Factors Participating in the Occurrence of Inflammation of the Lips (Cheilitis) and Perioral Skin
Abstract
:1. Background
2. Most Common Causes of Lip and Perioral Inflammation
3. Accompanying Nonpsychiatric Diseases and Conditions in Patients with Lip and Perioral Inflammation
4. Psychiatric Diseases and Conditions and Common Behavioral Attitudes in Patients with Lip and Perioral Inflammation
5. Allergies Associated with Lip and Perioral Inflammation
6. Nutritional Deficiencies and Microbiome Changes in Patients with Lip and Perioral Inflammation
7. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Greenberg, S.A.; Schlosser, B.J.; Mirowski, G.W. Diseases of the lips. Clin. Dermatol. 2017, 35, e1–e14. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lugović-Mihić, L.; Pilipović, K.; Crnarić, I.; Šitum, M.; Duvančić, T. Differential diagnosis od cheilitis: How to classify cheilitis? Acta Clin. Croat. 2018, 57, 342–351. [Google Scholar] [PubMed]
- Lugović-Mihić, L.; Blagec, T.; Japundžić, I.; Skroza, N.; Delaš Adžajić, M.; Mravak-Stipetić, M. Diagnostic management of cheilitis: An approach based on a recent proposal for cheilitis classification. Acta Dermatovenerol. Alp. Pannonica Adriat. 2020, 29, 67–72. [Google Scholar] [CrossRef] [PubMed]
- Litaiem, N.; Ben Slimane, M.; Bacha, T.; Rammeh, S.; Zeglaoui, F. Cheilitis with Hemorrhagic Crusts of the Vermilion Lips. Int. J. Dermatol. 2020, 59, e234–e236. [Google Scholar] [CrossRef]
- Opstelten, W.; Neven, A.K.; Eekhof, J. Treatment and prevention of herpes labialis. Can. Fam. Phys. 2008, 54, 1683–1687. [Google Scholar]
- Scully, C. Dermatoses of the Oral Cavity and Lips. In Rook’s Textbook of Dermatology; Griffiths, C., Barker, J., Bleiker, T., Chalmers, R., Creamer, D., Eds.; Wiley Blackwell: Chichester, UK, 2016; pp. 110.1–110.94. [Google Scholar]
- Orion, E.; Wolf, R. Psychologic factors in the development of facial dermatoses. Clin. Dermatol. 2014, 32, 763–766. [Google Scholar] [CrossRef]
- Hitz Lindenmüller, I.; Itin, P.H.; Fistarol, S.K. Dermatology of the lips: Inflammatory diseases. Quintessence Int. 2014, 45, 875–883. [Google Scholar]
- Samimi, M. Cheilitis: Diagnosis and treatment. Presse Med. 2016, 45, 240–250. [Google Scholar] [CrossRef]
- Singh, M.; Pawar, M.; Bothra, A.; Maheshwari, A.; Dubey, V.; Tiwari, A.; Kelati, A. Personal protective equipment induced facial dermatoses in healthcare workers managing Coronavirus disease 2019. J. Eur. Acad. Dermatol. Venereol. 2020, 34, e378–e380. [Google Scholar] [CrossRef]
- Bhutta, B.S.; Hafsi, W. Cheilitis. Available online: https://www.statpearls.com/articlelibrary/viewarticle/37546/ (accessed on 17 November 2022).
- Thongprasom, K. Glycerin borax treatment of exfoliative cheilitis induced by sodium lauryl sulfate: A case report. Acta Stomatol. Croat. 2016, 50, 158–161. [Google Scholar] [CrossRef]
- Parker, J.; Neill, B.; Whitsitt, J.; Rajpara, A.; Aires, D. Exacerbation of Pediatric Periorificial Dermatitis: A Novel Adverse Reaction. J. Drugs Dermatol. 2020, 19, 428. [Google Scholar] [PubMed]
- Maarouf, M.; Saberian, C.; Lio, P.A.; Shi, V.Y. Head-and-neck dermatitis: Diagnostic difficulties and management pearls. Pediatr. Dermatol. 2018, 35, 748–753. [Google Scholar] [CrossRef] [PubMed]
- Oakley, A. Cheilitis. Avaliable online: https://www.dermnetnz.org/topics/cheilitis/ (accessed on 17 November 2022).
