Isolation of Antidiabetic Withanolides from Withania coagulans Dunal and Their In Vitro and In Silico Validation
Abstract
1. Introduction
2. Experimental
2.1. General Experimental Method
2.2. Plant Material
2.3. Extraction and Isolation
2.4. Acidic Hydrolysis of Compound 1
2.5. In Vitro α-Glucosidase Inhibition Assay
2.6. In Vitro Antiglycation Assay
2.7. Software Used/Statistical Analysis
2.8. Homology Modeling and Molecular Docking
2.9. Molecular Dynamics Simulation and Binding Free Energy Calculations
3. Results and Discussion
3.1. Characterization of the Isolated Compounds
3.2. Biological Activity
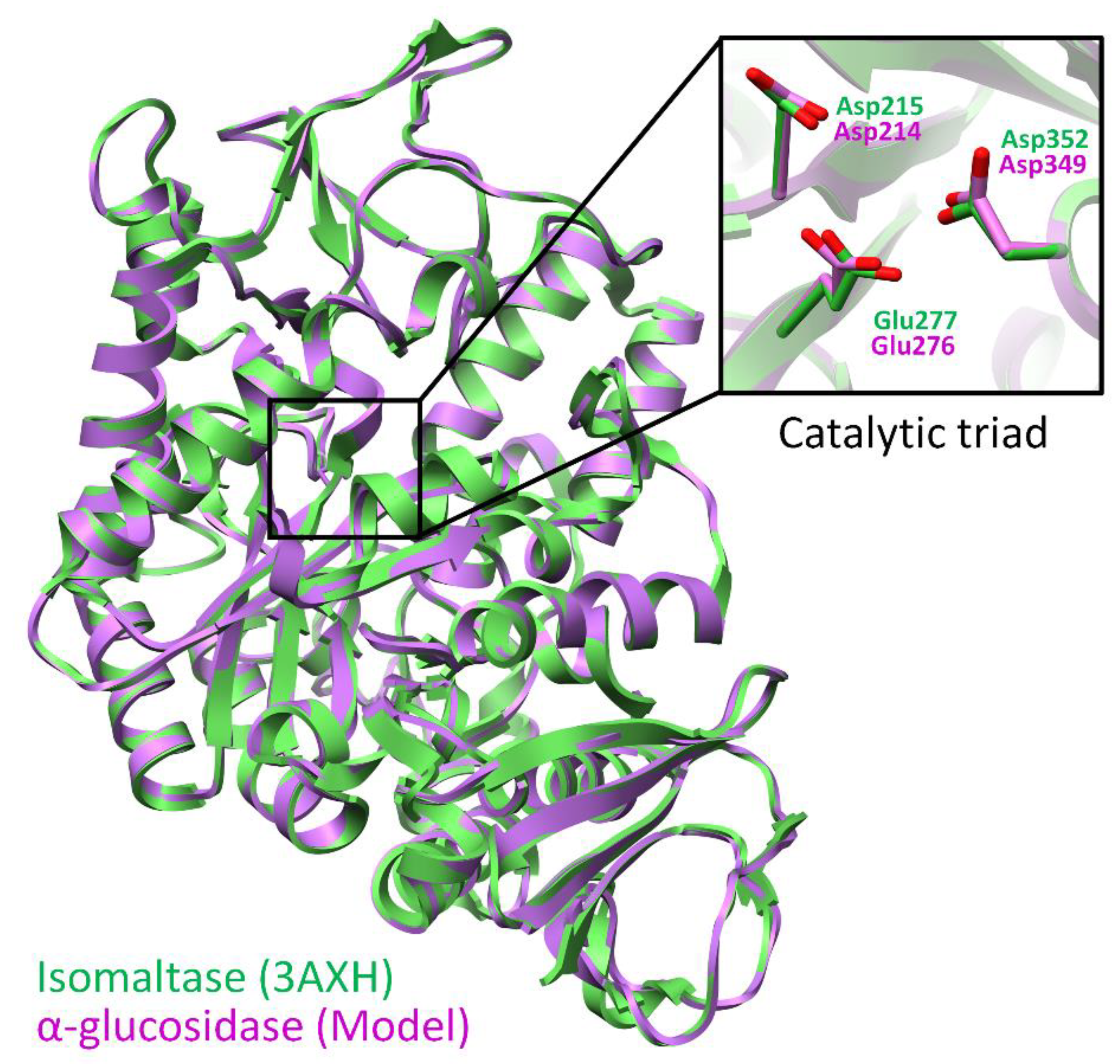
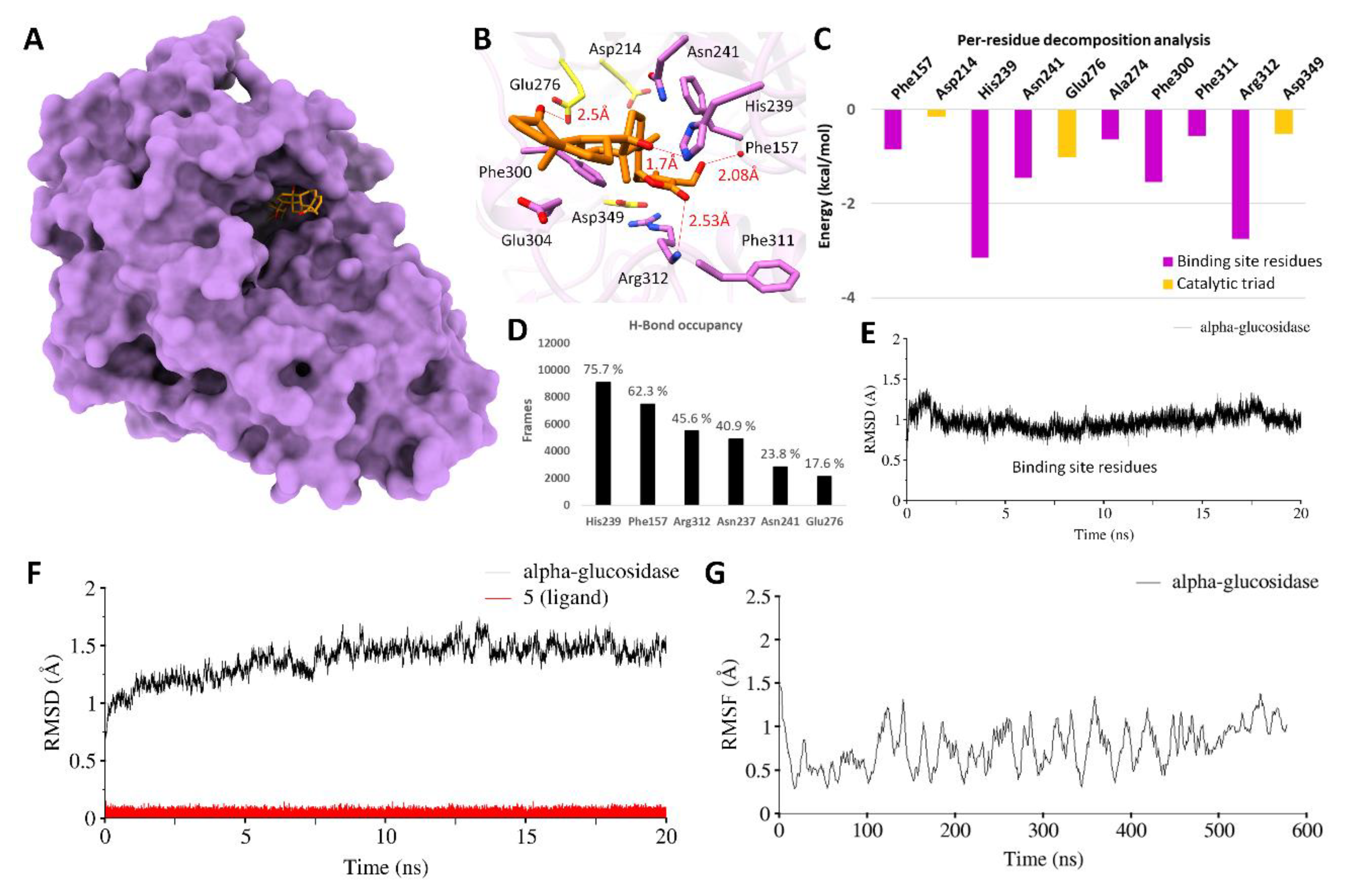
3.3. Molecular Modeling and Simulations
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Chadha, Y.R. The wealth of India, New Delhi: Publications and informations directorate. CSIR 1976, X, 582. [Google Scholar]
- Yousaf, Z.; Masood, S.; Shinwari, Z.K.; Khan, M.A.; Rabani, A. Evaluation of taxonomic status of medicinal species of the genus Hyoscyamous, Withania, Atropa and Datura based on polyacrylamide gel electrophoresis. Pak. J. Bot. 2008, 40, 2289–2297. [Google Scholar]
- Watt, G.A. Dictionary of the Economic Products of India; Cosmo Publications: Delhi, India, 1972; Volume 6, p. 309. [Google Scholar]
- Mathur, D.; Agrawal, R. Withania coagulans: A review on the morphological and pharmacological properties of the shrub. Sci. World J. 2011, 1, 30–37. [Google Scholar]
- Rahman, A.U.; Abbas, S.; Shahwar, D.E.; Jamal, S.; Choudhary, M. New withanolides from Withania spp. J. Nat. Prod. 1993, 56, 1000–1006. [Google Scholar] [CrossRef]
- Atta-ur-Rahman; Choudhary, M.I.; Qureshi, S.; Gul, W.; Yousaf, M. Two new ergostane-type steroidal lactones from Withania coagulans. J. Nat. Prod. 1998, 61, 812–814. [Google Scholar] [CrossRef]
