3D Printed Hollow Microneedles for Treating Skin Wrinkles Using Different Anti-Wrinkle Agents: A Possible Futuristic Approach
Abstract
:1. Introduction
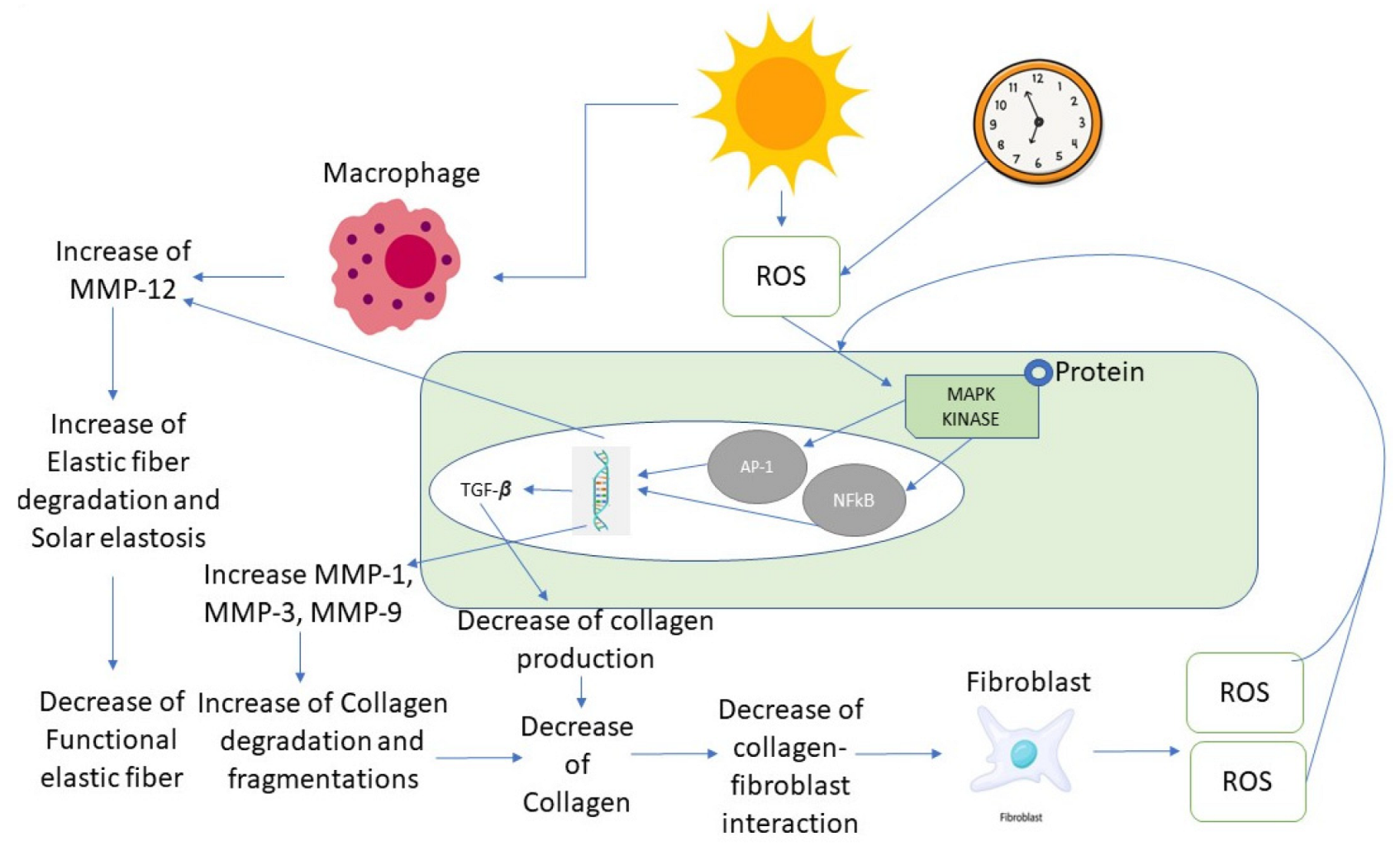
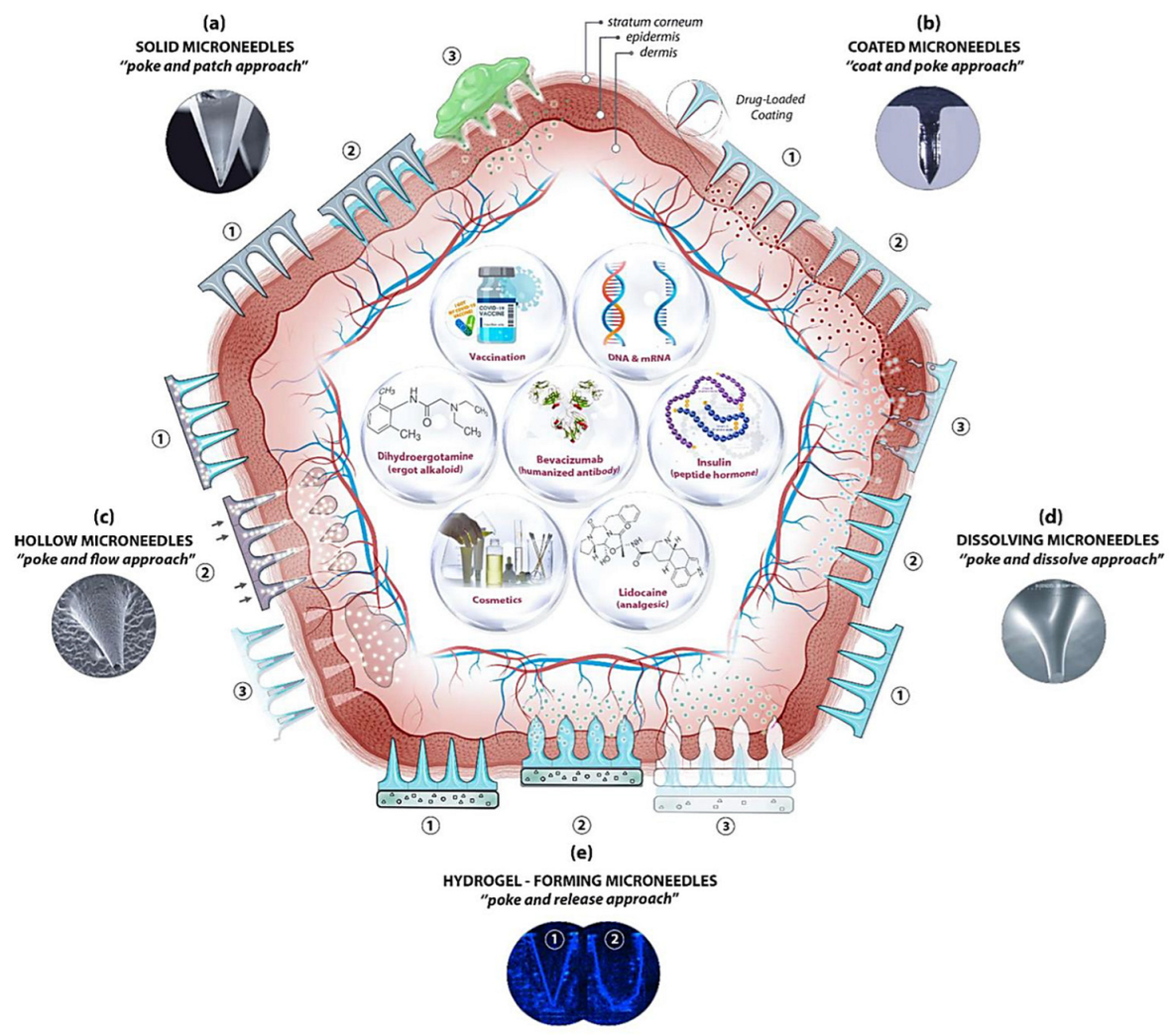
Microneedle-Mediated Anti−Wrinkle Therapy
2. Scope of Treatment for Skin Wrinkles
3. The Potential of HMNs to Treat Skin Wrinkles
4. Fabrication of 3D Printed HMNs
5. Techniques Involved in 3D Printed HMNs
6. Future Directions
7. Discussion
8. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Hamer, M.A.; Pardo, L.M.; Jacobs, L.C.; Deelen, J.; Uitterlinden, A.G.; Slagboom, E.; van Heemst, D.; Uh, H.-W.; Beekman, M.; Kayser, M.; et al. Facial Wrinkles in Europeans: A Genome-Wide Association Study. J. Investig. Dermatol. 2018, 138, 1877–1880. [Google Scholar] [CrossRef] [PubMed]
- Sandulescu, T.; Franzmann, M.; Jast, J.; Blaurock-Sandulescu, T.; Spilker, L.; Klein, C.; Naumova, E.A.; Arnold, W.H. Facial fold and crease development: A new morphological approach and classification. Clin. Anat. 2019, 32, 573–584. [Google Scholar] [CrossRef] [PubMed]
- Addor, F.A.S. Beyond photoaging: Additional factors involved in the process of skin aging. Clin. Cosmet. Investig. Dermatol. 2018, 11, 437–443. [Google Scholar] [CrossRef] [PubMed]
- Strnadova, K.; Sandera, V.; Dvorankova, B.; Kodet, O.; Duskova, M.; Smetana, K.; Lacina, L. Skin aging: The dermal perspective. Clin. Dermatol. 2019, 37, 326–335. [Google Scholar] [CrossRef]
- Singh, M.; Griffiths, C.E.M. The use of retinoids in the treatment of photoaging. Dermatol. Ther. 2006, 19, 297–305. [Google Scholar] [CrossRef]
- Huang, A.H.; Chien, A.L. Photoaging: A Review of Current Literature. Curr. Dermatol. Rep. 2020, 9, 22–29. [Google Scholar] [CrossRef]
- Yasui, T.; Yonetsu, M.; Tanaka, R.; Tanaka, Y.; Fukushima, S.-I.; Yamashita, T.; Ogura, Y.; Hirao, T.; Murota, H.; Araki, T. In vivo observation of age-related structural changes of dermal collagen in human facial skin using collagen-sensitive second harmonic generation microscope equipped with 1250-nm mode-locked Cr:Forsterite laser. J. Biomed. Opt. 2012, 18, 031108. [Google Scholar] [CrossRef]
