Plants from Arid Zones of Mexico: Bioactive Compounds and Potential Use for Food Production
Abstract
:1. Introduction
2. Data Collection and Structure
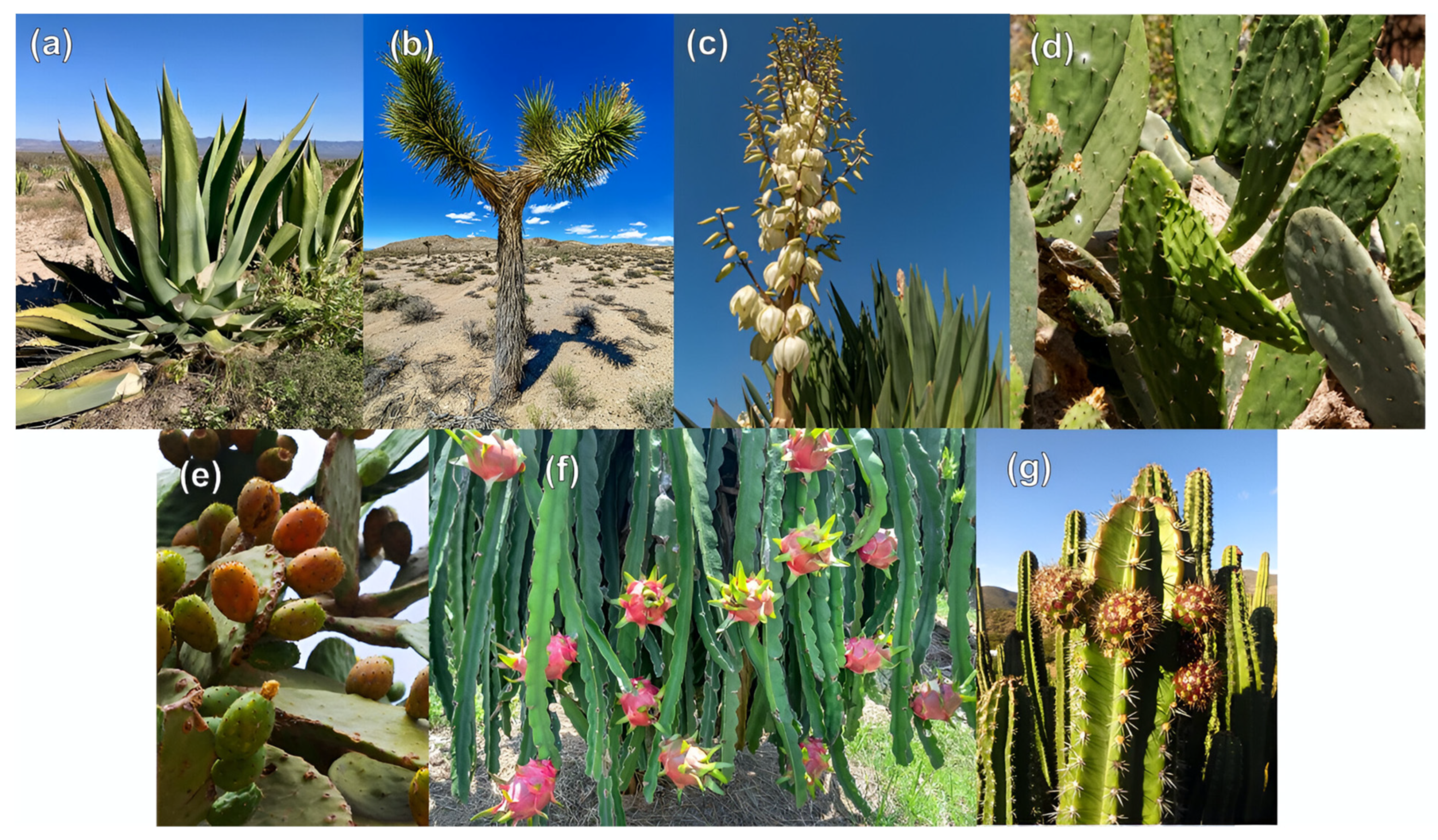
3. Arid Zone Plants in Mexico
4. Nutritional Benefits and Importance of Arid Zone Plants
5. The Importance and Traditional Use of Arid Zone Plants
6. Bioactive Compounds in Arid Zone Plants and Their Usage as Foodstuff
6.1. Agave Leaves
6.2. Pulque and Agave Syrup
6.3. Yucca Bark, Flowers, and Fruits
6.4. Opuntia Cladodes and Fruits
6.5. Pitahaya and Pitaya Fruits
6.6. Biological Activities
7. The Use of Traditional Crops for the Development of Functional Foods
8. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Morte, A.; Kagan-Zur, V.; Navarro-Ródenas, A.; Sitrit, Y. Cultivation of Desert Truffles—A Crop Suitable for Arid and Semi-Arid Zones. Agronomy 2021, 11, 1462. [Google Scholar] [CrossRef]
- Gaur, M.K.; Squires, V.R. Climate Variability Impacts on Land Use and Livelihoods in Drylands; Springer International Publishing: New York, NY, USA, 2017; pp. 1–20. ISBN 9783319566818. [Google Scholar]
- Ruiz-Nieto, J.E.; Hernández-Ruiz, J.; Hernández-Marín, J.; Mendoza-Carrillo, J.; Abraham-Juárez, M.; Isiordia-Lachica, P.M.; Mireles-Arriaga, A.I. Mesquite (Prosopis spp.) Tree as a Feed Resource for Animal Growth. Agrofor. Syst. 2020, 94, 1139–1149. [Google Scholar] [CrossRef]
- Tan, M.; Zheng, L. Increase in Economic Efficiency of Water Use Caused by Crop Structure Adjustment in Arid Areas. J. Environ. Manag. 2019, 230, 386–391. [Google Scholar] [CrossRef] [PubMed]
- Jiang, L.; Hu, D.; Wang, H.; Lv, G. Discriminating Ecological Processes Affecting Different Dimensions of α- and β-Diversity in Desert Plant Communities. Ecol. Evol. 2022, 12, e8710. [Google Scholar] [CrossRef] [PubMed]
- Muluneh, M.G. Impact of Climate Change on Biodiversity and Food Security: A Global Perspective—A Review Article. Agric. Food Secur. 2021, 10, 36. [Google Scholar] [CrossRef]
- Dobler-Morales, C.; Bocco, G. Social and Environmental Dimensions of Drought in Mexico: An Integrative Review. Int. J. Disaster Risk Reduct. 2021, 55, 102067. [Google Scholar] [CrossRef]
- Jimenez-Torres, J.A.; Peña-Valdivia, C.B.; Padilla-Chacón, D.; García-Nava, R. Physiological and Biochemical Responses of Agave to Temperature and Climate of Their Native Environment. Flora Morphol. Distrib. Funct. Ecol. Plants 2021, 278, 151797. [Google Scholar] [CrossRef]
- Marasco, R.; Mosqueira, M.J.; Cherif, A.; Daffonchio, D. Diversity and Plant Growth-Promoting Properties of Microbiomes Associated with Plants in Desert Soils BT. In Microbiology of Hot Deserts; Ramond, J.-B., Cowan, D.A., Eds.; Springer International Publishing: Cham, Switzerland, 2022; pp. 205–233. ISBN 978-3-030-98415-1. [Google Scholar]
- Mohanta, T.K.; Mohanta, Y.K.; Kaushik, P.; Kumar, J. Physiology, Genomics, and Evolutionary Aspects of Desert Plants. J. Adv. Res. 2023, 58, 63–78. [Google Scholar] [CrossRef] [PubMed]
- Yang, X.D.; Wu, N.C.; Gong, X.W. Plant Adaptation to Extreme Environments in Drylands. Forests 2023, 14, 390. [Google Scholar] [CrossRef]
- Nabhan, G.P.; Colunga-GarcíaMarín, P.; Zizumbo-Villarreal, D. Comparing Wild and Cultivated Food Plant Richness Between the Arid American and the Mesoamerican Centers of Diversity, as Means to Advance Indigenous Food Sovereignty in the Face of Climate Change. Front. Sustain. Food Syst. 2022, 6, 1–11. [Google Scholar] [CrossRef]
- Arba, M. The Potential of Cactus Pear (Opuntia ficus-indica (L.) Mill.) as Food and Forage Crop. In Emerging Research in Alternative Crops; Hirich, A., Choukr-Allah, R., Ragab, R., Eds.; Springer: Cham, Switzerland, 2020; Volume 58, pp. 335–357. ISBN 978-3-319-90471-9. [Google Scholar]
- Daniloski, D.; D’Cunha, N.M.; Speer, H.; McKune, A.J.; Alexopoulos, N.; Panagiotakos, D.B.; Petkoska, A.T.; Naumovski, N. Recent Developments on Opuntia spp., Their Bioactive Composition, Nutritional Values, and Health Effects. Food Biosci. 2022, 47, 101665. [Google Scholar] [CrossRef]
- Aldughaylibi, F.S.; Raza, M.A.; Naeem, S.; Rafi, H.; Alam, M.W.; Souayeh, B.; Farhan, M.; Aamir, M.; Zaidi, N.; Mir, T.A. Extraction of Bioactive Compounds for Antioxidant, Antimicrobial, and Antidiabetic Applications. Molecules 2022, 27, 5935. [Google Scholar] [CrossRef] [PubMed]
- Rodrigues, C.; Paula, C.D.d.; Lahbouki, S.; Meddich, A.; Outzourhit, A.; Rashad, M.; Pari, L.; Coelhoso, I.; Fernando, A.L.; Souza, V.G.L. Opuntia spp.: An Overview of the Bioactive Profile and Food Applications of This Versatile Crop Adapted to Arid Lands. Foods 2023, 12, 1465. [Google Scholar] [CrossRef] [PubMed]
- Torres-León, C.; Rebolledo Ramírez, F.; Aguirre-Joya, J.A.; Ramírez-Moreno, A.; Chávez-González, M.L.; Aguillón-Gutierrez, D.R.; Camacho-Guerra, L.; Ramírez-Guzmán, N.; Hernández Vélez, S.; Aguilar, C.N. Medicinal Plants Used by Rural Communities in the Arid Zone of Viesca and Parras Coahuila in Northeast Mexico. Saudi Pharm. J. 2023, 31, 21–28. [Google Scholar] [CrossRef]