- Kuroyanagi, M.; Murata, M.; Nakane, T.; Shirota, O.; Sekita, S.; Fuchino, H.; Shinwari, Z.K. Leishmanicidal active withanolides from a Pakistani medicinal plant, Withania coagulans. Chem. Pharm. Bull. 2012, 60, 892–897. [Google Scholar] [CrossRef]
- Verma, P.K.; Rajurkar, S.; Gaikwad, N.; Kamboj, V. The isolation and structure elucidation of new Withanaoloides from Withania cogulance with antidiabetic activity. Acta Pharm. Sci. 2010, 52, 155–157. [Google Scholar]
- Ali, A.; Maher, S.; Khan, S.A.; Chaudhary, M.I.; Musharraf, S.G. Sensitive quantification of six steroidal lactones in Withania coagulans extract by UHPLC electrospray tandem mass spectrometry. Steroids 2015, 104, 176–181. [Google Scholar] [CrossRef]
- Khan, S.; Khan, S.; Shah, Z.; Asiri, A. Withanolides: Biologically active constituents in the treatment of Alzheimer disease. Med. Chem. 2016, 12, 238–256. [Google Scholar] [CrossRef]
- Adam, G.; Chien, N.; Khoi, N. Dunawithanine A and B, first plant withanolide glycosides from Dunalia australis. Naturwissenschaften 1981, 68, 425–426. [Google Scholar] [CrossRef]
- Chen, L.-X.; He, H.; Qiu, F. Natural withanolides: An overview. Nat. Prod. Rep. 2011, 28, 705–740. [Google Scholar] [CrossRef] [PubMed]
- Glotter, E. Withanolides and related ergostane-type steroids. Nat. Prod. Rep. 1991, 8, 415–440. [Google Scholar] [CrossRef] [PubMed]
- Kimura, A.; Takata, M.; Fukushi, Y.; Mori, H.; Matsui, H.; Chiba, S. A catalytic amino acid and primary structure of active site in Aspevgillus niger α-glucosidase. Biosci. Biotechnol. Biochem. 1997, 61, 1091–1098. [Google Scholar] [CrossRef]
- Shim, Y.-J.; Doo, H.-K.; Ahn, S.-Y.; Kim, Y.-S.; Seong, J.-K.; Park, I.-S.; Min, B.-H. Inhibitory effect of aqueous extract from the gall of Rhus chinensis on alpha-glucosidase activity and postprandial blood glucose. J. Ethnopharmacol. 2003, 85, 283–287. [Google Scholar] [CrossRef]
- Braun, C.; Brayer, G.D.; Withers, S.G. Mechanism-based inhibition of yeast α-glucosidase and human pancreatic α-amylase by a new class of inhibitors 2-deoxy-2,2-difluoro-α-glycosides. J. Biol. Chem. 1995, 270, 26778–26781. [Google Scholar] [CrossRef]