- Fang, J.-Y.; Wang, P.-W.; Huang, C.-H.; Chen, M.-H.; Wu, Y.-R.; Pan, T.-L. Skin aging caused by intrinsic or extrinsic processes characterized with functional proteomics. Proteomics 2016, 16, 2718–2731. [Google Scholar] [CrossRef]
- El-Domyati, M.; Attia, S.; Saleh, F.; Brown, D.; Birk, D.E.; Gasparro, F.; Ahmad, H.; Uitto, J. Intrinsic aging vs. photoaging: A comparative histopathological, immunohistochemical, and ultrastructural study of skin. Exp. Dermatol. 2002, 11, 398–405. [Google Scholar] [CrossRef]
- Zouboulis, C.C.; Makrantonaki, E.; Nikolakis, G. When the skin is in the center of interest: An aging issue. Clin. Dermatol. 2019, 37, 296–305. [Google Scholar] [CrossRef]
- Chien, A.L.; Suh, J.; Cesar, S.S.A.; Fischer, A.H.; Cheng, N.; Poon, F.; Rainer, B.; Leung, S.; Martin, J.; Okoye, G.A.; et al. Pigmentation in African American skin decreases with skin aging. J. Am. Acad. Dermatol. 2016, 75, 782–787. [Google Scholar] [CrossRef] [PubMed]
- Alexis, A.F.; Obioha, J.O. Ethnicity and Aging Skin. J. Drugs Dermatol. 2017, 16, s77–s80. [Google Scholar]
- Hwang, W.; Kim, D.; Kwon, O.S.; Kim, Y.-S.; Ahn, B.; Kang, N.-G. Topical application of Zanthoxylum piperitum extract improves lateral canthal rhytides by inhibiting muscle contractions. Sci. Rep. 2020, 10, 21514. [Google Scholar] [CrossRef] [PubMed]
- Zouboulis, C.C.; Ganceviciene, R.; Liakou, A.I.; Theodoridis, A.; Elewa, R.; Makrantonaki, E. Aesthetic aspects of skin aging, prevention, and local treatment. Clin. Dermatol. 2019, 37, 365–372. [Google Scholar] [CrossRef] [PubMed]
- Shin, J.-W.; Kwon, S.-H.; Choi, J.-Y.; Na, J.-I.; Huh, C.-H.; Choi, H.-R.; Park, K.-C. Molecular Mechanisms of Dermal Aging and Antiaging Approaches. Int. J. Mol. Sci. 2019, 20, 2126. [Google Scholar] [CrossRef]
- Chavoshnejad, P.; More, S.; Razavi, M.J. From surface microrelief to big wrinkles in skin: A mechanical in-silico model. Extreme Mech. Lett. 2020, 36, 100647. [Google Scholar] [CrossRef]