- CONAFOR. Las Zonas Áridas Son Más Que Desierto. Available online: https://www.gob.mx/conafor/es/articulos/las-zonas-aridas-son-mas-que-desierto?idiom=es (accessed on 5 January 2025).
- Álvarez-Chávez, J.; Santos-Zea, L.; Ramírez-Jiménez, A.K.; Kleinschek, K.S. Agave By-Products: An Overview of Their Nutraceutical Value, Current Applications, and Processing Methods. Polysaccharides 2021, 2, 720–743. [Google Scholar] [CrossRef]
- Martínez, Y.; Iser, M.; Valdivié, M.; Rosales, M.; Albarrán, E.; Sánchez, D. Dietary Supplementation with Agave Tequilana (Weber Var. Blue) Stem Powder Improves the Performance and Intestinal Integrity of Broiler Rabbits. Animals 2022, 12, 1117. [Google Scholar] [CrossRef] [PubMed]
- Pérez-Zavala, M.d.L.; Hernández-Arzaba, J.C.; Bideshi, D.K.; Barboza-Corona, J.E. Agave: A Natural Renewable Resource with Multiple Applications. J. Sci. Food Agric. 2020, 100, 5324–5333. [Google Scholar] [CrossRef] [PubMed]
- Jiménez, G.G.; Durán, A.G.; Macías, F.A.; Simonet, A.M. Structure, Bioactivity and Analytical Methods for the Determination of Yucca Saponins. Molecules 2021, 26, 5251. [Google Scholar] [CrossRef] [PubMed]
- López-Ramírez, Y.; Cabañas-García, E.; Areche, C.; Trejo-Tapia, G.; Pérez-Molphe-Balch, E.; Gómez-Aguirre, Y.A. Callus Induction and Phytochemical Profiling of Yucca Carnerosana (Trel.) McKelvey Obtained from in Vitro Cultures. Rev. Mex. Ing. Quim. 2021, 20, 823–837. [Google Scholar] [CrossRef]
- Al-Sulbi, A.O.; Alghanem, A.A. Synchronous Management of Public Green Spaces: The Case of Imam Abdulrahman Bin Faisal University’s Eastern Campus—Dammam, Saudi Arabia. Ain Shams Eng. J. 2022, 13, 101605. [Google Scholar] [CrossRef]
- Krümpel, J.; George, T.; Gasston, B.; Francis, G.; Lemmer, A. Suitability of Opuntia ficus-indica (L) Mill. and Euphorbia tirucalli L. as Energy Crops for Anaerobic Digestion. J. Arid. Environ. 2020, 174, 104047. [Google Scholar] [CrossRef]
- Eleojo, C.; Amoo, S.O.; Kudanga, T. Opuntia (Cactaceae) Plant Compounds, Biological Activities and Prospects—A Comprehensive Review. Food Res. Int. 2018, 112, 328–344. [Google Scholar] [CrossRef]
- du Toit, A.; de Wit, M.; Osthoff, G.; Hugo, A. Antioxidant Properties of Fresh and Processed Cactus Pear Cladodes from Selected Opuntia ficus-indica and O. robusta Cultivars. S. Afr. J. Bot. 2018, 118, 44–51. [Google Scholar] [CrossRef]
- Besné-Eseverri, I.; Trepiana, J.; Gómez-Zorita, S.; Antunes-Ricardo, M.; Cano, M.P.; Portillo, M.P. Beneficial Effects of Opuntia spp. on Liver Health. Antioxidants 2023, 12, 1174. [Google Scholar] [CrossRef]
- Regalado-Rentería, E.; Aguirre-Rivera, J.R.; González-Chávez, M.M.; Sánchez-Sánchez, R.; Martínez-Gutiérrez, F.; Juárez-Flores, B.I. Assessment of Extraction Methods and Biological Value of Seed Oil from Eight Variants of Prickly Pear Fruit (Opuntia spp.). Waste Biomass Valorization 2018, 11, 1181–1189. [Google Scholar] [CrossRef]
- Manzur-Valdespino, S.; Arias-Rico, J.; Ramírez-Moreno, E.; Sánchez-Mata, M.d.C.; Jaramillo-Morales, O.A.; Angel-García, J.; Zafra-Rojas, Q.Y.; Barrera-Gálvez, R.; Cruz-Cansino, N.D.S. Applications and Pharmacological Properties of Cactus Pear (Opuntia spp.) Peel: A Review. Life 2022, 12, 1903. [Google Scholar] [CrossRef]
- Balendres, M.A.; Bengoa, J.C. Diseases of Dragon Fruit (Hylocereus Species): Etiology and Current Management Options. Crop Prot. 2019, 126, 104920. [Google Scholar] [CrossRef]
- Attar, S.H.; Urün, I.; Kafkas, S.; Kafkas, N.E.; Ercisli, S.; Ge, C.; Mlcek, J.; Adamkova, A. Nutritional Analysis of Red-Purple and White-Fleshed Pitaya (Hylocereus) Species. Molecules 2022, 27, 808. [Google Scholar] [CrossRef]
- Tang, W.; Li, W.; Yang, Y.; Lin, X.; Wang, L.; Li, C.; Yang, R. Phenolic Compounds Profile and Antioxidant Capacity Of. Foods 2021, 10, 1183. [Google Scholar] [CrossRef] [PubMed]
- García-Cruz, L.; Dueñas, M.; Santos-Buelgas, C.; Valle-Guadarrama, S.; Salinas-Moreno, Y. Betalains and Phenolic Compounds Profiling and Antioxidant Capacity of Pitaya (Stenocereus spp.) Fruit from Two Species (S. pruinosus and S. stellatus). Food Chem. 2017, 234, 111–118. [Google Scholar] [CrossRef] [PubMed]
- García Ordoñez, T.E.; Díaz Castro, F.; Castellanos Suárez, J.A.; Sedano Castro, G.; Almeraya Quintero, S.X. Characterization of the Pitaya Crop Stenocereus Pruinosus in the Community of Dolores Hidalgo, Huitziltepec, Puebla. Rev. Científica y Académica 2024, 5, 632–648. [Google Scholar] [CrossRef]
- Escobedo-García, S.; Salas-Tovar, J.A.; Flores-Gallegos, A.C.; Contreras-Esquivel, J.C.; González-Montemayor, Á.M.; López, M.G.; Rodríguez-Herrera, R. Functionality of Agave Bagasse as Supplement for the Development of Prebiotics-Enriched Foods. Plant Foods Hum. Nutr. 2019, 75, 96–102. [Google Scholar] [CrossRef] [PubMed]
- Castañeda-Rodríguez, R.; Quiles, A.; Llorca, E.; Ozuna, C. How to Cook Yucca spp. Flowers? An Analysis of Their Chemical Composition, Microstructure, and Bioactive Compound Bioaccessibility. Appl. Food Res. 2024, 4, 100414. [Google Scholar] [CrossRef]