- Reddy, V.P.; Beyaz, A. Inhibitors of the Maillard reaction and AGE breakers as therapeutics for multiple diseases. Drug Discov. Today 2006, 11, 646–654. [Google Scholar] [CrossRef]
- Talele, T.T.; Khedkar, S.A.; Rigby, A.C. Successful applications of computer aided drug discovery: Moving drugs from concept to the clinic. Curr. Top. Med. Chem. 2010, 10, 127–141. [Google Scholar] [CrossRef]
- Kubinyi, H. Success stories of computer-aided design. Comput. Appl. Pharm. Res. Dev. 2006, 2, 377. [Google Scholar]
- Ma, H.; Wang, L.; Niesen, D.B.; Cai, A.; Cho, B.P.; Tan, W.; Gu, Q.; Xu, J.; Seeram, N.P. Structure activity related, mechanistic, and modeling studies of gallotannins containing a glucitol-core and α-glucosidase. RSC Adv. 2015, 5, 107904–107915. [Google Scholar] [CrossRef]
- Bharatham, K.; Bharatham, N.; Park, K.H.; Lee, K.W. Binding mode analyses and pharmacophore model development for sulfonamide chalcone derivatives, a new class of α-glucosidase inhibitors. J. Mol. Graph. Model. 2008, 26, 1202–1212. [Google Scholar] [CrossRef]
- Park, H.; Hwang, K.Y.; Kim, Y.H.; Oh, K.H.; Lee, J.Y.; Kim, K. Discovery and biological evaluation of novel α-glucosidase inhibitors with in vivo antidiabetic effect. Bioorg. Med. Chem. Lett. 2008, 18, 3711–3715. [Google Scholar] [CrossRef] [PubMed]
- Park, H.; Hwang, K.Y.; Oh, K.H.; Kim, Y.H.; Lee, J.Y.; Kim, K. Discovery of novel α-glucosidase inhibitors based on the virtual screening with the homology-modeled protein structure. Bioorg. Med. Chem. 2008, 16, 284–292. [Google Scholar] [CrossRef] [PubMed]
- Park, J.-H.; Ko, S.-M.; Park, H. Toward the virtual screening of α-glucosidase inhibitors with the homology-modeled protein structure. Bull. Korean Chem. Soc. 2008, 29, 921–927. [Google Scholar]
