Which Zebrafish Strains Are More Suitable to Perform Behavioral Studies? A Comprehensive Comparison by Phenomic Approach
Abstract
:1. Introduction
2. Materials and Methods
2.1. Zebrafish Strains Used in This Study
2.2. Zebrafish Behavioral Tests and Ethics
2.3. Video-Tracking and Statistical Data Analysis
2.4. Principal Component Analysis (PCA), Heatmap, and Hierarchical Clustering Analysis
3. Results
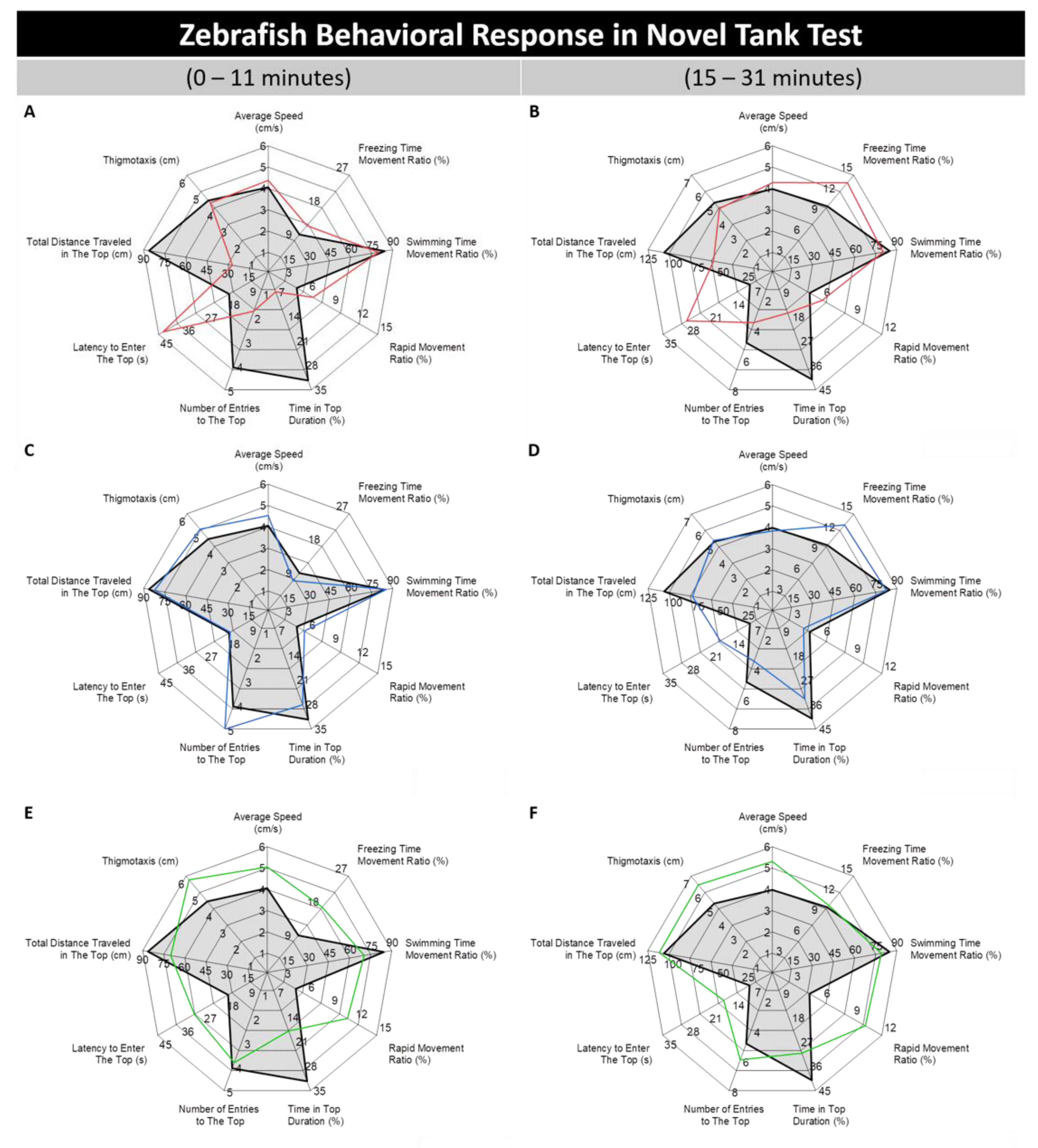
3.1. Absolute, Golden, and Pet Store-Purchased (PET) Zebrafish Displayed Different Behaviors in the Novel Tank Test
3.2. Different Level of Aggressiveness from AB Zebrafish Was Displayed by Absolute, Tübingen Long Fin (TL), Golden, and Wild Indian Karyotype (WIK) Zebrafish
3.3. Absolute, Tübingen Long Fin (TL), and Wild Indian Karyotype (WIK) Zebrafish Displayed Less Predator Avoidance Behaviors than the AB Zebrafish in the Predator Avoidance Test
3.4. Absolute, Pet Store-Purchased (PET), and Wild Indian Karyotype (WIK) Zebrafish Displayed Less Interest to the Conspecific than the AB Zebrafish in the Social Interaction Test
3.5. Loose Shoal Was Formed by Pet Store-Purchased (PET) and Wild Indian Karyotype (WIK) Zebrafishes in the Shoaling Test
3.6. Principal Component Analysis (PCA) Analysis and Hierarchical Clustering Analysis of Several Fish Behavioral Endpoints
4. Discussions and Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Meyer, B.M.; Froehlich, J.M.; Galt, N.J.; Biga, P.R. Inbred strains of zebrafish exhibit variation in growth performance and myostatin expression following fasting. Comp. Biochem. Physiol. Part A Mol. Integr. Physiol. 2013, 164, 1–9. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chakraborty, R.; Vepuri, V.; Mhatre, S.D.; Paddock, B.E.; Miller, S.; Michelson, S.J.; Delvadia, R.; Desai, A.; Vinokur, M.; Melicharek, D.J. Characterization of a Drosophila Alzheimer’s disease model: Pharmacological rescue of cognitive defects. PLoS ONE 2011, 6, e20799. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sarasamma, S.; Varikkodan, M.M.; Liang, S.-T.; Lin, Y.-C.; Wang, W.-P.; Hsiao, C.-D. Zebrafish: A premier vertebrate model for biomedical research in indian scenario. Zebrafish 2017, 14, 589–605. [Google Scholar] [CrossRef] [PubMed]
- Liang, S.-T.; Audira, G.; Juniardi, S.; Chen, J.-R.; Lai, Y.-H.; Du, Z.-C.; Lin, D.-S.; Hsiao, C.-D. Zebrafish carrying pycr1 gene deficiency display aging and multiple behavioral abnormalities. Cells 2019, 8, 453. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Meredith, G.E.; Kang, U.J. Behavioral models of Parkinson’s disease in rodents: A new look at an old problem. Mov. Disord. 2006, 21, 1595–1606. [Google Scholar] [CrossRef] [PubMed]
- Crawley, J.N. Behavioral phenotyping of rodents. Comp. Med. 2003, 53, 140–146. [Google Scholar]
- Pan, Y.; Chatterjee, D.; Gerlai, R. Strain dependent gene expression and neurochemical levels in the brain of zebrafish: Focus on a few alcohol related targets. Physiol. Behav. 2012, 107, 773–780. [Google Scholar] [CrossRef] [Green Version]