- Juárez-Trujillo, N.; Monribot-Villanueva, J.L.; Jiménez-Fernández, V.M.; Suárez-Montaño, R.; Aguilar-Colorado, Á.S.; Guerrero-Analco, J.A.; Jiménez, M. Phytochemical Characterization of Izote (Yucca Elephantipes) Flowers. J. Appl. Bot. Food Qual. 2018, 91, 202–210. [Google Scholar] [CrossRef]
- Rezende, F.M.; Véras, A.S.C.; Siqueira, M.C.B.; Conceição, M.G.; Lima, C.L.; Almeida, M.P.; Mora-Luna, R.E.; Neves, M.L.M.W.; Monteiro, C.C.F.; Ferreira, M.A. Nutritional Effects of Using Cactus Cladodes (Opuntia Stricta Haw Haw) to Replace Sorghum Silage in Sheep Diet. Trop. Anim. Health Prod. 2020, 52, 1875–1880. [Google Scholar] [CrossRef] [PubMed]
- De Santiago, E.; Domínguez-Fernández, M.; Cid, C.; De Peña, M.P. Impact of Cooking Process on Nutritional Composition and Antioxidants of Cactus Cladodes (Opuntia ficus-indica). Food Chem. 2018, 240, 1055–1062. [Google Scholar] [CrossRef] [PubMed]
- Chahdoura, H.; Mzoughi, Z.; Ellouze, I.; Generalić Mekinić, I.; Čmiková, N.; El Bok, S.; Majdoub, H.; Ben Hsouna, A.; Ben Saad, R.; Mnif, W.; et al. Opuntia Species: A Comprehensive Review of Chemical Composition and Bio-Pharmacological Potential with Contemporary Applications. S. Afr. J. Bot. 2024, 174, 645–677. [Google Scholar] [CrossRef]
- Pulido-Hornedo, N.A.; Ventura-Juárez, J.; Guevara-Lara, F.; González-Ponce, H.A.; Sánchez-Alemán, E.; Buist-Homan, M.; Moshage, H.; Martínez-Saldaña, M.C. Hepatoprotective Effect of Opuntia Robusta Fruit Biocomponents in a Rat Model of Thioacetamide-Induced Liver Fibrosis. Plants 2022, 11, 2039. [Google Scholar] [CrossRef] [PubMed]
- Ibrahim, S.R.M.; Mohamed, G.A.; Khedr, A.I.M.; Zayed, M.F.; El-Kholy, A.A.E.S. Genus Hylocereus: Beneficial Phytochemicals, Nutritional Importance, and Biological Relevance-A Review. J. Food Biochem. 2018, 42, e12491. [Google Scholar] [CrossRef]
- Rathi, K.M.; Singh, S.L.; Gigi, G.G.; Shekade, S.V. Nutrition and Therapeutic Potential of the Dragon Fruit: A Qualitative Approach. Pharmacogn. Res. 2023, 16, 1–9. [Google Scholar] [CrossRef]
- Corzo-Rios, L.J.; Bautista-Ramírez, M.E.; Gómez y Gómez, Y.d.l.M.; Torres-Bustillos, L.G. Frutas de Cactáceas: Compuestos Bioactivos y Sus Propiedades Nutracéuticas. Propiedades Funcionales Hoy 2016, 35–66. [Google Scholar] [CrossRef]
- García-Cruz, L.; Valle-Guadarrama, S.; Guerra-Ramírez, D.; Martínez-Damián, M.T.; Zuleta-Prada, H. Cultivation, Quality Attributes, Postharvest Behavior, Bioactive Compounds, and Uses of Stenocereus: A Review. Sci. Hortic. 2022, 304, 111336. [Google Scholar] [CrossRef]
- Arellano-Plaza, M.; Paez-Lerma, J.B.; Soto-Cruz, N.O.; Kirchmayr, M.R.; Gschaedler Mathis, A. Mezcal Production in Mexico: Between Tradition and Commercial Exploitation. Front. Sustain. Food Syst. 2022, 6, 832532. [Google Scholar] [CrossRef]
- de la Rosa, O.; Flores-Gallegos, A.C.; Muñíz-Márquez, D.; Contreras-Esquivel, J.C.; Teixeira, J.A.; Nobre, C.; Aguilar, C.N. Successive Fermentation of Aguamiel and Molasses by Aspergillus Oryzae and Saccharomyces Cerevisiae to Obtain High Purity Fructooligosaccharides. Foods 2022, 11, 1786. [Google Scholar] [CrossRef] [PubMed]
- Aldrete-Herrera, P.I.; López, M.G.; Medina-Torres, L.; Ragazzo-Sánchez, J.A.; Calderón-Santoyo, M.; González-Ávila, M.; Ortiz-Basurto, R.I. Physicochemical Composition and Apparent Degree of Polymerization of Fructans in Five Wild Agave Varieties: Potential Industrial Use. Foods 2019, 8, 404. [Google Scholar] [CrossRef] [PubMed]
- Vázquez-Vuelvas, O.F.; Chávez-Camacho, F.A.; Meza-Velázquez, J.A.; Mendez-Merino, E.; Ríos-Licea, M.M.; Contreras-Esquivel, J.C. A Comparative FTIR Study for Supplemented Agavin as Functional Food. Food Hydrocoll. 2020, 103, 105642. [Google Scholar] [CrossRef]
- Martinez Gamiño, D.; Garcia Soto, M.J.; Gonzalez Acevedo, O.; Godinez Hernandez, C.; Juarez Flores, B.; Ortiz Basurto, R.I.; Rodriguez Aguilar, M.; Flores Ramirez, R.; Martinez Martinez, M.; Ratering, S.; et al. Prebiotic Effect of Fructans from Agave Salmiana on Probiotic Lactic Acid Bacteria and in Children as a Supplement for Malnutrition. Food Funct. 2022, 13, 4184–4193. [Google Scholar] [CrossRef]
- Mulík, S.; Ozuna, C. Mexican Edible Flowers: Cultural Background, Traditional Culinary Uses, and Potential Health Benefits. Int. J. Gastron. Food Sci. 2020, 21, 100235. [Google Scholar] [CrossRef]
- Attanzio, A.; Diana, P.; Barraja, P.; Carbone, A.; Spanò, V.; Parrino, B.; Cascioferro, S.M.; Allegra, M.; Cirrincione, G.; Tesoriere, L.; et al. Quality, Functional and Sensory Evaluation of Pasta Fortified with Extracts from Opuntia ficus-indica Cladodes. J. Sci. Food Agric. 2019, 99, 4242–4247. [Google Scholar] [CrossRef] [PubMed]
- Gouws, C.; Mortazavi, R.; Mellor, D.; McKune, A.; Naumovski, N. The Effects of Prickly Pear Fruit and Cladode (Opuntia spp.) Consumption on Blood Lipids: A Systematic Review. Complement. Ther. Med. 2020, 50, 102384. [Google Scholar] [CrossRef]