- Hemalatha, S.; Wahi, A.K.; Singh, P.N.; Chansouria, J.P.N. Hypoglycemic activity of Withania coagulans Dunal in streptozotocin induced diabetic rats. J. Ethnopharmacol. 2004, 93, 261–264. [Google Scholar] [CrossRef]
- Rahman, A.-U.; Choudhary, M.I.; Yousaf, M.; Gul, W.; Qureshi, S. New withanolides from Withania coagulans. Chem. Pharm. Bull. 1998, 46, 1853–1856. [Google Scholar] [CrossRef]
- Bedir, E.; Khan, I.A. New steroidal glycosides from the fruits of Tribulus terrestris. J. Nat. Prod. 2000, 63, 1699–1701. [Google Scholar] [CrossRef]
- Choudhary, M.I.; Adhikari, A.; Rasheed, S.; Marasini, B.P.; Hussain, N.; Kaleem, W.A. Cyclopeptide alkaloids of Ziziphus oxyphylla Edgw as novel inhibitors of α-glucosidase enzyme and protein glycation. Phytochem. Lett. 2011, 4, 404–406. [Google Scholar] [CrossRef]
- Trott, O.; Olson, A.J. AutoDock Vina: Improving the speed and accuracy of docking with a new scoring function, efficient optimization, and multithreading. J. Comput. Chem. 2010, 31, 455–461. [Google Scholar] [CrossRef]
- Case, D.A.; Ben-Shalom, I.Y.; Brozell, S.R.; Cerutti, D.S.; Cheatham, T.E., III; Cruzeiro, V.W.D.; Darden, T.A.; Duke, R.E.; Ghoreishi, D.; Gilson, M.K. AMBER 2018; University of California: San Francisco, CA, USA, 2018. [Google Scholar]
- Ikram, N.; Mirza, M.U.; Vanmeert, M.; Froeyen, M.; Salo-Ahen, O.M.H.; Tahir, M.; Qazi, A.; Ahmad, S. Inhibition of Oncogenic Kinases: An In Vitro Validated Computational Approach Identified Potential Multi-Target Anticancer Compounds. Biomolecules 2019, 9, 124. [Google Scholar] [CrossRef]
- Jabbar, B.; Rafique, S.; Salo-Ahen, O.M.H.; Ali, A.; Munir, M.; Idrees, M.; Mirza, M.U.; Vanmeert, M.; Shah, S.Z.; Jabbar, I. Antigenic Peptide Prediction From E6 and E7 Oncoproteins of HPV Types 16 and 18 for Therapeutic Vaccine Design Using Immunoinformatics and MD Simulation Analysis. Front. Immunol. 2018, 9, 3000. [Google Scholar] [CrossRef]