- Ganceviciene, R.; Liakou, A.I.; Theodoridis, A.; Makrantonaki, E.; Zouboulis, C.C. Skin anti-aging strategies. Derm. Endocrinol. 2012, 4, 308–319. [Google Scholar] [CrossRef]
- Laronha, H.; Caldeira, J. Structure and Function of Human Matrix Metalloproteinases. Cells 2020, 9, 1076. [Google Scholar] [CrossRef]
- Verma, R.P.; Hansch, C. Matrix metalloproteinases (MMPs): Chemical–biological functions and (Q)SARs. Bioorg. Med. Chem. 2007, 15, 2223–2268. [Google Scholar] [CrossRef]
- Quan, T.; Fisher, G.J. Role of Age-Associated Alterations of the Dermal Extracellular Matrix Microenvironment in Human Skin Aging: A Mini-Review. Gerontology 2015, 61, 427–434. [Google Scholar] [CrossRef]
- Fisher, G.J.; Varani, J.; Voorhees, J.J. Looking Older: Fibroblast Collapse and Therapeutic Implications. Arch. Dermatol. 2008, 144, 666–672. [Google Scholar] [CrossRef] [PubMed]
- Nagase, H.; Visse, R.; Murphy, G. Structure and function of matrix metalloproteinases and TIMPs. Cardiovasc. Res. 2006, 69, 562–573. [Google Scholar] [CrossRef] [PubMed]
- Newby, A.C. Dual Role of Matrix Metalloproteinases (Matrixins) in Intimal Thickening and Atherosclerotic Plaque Rupture. Physiol. Rev. 2005, 85, 1–31. [Google Scholar] [CrossRef] [PubMed]
- Yokose, U.; Hachiya, A.; Sriwiriyanont, P.; Fujimura, T.; Visscher, M.O.; Kitzmiller, W.J.; Bello, A.; Tsuboi, R.; Kitahara, T.; Kobinger, G.P.; et al. The Endogenous Protease Inhibitor TIMP-1 Mediates Protection and Recovery from Cutaneous Photodamage. J. Investig. Dermatol. 2012, 132, 2800–2809. [Google Scholar] [CrossRef] [PubMed]
- Golden, T.R.; Hinerfeld, D.A.; Melov, S. Oxidative stress and aging: Beyond correlation. Aging Cell 2002, 1, 117–123. [Google Scholar] [CrossRef]
- Brennan, M.; Bhatti, H.; Nerusu, K.C.; Bhagavathula, N.; Kang, S.; Fisher, G.J.; Varani, J.; Voorhees, J.J. Matrix Metalloproteinase-1 is the Major Collagenolytic Enzyme Responsible for Collagen Damage in UV-irradiated Human Skin. Photochem. Photobiol. 2003, 78, 43–48. [Google Scholar] [CrossRef]