- García-Morales, S.; Corzo-Jiménez, I.J.; Silva-Córdova, N.F.; Soto-Cordero, A.M.; Rodríguez-Mejía, D.I.; Pardo-Núñez, J.; León-Morales, J.M. Comparative Study of Steroidal Sapogenins Content in Leaves of Five Agave Species. J. Sci. Food Agric. 2022, 102, 5653–5659. [Google Scholar] [CrossRef] [PubMed]
- González-Llanes, M.D.; Hernández-Calderón, O.M.; Rios-Iribe, E.Y.; Alarid-García, C.; Castro Montoya, A.J.; Escamilla-Silva, E.M. Fermentable Sugars Production by Enzymatic Processing of Agave Leaf Juice. Can. J. Chem. Eng. 2018, 96, 639–650. [Google Scholar] [CrossRef]
- López-Romero, J.C.; Ayala-Zavala, J.F.; González-Aguilar, G.A.; Peña-Ramos, E.A.; González-Rios, H. Biological Activities of Agave-by-Products and Their Possible Applications in Food and Pharmaceuticals. J. Sci. Food Agric. 2017, 98, 2461–2474. [Google Scholar] [CrossRef]
- Vernon-Carter, E.J.; Garcia-Diaz, S.; Reyes, I.; Carrillo-Navas, H.; Alvarez-Ramirez, J. Rheological and Thermal Properties of Dough and Textural and Microstructural Characteristics of Bread with Pulque as Leavening Agent. Int. J. Gastron. Food Sci. 2017, 9, 39–48. [Google Scholar] [CrossRef]
- Aruwa, C.E.; Amoo, S.O.; Kudanga, T. Extractable and Macromolecular Antioxidants of Opuntia ficus-indica Cladodes: Phytochemical Profiling, Antioxidant and Antibacterial Activities. S. Afr. J. Bot. 2019, 125, 402–410. [Google Scholar] [CrossRef]
- Khaled, S.; Dahmoune, F.; Madani, K.; Urieta, J.S.; Mainar, A.M. Supercritical Fractionation of Antioxidants from Algerian Opuntia ficus-indica (L.) Mill. Seeds. J. Food Process. Preserv. 2019, 44, e14343. [Google Scholar] [CrossRef]
- Bakar, B.; Çakmak, M.; Ibrahim, M.S.; Özer, D.; Saydam, S.; Karatas, F. Investigation of Amounts of Vitamins, Lycopene, and Elements in the Fruits of Opuntia ficus-indica Subjected to Different Pretreatments. Biol. Trace Elem. Res. 2020, 198, 315–323. [Google Scholar] [CrossRef] [PubMed]
- Diboune, N.; Nancib, A.; Nancib, N.; Aníbal, J.; Boudrant, J. Utilization of Prickly Pear Waste for Baker’s Yeast Production. Biotechnol. Appl. Biochem. 2019, 66, 744–755. [Google Scholar] [CrossRef] [PubMed]
- Rivera, G.; Bocanegra-García, V.; Monge, A. Traditional Plants as Source of Functional Foods: A Review. CYTA J. Food 2010, 8, 159–167. [Google Scholar] [CrossRef]
- Sharma, B.R.; Jaiswal, S.; Ravindra, P.V. Modulation of Gut Microbiota by Bioactive Compounds for Prevention and Management of Type 2 Diabetes. Biomed. Pharmacother. 2022, 152, 113148. [Google Scholar] [CrossRef]
- Luo, M.; Zhou, D.D.; Shang, A.; Gan, R.Y.; Li, H. Bin Influences of Food Contaminants and Additives on Gut Microbiota as Well as Protective Effects of Dietary Bioactive Compounds. Trends Food Sci. Technol. 2021, 113, 180–192. [Google Scholar] [CrossRef]
- Puente-Garza, C.A.; Meza-Miranda, C.; Ochoa-Martínez, D.; García-Lara, S. Effect of in Vitro Drought Stress on Phenolic Acids, Flavonols, Saponins, and Antioxidant Activity in Agave Salmiana. Plant Physiol. Biochem. 2017, 115, 400–407. [Google Scholar] [CrossRef] [PubMed]
- Bermúdez-Bazán, M.; Estarrón-Espinosa, M.; Castillo-Herrera, G.A.; Escobedo-Reyes, A.; Urias-Silvas, J.E.; Lugo-Cervantes, E.; Gschaedler-Mathis, A. Agave Angustifolia Haw. Leaves as a Potential Source of Bioactive Compounds: Extraction Optimization and Extract Characterization. Molecules 2024, 29, 1137. [Google Scholar] [CrossRef] [PubMed]
- Montoro, P.; Skhirtladze, A.; Bassarello, C.; Perrone, A.; Kemertelidze, E.; Pizza, C.; Piacente, S. Determination of Phenolic Compounds in Yucca Gloriosa Bark and Root by LC-MS/MS. J. Pharm. Biomed. Anal. 2008, 47, 854–859. [Google Scholar] [CrossRef] [PubMed]
- Montoro, P.; Skhirtladze, A.; Perrone, A.; Benidze, M.; Kemertelidze, E.; Piacente, S. Determination of Steroidal Glycosides in Yucca Gloriosa Flowers by LC/MS/MS. J. Pharm. Biomed. Anal. 2010, 52, 791–795. [Google Scholar] [CrossRef] [PubMed]
- de Sousa Rocha, S.; da Silva, S.R.F.; da Silva, J.Y.P.; de Medeiros, V.P.B.; Aburjaile, F.F.; de Oliveira Carvalho, R.D.; da Silva, M.S.; Tavares, J.F.; do Nascimento, Y.M.; dos Santos Lima, M.; et al. Exploring the Potential Prebiotic Effects of Opuntia Dillenii (Ker Gawl). Haw (Cactaceae) Cladodes on Human Intestinal Microbiota. J. Funct. Foods 2024, 118, 106259. [Google Scholar] [CrossRef]
- García-Cayuela, T.; Gómez-Maqueo, A.; Guajardo-Flores, D.; Welti-Chanes, J.; Cano, M.P. Characterization and Quantification of Individual Betalain and Phenolic Compounds in Mexican and Spanish Prickly Pear (Opuntia ficus-indica L. Mill) Tissues: A Comparative Study. J. Food Compos. Anal. 2019, 76, 1–13. [Google Scholar] [CrossRef]
- Rodrigues Vieira, T.R.; Lima, A.B.; Ribeiro, C.M.C.M.; de Medeiros, P.V.Q.; Converti, A.; dos Santos Lima, M.; Maciel, M.I.S. Red Pitaya (Hylocereus polyrhizus) as a Source of Betalains and Phenolic Compounds: Ultrasound Extraction, Microencapsulation, and Evaluation of Stability. LWT 2024, 196, 115755. [Google Scholar] [CrossRef]
- Sen, R.; Baruah, A.M. Phenolic Profile and Pigment Stability of Hylocereus Species Grown in North-East India. J. Food Compos. Anal. 2023, 116, 105078. [Google Scholar] [CrossRef]