- Tolaymat, L.; Hall, M.R. Perioral Dermatitis. Available online: https://www.ncbi.nlm.nih.gov/books/NBK525968/ (accessed on 17 November 2022).
- Contento, M.; Maher, J.; Cline, A.; Rose, S. Why Does Facial Eczema Differ from Body Eczema? J. Drugs Dermatol. 2022, 21, 1119–1123. [Google Scholar] [CrossRef] [PubMed]
- Zheng, Y.; Wang, Q.; Ma, L.; Chen, Y.; Gao, Y.; Zhang, G.; Cui, S.; Liang, H.; He, C.; Song, L. Alterations in the skin microbiome are associated with disease severity and treatment in the perioral zone of the skin of infants with atopic dermatitis. Eur. J. Clin. Microbiol. Infect. Dis. 2019, 38, 1677–1685. [Google Scholar] [CrossRef] [PubMed]
- Ferček, I.; Lugović-Mihić, L.; Tambić-Andrašević, A.; Ćesić, D.; Grginić, A.G.; Bešlić, I.; Mravak-Stipetić, M.; Mihatov-Štefanović, I.; Buntić, A.-M.; Čivljak, R. Features of the Skin Microbiota in Common Inflammatory Skin Diseases. Life 2021, 11, 962. [Google Scholar] [CrossRef]
- Ye, C.; Chen, J.; Yang, S.; Yi, J.; Chen, H.; Li, M.; Yin, S.; Lai, W.; Zheng, Y. Skin sensitivity evaluation: What could impact the assessment results? J. Cosmet. Dermatol. 2020, 19, 1231–1238. [Google Scholar] [CrossRef]
- Ehnis-Pérez, A.; Torres-Álvarez, B.; Cortés-García, D.; Hernández-Blanco, D.; Fuentes-Ahumada, C.; Castanedo-Cázares, J.P. Relationship between transient receptor potential vanilloid-1 expression and the intensity of sensitive skin symptoms. J. Cosmet. Dermatol. 2016, 15, 231–237. [Google Scholar] [CrossRef]
- Blicharz, L.; Rudnicka, L.; Samochocki, Z. Staphylococcus aureus: An Underestimated Factor in the Pathogenesis of Atopic Dermatitis? Postepy Dermatol. Alergol. 2019, 36, 11–17. [Google Scholar] [CrossRef]
- Abdi, F.; Kashani, H.; Naeini, F.; Narimani, T.; Khorvash, F. Staphylococcus aureus in Acne Pathogenesis: A Case-Control Study. N. Am. J. Med. Sci. 2012, 4, 573. [Google Scholar] [CrossRef] [Green Version]