- Mirza, M.U.; Rafique, S.; Ali, A.; Munir, M.; Ikram, N.; Manan, A.; Salo-Ahen, O.M.H.; Idrees, M. Towards peptide vaccines against Zika virus: Immunoinformatics combined with molecular dynamics simulations to predict antigenic epitopes of Zika viral proteins. Sci. Rep. 2016, 6, 37313. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Wolf, R.M.; Caldwell, J.W.; Kollman, P.A.; Case, D.A. Development and testing of a general amber force field. J. Comput. Chem. 2004, 25, 1157–1174. [Google Scholar] [CrossRef]
- Roe, D.R.; Cheatham, T.E., III. PTRAJ and CPPTRAJ: Software for processing and analysis of molecular dynamics trajectory data. J. Chem. Theory Comput. 2013, 9, 3084–3095. [Google Scholar] [CrossRef]
- Hou, T.; Wang, J.; Li, Y.; Wang, W. Assessing the performance of the MM/PBSA and MM/GBSA methods. 1. The accuracy of binding free energy calculations based on molecular dynamics simulations. J. Chem. Inf. Model. 2011, 51, 69–82. [Google Scholar] [CrossRef] [PubMed]
- Iman, K.; Mirza, M.U.; Mazhar, N.; Vanmeert, M.; Irshad, I.; Kamal, M.A. In silico Structure-based Identification of Novel Acetylcholinesterase Inhibitors Against Alzheimer’s Disease. CNS Neurol. Disord.-Drug Targets (Former. Curr. Drug Targets-CNS Neurol. Disord.) 2018, 17, 54–68. [Google Scholar] [CrossRef] [PubMed]
- Chen, F.; Liu, H.; Sun, H.; Pan, P.; Li, Y.; Li, D.; Hou, T. Assessing the performance of the MM/PBSA and MM/GBSA methods. 6. Capability to predict protein–protein binding free energies and re-rank binding poses generated by protein–protein docking. Phys. Chem. Chem. Phys. 2016, 18, 22129–22139. [Google Scholar] [CrossRef]
- Sirin, S.; Kumar, R.; Martinez, C.; Karmilowicz, M.J.; Ghosh, P.; Abramov, Y.A.; Martin, V.; Sherman, W. A computational approach to enzyme design: Predicting ω-aminotransferase catalytic activity using docking and MM-GBSA scoring. J. Chem. Inf. Model. 2014, 54, 2334–2346. [Google Scholar] [CrossRef]
- Ma, L.; Xie, C.M.; Li, J.; Lou, F.C.; Hu, L.H. Daturametelins H, I, and J: Three new withanolide glycosides from Datura metel L. Chem. Biodivers. 2006, 3, 180–186. [Google Scholar] [CrossRef]
