Endoscopy Lifetime Systems Architecture: Scoping Out the Past to Diagnose the Future Technology
Abstract
:1. Introduction
2. Historical Overview
2.1. The Origin Story
2.2. A Bright Idea
2.3. “Savings” When You (Fiber) Bundle
2.4. Smile, You’re on Camera
3. Presenting, the Endoscope
3.1. What’s Trending

3.2. Endoscopy: The Next Generation
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Acknowledgments
Conflicts of Interest
References
- Boehm, B.W. A Spiral Model of Software Development and Enhancement. Computer 1988, 21, 61–72. [Google Scholar] [CrossRef]
- Hossain, N.U.I.; Dayarathna, V.L.; Nagahi, M.; Jaradat, R. Systems Thinking: A Review and Bibliometric Analysis. Systems 2020, 8, 23. [Google Scholar] [CrossRef]
- Lieberman, D.A.; Garewal, H. Use of Colonoscopy to Screen Asymptomatic Adults for Colorectal Cancer. N. Engl. J. Med. 2000, 343, 162–168. [Google Scholar] [CrossRef]
- Kaltenbach, T.; Friedland, S.; Soetikno, R. A Randomised Tandem Colonoscopy Trial of Narrow Band Imaging versus White Light Examination to Compare Neoplasia Miss Rates. Gut 2008, 57, 1406. [Google Scholar] [CrossRef] [PubMed]
- Levin, B.; Murphy, G.P. Revision in American Cancer Society Recommendations for the Earlydetection of Colorectal Cancer. CA Cancer J. Clin. 1992, 42, 296–299. [Google Scholar] [CrossRef]
- Chiu, H.-M.; Chang, C.-Y.; Chen, C.-C.; Lee, Y.-C.; Wu, M.-S.; Lin, J.-T.; Shun, C.-T.; Wang, H.-P. A Prospective Comparative Study of Narrow-Band Imaging, Chromoendoscopy, and Conventional Colonoscopy in the Diagnosis of Colorectal Neoplasia. Gut 2007, 56, 373–379. [Google Scholar] [CrossRef] [PubMed]
- Cho, J.-H. Advanced Imaging Technology Other than Narrow Band Imaging. Clin. Endosc. 2015, 48, 503. [Google Scholar] [CrossRef] [PubMed]
- Negreanu, L.; Preda, C.; Ionescu, D.; Ferechide, D. Progress in Digestive Endoscopy: Flexible Spectral Imaging Colour Enhancement (FICE)-Technical Review. J. Med. Life 2015, 8, 416–422. [Google Scholar]
- Yung, D.E.; Carvalho, P.B.; Giannakou, A.; Kopylov, U.; Rosa, B.; Rondonotti, E.; Toth, E.; Plevris, J.N.; Koulaouzidis, A. Clinical Validity of Flexible Spectral Imaging Color Enhancement (FICE) in Small-Bowel Capsule Endoscopy: A Systematic Review and Meta-Analysis. Endoscopy 2017, 49, 258–269. [Google Scholar] [CrossRef] [PubMed]
- Blachar, A.; Sosna, J. CT Colonography (Virtual Colonoscopy): Technique, Indications and Performance. Digestion 2007, 76, 34–41. [Google Scholar] [CrossRef] [PubMed]
- Bressler, B.; Paszat, L.F.; Chen, Z.; Rothwell, D.M.; Vinden, C.; Rabeneck, L. Rates of New or Missed Colorectal Cancers After Colonoscopy and Their Risk Factors: A Population-Based Analysis. Gastroenterology 2007, 132, 96–102. [Google Scholar] [CrossRef] [PubMed]
- Chung, S.J.; Kim, D.; Song, J.H.; Kang, H.Y.; Chung, G.E.; Choi, J.; Kim, Y.S.; Park, M.J.; Kim, J.S. Comparison of Detection and Miss Rates of Narrow Band Imaging, Flexible Spectral Imaging Chromoendoscopy and White Light at Screening Colonoscopy: A Randomised Controlled Back-to-Back Study. Gut 2014, 63, 785–791. [Google Scholar] [CrossRef] [PubMed]
- Kim, N.H.; Jung, Y.S.; Jeong, W.S.; Yang, H.-J.; Park, S.-K.; Choi, K.; Park, D.I. Miss Rate of Colorectal Neoplastic Polyps and Risk Factors for Missed Polyps in Consecutive Colonoscopies. Intest. Res. 2017, 15, 411–418. [Google Scholar] [CrossRef] [Green Version]
- Siegel, R.; Desantis, C.; Jemal, A. Colorectal Cancer Statistics, 2014. CA Cancer J. Clin. 2014, 64, 104–117. [Google Scholar] [CrossRef] [PubMed]