- Takiwaki, H.; Tsuda, H.; Arase, S.; Takeichi, H. Differences between Intrafollicular Microorganism Profiles in Perioral and Seborrhoeic Dermatitis. Clin. Exp. Dermatol. 2003, 28, 531–534. [Google Scholar] [CrossRef]
- Ishiguro, N.; Maeda, A.; Suzuki, K.; Yamana, Y.; Fukuya, Y.; Kawashima, M. Three Cases of Perioral Dermatitis Related to Fusobacteria Treated with β-Lactam Antibiotics. J. Dermatol. Treat. 2013, 25, 507–509. [Google Scholar] [CrossRef] [PubMed]
- Teo, W. The “Maskne” Microbiome—Pathophysiology and Therapeutics. Int. J. Dermatol. 2021, 60, 799–809. [Google Scholar] [CrossRef] [PubMed]
- Ayesh, M.H. Angular cheilitis induced by iron deficiency anemia. Cleve Clin. J. Med. 2018, 85, 581–582. [Google Scholar] [CrossRef] [PubMed]
- Phatak, S.; Redkar, N.; Patil, M.A.; Kuwar, A. Plummer-Vinson Syndrome. Case Rep. 2012, 2012, bcr2012006403. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Demir, N.; Doğan, M.; Koç, A.; Kaba, S.; Bulan, K.; Ozkol, H.U.; Doğan, S.Z. Dermatological findings of vitamin B12 deficiency and resolving time of these symptoms. Cutan. Ocul. Toxicol. 2014, 33, 70–73. [Google Scholar] [CrossRef]
- Nico, M.M.S.; Dwan, A.J.; Lourenço, S.V. Ointment pseudo-cheilitis: A disease distinct from factitial cheilitis. A series of 13 patients from São Paolo, Brazil. J. Cutan. Med. Surg. 2019, 23, 277–281. [Google Scholar] [CrossRef]
- Panico, R.; Piemonte, E.; Lazos, J.; Gilligan, G.; Zampini, A.; Lanfranchi, H. Oral mucosal lesions in anorexia nervosa, bulimia nervosa and EDNOS. J. Psychiatr. Res. 2018, 96, 178–182. [Google Scholar] [CrossRef]
- Seghers, A.K.; Grosber, M.; Urbain, D.; Mana, F. Cheilitis granulomatosa and Crohn’s disease: A case report. Acta Gastroenterol. Belg. 2019, 82, 326–328. [Google Scholar]
- Mathelier-Fusade, P. Cheilitis: A new manifestation of gastro-oesophageal reflux? Ann. Dermatol. Venereol. 2009, 136, 887–889. [Google Scholar] [CrossRef]