- Võikar, V.; Kõks, S.; Vasar, E.; Rauvala, H. Strain and gender differences in the behavior of mouse lines commonly used in transgenic studies. Physiol. Behav. 2001, 72, 271–281. [Google Scholar] [CrossRef]
- Hendley, E.D.; Wessel, D.J.; Atwater, D.G.; Gellis, J.; Whitehorn, D.; Low, W.C. Age, sex and strain differences in activity and habituation in SHR and WKY rats. Physiol. Behav. 1985, 34, 379–383. [Google Scholar] [CrossRef]
- Sagvolden, T.; Hendley, E.D.; Knardahl, S. Behavior of hypertensive and hyperactive rat strains: Hyperactivity is not unitarily determined. Physiol. Behav. 1992, 52, 49–57. [Google Scholar] [CrossRef]
- McCarty, R.; Kirby, R.F. Spontaneous hypertension and open-field behavior. Behav. Neural Biol. 1982, 34, 450–452. [Google Scholar] [CrossRef]
- Bouwknecht, J.A.; Paylor, R. Behavioral and physiological mouse assays for anxiety: A survey in nine mouse strains. Behav. Brain Res. 2002, 136, 489–501. [Google Scholar] [CrossRef]
- Rodgers, R.; Cole, J. Influence of social isolation, gender, strain, and prior novelty on plus-maze behaviour in mice. Physiol. Behav. 1993, 54, 729–736. [Google Scholar] [CrossRef]
- Parker, M.O.; Brock, A.J.; Millington, M.E.; Brennan, C.H. Behavioral phenotyping of casper mutant and 1-pheny-2-thiourea treated adult zebrafish. Zebrafish 2013, 10, 466–471. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- De Esch, C.; van der Linde, H.; Slieker, R.; Willemsen, R.; Wolterbeek, A.; Woutersen, R.; De Groot, D. Locomotor activity assay in zebrafish larvae: Influence of age, strain and ethanol. Neurotoxicology Teratol. 2012, 34, 425–433. [Google Scholar] [CrossRef] [PubMed]
- Lieschke, G.J.; Currie, P.D. Animal models of human disease: Zebrafish swim into view. Nat. Rev. Genet. 2007, 8, 353. [Google Scholar] [CrossRef]
- Volgin, A.D.; Yakovlev, O.A.; Demin, K.A.; de Abreu, M.S.; Alekseeva, P.A.; Friend, A.J.; Lakstygal, A.M.; Amstislavskaya, T.G.; Bao, W.; Song, C. Zebrafish models for personalized psychiatry: Insights from individual, strain and sex differences, and modeling gene x environment interactions. J. Neurosci. Res. 2019, 97, 402–413. [Google Scholar] [CrossRef]
- Van den Bos, R.; Mes, W.; Galligani, P.; Heil, A.; Zethof, J.; Flik, G.; Gorissen, M. Further characterisation of differences between TL and AB zebrafish (Danio rerio): Gene expression, physiology and behaviour at day 5 of the larval stage. PLoS ONE 2017, 12, e0175420. [Google Scholar] [CrossRef]
- Vignet, C.; Bégout, M.-L.; Péan, S.; Lyphout, L.; Leguay, D.; Cousin, X. Systematic screening of behavioral responses in two zebrafish strains. Zebrafish 2013, 10, 365–375. [Google Scholar] [CrossRef] [Green Version]
- Gorissen, M.; Manuel, R.; Pelgrim, T.N.; Mes, W.; de Wolf, M.J.; Zethof, J.; Flik, G.; van den Bos, R. Differences in inhibitory avoidance, cortisol and brain gene expression in TL and AB zebrafish. GenesBrain Behav. 2015, 14, 428–438. [Google Scholar] [CrossRef]
