholar] [CrossRef] [PubMed]
- Brennan, T.; Frenkel, C. Involvement of hydrogen peroxide in the regulation of senescence in pear. Plant Physiol. 1977, 59, 411–416. [Google Scholar] [CrossRef] [Green Version]
- DuBois, M.; Gilles, K.A.; Hamilton, J.K.; Rebers, P.A.; Smith, F. Colorimetric method for determination of sugars and related substances. Anal. Chem. 1956, 28, 350–356. [Google Scholar] [CrossRef]
- McCready, R.M.; Guggolz, J.; Silviera, V.; Owens, H.S. Determination of starch and amylase in vegetables. Anal. Chem. 1950, 2, 1156–1158. [Google Scholar] [CrossRef]
- Tedesco, M.J.; Gianello, C.; Bissani, C.A.; Bohnen, H.; Volkweiss, S.J. Análises de Solo, Plantas e Outros Materiais, 2nd ed.; Universidade Federal do Rio Grande do Sul: Porto Alegre, Brazil, 1995. [Google Scholar]
- Doyle, J.J.; Doyle, J.L. Isolation of plant DNA from fresh tissue. Focus 1990, 12, 13–14. [Google Scholar]
- Langfinger, D.; Sonntag, C.V. C-radiolysis of 2-deoxyguanosine. The structure of the malondialdehyde like product. Z. Naturforch. C Biosci. 1985, 40, 446–448. [Google Scholar] [CrossRef]
- Robards, A.W. An introduction to techniques for scanning electron microscopy of plant cells. In Electron Microscopy and Cytochemistry of Plant Cells; Hall, J.L., Ed.; Elsevier: New York, NY, USA, 1978; pp. 343–444. [Google Scholar]
- Mojena, R. Hierárquical grouping method and stopping rules: An evaluation. Comput. J. 1977, 20, 359–363. [Google Scholar] [CrossRef] [Green Version]
- Singh, D. The relative importance of characters affecting genetic divergence. Indian J. Genet. Plant Breed. 1981, 41, 237–245. [Google Scholar]
- Ayala, A.; Munoz, M.F.; Arguelles, S. Lipid peroxidation: Production, metabolism, and signaling mechanisms of malondialdehyde and 4-Hydroxy-2-Nonenal. Oxid. Med. Cell Longev. 2014, 2014, 360438. [Google Scholar] [CrossRef]
- Ning, F.; Wu, X.; Zhang, H.; Wu, Z.; Niu, L.; Yang, H.; Wang, W. Accumulation profiles of embryonic salt-soluble proteins in maize hybrids and parental lines indicate matroclinous inheritance: A proteomic analysis. Front. Plant Sci. 2017, 25. [Google Scholar] [CrossRef]
- Chen, H.; Osuna, D.; Colville, L.; Lorenzo, O.; Graeber, K.; Ku, H.; Leubner-Metzger, G.; Kranner, I. Transcriptome-wide mapping of pea seed ageing reveals a pivotal role for genes related to oxidative stress and programmed cell death. PLoS ONE 2013, 8, e78471. [Google Scholar] [CrossRef] [Green Version]
- Gidrol, X.; Noubhani, A.; Mocquot, B.; Fournier, A.; Pradet, A. Effect of accelerated aging on protein synthesis in two legume seeds. Plant Physiol. Biochem. 1988, 26, 281–288. [Google Scholar]
- Daum, B.; Walter, A.; Horst, A.; Osiewacz, H.D.; Kühlbrandt, W. Age-dependent dissociation of ATP synthase dimers and loss of inner-membrane cristae in mitochondria. Proc. Natl. Acad. Sci. USA 2013, 110, 15301–15306. [Google Scholar] [CrossRef] [Green Version]
- Yin, G.; Whelan, J.; Wu, S.; Zhou, J.; Chen, B.; Chen, X.; Zhang, J.; He, J.; Xin, X.; Lu, X. Comprehensive mitochondrial metabolic shift during the critical node of seed ageing in rice. PLoS ONE 2016, 11, e0148013. [Google Scholar] [CrossRef] [PubMed]
- Murthy, U.M.; Sun, W.Q. Protein modification by Amadori and Maillard reactions during seed storage: Roles of sugar hydrolysis and lipid peroxidation. J. Exp. Bot. 2000, 51, 1221–1228. [Google Scholar] [CrossRef] [PubMed]
- Silva, P.A.; Diniz, K.A.; Oliveira, J.A.; Pinho, E.V.R.V. Ultra-structural and physiological analysis during the development and drying of soybean seeds. Rev. Bras. Sementes 2007, 29, 15–22. [Google Scholar] [CrossRef] [Green Version]
- Bewley, J.D. Membrane changes in seeds as related to germination and the perturbations resulting from deterioration in storage. In Physiology of Seed Deterioration; McDonald, J.R., Nelson, C.J., Eds.; Crop Science Society of America: Madison, WI, USA, 1986; pp. 1–25. [Google Scholar]