- Siegel, R.L.; Miller, K.D.; Jemal, A. Cancer Statistics, 2016. CA Cancer J. Clin. 2016, 66, 7–30. [Google Scholar] [CrossRef] [Green Version]
- Siegel, R.L.; Miller, K.D.; Jemal, A. Cancer Statistics, 2017. CA Cancer J. Clin. 2017, 67, 7–30. [Google Scholar] [CrossRef] [Green Version]
- Siegel, R.L.; Miller, K.D.; Jemal, A. Cancer Statistics, 2019. CA Cancer J. Clin. 2019, 69, 7–34. [Google Scholar] [CrossRef] [Green Version]
- Bolin, S.; Nilsson, E.; Sjödahl, R. Carcinoma of the Colon and Rectum--Growth Rate. Ann. Surg. 1983, 198, 151–158. [Google Scholar] [CrossRef] [PubMed]
- Burke, J.R.; Brown, P.; Quyn, A.; Lambie, H.; Tolan, D.; Sagar, P. Tumour Growth Rate of Carcinoma of the Colon and Rectum: Retrospective Cohort Study. BJS Open 2020, 4, 1200–1207. [Google Scholar] [CrossRef] [PubMed]
- Comroe, J.H. Retrospectroscope: Insights into Medical Discovery; Von Gehr Press: Menlo Park, CA, USA, 1977. [Google Scholar]
- Lau, W.Y.; Leow, C.K.; Li, A.K.C. History of Endoscopic and Laparoscopic Surgery. World J. Surg. 1997, 21, 444–453. [Google Scholar] [CrossRef]
- Berci, G.; Forde, K. History of Endoscopy. Surg. Endosc. 2000, 14, 5–15. [Google Scholar] [CrossRef] [PubMed]
- De Groen, P.C. History of the Endoscope [Scanning Our Past]. Proc. IEEE 2017, 105, 1987–1995. [Google Scholar] [CrossRef]
- Abbott, R. History of Neuroendoscopy. Neurosurg. Clin. N. Am. 2004, 15, 1–7. [Google Scholar] [CrossRef]
- Achord, J.L.; Muthusamy, V.R. The History of Gastrointestinal Endoscopy. In Clinical Gastrointestinal Endoscopy; Elsevier: Amsterdam, The Netherlands, 2019; pp. 2–11.e1. [Google Scholar] [CrossRef]
- Bozzini, P. Lichtleiter, Eine Erfindung Zur Anschauung Innerer Theile Und Krankheiten Nebst Der Abbildung. J. Pract. Heilkd. Berl. 1806, 24, 107–124. [Google Scholar]
- Doglietto, F.; Prevedello, D.M.; Jane, J.A.; Han, J.; Laws, E.R. A Brief History of Endoscopic Transsphenoidal Surgery—From Philipp Bozzini to the First World Congress of Endoscopic Skull Base Surgery. Neurosurg. Focus 2005, 19, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Mann, G.; Bozzini, P.; Bozzini, D. Der Frankfurter Lichtleiter: Neues Über Philipp Bozzini Und Sein Endoskop. Medizinhist. J. 1973, 8, 105–130. [Google Scholar]
- Cecchini, R.; Pelosi, G. Alessandro Volta and His Battery. IEEE Antennas Propag. Mag. 1992, 34, 30–37. [Google Scholar] [CrossRef]
- Chatterjee, S. Michael Faraday: Discovery of Electromagnetic Induction. Resonance 2002, 7, 35–45. [Google Scholar] [CrossRef]
- Holmes, R. Humphry Davy and the Chemical Moment. Clin. Chem. 2011, 57, 1625–1631. [Google Scholar] [CrossRef] [Green Version]
- Rue, W.D.L.; Muller, H.; Spottiswoode, W., II. Experiments to Ascertain the Cause of Stratification Electrical Discharges in Vacuo. Proc. R. Soc. Lond. 1875, 23, 356–361. [Google Scholar]
- Zajaczkowski, T.; Zamann, A.P. Julius Bruck (1840?1902) and His Influence on the Endoscopy of Today. World J. Urol. 2004, 22, 293–303. [Google Scholar] [CrossRef] [PubMed]
- Zissis, G.; Kitsinelis, S. State of Art on the Science and Technology of Electrical Light Sources: From the Past to the Future. J. Phys. Appl. Phys. 2009, 42, 173001. [Google Scholar] [CrossRef]
- Chirnside, R.C. Sir Joseph Swan and the Invention of the Electric Lamp. Electron. Power 1979, 25, 96–100. [Google Scholar] [CrossRef]
- Spear, B. Let There Be Light! Sir Joseph Swan and the Incandescent Light Bulb. World Pat. Inf. 2013, 35, 38–41. [Google Scholar] [CrossRef]
- The Light Bulb, Cystoscopy, and Thomas Alva Edison. Available online: http://www.liebertpub.com/doi/epub/10.1089/end.2010.0420 (accessed on 15 July 2022).