- Egan, R.J.; Bergner, C.L.; Hart, P.C.; Cachat, J.M.; Canavello, P.R.; Elegante, M.F.; Elkhayat, S.I.; Bartels, B.K.; Tien, A.K.; Tien, D.H. Understanding behavioral and physiological phenotypes of stress and anxiety in zebrafish. Behav. Brain Res. 2009, 205, 38–44. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Barba-Escobedo, P.A.; Gould, G.G. Visual social preferences of lone zebrafish in a novel environment: Strain and anxiolytic effects. GenesBrain Behav. 2012, 11, 366–373. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sackerman, J.; Donegan, J.J.; Cunningham, C.S.; Nguyen, N.N.; Lawless, K.; Long, A.; Benno, R.H.; Gould, G.G. Zebrafish behavior in novel environments: Effects of acute exposure to anxiolytic compounds and choice of Danio rerio line. Int. J. Comp. Psychol.Iscp Spons. By Int. Soc. Comp. Psychol. Univ. Calabr. 2010, 23, 43. [Google Scholar]
- Audira, G.; Sampurna, B.; Juniardi, S.; Liang, S.-T.; Lai, Y.-H.; Hsiao, C.-D. A versatile setup for measuring multiple behavior endpoints in zebrafish. Inventions 2018, 3, 75. [Google Scholar] [CrossRef] [Green Version]
- Jarret, R.L.; McCluskey, K. The Biological Resources of Model Organisms: Collection, Characterization and Applications; CRC Press: Boca Raton, FL, USA, 2019. [Google Scholar]
- Haffter, P.; Granato, M.; Brand, M.; Mullins, M.C.; Hammerschmidt, M.; Kane, D.A.; Odenthal, J.; Van Eeden, F.; Jiang, Y.-J.; Heisenberg, C.-P. The identification of genes with unique and essential functions in the development of the zebrafish, Danio rerio. Development 1996, 123, 1–36. [Google Scholar] [PubMed]
- Brown, A.R.; Bickley, L.K.; Ryan, T.A.; Paull, G.C.; Hamilton, P.B.; Owen, S.F.; Sharpe, A.D.; Tyler, C.R. Differences in sexual development in inbred and outbred zebrafish (Danio rerio) and implications for chemical testing. Aquat. Toxicol. 2012, 112, 27–38. [Google Scholar] [CrossRef] [PubMed]
- Sturm, R.A. A golden age of human pigmentation genetics. Trends Genet. 2006, 22, 464–468. [Google Scholar] [CrossRef]
- Cook, A.L.; Chen, W.; Thurber, A.E.; Smit, D.J.; Smith, A.G.; Bladen, T.G.; Brown, D.L.; Duffy, D.L.; Pastorino, L.; Bianchi-Scarra, G. Analysis of cultured human melanocytes based on polymorphisms within the SLC45A2/MATP, SLC24A5/NCKX5, and OCA2/P loci. J. Investig. Dermatol. 2009, 129, 392–405. [Google Scholar] [CrossRef] [Green Version]
- Seguin, D.; Gerlai, R. Zebrafish prefer larger to smaller shoals: Analysis of quantity estimation in a genetically tractable model organism. Anim. Cogn. 2017, 20, 813–821. [Google Scholar] [CrossRef]
- Avdesh, A.; Chen, M.; Martin-Iverson, M.T.; Mondal, A.; Ong, D.; Rainey-Smith, S.; Taddei, K.; Lardelli, M.; Groth, D.M.; Verdile, G. Regular care and maintenance of a zebrafish (Danio rerio) laboratory: An introduction. Jove J. Vis. Exp. 2012, 18, e4196. [Google Scholar] [CrossRef] [Green Version]