- Dorocka-Bobkowska, B.; Zozulinska-Ziolkiewicz, D.; Wierusz-Wysocka, B.; Hedzelek, W.; Szumala-Kakol, A.; Budtz-Jörgensen, E. Candida-associated denture stomatitis in type 2 diabetes mellitus. Diabetes Res. Clin. Pract. 2010, 90, 81–86. [Google Scholar] [CrossRef]
- Charpentier, C.; Kottler, D.; Fite, C.; Pelletier, A.L.; Deschamps, L.; Descamps, V. A surprising granulomatous cheilitis. Gastroenterology 2018, 154, 1239–1240. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jellali, K.; Mellouki, I.; Ibrahimi, A. Cheilitis granulomatosa revealing Crohn’s disease. Pan Afr. Med. J. 2018, 30, 147. [Google Scholar] [PubMed]
- Blagec, T.; Glavina, A.; Špiljak, B.; Bešlić, I.; Bulat, V.; Lugović-Mihić, L. Cheilitis: A Cross-Sectional Study—Multiple Factors Involved in the Aetiology and Clinical Features. Oral Dis. 2022. ahead of print. [Google Scholar] [CrossRef] [PubMed]
- Balighi, K.; Daneshpazhooh, M.; Lajevardi, V.; Talebi, S.; Azizpour, A. Cheilitis in acne vulgaris patients with no previous use of systemic retionoid products. Australas. J. Dermatol. 2017, 58, 211–213. [Google Scholar] [CrossRef]
- Liu, Y.; Zhang, Q.; Hu, X.; Chen, F.; Hua, H. Characteristics of the Salivary Microbiota in Cheilitis Granulomatosa. Med. Oral Patol. Oral Cir. Bucal 2019, 24, 719–725. [Google Scholar] [CrossRef]
- Freeman, S.; Stephens, R. Cheilitis: Analysis of 75 cases referred to a contact dermatitis clinic. Am. J. Contact Dermat. 1999, 10, 198–200. [Google Scholar]
- Lim, S.W.; Goh, C.L. Epidemiology of eczematous cheilitis at a tertiary dermatological refferal centre in Singapore. Contact Dermat. 2000, 43, 322–326. [Google Scholar] [CrossRef]
- Almazrooa, S.A.; Woo, S.B.; Mawardi, H.; Treister, N. Characterization and mangement of exfoliative cheilitis: A single-centre experience. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. 2013, 116, 485–489. [Google Scholar] [CrossRef]