- Bagchi, A.; Neogi, P.; Sahai, M.; Ray, A.B.; Oshima, Y.; Hikino, H. Withaperuvin E and nicandrin B, withanolides from Physalis peruviana and Nicandra physaloides. Phytochemistry 1984, 23, 853–855. [Google Scholar] [CrossRef]
- Velde, V.V.; Lavie, D. New withanolides of biogenetic interest from Withania somnifera. Phytochemistry 1981, 20, 1359–1364. [Google Scholar] [CrossRef]
- Khan, P.M.; Malik, A.; Ahmad, S.; Nawaz, H.R. Withanolides from Ajuga parviflora. J. Nat. Prod. 1999, 62, 1290–1292. [Google Scholar] [CrossRef] [PubMed]
- Takimoto, T.; Kanbayashi, Y.; Toyoda, T.; Adachi, Y.; Furuta, C.; Suzuki, K.; Miwa, T.; Bannai, M. 4β-Hydroxywithanolide E isolated from Physalis pruinosa calyx decreases inflammatory responses by inhibiting the NF-κB signaling in diabetic mouse adipose tissue. Int. J. Obes. 2014, 38, 1432–1439. [Google Scholar] [CrossRef]
- Brayer, G.D.; Luo, Y.; Withers, S.G. The structure of human pancreatic α-amylase at 1.8 Å resolution and comparisons with related enzymes. Protein Sci. 1995, 4, 1730–1742. [Google Scholar] [CrossRef] [PubMed]
- Machius, M.; Wiegand, G.; Huber, R.J.J.o.m.b. Crystal Structure of Calcium-depletedBacillus licheniformisα-amylase at 2.2 Å Resolution. J. Mol. Biol. 1995, 246, 545–559. [Google Scholar] [CrossRef]
- Yamamoto, K.; Miyake, H.; Kusunoki, M.; Osaki, S. Crystal structures of isomaltase from Saccharomyces cerevisiae and in complex with its competitive inhibitor maltose. FEBS J. 2010, 277, 4205–4214. [Google Scholar] [CrossRef] [PubMed]
- Seo, W.D.; Kim, J.H.; Kang, J.E.; Ryu, H.W.; Curtis-Long, M.J.; Lee, H.S.; Yang, M.S.; Park, K.H. Sulfonamide chalcone as a new class of α-glucosidase inhibitors. Bioorg. Med. Chem. Lett. 2005, 15, 5514–5516. [Google Scholar] [CrossRef] [PubMed]