- Spence, R.; Gerlach, G.; Lawrence, C.; Smith, C. The behaviour and ecology of the zebrafish, Danio rerio. Biol. Rev. 2008, 83, 13–34. [Google Scholar] [CrossRef] [PubMed]
- Pérez-Escudero, A.; Vicente-Page, J.; Hinz, R.C.; Arganda, S.; De Polavieja, G.G. idTracker: Tracking individuals in a group by automatic identification of unmarked animals. Nat. Methods 2014, 11, 743. [Google Scholar] [CrossRef] [PubMed]
- Adams, D.C.; Anthony, C.D. Using randomization techniques to analyse behavioural data. Anim. Behav. 1996, 51, 733–738. [Google Scholar] [CrossRef] [Green Version]
- Mustafa, A.; Roman, E.; Winberg, S. Boldness in male and female zebrafish (Danio rerio) is dependent on strain and test. Front. Behav. Neurosci. 2019, 13, 248. [Google Scholar] [CrossRef]
- Gerlai, R.; Ahmad, F.; Prajapati, S. Differences in acute alcohol-induced behavioral responses among zebrafish populations. Alcohol. Clin. Exp. Res. 2008, 32, 1763–1773. [Google Scholar] [CrossRef]
- Metsalu, T.; Vilo, J. ClustVis: A web tool for visualizing clustering of multivariate data using Principal Component Analysis and heatmap. Nucleic Acids Res. 2015, 43, W566–W570. [Google Scholar] [CrossRef]
- Blaser, R.E.; Rosemberg, D.B. Measures of anxiety in zebrafish (Danio rerio): Dissociation of black/white preference and novel tank test. PLoS ONE 2012, 7, e36931. [Google Scholar] [CrossRef] [Green Version]
- Pham, M.; Raymond, J.; Hester, J.; Kyzar, E.; Gaikwad, S.; Bruce, I.; Fryar, C.; Chanin, S.; Enriquez, J.; Bagawandoss, S. Assessing social behavior phenotypes in adult zebrafish: Shoaling, social preference, and mirror biting tests. In Zebrafish Protocols for Neurobehavioral Research; Springer: Berlin/Heidelberg, Germany, 2012; pp. 231–246. [Google Scholar]
- Moretz, J.A.; Martins, E.P.; Robison, B.D. Behavioral syndromes and the evolution of correlated behavior in zebrafish. Behav. Ecol. 2007, 18, 556–562. [Google Scholar] [CrossRef] [Green Version]
- Ahmed, O.; Seguin, D.; Gerlai, R. An automated predator avoidance task in zebrafish. Behav. Brain Res. 2011, 216, 166–171. [Google Scholar] [CrossRef] [Green Version]
- Miller, N.; Gerlai, R. Quantification of shoaling behaviour in zebrafish (Danio rerio). Behav. Brain Res. 2007, 184, 157–166. [Google Scholar] [CrossRef]
- Engeszer, R.E.; Da Barbiano, L.A.; Ryan, M.J.; Parichy, D.M. Timing and plasticity of shoaling behaviour in the zebrafish, Danio rerio. Anim. Behav. 2007, 74, 1269–1275. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dlugos, C.A.; Rabin, R.A. Ethanol effects on three strains of zebrafish: Model system for genetic investigations. Pharmacol. Biochem. Behav. 2003, 74, 471–480. [Google Scholar] [CrossRef]
- Kim, L.; He, L.; Maaswinkel, H.; Zhu, L.; Sirotkin, H.; Weng, W. Anxiety, hyperactivity and stereotypy in a zebrafish model of fragile X syndrome and autism spectrum disorder. Prog. Neuro Psychopharmacol. Biol. Psychiatry 2014, 55, 40–49. [Google Scholar] [CrossRef] [PubMed]