- Rodríguez-Blanco, I.; Flórez; Paredes-Suárez, C.; Rodríguez-Lojo, R.; González-Vilas, D.; Ramírez-Santos, A.; Paradela, S.; Conde, I.; Pereiro-Ferreirós, M. Actinic Cheilitis Prevalence and Risk Factors: A Cross-Sectional, Multicentre Study in a Population Aged 45 Years and over in North-West Spain. Acta Derm. Venereol. 2018, 98, 970–974. [Google Scholar] [CrossRef] [Green Version]
- Gheno, J.N.; Martins, M.A.T.; Munerato, M.C.; Hugo, F.N.; Sant’ana Filho, M.; Weissheimer, C.; Carrard, V.C.; Martins, M.D. Oral Mucosal Lesions and Their Association with Sociodemographic, Behavioral, and Health Status Factors. Braz. Oral Res. 2015, 29, S1806-83242015000100289. [Google Scholar] [CrossRef]
- Lopes, M.L.D.d.S.; da Silva Júnior, F.L.; Lima, K.C.; de Oliveira, P.T.; da Silveira, É.J.D. Clinicopathological Profile and Management of 161 Cases of Actinic Cheilitis. An. Bras. Dermatol. 2015, 90, 505–512. [Google Scholar] [CrossRef] [PubMed]
- Bakirtzi, K.; Papadimitriou, I.; Andreadis, D.; Sotiriou, E. Treatment Options and Post-Treatment Malignant Transformation Rate of Actinic Cheilitis: A Systematic Review. Cancers 2021, 13, 3354. [Google Scholar] [CrossRef] [PubMed]
- Vasilovici, A.; Ungureanu, L.; Grigore, L.; Cojocaru, E.; Şenilă, S. Actinic Cheilitis—From Risk Factors to Therapy. Front. Med. 2022, 9, 805425. [Google Scholar] [CrossRef] [PubMed]
- De Lucena, I.M.; Santos, I.d.S.; Daroit, N.B.; Salgueiro, A.P.; Cavagni, J.; Haas, A.N.; Rados, P.V. Sun Protection as a Protective Factor for Actinic Cheilitis: Cross-Sectional Population-Based Study. Oral Dis. 2021, 28, 1802–1810. [Google Scholar] [CrossRef] [PubMed]
- Lupu, M.; Caruntu, A.; Caruntu, C.; Boda, D.; Moraru, L.; Voiculescu, V.; Bastian, A. Non-Invasive Imaging of Actinic Cheilitis and Squamous Cell Carcinoma of the Lip. Mol. Clin. Oncol. 2018, 8, 640–646. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Brown, G.E.; Malakouti, M.; Sorenson, E.; Gupta, R.; Koo, J.Y. Psychodermatology. Adv. Psychosom. Med. 2015, 34, 123–134. [Google Scholar] [PubMed]
- Daley, T.D.; Gupta, A.K. Exfoliative cheilitis. J. Oral Pathol. Med. 1995, 24, 177–179. [Google Scholar] [CrossRef]