- Wise, G. Innovation: Swan’s Way: A Study in Style: With the Birth of the Electric Light Bulb, Two Modern Styles of Inventing Emerged: Thomas Edison’s and Joseph Swan’s. IEEE Spectr. 1982, 19, 66–71. [Google Scholar] [CrossRef]
- Smith, N. The Whole Story Of… Light Bulbs. Eng. Technol. 2018, 13, 54–59. [Google Scholar] [CrossRef]
- Mouton, W.G.; Bessell, J.R.; Maddern, G.J. Looking Back to the Advent of Modern Endoscopy: 150th Birthday of Maximilian Nitze. World J. Surg. 1998, 22, 1256–1258. [Google Scholar] [CrossRef]
- Rehnberg, V.; Walters, E. The Life and Work of Adolph Kussmaul 1822–1902: ‘Sword Swallowers in Modern Medicine. J. Intensive Care Soc. 2017, 18, 71–72. [Google Scholar] [CrossRef] [Green Version]
- Payne, S.; Eardley, I.; O’Flynn, K. (Eds.) Imaging and Technology in Urology; Springer: London, UK, 2012. [Google Scholar] [CrossRef]
- Gow, J.G. Harold hopkins and optical systems for urology—An appreciation. Urology 1998, 52, 152–157. [Google Scholar]
- Dobson, S.J.; Hopkins, H.H. A New Rod-Lens Relay System Offering Improved Image Quality. J. Phys. [E] 1989, 22, 450–455. [Google Scholar] [CrossRef]
- Commissioner, O. FDA History. Available online: https://www.fda.gov/about-fda/fda-history (accessed on 22 September 2022).
- Roux, G.; Halstead, J.A. Issues and Trends in Nursing; Jones & Bartlett Learning: Burlington, MA, USA, 2017; pp. 1–26. [Google Scholar]
- Shah, J. Endoscopy through the Ages. BJU Int. 2002, 89, 645–652. [Google Scholar] [CrossRef] [PubMed]
- Reuter, M. The Historical Development of Endophotography. World J. Urol. 2000, 18, 299–302. [Google Scholar] [CrossRef] [PubMed]
- Beer, J.J. Capitalizing on Invention. Science 1976, 192, 658–660. [Google Scholar] [CrossRef]
- Evans, R.M. Maxwell’s Color Photograph. Sci. Am. 1961, 205, 118–131. [Google Scholar] [CrossRef]
- Epstean, E.; Tennant, J.A. Frederic Eugene Ives. J. Appl. Phys. 1938, 9, 226–236. [Google Scholar] [CrossRef]
- Nudds, J.R. The Life and Work of John Joly (1857–1933). Ir. J. Earth Sci. 1986, 8, 81–94. [Google Scholar]