- Puente-Garza, C.A.; García-Lara, S.; Gutiérrez-Uribe, J.A. Enhancement of Saponins and Flavonols by Micropropagation of Agave Salmiana. Ind. Crops Prod. 2017, 105, 225–230. [Google Scholar] [CrossRef]
- El-Hawary, S.S.; El-Kammar, H.A.; Farag, M.A.; Saleh, D.O.; El Dine, R.S. Metabolomic Profiling of Five Agave Leaf Taxa via UHPLC/PDA/ESI-MS Inrelation to Their Anti-Inflammatory, Immunomodulatory and Ulceroprotective Activities. Steroids 2020, 160. [Google Scholar] [CrossRef]
- Shegute, T.; Wasihun, Y. Antibacterial Activity and Phytochemical Components of Leaf Extracts of Agave Americana. J. Exp. Pharmacol. 2020, 12, 447–454. [Google Scholar] [CrossRef] [PubMed]
- Pérez-Armendáriz, B.; Cardoso-Ugarte, G.A. Traditional Fermented Beverages in Mexico: Biotechnological, Nutritional, and Functional Approaches. Food Res. Int. 2020, 136, 109307. [Google Scholar] [CrossRef] [PubMed]
- de la Rosa, O.; Flores-Gallegos, A.C.; Muñíz-Marquez, D.; Nobre, C.; Contreras-Esquivel, J.C.; Aguilar, C.N. Fructooligosaccharides Production from Agro-Wastes as Alternative Low-Cost Source. Trends Food Sci. Technol. 2019, 91, 139–146. [Google Scholar] [CrossRef]
- Martinez-Gutierrez, F.; Ratering, S.; Juárez-Flores, B.; Godinez-Hernandez, C.; Geissler-Plaum, R.; Prell, F.; Zorn, H.; Czermak, P.; Schnell, S. Potential Use of Agave Salmiana as a Prebiotic That Stimulates the Growth of Probiotic Bacteria. LWT 2017, 84, 151–159. [Google Scholar] [CrossRef]
- Sáyago-Ayerdi, S.G.; Zamora-Gasga, V.M.; Venema, K. Changes in Gut Microbiota in Predigested Hibiscus Sabdariffa L Calyces and Agave (Agave Tequilana Weber) Fructans Assessed in a Dynamic in Vitro Model (TIM-2) of the Human Colon. Food Res. Int. 2020, 132, 109036. [Google Scholar] [CrossRef] [PubMed]
- Enríquez-Salazar, M.I.; Veana, F.; Aguilar, C.N.; De la Garza-Rodríguez, I.M.; López, M.G.; Rutiaga-Quiñones, O.M.; Morlett-Chávez, J.A.; Rodríguez-Herrera, R. Microbial Diversity and Biochemical Profile of Aguamiel Collected from Agave Salmiana and A. Atrovirens during Different Seasons of Year. Food Sci. Biotechnol. 2017, 26, 1003–1011. [Google Scholar] [CrossRef] [PubMed]
- Gutiérrez-Uribe, J.A.; Figueroa, L.M.; Martín-del-Campo, S.T.; Escalante, A. Pulque; Elsevier Inc.: Amsterdam, The Netherlands, 2017; ISBN 9780128023099. [Google Scholar]
- Villarreal-Morales, S.L.; Muñiz-Márquez, D.B.; Michel-Michel, M.; González-Montemayor, Á.M.; Escobedo-García, S.; Salas-Tovar, J.A.; Flores-Gallegos, A.C.; Rodríguez-Herrera, R. Aguamiel a Fresh Beverage from Agave spp. Sap with Functional Properties. Nat. Beverages 2019, 13, 179–208. [Google Scholar] [CrossRef]
- Bafundo, K.W.; Duerr, I.; McNaughton, J.L.; Johnson, A.B. The Effects of a Quillaja and Yucca Combination on Performance and Carcass Traits of Coccidia-Vaccinated Broilers Exposed to an Enteric Disease Challenge. Poult. Sci. 2021, 100, 101391. [Google Scholar] [CrossRef]
- Stefanello, C.; Moreira, B.; Gräf, W.M.; Robalo, S.; Costa, S.T.; Vieira, I.M.; Miranda, D.J. Effects of a Proprietary Blend of Quillaja and Yucca on Growth Performance, Nutrient Digestibility, and Intestinal Measurements of Broilers. J. Appl. Poult. Res. 2022, 31, 100251. [Google Scholar] [CrossRef]
- Attallah, N.G.M.; El-Sherbeni, S.A.; El-Kadem, A.H.; Elekhnawy, E.; El-Masry, T.A.; Elmongy, E.I.; Altwaijry, N.; Negm, W.A. Elucidation of the Metabolite Profile of Yucca Gigantea and Assessment of Its Cytotoxic, Antimicrobial, and Anti-Inflammatory Activities. Molecules 2022, 27, 1329. [Google Scholar] [CrossRef] [PubMed]
- Morales-Figueroa, G.-G.; Pereo-vega, G.D.; Reyna-murrieta, M.E.; Pérez-morales, R.; López-mata, M.A.; Sánchez-escalante, J.J.; Tapia-rodriguez, M.R.; Ayala-zavala, J.F.; Juárez, J.; Quihui-cota, L. Antibacterial and Antioxidant Properties of Extracts of Yucca baccata, a Plant of Northwestern Mexico, against Pathogenic Bacteria. BioMed Res. Int. 2022, 2022, 9158836. [Google Scholar] [CrossRef] [PubMed]
- Mokbli, S.; Nehdi, I.A.; Sbihi, H.M.; Tan, C.P.; Al-Resayes, S.I.; Rashid, U. Yucca Aloifolia Seed Oil: A New Source of Bioactive Compounds. Waste Biomass Valorization 2018, 9, 1087–1093. [Google Scholar] [CrossRef]
- Boutakiout, A.; Elothmani, D.; Hanine, H.; Mahrouz, M.; Le Meurlay, D.; Hmid, I.; Ennahli, S. Effects of Different Harvesting Seasons on Antioxidant Activity and Phenolic Content of Prickly Pear Cladode Juice. J. Saudi Soc. Agric. Sci. 2018, 17, 471–480. [Google Scholar] [CrossRef]
- González-Monroy, A.D.; Kaur Kataria, T.; Olvera-Cervantes, J.L.; Corona-Chávez, A.; Ozuna, C.; Rodríguez-Hernández, G.; Sosa-Morales, M.E. Dielectric Properties of Beverages (Tamarind and Green) Relevant to Microwave-Assisted Pasteurization. J. Food Sci. 2018, 83, 2317–2323. [Google Scholar] [CrossRef] [PubMed]