- Girijala, R.L.; Falkner, L.; Dalton, S.R.; Martin, B.D. Exfoliative cheilitis as a manifestation of factitial cheilitis. Cureus 2018, 10, 2565. [Google Scholar] [CrossRef] [Green Version]
- Lugović-Mihić, L.; Meštrović-Štefekov, J.; Ferček, I.; Pondeljak, N.; Lazić-Mosler, E.; Gašić, A. Atopic Dermatitis Severity, Patient Perception of the Disease, and Personality Characteristics: How Are They Related to Quality of Life? Life 2021, 11, 1434. [Google Scholar] [CrossRef]
- Fishbein, A.B.; Silverberg, J.I.; Wilson, E.J.; Ong, P.Y. Update on Atopic Dermatitis: Diagnosis, Severity Assessment, and Treatment Selection. J. Allergy Clin. Immunol. Pract. 2020, 8, 91–101. [Google Scholar] [CrossRef]
- Schmidt, D.D.; Zyzansky, S.; Ellner, J.; Kumar, M.L.; Arno, J. Stress as a precipitating factor in subjects with recurrent herpes labialis. J. Fam. Pract. 1985, 20, 359–366. [Google Scholar]
- Collet, E.; Jeudy, G.; Dalac, S. Cheilitis, perioral dermatitis and contact allergy. Eur. J. Dermatol. 2013, 23, 303–307. [Google Scholar] [CrossRef] [PubMed]
- Blagec, T.; Crnarić, I.; Homolak, D.; Pondeljak, N.; Buljan, M.; Lugović-Mihić, L. The association between allergic reactions and lip inflammatory lesions (cheilitis). Acta Clin. Croat. 2022, in press. [Google Scholar]
- O’Gorman, S.M.; Torgerson, R.R. Contact allergy in cheilitis. Int. J. Dermatol. 2016, 55, 386–391. [Google Scholar] [CrossRef] [PubMed]
- Zoli, V.; Silvani, S.; Vincenzi, C.; Tosti, A. Allergic contact cheilitis. Contact Dermat. 2006, 54, 296–297. [Google Scholar] [CrossRef]
- Bakula, A.; Lugović-Mihić, L.; Šitum, M.; Turčin, J.; Sinković, A. Contact allergy in the mouth: Diversity of clinical presentations and diagnosis of common allergens relevant to dental practice. Acta Clin. Croat. 2011, 50, 553–561. [Google Scholar]
- Kim, T.W.; Kim, W.I.; Mun, J.H.; Song, M.; Kim, H.S.; Kim, B.S.; Kim, M.B.; Ko, H.C. Patch testing with dental screening series in oral disease. Ann. Dermatol. 2015, 27, 389–393. [Google Scholar] [CrossRef]