- Yamamoto, K.; Nakayama, A.; Yamamoto, Y.; Tabata, S. Val216 decides the substrate specificity of α-glucosidase in Saccharomyces cerevisiae. Eur. J. Biochem. 2004, 271, 3414–3420. [Google Scholar] [CrossRef] [PubMed]
- Saleem, F.; Mehmood, R.; Mehar, S.; Khan, M.T.J.; Khan, Z.-U.-D.; Ashraf, M.; Ali, M.S.; Abdullah, I.; Froeyen, M.; Mirza, M.U.; et al. Bioassay Directed Isolation, Biological Evaluation and in Silico Studies of New Isolates from Pteris cretica L. Antioxidants 2019, 8, 231. [Google Scholar] [CrossRef]
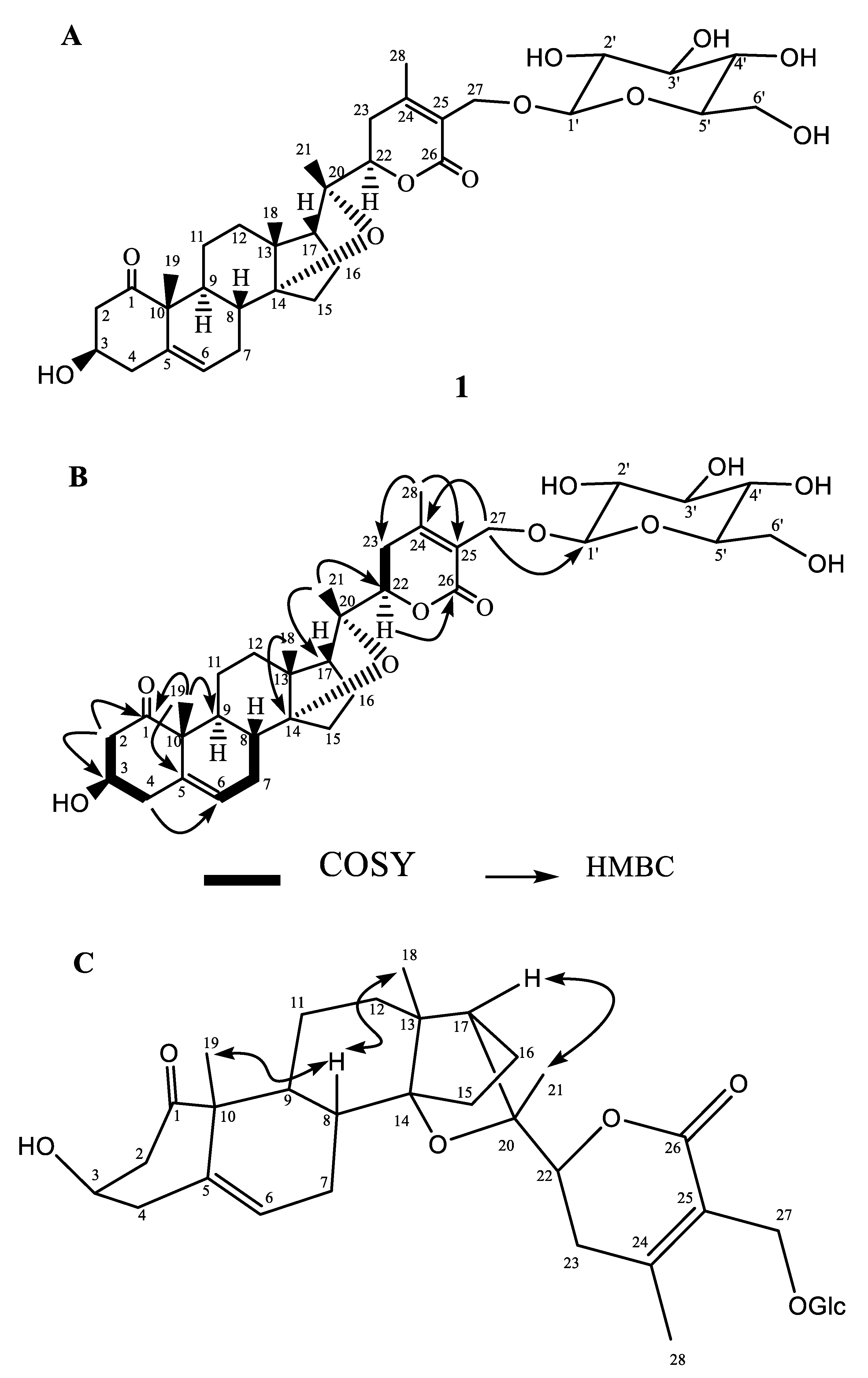
| C. No. | δC | δH (J, Hz) | C. No. | δC | δH (J, Hz) |
|---|---|---|---|---|---|
| 1 | 220.4 | - | 18 | 20.7 | 1.35 s |
| 2 | 40.5 | 2.31, 2.12 | 19 | 18.2 | 1.27 s |
| 3 | 75.0 | 3.84 br m, (W1/2 = 14.5) | 20 | 78.1 | - |
| 4 | 41.0 | 2.04, 2.01 m | 21 | 21.1 | 1.59 |
| 5 | 136.3 | - | 22 | 83.1 | 4.23 dd (13.2, 3.4) |
| 6 | 123.6 | 4.58 br s | 23 | 32.9 | 1.60, 1.84 overlap |
| 7 | 32.6 | 1.77, 1.75 m | 24 | 160.1 | - |
| 8 | 22.1 | 1.63 m | 25 | 126.4 | - |
| 9 | 21.0 | 1.61 m | 26 | 168.0 | - |
| 10 | 52.7 | - | 27 | 62.7 | 4.30 d (11.4), 4.31 d (11.7) |
| 11 | 21.1 | 1.27, 1.35 m | 28 | 18.0 | 2.01 s |
| 12 | 20.8 | 1.61, 2.01 m | 1′ | 104.0 | 4.36 d (7.6) |
| 13 | 49.0 | - | 2′ | 75.0 | 3.31 t (8.1) |
| 14 | 85.5 | - | 3′ | 74.0 | 3.65 overlap |
| 15 | 22.3 | 1.63, 1.75 m | 4′ | 71.6 | 3.33 overlap |
| 16 | 32.8 | 1.35, 1.09 m | 5′ | 78.0 | 3.33 overlap |
| 17 | 50.5 | 2.30 t (9.4) | 6′ | 63.5 | 3.64 dd (11.8, 4.8), 3.84 d (11.8) |
| Compound | α-Glucosidase Inhibition | Antiglycation |
|---|---|---|
| IC50 (µM) ± (SEM) | IC50 (µM) ± (SEM) | |
| 1 | Inactive | Inactive |
| 2 | 683 ± 0.94 | Inactive |
| 3 | Inactive | Inactive |
| 4 | 407 ± 4.5 | Inactive |
| 5 | 66.7 ± 3.6 | Inactive |
| 6 | Inactive | Inactive |
| Standard | 440.99 ± 0.01 a | 288.9 ± 1.84 b |
| Template (S. cerevisiae Isomaltase) | Model (S. cerevisiae α-Glucosidase) |
|---|---|
| Catalytic residues | |
| Asp215 | Asp214 |
| Glu277 | Glu276 |
| Asp352 | Asp349 |