- Liguori, G.; Gentile, C.; Gaglio, R.; Perrone, A.; Guarcello, R.; Francesca, N.; Fretto, S.; Inglese, P.; Settanni, L. Effect of Addition of Opuntia ficus-indica Mucilage on the Biological Leavening, Physical, Nutritional, Antioxidant and Sensory Aspects of Bread. J. Biosci. Bioeng. 2020, 129, 184–191. [Google Scholar] [CrossRef] [PubMed]
- Dick, M.; Limberger, C.; Cruz Silveira Thys, R.; de Oliveira Rios, A.; Hickmann Flôres, S. Mucilage and Cladode Flour from Cactus (Opuntia monacantha) as Alternative Ingredients in Gluten-Free Crackers. Food Chem. 2020, 314, 126178. [Google Scholar] [CrossRef]
- Song, W.; Lagmay, V.; Jeong, B.G.; Jung, J.; Chun, J. Changes in Physicochemical and Functional Properties of Opuntia Humifusa during Fermentation with Cellulolytic Enzyme and Lactic Acid Bacteria. LWT 2022, 159, 113192. [Google Scholar] [CrossRef]
- Abd El-Moaty, H.I.; Sorour, W.A.; Youssef, A.K.; Gouda, H.M. Structural Elucidation of Phenolic Compounds Isolated from Opuntia Littoralis and Their Antidiabetic, Antimicrobial and Cytotoxic Activity. S. Afr. J. Bot. 2020, 131, 320–327. [Google Scholar] [CrossRef]
- Betancourt, C.; Cejudo-Bastante, M.J.; Heredia, F.J.; Hurtado, N. Pigment Composition and Antioxidant Capacity of Betacyanins and Betaxanthins Fractions of Opuntia Dillenii (Ker Gawl) Haw Cactus Fruit. Food Res. Int. 2017, 101, 173–179. [Google Scholar] [CrossRef]
- Otálora, M.C.; de Jesús Barbosa, H.; Perilla, J.E.; Osorio, C.; Nazareno, M.A. Encapsulated Betalains (Opuntia ficus-indica) as Natural Colorants. Case Study: Gummy Candies. LWT 2019, 103, 222–227. [Google Scholar] [CrossRef]
- Bouazizi, S.; Montevecchi, G.; Antonelli, A.; Hamdi, M. Effects of Prickly Pear (Opuntia ficus-indica L.) Peel Flour as an Innovative Ingredient in Biscuits Formulation. LWT 2020, 124, 109155. [Google Scholar] [CrossRef]
- Yao, X.; Hu, H.; Qin, Y.; Liu, J. Development of Antioxidant, Antimicrobial and Ammonia-Sensitive Films Based on Quaternary Ammonium Chitosan, Polyvinyl Alcohol and Betalains-Rich Cactus Pears (Opuntia ficus-indica) Extract. Food Hydrocoll. 2020, 106, 105896. [Google Scholar] [CrossRef]
- Chahdoura, H.; Barreira, J.C.M.; Barros, L.; Dias, M.I.; Calhelha, R.C.; Flamini, G.; Soković, M.; Achour, L.; Ferreira, I.C.F.R. Bioactivity, Hydrophilic, Lipophilic and Volatile Compounds in Pulps and Skins of Opuntia Macrorhiza and Opuntia Microdasys Fruits. LWT 2019, 105, 57–65. [Google Scholar] [CrossRef]
- Som, A.M.; Ahmat, N.; Abdul Hamid, H.A.; Azizuddin, N.M. A Comparative Study on Foliage and Peels of Hylocereus Undatus (White Dragon Fruit) Regarding Their Antioxidant Activity and Phenolic Content. Heliyon 2019, 5, e01244. [Google Scholar] [CrossRef]
- Zambrano, C.; Kerekes, E.B.; Kotogán, A.; Papp, T.; Vágvölgyi, C.; Krisch, J.; Takó, M. Antimicrobial Activity of Grape, Apple and Pitahaya Residue Extracts after Carbohydrase Treatment against Food-Related Bacteria. LWT 2019, 100, 416–425. [Google Scholar] [CrossRef]
- da Silveira Agostini-Costa, T. Bioactive Compounds and Health Benefits of Pereskioideae and Cactoideae: A Review. Food Chem. 2020, 327, 126961. [Google Scholar] [CrossRef]
- Cejudo-Bastante, M.J.; Hurtado, N.; Muñoz-Burguillos, P.; Heredia, F.J. Stenocereus Griseus (Haw) Pitaya as Source of Natural Colourant: Technological Stability of Colour and Individual Betalains. Int. J. Food Sci. Technol. 2019, 54, 3024–3031. [Google Scholar] [CrossRef]
- Sandate-Flores, L.; Rodríguez-Rodríguez, J.; Velázquez, G.; Mayolo-Deloisa, K.; Rito-Palomares, M.; Torres, J.A.; Parra-Saldívar, R. Low-Sugar Content Betaxanthins Extracts from Yellow Pitaya (Stenocereus pruinosus). Food Bioprod. Process. 2020, 121, 178–185. [Google Scholar] [CrossRef]
- Zhang, Q.; Xu, Y.; Bukvicki, D.; Peng, Y.; Li, F.; Zhang, Q.; Yan, J.; Lin, S.; Liu, S.; Qin, W. Phenolic Compounds in Dietary Target the Regulation of Gut Microbiota: Role in Health and Disease. Food Biosci. 2024, 62, 105107. [Google Scholar] [CrossRef]
- Chuaykarn, N.; Tampanna, N.; Wangkulangkul, P.; Wanitsuwan, W.; Yolsuriyanwong, K.; Wichienchot, S. Comparative Effectiveness of Indigestible Carbohydrates and Plant Polyphenols on the Gut Microbiota Profile and Metabolite Alterations of Obese Patients. Bioact. Carbohydr. Diet. Fibre 2024, 32, 100443. [Google Scholar] [CrossRef]
- Bouyahya, A.; Bakrim, S.; Aboulaghras, S.; El Kadri, K.; Aanniz, T.; Khalid, A.; Abdalla, A.N.; Abdallah, A.A.; Ardianto, C.; Ming, L.C.; et al. Bioactive Compounds from Nature: Antioxidants Targeting Cellular Transformation in Response to Epigenetic Perturbations Induced by Oxidative Stress. Biomed. Pharmacother. 2024, 174, 116432. [Google Scholar] [CrossRef] [PubMed]
- König, J. Functional Foods. Encycl. Imdustrial Chem. 2016, 1, 492–499. [Google Scholar] [CrossRef]
- Jiang, L.L.; Gong, X.; Ji, M.Y.; Wang, C.C.; Wang, J.H.; Li, M.H. Bioactive Compounds from Plant-Based Functional Foods: A Promising Choice for the Prevention and Management of Hyperuricemia. Foods 2020, 9, 973. [Google Scholar] [CrossRef] [PubMed]