- Cachat, J.; Stewart, A.; Grossman, L.; Gaikwad, S.; Kadri, F.; Chung, K.M.; Wu, N.; Wong, K.; Roy, S.; Suciu, C. Measuring behavioral and endocrine responses to novelty stress in adult zebrafish. Nat. Protoc. 2010, 5, 1786. [Google Scholar] [CrossRef]
- Robison, B.D.; Rowland, W. A potential model system for studying the genetics of domestication: Behavioral variation among wild and domesticated strains of zebra danio (Danio rerio). Can. J. Fish. Aquat. Sci. 2005, 62, 2046–2054. [Google Scholar] [CrossRef]
- Maximino, C.; de Brito, T.M.; da Silva Batista, A.W.; Herculano, A.M.; Morato, S.; Gouveia, A., Jr. Measuring anxiety in zebrafish: A critical review. Behav. Brain Res. 2010, 214, 157–171. [Google Scholar] [CrossRef]
- Kalueff, A.V.; Stewart, A.M.; Gerlai, R. Zebrafish as an emerging model for studying complex brain disorders. Trends Pharmacol. Sci. 2014, 35, 63–75. [Google Scholar] [CrossRef] [Green Version]
- Cachat, J.; Kyzar, E.J.; Collins, C.; Gaikwad, S.; Green, J.; Roth, A.; El-Ounsi, M.; Davis, A.; Pham, M.; Landsman, S. Unique and potent effects of acute ibogaine on zebrafish: The developing utility of novel aquatic models for hallucinogenic drug research. Behav. Brain Res. 2013, 236, 258–269. [Google Scholar] [CrossRef]
- Sarasamma, S.; Audira, G.; Siregar, P.; Malhotra, N.; Lai, Y.-H.; Liang, S.-T.; Chen, J.-R.; Chen, K.H.-C.; Hsiao, C.-D. Nanoplastics cause neurobehavioral impairments, reproductive and oxidative damages, and biomarker responses in zebrafish: Throwing up alarms of wide spread health risk of exposure. Int. J. Mol. Sci. 2020, 21, 1410. [Google Scholar] [CrossRef] [Green Version]
- Cheresiz, S.V.; Volgin, A.D.; Kokorina Evsyukova, A.; Bashirzade, A.A.; Demin, K.A.; de Abreu, M.S.; Amstislavskaya, T.G.; Kalueff, A.V. Understanding neurobehavioral genetics of zebrafish. J. Neurogenet. 2020, 34, 203–215. [Google Scholar] [CrossRef]
- Canavello, P.R.; Cachat, J.M.; Beeson, E.C.; Laffoon, A.L.; Grimes, C.; Haymore, W.A.; Elegante, M.F.; Bartels, B.K.; Hart, P.C.; Elkhayat, S.I. Measuring endocrine (cortisol) responses of zebrafish to stress. In Zebrafish Neurobehavioral Protocols; Springer: Berlin/Heidelberg, Germany, 2011; pp. 135–142. [Google Scholar]
- Larson, E.T.; O’Malley, D.M.; Melloni, R.H., Jr. Aggression and vasotocin are associated with dominant–subordinate relationships in zebrafish. Behav. Brain Res. 2006, 167, 94–102. [Google Scholar] [CrossRef] [PubMed]
- Kalueff, A.V.; Gebhardt, M.; Stewart, A.M.; Cachat, J.M.; Brimmer, M.; Chawla, J.S.; Craddock, C.; Kyzar, E.J.; Roth, A.; Landsman, S. Towards a comprehensive catalog of zebrafish behavior 1.0 and beyond. Zebrafish 2013, 10, 70–86. [Google Scholar] [CrossRef] [PubMed]
- Nielsen, S.V.; Kellner, M.; Henriksen, P.G.; Olsén, H.; Hansen, S.H.; Baatrup, E. The psychoactive drug Escitalopram affects swimming behaviour and increases boldness in zebrafish (Danio rerio). Ecotoxicology 2018, 27, 485–497. [Google Scholar] [CrossRef] [PubMed]