- Quan, T.; Little, E.; Quan, H.; Qin, Z.; Voorhees, J.J.; Fisher, G.J. Elevated Matrix Metalloproteinases and Collagen Fragmentation in Photodamaged Human Skin: Impact of Altered Extracellular Matrix Microenvironment on Dermal Fibroblast Function. J. Investig. Dermatol. 2013, 133, 1362–1366. [Google Scholar] [CrossRef]
- Qin, Z.; Balimunkwe, R.; Quan, T. Age-related reduction of dermal fibroblast size upregulates multiple matrix metalloproteinases as observed in aged human skin in vivo. Br. J. Dermatol. 2017, 177, 1337–1348. [Google Scholar] [CrossRef]
- Tewari, A.; Grys, K.; Kollet, J.; Sarkany, R.; Young, A.R. Upregulation of MMP12 and Its Activity by UVA1 in Human Skin: Potential Implications for Photoaging. J. Investig. Dermatol. 2014, 134, 2598–2609. [Google Scholar] [CrossRef]
- Parkinson, L.G.; Toro, A.; Zhao, H.; Brown, K.; Tebbutt, S.J.; Granville, D.J. Granzyme B mediates both direct and indirect cleavage of extracellular matrix in skin after chronic low-dose ultraviolet light irradiation. Aging Cell 2015, 14, 67–77. [Google Scholar] [CrossRef]
- Kim, J.; Lee, C.-W.; Kim, E.K.; Lee, S.-J.; Park, N.-H.; Kim, H.-S.; Kim, H.-K.; Char, K.; Jang, Y.P.; Kim, J.-W. Inhibition effect of Gynura procumbens extract on UV-B-induced matrix-metalloproteinase expression in human dermal fibroblasts. J. Ethnopharmacol. 2011, 137, 427–433. [Google Scholar] [CrossRef] [PubMed]
- Chiang, H.-M.; Chen, H.-C.; Chiu, H.-H.; Chen, C.-W.; Wang, S.-M.; Wen, K.-C. Neonauclea reticulata (Havil.) Merr Stimulates Skin Regeneration after UVB Exposure via ROS Scavenging and Modulation of the MAPK/MMPs/Collagen Pathway. Evidence-Based Complement. Altern. Med. 2013, 2013, 324864. [Google Scholar] [CrossRef] [PubMed]
- Shaulian, E.; Karin, M. AP-1 as a regulator of cell life and death. Nat. Cell Biol. 2002, 4, E131–E136. [Google Scholar] [CrossRef] [PubMed]
- Park, J.-E.; Pyun, H.-B.; Woo, S.W.; Jeong, J.-H.; Hwang, J.-K. The protective effect of Kaempferia parviflora extract on UVB-induced skin photoaging in hairless mice. Photodermatol. Photoimmunol. Photomed. 2014, 30, 237–245. [Google Scholar] [CrossRef] [PubMed]