- Fechete, I. Jonas Ferdinand Gabriel Lippmann: The Pioneer of Color Photography or Primus Inter Pares. Comptes Rendus Chim. 2016, 19, 903–908. [Google Scholar] [CrossRef] [Green Version]
- Leskosky, R.J. Phenakiscope: 19th Century Science Turned to Animation. Film Hist. 1993, 5, 176–189. [Google Scholar]
- Spehr, P. The Man Who Made Movies; Dickson, W.K.L., Ed.; Indiana University Press: Bloomington, Indiana, 2008. [Google Scholar]
- Salazard, B.; Desouches, C.; Magalon, G. Auguste and Louis Lumière, Inventors at the Service of the Suffering. Eur. J. Plast. Surg. 2006, 28, 441–447. [Google Scholar] [CrossRef]
- Berci, G.; Davids, J. Endoscopy and Television. Br. Med. J. 1962, 1, 1610–1613. [Google Scholar] [CrossRef] [Green Version]
- Smith, G.E. The Invention and Early History of the CCD. J. Appl. Phys. 2011, 109, 102421. [Google Scholar] [CrossRef]
- Fossum, E.R. The Invention of CMOS Image Sensors: A Camera in Every Pocket. In Proceedings of the 2020 Pan Pacific Microelectronics Symposium (Pan Pacific), Kahuku, HI, USA, 10–13 February 2020; pp. 1–6. [Google Scholar] [CrossRef]
- Abe, H. Device Technologies for High Quality and Smaller Pixel in CCD and CMOS Image Sensors. In Proceedings of the IEDM Technical Digest. IEEE International Electron Devices Meeting, San Francisco, CA, USA, 13–15 December 2004; pp. 989–992. [Google Scholar] [CrossRef]
- Gouveia, L.C.P.; Choubey, B. On Evolution of CMOS Image Sensors. Int. J. Smart Sens. Intell. Syst. 2014, 7, 1–6. [Google Scholar] [CrossRef] [Green Version]
- Gouveia, L.C.P.; Choubey, B. Advances on CMOS Image Sensors. Sens. Rev. 2016, 36, 231–239. [Google Scholar] [CrossRef]
- Suzuki, T. Challenges of Image-Sensor Development. In Proceedings of the 2010 IEEE International Solid-State Circuits Conference—(ISSCC), San Francisco, CA, USA, 7–11 February 2010; pp. 27–30. [Google Scholar] [CrossRef]
- Liu, Y.T. Review and Design a Mobile Phone Camera Lens for 21.4 Mega. Ph.D. Dissertation, University of Arizona, Tucson, AZ, USA, 2017. [Google Scholar]
- Morrissey, S. IOS Forensic Analysis for IPhone, IPad, and IPod Touch; Apress: Berkeley, CA, USA, 2010. [Google Scholar] [CrossRef]