- Budimir, J.; Mravak-Stipetić, M.; Bulat, V.; Ferček, I.; Japundžić, I.; Lugović-Mihić, L. Allergic reactions in oral and perioral diseases- what do allergy skin test results show? Oral Surg. Oral Med. Oral Pathol. Oral Radiol. 2019, 127, 40–48. [Google Scholar] [CrossRef]
- Khamaysi, Z.; Bergman, R.; Weltfriend, S. Positive patch test reactions to allergens of the dental series and the relation to the clinical presentations. Contact Dermat. 2006, 55, 216–218. [Google Scholar] [CrossRef]
- Critchlow, W.A.; Chang, D. Cheilitis granulomatosa: A review. Head Neck Pathol. 2014, 8, 209–213. [Google Scholar] [CrossRef]
- Torgerson, R.R.; Davis, M.D.P.; Bruce, A.J.; Farmer, S.A.; Rogers, R.S., 3rd. Contact allergy in oral disease. J. Am. Acad. Dermatol. 2007, 57, 315–321. [Google Scholar] [CrossRef] [PubMed]
- Cheng, H.S.; Konya, J.; Lobel, E.; Fernandez-Penas, P. Patch testing for cheilitis: A 10-year series. Dermatitis 2019, 30, 347–351. [Google Scholar] [CrossRef] [PubMed]
- Tomljanović-Veselski, M.; Jovanović, I. Najčešći kontaktni alergeni u bolesnika s kontaktnim dermatitisima u području Slavonskog Broda. Med. Jadertina 2006, 36, 45–52. [Google Scholar]
- Domić, I.; Budimir, J.; Novak, I.; Mravak-Stipetić, M.; Lugović-Mihić, L. Assessment of allergies to food and additives in patients with angioedema, burning mouth syndrome, cheilitis, gingivostomatitis, oral lichenoid reactions, and perioral dermatitis. Acta Clin. Croat. 2021, 60, 276–281. [Google Scholar] [CrossRef]
- Holmes, G.; Freeman, S. Cheilitis caused by contact urticaria to mint flavoured toothpaste. Australas J. Dermatol. 2001, 42, 43–45. [Google Scholar] [CrossRef] [PubMed]
- Schlosser, B.J.; Pirigyi, M.; Mirowski, G.W. Oral manifestations of hematologic and nutritional diseases. Otolaryngol. Clin. N. Am. 2011, 44, 183–203. [Google Scholar] [CrossRef] [PubMed]