- Quan, T.; Shao, Y.; He, T.; Voorhees, J.J.; Fisher, G.J. Reduced Expression of Connective Tissue Growth Factor (CTGF/CCN2) Mediates Collagen Loss in Chronologically Aged Human Skin. J. Investig. Dermatol. 2010, 130, 415–424. [Google Scholar] [CrossRef]
- Sun, Z.-W.; Hwang, E.; Lee, H.J.; Lee, T.Y.; Song, H.G.; Park, S.-Y.; Shin, H.-S.; Lee, D.-G.; Yi, T.H. Effects of Galla chinensis extracts on UVB-irradiated MMP-1 production in hairless mice. J. Nat. Med. 2015, 69, 22–34. [Google Scholar] [CrossRef]
- Chen, B.; Li, R.; Yan, N.; Chen, G.; Qian, W.; Jiang, H.-L.; Ji, C.; Bi, Z.-G. Astragaloside IV controls collagen reduction in photoaging skin by improving transforming growth factor-β/Smad signaling suppression and inhibiting matrix metalloproteinase-1. Mol. Med. Rep. 2015, 11, 3344–3348. [Google Scholar] [CrossRef]
- Cipriani, E.; Bernardi, S.; Continenza, M.A. Wrinkles: Origins and Treatments. Adv. Cosmet. Dermatol. 2016, 2, 01–07. [Google Scholar]
- Farage, M.A.; Miller, K.W.; Berardesca, E.; Maibach, H.I. Psychological and Social Implications of Aging Skin: Normal Aging and the Effects of Cutaneous Disease. In Textbook of Aging Skin; Springer: Berlin/Heidelberg, Germany, 2015; pp. 1–14. [Google Scholar] [CrossRef]
- Atiyeh, B.S.; Ghanem, O.A.; Chahine, F. Microneedling: Percutaneous Collagen Induction (PCI) Therapy for Management of Scars and Photoaged Skin—Scientific Evidence and Review of the Literature. Aesthetic Plast. Surg. 2021, 45, 296–308. [Google Scholar] [CrossRef]
- He, M.; Yang, G.; Zhang, S.; Zhao, X.; Gao, Y. Dissolving Microneedles Loaded With Etonogestrel Microcrystal Particles for Intradermal Sustained Delivery. J. Pharm. Sci. 2018, 107, 1037–1045. [Google Scholar] [CrossRef]
- Jung, G.S.; Kim, H.S. A Novel Technique to Reduce Pain from Intradermal Injection of Botulinum Toxin Type A. Plast. Reconstr. Surg. Glob. Open 2021, 9, e3417. [Google Scholar] [CrossRef] [PubMed]