- Abdallah, S.; Saleh, B.; Aboulsoud, A.K. A General Overview of Solid State Imaging Sensors Types. In Proceedings of the Third Workshop on Photonics and Its Application at Egyptian Engineering Faculties and Institutes (Cat. No.02EX509), Giza, Egypt, 5 January 2002; pp. 1–10. [Google Scholar] [CrossRef]
- Oto, A. Virtual Endoscopy. Eur. J. Radiol. 2002, 42, 231–239. [Google Scholar] [CrossRef]
- Adler, S.N.; Metzger, Y.C. PillCam COLON Capsule Endoscopy: Recent Advances and New Insights. Ther. Adv. Gastroenterol. 2011, 4, 265–268. [Google Scholar] [CrossRef] [Green Version]
- Gossum, A.V.; Fernandez-Urien, I.; Delvaux, M.; Neuhaus, H.; Riccioni, M.E.; Fraser, C.; Hagenmuller, F.; Devière, J. Capsule Endoscopy versus Colonoscopy for the Detection of Polyps and Cancer. N. Engl. J. Med. 2009, 361, 264–270. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Azevedo, I.L.; Morgan, M.G.; Morgan, F. The Transition to Solid-State Lighting. Proc. IEEE 2009, 97, 481–510. [Google Scholar] [CrossRef]
- Smith, S. The Scientist & Engineer’s Guide to Digital Signal Processing; California Technical Pub: Los Angeles, CA, USA, 1997. [Google Scholar]
- Tsai, T.-H.; Fujimoto, J.G.; Mashimo, H. Endoscopic Optical Coherence Tomography for Clinical Gastroenterology. Diagnostics 2014, 4, 57–93. [Google Scholar] [CrossRef] [Green Version]
- Gaab, M.R. Instrumentation: Endoscopes and Equipment. World Neurosurg. 2013, 79, S14.e11–S14.e21. [Google Scholar] [CrossRef]
- Swain, P. Role of Video Endoscopy in Managing Small Bowel Disease. Gut 2004, 53, 1866–1875. [Google Scholar] [CrossRef]
- Iakovidis, D.K. Software Engineering Applications in Gastroenterology. Glob. J. Gastroenterol. Hepatol. 2014, 2, 11–18. [Google Scholar] [CrossRef] [Green Version]
- van der Sommen, F.; de Groof, J.; Struyvenberg, M.; van der Putten, J.; Boers, T.; Fockens, K.; Schoon, E.J.; Curvers, W.; de With, P.; Mori, Y.; et al. Machine Learning in GI Endoscopy: Practical Guidance in How to Interpret a Novel Field. Gut 2020, 69, 2035–2045. [Google Scholar] [CrossRef] [PubMed]
- Hounnou, G.; Destrieux, C.; Desme, J.; Bertrand, P.; Velut, S. Anatomical Study of the Length of the Human Intestine. Surg. Radiol. Anat. 2002, 24, 290–294. [Google Scholar] [PubMed]