- Stewart, A.; Gaikwad, S.; Kyzar, E.; Green, J.; Roth, A.; Kalueff, A.V. Modeling anxiety using adult zebrafish: A conceptual review. Neuropharmacology 2012, 62, 135–143. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Manuel, R.; Gorissen, M.; van den Bos, R. Relevance of test-and subject-related factors on inhibitory avoidance (performance) of zebrafish for psychopharmacology studies. Curr. Psychopharmacol. 2016, 5, 152–168. [Google Scholar] [CrossRef]
- Van den Bos, R.; Flik, G.; Gorissen, M. Behavioral research in zebrafish (Danio rerio): Strain as source of variation. In Behavioral and Neural Genetics of Zebrafish; Elsevier: Amsterdam, The Netherlands, 2020; pp. 245–262. [Google Scholar]
- Muto, A.; Orger, M.B.; Wehman, A.M.; Smear, M.C.; Kay, J.N.; Page-McCaw, P.S.; Gahtan, E.; Xiao, T.; Nevin, L.M.; Gosse, N.J. Forward genetic analysis of visual behavior in zebrafish. PLoS Genet. 2005, 1, e66. [Google Scholar] [CrossRef]
- Lange, M.; Neuzeret, F.; Fabreges, B.; Froc, C.; Bedu, S.; Bally-Cuif, L.; Norton, W.H. Inter-individual and inter-strain variations in zebrafish locomotor ontogeny. PLoS ONE 2013, 8, e70172. [Google Scholar] [CrossRef] [Green Version]
- Gerlai, R. Can paradise fish (Macropodus opercularis, Anabantidae) recognize a natural predator? An ethological analysis. Ethology 1993, 94, 127–136. [Google Scholar] [CrossRef]
- Dugatkin, L.; McCall, M.; Gregg, R.; Cavanaugh, A.; Christensen, C.; Unseld, M. Zebrafish (Danio rerio) exhibit individual differences in risk-taking behavior during predator inspection. Ethol. Ecol. Evol. 2005, 17, 77–81. [Google Scholar] [CrossRef]
- Pannia, E.; Tran, S.; Rampersad, M.; Gerlai, R. Acute ethanol exposure induces behavioural differences in two zebrafish (Danio rerio) strains: A time course analysis. Behav. Brain Res. 2014, 259, 174–185. [Google Scholar] [CrossRef] [Green Version]
- Scerbina, T.; Chatterjee, D.; Gerlai, R. Dopamine receptor antagonism disrupts social preference in zebrafish: A strain comparison study. Amino Acids 2012, 43, 2059–2072. [Google Scholar] [CrossRef] [PubMed]
- Zala, S.M.; Määttänen, I.; Penn, D.J. Different social-learning strategies in wild and domesticated zebrafish, Danio rerio. Anim. Behav. 2012, 83, 1519–1525. [Google Scholar] [CrossRef]
- Suriyampola, P.S.; Shelton, D.S.; Shukla, R.; Roy, T.; Bhat, A.; Martins, E.P. Zebrafish social behavior in the wild. Zebrafish 2016, 13, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Koide, T.; Moriwaki, K.; Ikeda, K.; Niki, H.; Shiroishi, T. Multi-phenotype behavioral characterization of inbred strains derived from wild stocks of Mus musculus. Mamm. Genome 2000, 11, 664–670. [Google Scholar] [CrossRef]
- Monson, C.A.; Sadler, K.C. Inbreeding depression and outbreeding depression are evident in wild-type zebrafish lines. Zebrafish 2010, 7, 189–197. [Google Scholar] [CrossRef]
- Nunes, A.R.; Ruhl, N.; Winberg, S.; Oliveira, R.F. Social phenotypes in zebrafish. In The Rights and Wrongs of Zebrafish: Behavioral Phenotyping of Zebrafish; Springer: Berlin/Heidelberg, Germany, 2017; pp. 95–130. [Google Scholar]