- Hassan, J.; Haigh, C.; Ahmed, T.; Uddin, J.; Das, D.B. Potential of Microneedle Systems for COVID-19 Vaccination: Current Trends and Challenges. Pharmaceutics 2022, 14, 1066. [Google Scholar] [CrossRef] [PubMed]
- Uddin, J.; Hassan, J.; Douroumis, D. Thermal Inkjet Printing: Prospects and Applications in the Development of Medicine. Technologies 2022, 10, 108. [Google Scholar] [CrossRef]
- Zhang, P.; Dalton, C.; Jullien, G.A. Design and fabrication of MEMS-based microneedle arrays for medical applications. Microsyst. Technol. 2009, 15, 1073–1082. [Google Scholar] [CrossRef]
- Lee, H.-Y.; Jeon, Y.; Kim, Y.K.; Jang, J.Y.; Cho, Y.S.; Bhak, J.; Cho, K.-H. Identifying molecular targets for reverse aging using integrated network analysis of transcriptomic and epigenomic changes during aging. Sci. Rep. 2021, 11, 12317. [Google Scholar] [CrossRef]
- Amani, H.; Shahbazi, M.-A.; D’Amico, C.; Fontana, F.; Abbaszadeh, S.; Santos, H.A. Microneedles for painless transdermal immunotherapeutic applications. J. Control. Release 2021, 330, 185–217. [Google Scholar] [CrossRef]
- Anjani, Q.K.; Permana, A.D.; Cárcamo-Martínez, Á.; Domínguez-Robles, J.; Tekko, I.A.; Larrañeta, E.; Vora, L.K.; Ramadon, D.; Donnelly, R.F. Versatility of hydrogel-forming microneedles in in vitro transdermal delivery of tuberculosis drugs. Eur. J. Pharm. Biopharm. 2021, 158, 294–312. [Google Scholar] [CrossRef]
- Turner, J.G.; White, L.R.; Estrela, P.; Leese, H.S. Hydrogel-Forming Microneedles: Current Advancements and Future Trends. Macromol. Biosci. 2021, 21, e2000307. [Google Scholar] [CrossRef]
- Nagarkar, R.; Singh, M.; Nguyen, H.X.; Jonnalagadda, S. A review of recent advances in microneedle technology for transdermal drug delivery. J. Drug Deliv. Sci. Technol. 2020, 59, 101923. [Google Scholar] [CrossRef]
- Ramadon, D.; McCrudden, M.T.C.; Courtenay, A.J.; Donnelly, R.F. Enhancement Strategies for Transdermal Drug Delivery Systems: Current Trends and Applications. Drug Deliv. Transl. Res. 2021, 12, 758–791. [Google Scholar] [CrossRef]