- Soulas, A.; Dubois De Montreynaud, J.M.; Edwards, R.J.; Gladu, A.J. Bronchoscopy and Television. Dis. Chest 1957, 31, 580–584. [Google Scholar] [CrossRef] [PubMed]
- Berci, G.; Shulman, A.; Morgenstern, L.; Paz-Partlow, M.; Cuschierei, A.; Wood, R. Television Choledochoscopy. Surg. Gynecol. Obstet. 1985, 160, 176–177. [Google Scholar] [PubMed]
- Baillie, J. The Endoscope. Gastrointest. Endosc. 2007, 65, 886–893. [Google Scholar] [CrossRef]
- Jacobson, M.C.; deVere White, R.W.; Demos, S.G. In Vivo Testing of a Prototype System Providing Simultaneous White Light and near Infrared Autofluorescence Image Acquisition for Detection of Bladder Cancer. J. Biomed. Opt. 2012, 17, 036011. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- McWade, M.A.; Paras, C.; White, L.M.; Phay, J.E.; Solórzano, C.C.; Broome, J.T.; Mahadevan-Jansen, A. Label-Free Intraoperative Parathyroid Localization With Near-Infrared Autofluorescence Imaging. J. Clin. Endocrinol. Metab. 2014, 99, 4574–4580. [Google Scholar] [CrossRef] [PubMed]
- Iseki, K.; Tatsuta, M.; Iishi, H.; Sakai, N.; Yano, H.; Ishiguro, S. Effectiveness of the Near-Infrared Electronic Endoscope for Diagnosis of the Depth of Involvement of Gastric Cancers. Gastrointest. Endosc. 2000, 52, 755–762. [Google Scholar] [CrossRef]
- Ortiz-Fernandez-Sordo, J.; Sami, S.S.; Mansilla-Vivar, R.; Subramanian, V.; Mannath, J.; Telakis, E.; Ragunath, K. Evaluation of a Novel Infra-Red Endoscopy System in the Assessment of Early Neoplasia in Barretts Esophagus: Pilot Study from a Single Center. Dis. Esophagus 2018, 31, dox137. [Google Scholar] [CrossRef]
- Betz, C.S.; Stepp, H.; Janda, P.; Arbogast, S.; Grevers, G.; Baumgartner, R.; Leunig, A. A Comparative Study of Normal Inspection, Autofluorescence and 5-ALA-Induced PPIX Fluorescence for Oral Cancer Diagnosis. Int. J. Cancer 2002, 97, 245–252. [Google Scholar] [CrossRef] [PubMed]
- Zhao, H.L.; Zhang, C.P.; Zhu, H.; Jiang, Y.F.; Fu, X.B. Autofluorescence of Collagen Fibres in Scar. Skin Res. Technol. 2017, 23, 588–592. [Google Scholar] [CrossRef] [PubMed]