- Ramírez-Pulido, B.; Bas-Bellver, C.; Betoret, N.; Barrera, C.; Seguí, L. Valorization of Vegetable Fresh-Processing Residues as Functional Powdered Ingredients. A Review on the Potential Impact of Pretreatments and Drying Methods on Bioactive Compounds and Their Bioaccessibility. Front. Sustain. Food Syst. 2021, 5, 654313. [Google Scholar] [CrossRef]
- Tanna, B.; Mishra, A. Metabolites Unravel Nutraceutical Potential of Edible Seaweeds: An Emerging Source of Functional Food. Compr. Rev. Food Sci. Food Saf. 2018, 17, 1613–1624. [Google Scholar] [CrossRef] [PubMed]
- Andrade, A.I.C.; Bautista, C.R.; Cabrera, M.A.R.; Guerra, R.E.S.; Chávez, E.G.; Ahumada, C.F.; Lagunes, A.G. Agave Salmiana Fructans as Gut Health Promoters: Prebiotic Activity and Inflammatory Response in Wistar Healthy Rats. Int. J. Biol. Macromol. 2019, 136, 785–795. [Google Scholar] [CrossRef]
- Santiago-García, P.A.; Mellado-Mojica, E.; León-Martínez, F.M.; López, M.G. Evaluation of Agave Angustifolia Fructans as Fat Replacer in the Cookies Manufacture. LWT Food Sci. Technol. 2017, 77, 100–109. [Google Scholar] [CrossRef]
- Palatnik, D.R.; Aldrete Herrera, P.; Rinaldoni, A.N.; Ortiz Basurto, R.I.; Campderrós, M.E. Development of Reduced-Fat Cheeses with the Addition of Agave Fructans. Int. J. Dairy Technol. 2017, 70, 212–219. [Google Scholar] [CrossRef]
- Pintor-Jardines, A.; Arjona-Román, J.L.; Totosaus-Sánchez, A.; Severiano-Pérez, P.; González-González, L.R.; Escalona-Buendia, H.B. The Influence of Agave Fructans on Thermal Properties of Low-Fat, and Low-Fat and Sugar Ice Cream. LWT 2018, 93, 679–685. [Google Scholar] [CrossRef]
- González-Herrera, S.M.; Rocha-Guzmán, N.E.; Simental-Mendía, L.E.; Rodríguez-Herrera, R.; Aguilar, C.N.; Rutiaga-Quiñones, O.M.; López, M.G.; Gamboa-Gómez, C.I. Dehydrated Apple-Based Snack Supplemented with Agave Fructans Exerts Prebiotic Effect Regulating the Production of Short-Chain Fatty Acid in Mice. J. Food Process. Preserv. 2019, 43, e14026. [Google Scholar] [CrossRef]
- Gutiérrez-García, G.J.; Quintana-Romero, L.A.; Morales-Figueroa, G.G.; Esparza-Romero, J.; Pérez-Morales, R.; López-Mata, M.A.; Juárez, J.; Sánchez-Escalante, J.J.; Peralta, E.; Quihui-Cota, L.; et al. Effect of Yucca baccata Butanolic Extract on the Shelf Life of Chicken and Development of an Antimicrobial Packaging for Beef. Food Control 2021, 127, 108142. [Google Scholar] [CrossRef]
- Gengatharan, A.; Dykes, G.A.; Choo, W.S. The Effect of PH Treatment and Refrigerated Storage on Natural Colourant Preparations (Betacyanins) from Red Pitahaya and Their Potential Application in Yoghurt. LWT Food Sci. Technol. 2017, 80, 437–445. [Google Scholar] [CrossRef]
- de Souza, A.C.; Fernandes, A.C.F.; Silva, M.S.; Schwan, R.F.; Dias, D.R. Antioxidant Activities of Tropical Fruit Wines. J. Inst. Brew. 2018, 124, 492–497. [Google Scholar] [CrossRef]
- Amjadi, S.; Ghorbani, M.; Hamishehkar, H.; Roufegarinejad, L. Improvement in the Stability of Betanin by Liposomal Nanocarriers: Its Application in Gummy Candy as a Food Model. Food Chem. 2018, 256, 156–162. [Google Scholar] [CrossRef] [PubMed]
- Hsu, C.T.; Chang, Y.H.; Shiau, S.Y. Color, Antioxidation, and Texture of Dough and Chinese Steamed Bread Enriched with Pitaya Peel Powder. Cereal Chem. 2019, 96, 76–85. [Google Scholar] [CrossRef]
- Utpott, M.; Ramos de Araujo, R.; Galarza Vargas, C.; Nunes Paiva, A.R.; Tischer, B.; de Oliveira Rios, A.; Hickmann Flôres, S. Characterization and Application of Red Pitaya (Hylocereus polyrhizus) Peel Powder as a Fat Replacer in Ice Cream. J. Food Process. Preserv. 2020, 44, e14420. [Google Scholar] [CrossRef]
- García-Lucas, K.A.; Méndez-Lagunas, L.L.; Rodríguez-Ramírez, J.; Campanella, O.H.; Patel, B.K.; Barriada-Bernal, L.G. Physical Properties of Spray Dryed Stenocereus Griseus Pitaya Juice Powder. J. Food Process Eng. 2016, 40, e12470. [Google Scholar] [CrossRef]

| Component (%) | Agave | Yucca Flowers | Opuntia | Opuntia Fruit | Hylocereus | Stenocereus |
|---|---|---|---|---|---|---|
| Moisture | 6.44–8.5 | 50–84.2 | 90.1–93.7 | 81.68–89.96 | 82.5–85 | 81–89 |
| Carbohydrate | 60–74 | 6.65–9.77 | 4.6–8.17 | 1.35 | 8.15–13.2 | 8.5–10.85 |
| Proteins | 2.5–8.35 | 0.30–0.31 | 0.36–1.1 | 1.18 | 0.18–1.1 | 1.08–1.30 |
| Lipids | 0.3 | 17.55 | 0.1–0.14 | 0.71–1.1 | 0.1–0.57 | 0.10–0.49 |
| Fiber | 5–8 | 19.23 | 2.75 | 2.84–9.49 | 0.45–11.34 | 0.53–7.10 |
| Ash | 6–8 | 1.60 | 0.5–1.32 | 0.43–16.5 | 0.96–1.18 | 0.46–0.81 |
| Authors | [19,36] | [37,38] | [39,40] | [26,41,42] | [43,44] | [45,46] |
| Plants | Bioactive Compounds | Concentrations | Biological Activities | Author |
|---|---|---|---|---|
| Agave salmiana and A. angustifolia | Phenolic compounds 1,* Flavonoid 2,* Saponins 3,* | 3850–8480 8.35 7630–171,450 | Prebiotic activity Antioxidant activity Anti-inflammatory activity Antimicrobial activity Antifungal activity | [66,67] |
| Yucca gloriosa and Y. schidigera | Phenolic compounds 4,* Saponins 4,* | 35,180–96,610 151.1 | Antioxidant activity Anti-inflammatory activity Antifungal activity | [68,69] |