- Baumgardner, D.J. Oral Fungal Microbiota: To Thrush and Beyond. J. Patient Cent. Res. Rev. 2019, 6, 252–261. [Google Scholar] [CrossRef] [Green Version]
- Kaur, S.; Goraya, J.S. Dermatologic findings of vitamin B(12) deiciency in infants. Pediatr. Dermatol. 2018, 35, 796–799. [Google Scholar] [CrossRef]
- Glutsch, V.; Hamm, H.; Goebeler, M. Zinc and Skin: An Update. J. Dtsch. Dermatol. Ges. 2019, 17, 589–596. [Google Scholar] [CrossRef] [Green Version]
- Gürtler, A.; Laurenz, S. The Impact of Clinical Nutrition on Inflammatory Skin Diseases. J. Dtsch. Dermatol. 2022, 20, 185–202. [Google Scholar] [CrossRef]
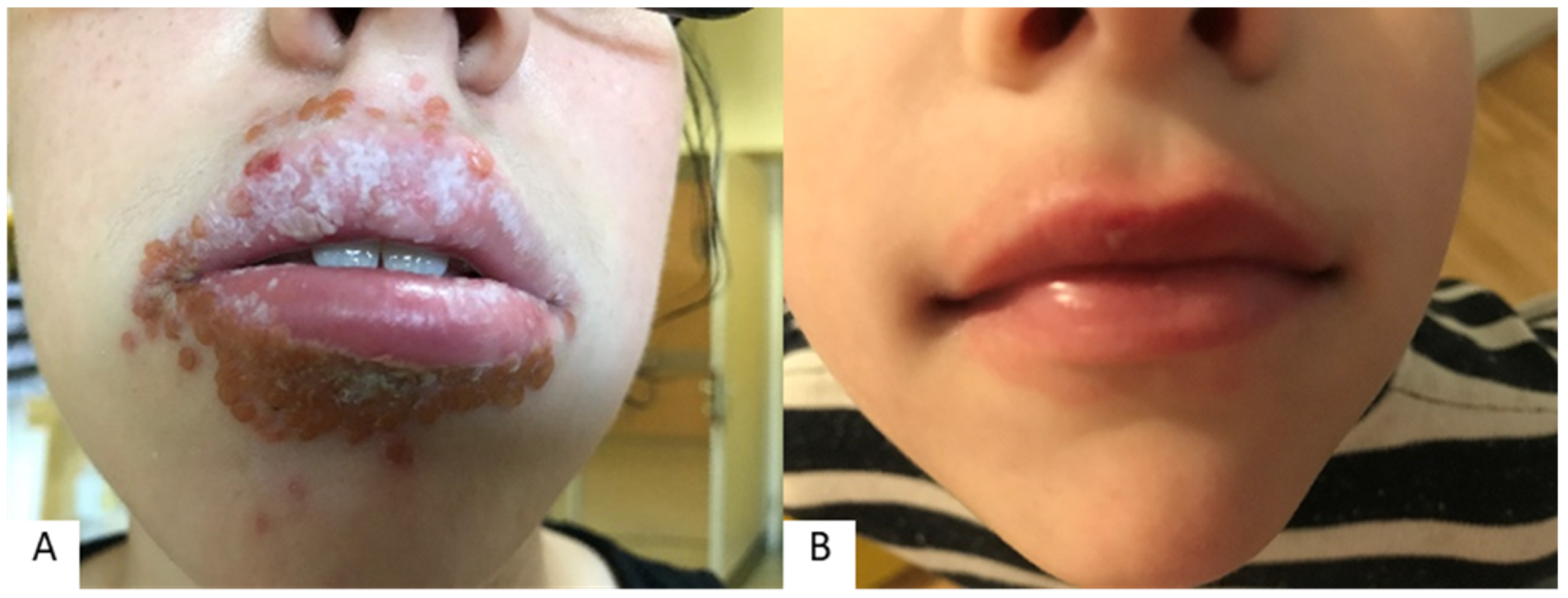


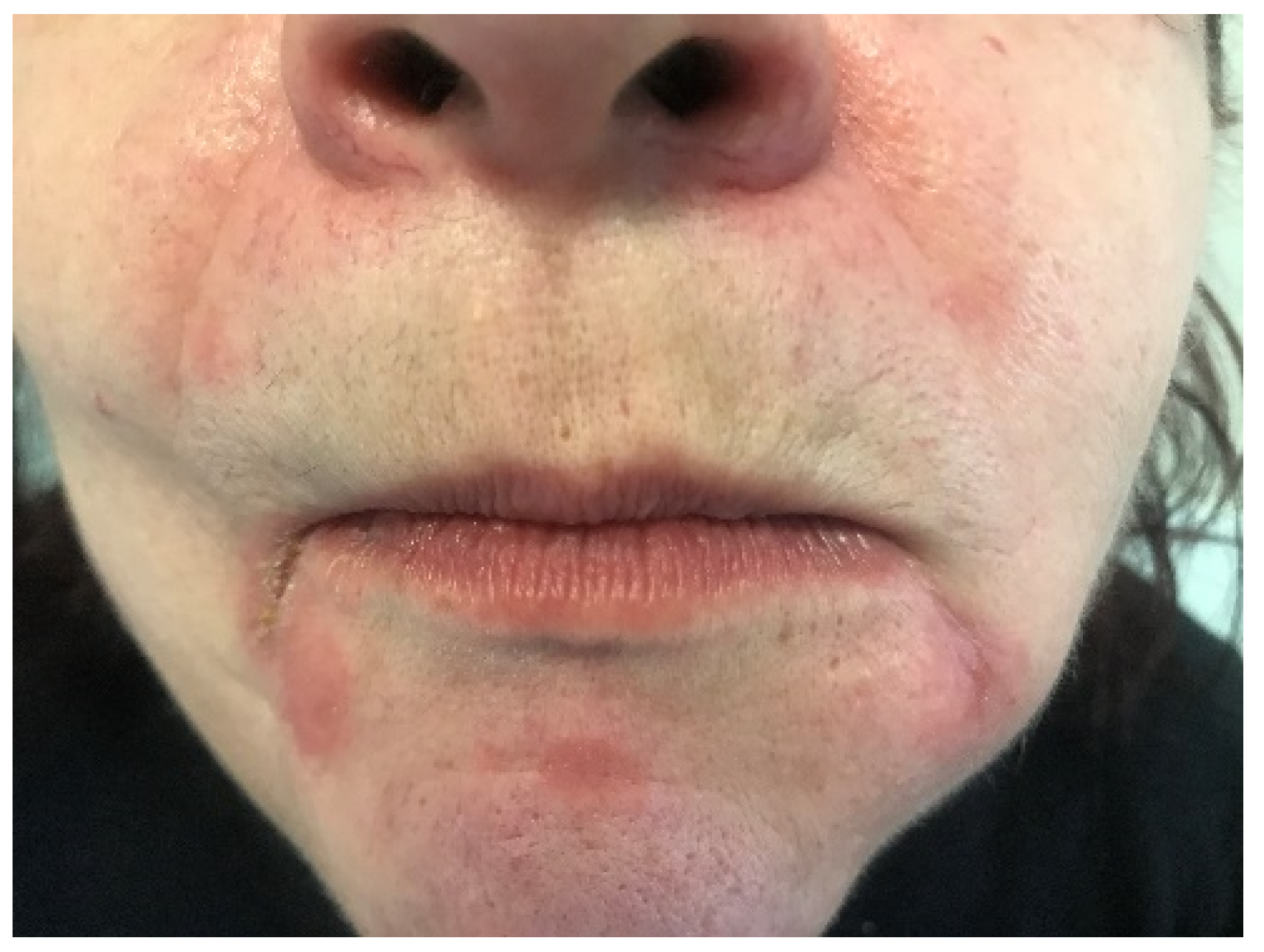
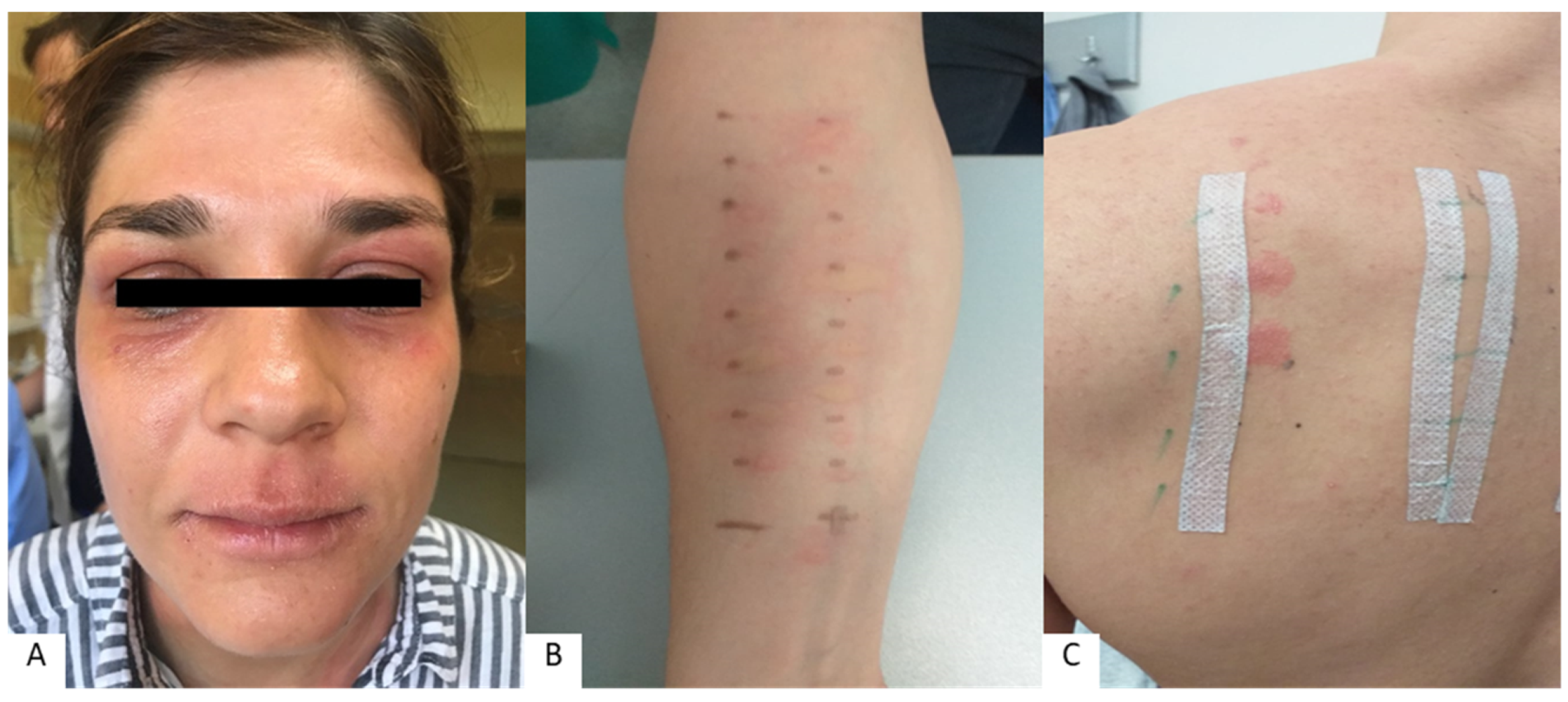
| Lip Inflammation | Perioral Dermatitis | |
|---|---|---|
| Involved factors |
|
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lugović-Mihić, L.; Špiljak, B.; Blagec, T.; Delaš Aždajić, M.; Franceschi, N.; Gašić, A.; Parać, E. Factors Participating in the Occurrence of Inflammation of the Lips (Cheilitis) and Perioral Skin. Cosmetics 2023, 10, 9. https://doi.org/10.3390/cosmetics10010009
Lugović-Mihić L, Špiljak B, Blagec T, Delaš Aždajić M, Franceschi N, Gašić A, Parać E. Factors Participating in the Occurrence of Inflammation of the Lips (Cheilitis) and Perioral Skin. Cosmetics. 2023; 10(1):9. https://doi.org/10.3390/cosmetics10010009
Chicago/Turabian StyleLugović-Mihić, Liborija, Bruno Špiljak, Tadeja Blagec, Marija Delaš Aždajić, Nika Franceschi, Ana Gašić, and Ena Parać. 2023. "Factors Participating in the Occurrence of Inflammation of the Lips (Cheilitis) and Perioral Skin" Cosmetics 10, no. 1: 9. https://doi.org/10.3390/cosmetics10010009
APA StyleLugović-Mihić, L., Špiljak, B., Blagec, T., Delaš Aždajić, M., Franceschi, N., Gašić, A., & Parać, E. (2023). Factors Participating in the Occurrence of Inflammation of the Lips (Cheilitis) and Perioral Skin. Cosmetics, 10(1), 9. https://doi.org/10.3390/cosmetics10010009