- Deal, J.; Mayes, S.; Browning, C.; Hill, S.; Rider, P.; Boudreaux, C.; Rich, T.C.; Leavesley, S.J. Identifying Molecular Contributors to Autofluorescence of Neoplastic and Normal Colon Sections Using Excitation-Scanning Hyperspectral Imaging. J. Biomed. Opt. 2018, 24, 021207. [Google Scholar] [CrossRef] [PubMed]
- Takeuchi, Y.; Hanaoka, N.; Hanafusa, M.; Ishihara, R.; Higashino, K.; Iishi, H.; Uedo, N. Autofluorescence Imaging of Early Colorectal Cancer. J. Biophotonics 2011, 4, 490–497. [Google Scholar] [CrossRef] [PubMed]
- Bae, S.-J.; Lee, D.-S.; Berezin, V.; Kang, U.; Lee, K.-H. Multispectral Autofluorescence Imaging for Detection of Cervical Lesions: A Preclinical Study. J. Obstet. Gynaecol. Res. 2016, 42, 1846–1853. [Google Scholar] [CrossRef]
- Chen, W.; Gao, X.; Tian, Q.; Chen, L. A Comparison of Autofluorescence Bronchoscopy and White Light Bronchoscopy in Detection of Lung Cancer and Preneoplastic Lesions: A Meta-Analysis. Lung Cancer 2011, 73, 183–188. [Google Scholar] [CrossRef] [PubMed]
- Ignjatovic, A.; East, J.; Guenther, T.; Hoare, J.; Morris, J.; Ragunath, K.; Shonde, A.; Simmons, J.; Suzuki, N.; Thomas-Gibson, S. What Is the Most Reliable Imaging Modality for Small Colonic Polyp Characterization? Study of White-Light, Autofluorescence, and Narrow-Band Imaging. Endoscopy 2011, 43, 94–99. [Google Scholar] [CrossRef]
- Falk, G.W. Autofluorescence Endoscopy. Gastrointest. Endosc. Clin. N. Am. 2009, 19, 209–220. [Google Scholar] [CrossRef]
- Becker, A.; Hessenius, C.; Licha, K.; Ebert, B.; Sukowski, U.; Semmler, W.; Wiedenmann, B.; Grötzinger, C. Receptor-Targeted Optical Imaging of Tumors with near-Infrared Fluorescent Ligands. Nat. Biotechnol. 2001, 19, 327–331. [Google Scholar] [CrossRef] [PubMed]
- Guo, Z.; Park, S.; Yoon, J.; Shin, I. Recent Progress in the Development of Near-Infrared Fluorescent Probes for Bioimaging Applications. Chem. Soc. Rev. 2014, 43, 16–29. [Google Scholar] [CrossRef]
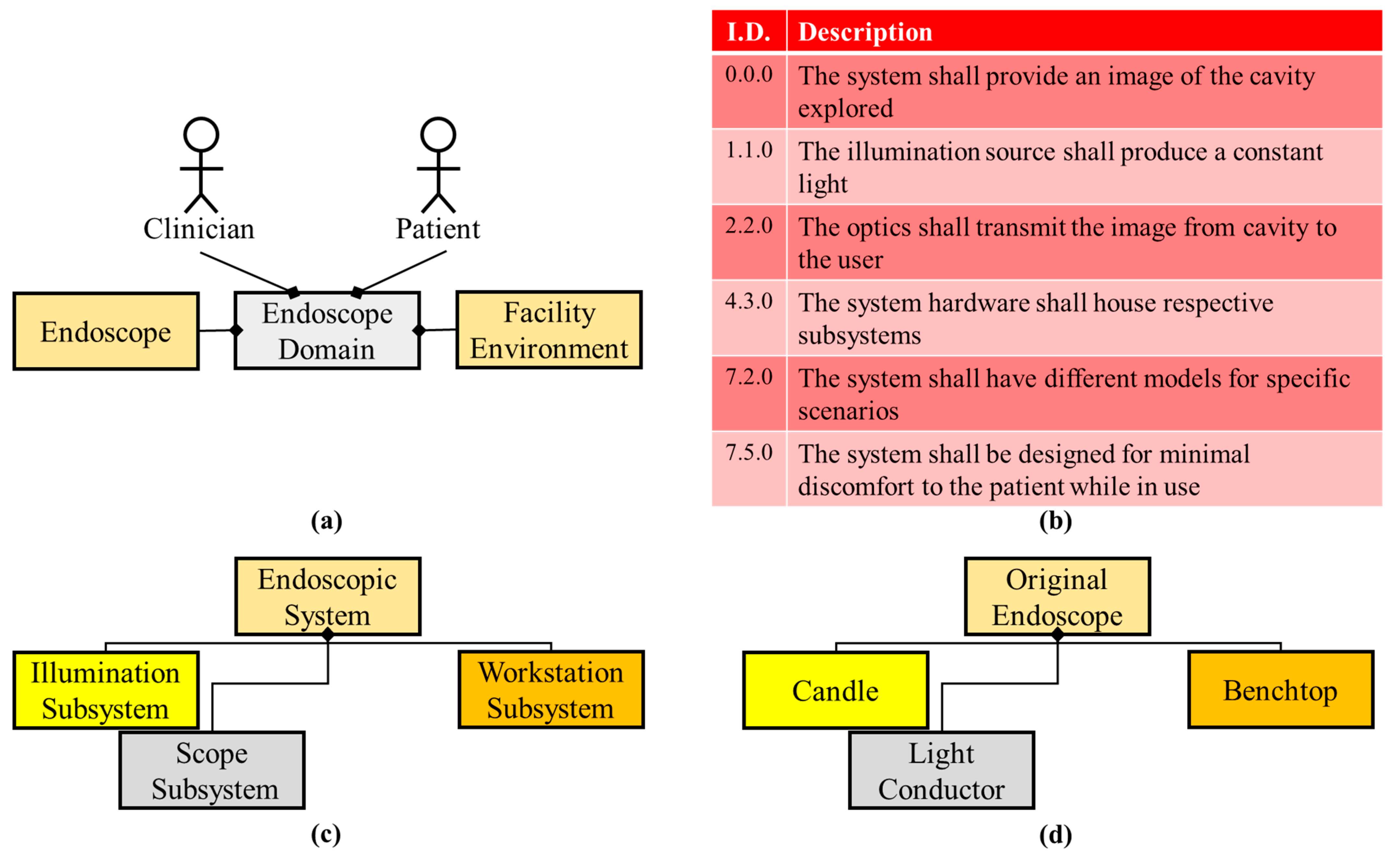





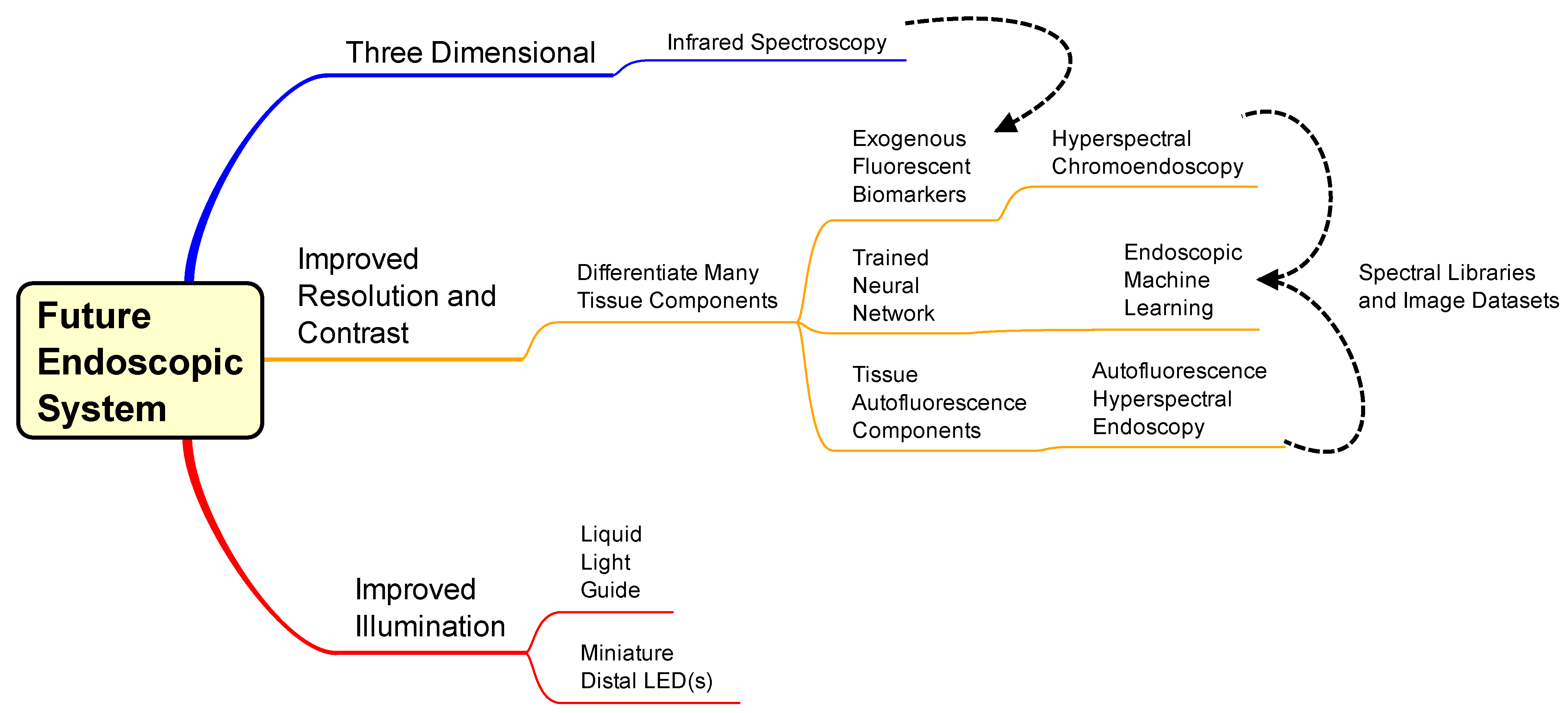
| Safety | Invasive | Patient Comfort | Operational Training | Example Image Training | Implementation * | Cost to Implement | Additional Image Data | Higher Contrast | Total | |