- Woodstock, L.W.; Furman, K.; Leffler, H.R. Relationship between weathering deterioration and germination, respiratory metabolism, and mineral leaching from cotton seeds. Crop Sci. 1985, 25, 459–466. [Google Scholar] [CrossRef]
- Yang, G.; Yang, L.; Jiang, H.; Li, Y.; Wang, P.; Chen, L. Physiological impacts of magnesium-deficiency in citrus seedlings: Photosynthesis, antioxidant system and carbohydrates. Trees 2012, 26, 1237–1250. [Google Scholar] [CrossRef]



| Independent Variables | SOD | CAT | APX | H2O2 | MAL | MAI | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Dependent Variables | GER | VIG | GER | VIG | GER | VIG | GER | VIG | GER | VIG | GER | VIG |
| Direct effect | −0.34 | 0.11 | 0.37 | 0.79 | −0.39 | 0.08 | −0.68 | −0.34 | −0.62 | 0.3 | −0.38 | −0.14 |
| Indirect effect by SOD | 1 | 1 | −0.15 | 0.05 | −0.01 | 0 | 0.01 | 0 | 0.09 | −0.03 | −0.12 | 0.04 |
| Indirect effect by CAT | 0.16 | 0.35 | 1 | 1 | 0.3 | 0.63 | −0.27 | −0.58 | −0.34 | −0.72 | 0.33 | 0.69 |
| Indirect effect by APX | −0.01 | 0 | −0.32 | 0.07 | 1 | 1 | 0.34 | −0.07 | 0.33 | −0.07 | −0.25 | 0.05 |
| Indirect effect by H2O2 | 0.01 | 0.01 | 0.49 | 0.25 | 0.58 | 0.29 | 1 | 1 | −0.5 | −0.25 | 0.33 | 0.17 |
| Indirect effect by MAL | 0.16 | −0.07 | 0.56 | −0.27 | 0.51 | −0.24 | −0.46 | 0.22 | 1 | 1 | 0.51 | −0.25 |
| Indirect effect by MAI | −0.14 | −0.05 | −0.34 | −0.12 | −0.25 | −0.09 | 0.19 | 0.07 | 0.32 | 0.12 | 1 | 1 |
| Correlation (r) | −0.16 | 0.35 | 0.63 | 0.84 | 0.74 | 0.68 | −0.87 | −0.74 | −0.72 | −0.62 | 0.41 | 0.55 |
| GER | VIG | |||||||||||
| Determinantion coefficient | 0.87 | 0.75 | ||||||||||
| Effect of the residual variable | 0.36 | 0.5 | ||||||||||
| Independent Variables | N | P | K | Ca | Mg | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| Dependent Variables | GER | VIG | GER | VIG | VIG | GER | VIG | GER | VIG | |
| Direct effect | 0.29 | −0.05 | −0.35 | 0.19 | 0.47 | −0.65 | 0.17 | 0.52 | 0.76 | |
| Indirect effect by N | 1 | 1 | 0.04 | −0.17 | 0.03 | −0.01 | 0.01 | −0.1 | 0.02 | |
| Indirect effect by Ca | −0.02 | −0.02 | 0.13 | 0.06 | 0.07 | 0.15 | 1 | 0.12 | 0.14 | |
| Indirect effect by P | −0.05 | −0.09 | 1 | −0.13 | −0.23 | 1 | −0.6 | −0.29 | −0.54 | |
| Indirect effect by K | −0.11 | −0.27 | 0.07 | 1 | 1 | 0.17 | 0.2 | 0.14 | 0.34 | |
| Indirect effect by Mg | −0.18 | −0.26 | 0.43 | 0.38 | 0.55 | 0.62 | 0.65 | 1 | 1 | |
| Correlation (r) | −0.05 | −0.69 | 0.29 | 0.36 | 0.89 | 0.29 | 0.42 | 0.44 | 0.72 | |
| GER | VIG | |||||||||
| Determination coefficient | 0.23 | 0.88 | ||||||||
| Effect of the residual variable | 0.88 | 0.34 | ||||||||
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ebone, L.A.; Caverzan, A.; Silveira, D.C.; Siqueira, L.d.O.; Lângaro, N.C.; Chiomento, J.L.T.; Chavarria, G. Biochemical Profile of the Soybean Seed Embryonic Axis and Its Changes during Accelerated Aging. Biology 2020, 9, 186. https://doi.org/10.3390/biology9080186
Ebone LA, Caverzan A, Silveira DC, Siqueira LdO, Lângaro NC, Chiomento JLT, Chavarria G. Biochemical Profile of the Soybean Seed Embryonic Axis and Its Changes during Accelerated Aging. Biology. 2020; 9(8):186. https://doi.org/10.3390/biology9080186
Chicago/Turabian StyleEbone, Luciano Antônio, Andréia Caverzan, Diógenes Cecchin Silveira, Luciano de Oliveira Siqueira, Nadia Canali Lângaro, José Luís Trevizan Chiomento, and Geraldo Chavarria. 2020. "Biochemical Profile of the Soybean Seed Embryonic Axis and Its Changes during Accelerated Aging" Biology 9, no. 8: 186. https://doi.org/10.3390/biology9080186
APA StyleEbone, L. A., Caverzan, A., Silveira, D. C., Siqueira, L. d. O., Lângaro, N. C., Chiomento, J. L. T., & Chavarria, G. (2020). Biochemical Profile of the Soybean Seed Embryonic Axis and Its Changes during Accelerated Aging. Biology, 9(8), 186. https://doi.org/10.3390/biology9080186