| Extended active site residues | |
| Asp69 | Asp68 |
| Tyr72 | Tyr71 |
| Val109 | Val108 |
| His112 | His111 |
| Tyr158 | Phe157 |
| Phe159 | Phe158 |
| Phe178 | Phe177 |
| Gln182 | Gln181 |
| Arg213 | Arg212 |
| Val216 | Thr215 |
| Gln279 | Ala278 |
| Phe303 | Phe300 |
| Arg315 | Arg312 |
| His351 | His348 |
| Gln353 | Gln350 |
| Glu411 | Asp408 |
| Arg442 | Arg439 |
| Arg446 | Arg443 |
| Interacting water molecules | |
| 1021, 1026, 1056, 1058, 1061, 1087, 1102, 1122, 1174, 1228 | 1021, 1026, 1056, 1058, 1061, 1087, 1102, 1122, 1174, 1228 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Maher, S.; Choudhary, M.I.; Saleem, F.; Rasheed, S.; Waheed, I.; Halim, S.A.; Azeem, M.; Abdullah, I.B.; Froeyen, M.; Mirza, M.U.; et al. Isolation of Antidiabetic Withanolides from Withania coagulans Dunal and Their In Vitro and In Silico Validation. Biology 2020, 9, 197. https://doi.org/10.3390/biology9080197
Maher S, Choudhary MI, Saleem F, Rasheed S, Waheed I, Halim SA, Azeem M, Abdullah IB, Froeyen M, Mirza MU, et al. Isolation of Antidiabetic Withanolides from Withania coagulans Dunal and Their In Vitro and In Silico Validation. Biology. 2020; 9(8):197. https://doi.org/10.3390/biology9080197
Chicago/Turabian StyleMaher, Saima, M. Iqbal Choudhary, Farooq Saleem, Saima Rasheed, Imran Waheed, Sobia Ahsan Halim, Muhammad Azeem, Iskandar Bin Abdullah, Matheus Froeyen, Muhammad Usman Mirza, and et al. 2020. "Isolation of Antidiabetic Withanolides from Withania coagulans Dunal and Their In Vitro and In Silico Validation" Biology 9, no. 8: 197. https://doi.org/10.3390/biology9080197
APA StyleMaher, S., Choudhary, M. I., Saleem, F., Rasheed, S., Waheed, I., Halim, S. A., Azeem, M., Abdullah, I. B., Froeyen, M., Mirza, M. U., & Ahmad, S. (2020). Isolation of Antidiabetic Withanolides from Withania coagulans Dunal and Their In Vitro and In Silico Validation. Biology, 9(8), 197. https://doi.org/10.3390/biology9080197