|---|---|---|---|---|---|---|---|---|---|---|
| Current Endoscopy | 4.3 ± 0.7 | 3.0 ± 1.6 | 4.0 ± 1.2 | 4.2 ± 0.7 | 3.7 ± 0.7 | 3.3 ± 1.4 | 2.8 ± 1.5 | 3.5 ± 1.4 | 3.0 ± 1.3 | 17.8 |
| Virtual Endoscopy | 4.7 ± 0.5 | 2.5 ± 1.5 | 4.5 ± 0.5 | 4.0 ± 0.8 | 4.2 ± 0.7 | 3.5 ± 0.8 | 3.5 ± 1.4 | 3.3 ± 1.1 | 3.0 ± 1.5 | 17.8 |
| Capsule Endoscopy | 4.2 ± 0.7 | 2.8 ± 1.2 | 4.0 ± 0.6 | 3.8 ± 0.7 | 4.2 ± 0.7 | 3.2 ± 1.3 | 3.5 ± 0.8 | 3.3 ± 1.1 | 2.8 ± 1.3 | 16.8 |
| Infrared Imaging Endoscopy | 3.7 ± 0.9 | 3.3 ± 1.2 | 4.0 ± 0.8 | 4.2 ± 0.4 | 4.2 ± 0.7 | 4.0 ± 0.8 | 3.8 ± 0.7 | 3.8 ± 0.7 | 3.2 ± 1.2 | 15.2 |
| Autofluorescence Hyperspectral Endoscopy | 4.0 ± 0.8 | 3.3 ± 1.2 | 4.0 ± 0.8 | 4.3 ± 0.7 | 4.7 ± 0.5 | 4.5 ± 0.8 | 3.7 ± 1.5 | 4.2 ± 0.9 | 4.0 ± 1.4 | 15.7 |
| Hyperspectral Chromoendoscopy | 3.8 ± 0.7 | 3.3 ± 1.2 | 4.2 ± 0.9 | 4.5 ± 0.8 | 4.7 ± 0.5 | 4.3 ± 0.7 | 3.8 ± 1.5 | 4.5 ± 0.8 | 4.3 ± 1.1 | 16.2 |
| Neural Network Endoscopy | 4.2 ± 0.9 | 3.5 ± 1.4 | 4.3 ± 0.9 | 4.7 ± 0.5 | 4.2 ± 0.9 | 4.2 ± 1.1 | 3.7 ± 1.9 | 4.2 ± 1.2 | 4.2 ± 1.2 | 16.7 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Browning, C.M.; Cloutier, R.; Rich, T.C.; Leavesley, S.J. Endoscopy Lifetime Systems Architecture: Scoping Out the Past to Diagnose the Future Technology. Systems 2022, 10, 189. https://doi.org/10.3390/systems10050189
Browning CM, Cloutier R, Rich TC, Leavesley SJ. Endoscopy Lifetime Systems Architecture: Scoping Out the Past to Diagnose the Future Technology. Systems. 2022; 10(5):189. https://doi.org/10.3390/systems10050189
Chicago/Turabian StyleBrowning, Craig M., Robert Cloutier, Thomas C. Rich, and Silas J. Leavesley. 2022. "Endoscopy Lifetime Systems Architecture: Scoping Out the Past to Diagnose the Future Technology" Systems 10, no. 5: 189. https://doi.org/10.3390/systems10050189
APA StyleBrowning, C. M., Cloutier, R., Rich, T. C., & Leavesley, S. J. (2022). Endoscopy Lifetime Systems Architecture: Scoping Out the Past to Diagnose the Future Technology. Systems, 10(5), 189. https://doi.org/10.3390/systems10050189