- Meshalkina, D.A.; Kysil, E.V.; Warnick, J.E.; Demin, K.A.; Kalueff, A.V. Adult zebrafish in CNS disease modeling: A tank that’s half-full, not half-empty, and still filling. Lab Anim. 2017, 46, 378–387. [Google Scholar] [CrossRef]
- Guo, S. Linking genes to brain, behavior and neurological diseases: What can we learn from zebrafish? Genes Brain Behav. 2004, 3, 63–74. [Google Scholar] [CrossRef]
- Stewart, A.M.; Braubach, O.; Spitsbergen, J.; Gerlai, R.; Kalueff, A.V. Zebrafish models for translational neuroscience research: From tank to bedside. Trends Neurosci. 2014, 37, 264–278. [Google Scholar] [CrossRef] [Green Version]
- INOUE-MURAYAMA, M. Genetic polymorphism as a background of animal behavior. Anim. Sci. J. 2009, 80, 113–120. [Google Scholar] [CrossRef]
- Holmes, A.; le Guisquet, A.M.; Vogel, E.; Millstein, R.A.; Leman, S.; Belzung, C. Early life genetic, epigenetic and environmental factors shaping emotionality in rodents. Neurosci. Biobehav. Rev. 2005, 29, 1335–1346. [Google Scholar] [CrossRef]
- Crews, D. Epigenetics, brain, behavior, and the environment. Hormones 2010, 9, 41–50. [Google Scholar] [CrossRef]
- Ren, J.Q.; McCarthy, W.R.; Zhang, H.; Adolph, A.R.; Li, L. Behavioral visual responses of wild-type and hypopigmented zebrafish. Vis. Res. 2002, 42, 293–299. [Google Scholar] [CrossRef] [Green Version]
- Neuhauss, S.C.; Biehlmaier, O.; Seeliger, M.W.; Das, T.; Kohler, K.; Harris, W.A.; Baier, H. Genetic disorders of vision revealed by a behavioral screen of 400 essential loci in zebrafish. J. Neurosci. 1999, 19, 8603–8615. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Granato, M.; Van Eeden, F.; Schach, U.; Trowe, T.; Brand, M.; Furutani-Seiki, M.; Haffter, P.; Hammerschmidt, M.; Heisenberg, C.-P.; Jiang, Y.-J. Genes controlling and mediating locomotion behavior of the zebrafish embryo and larva. Development 1996, 123, 399–413. [Google Scholar]
- Heisenberg, C.-P.; Brand, M.; Jiang, Y.-J.; Warga, R.M.; Beuchle, D.; Van Eeden, F.; Furutani-Seiki, M.; Granato, M.; Haffter, P.; Hammerschmidt, M. Genes involved in forebrain development in the zebrafish, Danio rerio. Development 1996, 123, 191–203. [Google Scholar] [PubMed]
- Kelsh, R.N.; Brand, M.; Jiang, Y.-J.; Heisenberg, C.-P.; Lin, S.; Haffter, P.; Odenthal, J.; Mullins, M.C.; van Eeden, F.J.; Furutani-Seiki, M. Zebrafish pigmentation mutations and the processes of neural crest development. Development 1996, 123, 369–389. [Google Scholar] [PubMed]
- Cachat, J.M.; Canavello, P.R.; Elegante, M.F.; Bartels, B.K.; Elkhayat, S.I.; Hart, P.C.; Tien, A.K.; Tien, D.H.; Beeson, E.; Mohnot, S. Modeling stress and anxiety in zebrafish. In Zebrafish Models in Neurobehavioral Research; Springer: Berlin/Heidelberg, Germany, 2011; pp. 73–88. [Google Scholar]
- Roeser, T.; Baier, H. Visuomotor behaviors in larval zebrafish after GFP-guided laser ablation of the optic tectum. J. Neurosci. 2003, 23, 3726–3734. [Google Scholar] [CrossRef] [Green Version]