| Opuntia ficus-indica and O. dillenii (Cladodes) | Organic acids 4,* Total chlorophyll 5,* Phenolic compounds 4,* Flavanols 4,* Flavonols 4,* Flavonones 4,* Stilbenes 4,* Phenolic acids 4,* | 259.7 9120 161.1–16,730 125–1660 8440 1690 700 35.6–4240 | Antioxidant activity Antidiabetic activity Anti-inflammatory activity Prebiotic activity | [40,70] |
| Opuntia ficus-indica (purple, red, and yellow fruits) | Phenolic compounds 1,* Flavonoids 2,* Total betalains 6,7,* Betacyanins 6,* Betaxanthins 7,* | 2387.1–3619.0 983.5–1447.8 310–2580 130–1830 180–760 | Antioxidant activity Anti-inflammatory activity Antidiabetic activity Anticancer activity | [71] |
| Hylocereus undatus, costaricensis, and polyrhizus | Phenolic compounds 4,** Total betalains 4,** Betacyanin 4,** Betacyanins 4,** Betaxanthins 4,** | 480–683.6 1080–1660 0–28,600 920–1100 160–560 | Antioxidant activity | [72,73] |
| Stenocereuspruinosus and S. stellatus | Phenolic compounds 4,* Hydroxycinnamoyl derivatives 4,* Flavonols 4,* Flavanones 4,* Betacyanins 4,* Betaxanthins 4,* | 53.75–121.86 7.51–15.61 4.08–10.59 14.89–16.25 162.07–5423.38 17,706.72–22,053.46678 | Antioxidant potential | [34,46] |
| Plants | Food or Additive | Functional Contribution | Bioactive Compounds | Author |
|---|---|---|---|---|
| Agave spp. | Powdered extract of Agave salmiana fructans | Prebiotic activity and anti-inflammatory activity | Fructans | [112] |
| Cookies with Agave angustifolia fructans as a fat substitute | Improved rheological properties | Fructans | [113] | |
| Reduced-fat cheeses | Improved nutritional qualities | Fructans | [114] | |
| Ice cream | Improved thermal properties | Fructans | [115] | |
| Dehydrated apple enriched with prebiotics | Prebiotic activity and sensory properties | Fructans | [116] | |
| Yucca spp. | Antimicrobial control in food Food packaging development | Antimicrobial activity | Saponins | [117] |
| Opuntia spp. (Cladodes) | Pasta with flour from Opuntia cladodes | Antioxidant activity and hypoglycemic activity | Fiber | [53] |
| Prickly pear cladode juice | Antioxidant activity | Gallic acid, epicatechin gallate, vanillic acid, procyanidin B2, epicatechin, p-Coumaric acid, epigallocatechin, ferulic acid, sinapic acid, benzoic acid, hyperoside, isoquercetin, rutin, and quercetin | [89] | |
| Gluten-free cookies with flour from cacti | Antioxidant activity | Soluble and insoluble fiber, flavonoids, phenolic acids, leutin, β-carotene, zeaxanthin, and α-carotene | [90] | |
| Bread with Opuntia ficus-indica mucilage | Antioxidant activity | Mucilage | [92] | |
| Pigment | Antioxidant activity | Betacyanins and betaxanthins | [95] | |
| Opuntia spp. (fruit) | Gummy candy | Antioxidant activity | Betalains | [97] |
| Cookies enriched with prickly pear peel flour | Antioxidant activity | Carotenoids, betalains, betacyanins, and betaxantins | [96] | |
| Edible films | Antioxidant activity and antimicrobial activity | Betalains | [98] | |
| Yogurt | Antioxidant activity | Betacyanins, betanin, isobetanin, betanidin, phyllocactin, and hyloccerenin | [118] | |
| Wine | Antioxidant activity | Succinic acid, citric acid, and acetic acid | [119] | |
| Hylocereus spp. | Gummy candy | Antioxidant activity | Betalains | [120] |
| Chinese steamed bread enriched with pitaya peel powder | Antioxidant activity | Batacyanin | [121] | |
| Reduced-fat ice cream | Antioxidant activity Technological and physicochemical properties | Betacyanins, fiber, and minerals | [122] | |
| Natural colorant | Antioxidant activity | Betalains, isobetanin, betanidin, 17-Decarboxy-neobetanin, isobetanidin, neobetanin, and 2-Decarbaxy-neobetanin | [103] | |
| Pitaya juice powder | Antioxidant activity | Betalains, fructose, glucose, sucrose, citric acid, malic acid, and tartaric acid | [123] | |
| Stenocereus spp. | Low-sugar food colorant | Antioxidant and antimicrobial activity | Betaxantinas | [104] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Márquez-Rangel, I.; Cruz, M.; Neira-Vielma, A.A.; Ramírez-Barrón, S.N.; Aguilar-Zarate, P.; Belmares, R. Plants from Arid Zones of Mexico: Bioactive Compounds and Potential Use for Food Production. Resources 2025, 14, 13. https://doi.org/10.3390/resources14010013
Márquez-Rangel I, Cruz M, Neira-Vielma AA, Ramírez-Barrón SN, Aguilar-Zarate P, Belmares R. Plants from Arid Zones of Mexico: Bioactive Compounds and Potential Use for Food Production. Resources. 2025; 14(1):13. https://doi.org/10.3390/resources14010013
Chicago/Turabian StyleMárquez-Rangel, Isabel, Mario Cruz, Alberto A. Neira-Vielma, Sonia N. Ramírez-Barrón, Pedro Aguilar-Zarate, and Ruth Belmares. 2025. "Plants from Arid Zones of Mexico: Bioactive Compounds and Potential Use for Food Production" Resources 14, no. 1: 13. https://doi.org/10.3390/resources14010013
APA StyleMárquez-Rangel, I., Cruz, M., Neira-Vielma, A. A., Ramírez-Barrón, S. N., Aguilar-Zarate, P., & Belmares, R. (2025). Plants from Arid Zones of Mexico: Bioactive Compounds and Potential Use for Food Production. Resources, 14(1), 13. https://doi.org/10.3390/resources14010013