- Eskova, A.; Frohnhöfer, H.G.; Nüsslein-Volhard, C.; Irion, U. Galanin Signaling in the Brain Regulates Color Pattern Formation in Zebrafish. Curr. Biol. 2020, 30, 298–303. [Google Scholar] [CrossRef] [Green Version]
- Peichel, C.L. Social behavior: How do fish find their shoal mate? Curr. Biol. 2004, 14, R503–R504. [Google Scholar] [CrossRef] [Green Version]
- Engeszer, R.E.; Ryan, M.J.; Parichy, D.M. Learned social preference in zebrafish. Curr. Biol. 2004, 14, 881–884. [Google Scholar] [CrossRef] [Green Version]
- Colón-Cruz, L.; Kristofco, L.; Crooke-Rosado, J.; Acevedo, A.; Torrado, A.; Brooks, B.W.; Sosa, M.A.; Behra, M. Alterations of larval photo-dependent swimming responses (PDR): New endpoints for rapid and diagnostic screening of aquatic contamination. Ecotoxicol. Environ. Saf. 2018, 147, 670–680. [Google Scholar] [CrossRef] [PubMed]
- Séguret, A.; Collignon, B.; Halloy, J. Strain differences in the collective behaviour of zebrafish (Danio rerio) in heterogeneous environment. R. Soc. Open Sci. 2016, 3, 160451. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Collier, A.D.; Kalueff, A.V.; Echevarria, D.J. Zebrafish models of anxiety-like behaviors. In The Rights and Wrongs of Zebrafish: Behavioral Phenotyping of Zebrafish; Springer: Berlin/Heidelberg, Germany, 2017; pp. 45–72. [Google Scholar]
- Ampatzis, K.; Dermon, C.R. Sexual dimorphisms in swimming behavior, cerebral metabolic activity and adrenoceptors in adult zebrafish (Danio rerio). Behav. Brain Res. 2016, 312, 385–393. [Google Scholar] [CrossRef] [PubMed]






| Multiple Fish Behavior Tests | ||||||
|---|---|---|---|---|---|---|
| Fish Strains | Locomotor Activity | Exploratory Behavior | Aggressiveness | Predator Avoidance | Conspecific Social Interaction | Shoaling Size |
| Absolute Zebrafish | - | ↓↓↓↓ | ↓↓ | ↓↓ | ↓↓↓ | - |
| TL Zebrafish | - | - | ↓ | ↓ | - | - |
| Golden Zebrafish | ↑↑ | ↓↓↓ | ↑ | - | - | - |
| PET Zebrafish | ↑↑ | ↓↓↓↓↓ | - | - | ↓↓ | ↑↑ |
| WIK Zebrafish | - | ↓ | ↓ | ↓↓ | ↓↓↓ | ↑ |
| Puntigrus tetrazona | ↑↑ | ↑↑↑ | ↑↑ | ↓↓ | ↓↓ | ↓ |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Audira, G.; Siregar, P.; Strungaru, S.-A.; Huang, J.-C.; Hsiao, C.-D. Which Zebrafish Strains Are More Suitable to Perform Behavioral Studies? A Comprehensive Comparison by Phenomic Approach. Biology 2020, 9, 200. https://doi.org/10.3390/biology9080200
Audira G, Siregar P, Strungaru S-A, Huang J-C, Hsiao C-D. Which Zebrafish Strains Are More Suitable to Perform Behavioral Studies? A Comprehensive Comparison by Phenomic Approach. Biology. 2020; 9(8):200. https://doi.org/10.3390/biology9080200
Chicago/Turabian StyleAudira, Gilbert, Petrus Siregar, Stefan-Adrian Strungaru, Jong-Chin Huang, and Chung-Der Hsiao. 2020. "Which Zebrafish Strains Are More Suitable to Perform Behavioral Studies? A Comprehensive Comparison by Phenomic Approach" Biology 9, no. 8: 200. https://doi.org/10.3390/biology9080200