- Al-Japairai, K.A.S.; Mahmood, S.; Almurisi, S.H.; Venugopal, J.R.; Hilles, A.R.; Azmana, M.; Raman, S. Current trends in polymer microneedle for transdermal drug delivery. Int. J. Pharm. 2020, 587, 119673. [Google Scholar] [CrossRef]
- Yang, Q.; Zhong, W.; Xu, L.; Li, H.; Yan, Q.; She, Y.; Yang, G. Recent progress of 3D-printed microneedles for transdermal drug delivery. Int. J. Pharm. 2021, 593, 120106. [Google Scholar] [CrossRef] [PubMed]
- Jang, M.; Baek, S.; Kang, G.; Yang, H.; Kim, S.; Jung, H. Dissolving microneedle with high molecular weight hyaluronic acid to improve skin wrinkles, dermal density and elasticity. Int. J. Cosmet. Sci. 2020, 42, 302–309. [Google Scholar] [CrossRef] [PubMed]
- Nestor, M.S.; Ablon, G.; Gade, A.; Han, H.; Fischer, D.L.; Do, A.G.; Do, H.H.; Do, M.D.L.F. Treatment options for androgenetic alopecia: Efficacy, side effects, compliance, financial considerations, and ethics. J. Cosmet. Dermatol. 2021, 20, 3759–3781. [Google Scholar] [CrossRef] [PubMed]
- Olowe, M.; Parupelli, S.K.; Desai, S. A Review of 3D-Printing of Microneedles. Pharmaceutics 2022, 14, 2693. [Google Scholar] [CrossRef]
- Hong, J.Y.; Ko, E.J.; Choi, S.Y.; Li, K.; Kim, A.R.; O Park, J.; Kim, B.J. Efficacy and safety of a novel, soluble microneedle patch for the improvement of facial wrinkle. J. Cosmet. Dermatol. 2018, 17, 235–241. [Google Scholar] [CrossRef]
- Lim, S.H.; Tiew, W.J.; Zhang, J.; Ho, P.C.-L.; Kachouie, N.N.; Kang, L. Geometrical optimisation of a personalised microneedle eye patch for transdermal delivery of anti-wrinkle small peptide. Biofabrication 2020, 12, 035003. [Google Scholar] [CrossRef]
- Ita, K. Dissolving microneedles for transdermal drug delivery: Advances and challenges. Biomed. Pharmacother. 2017, 93, 1116–1127. [Google Scholar] [CrossRef]
- Sadowski, G.; Sadowski, J. Safety and Efficacy of a Novel Antiaging Skin Care Regimen Containing Neutraceuticals and Growth Factors on the Facial Skin of Women: A 12-Week Open-label Study. J. Clin. Aesthetic Dermatol. 2020, 13, 24–34. [Google Scholar]
- Yang, H.; Kim, S.; Jang, M.; Kim, H.; Lee, S.; Kim, Y.; Eom, Y.A.; Kang, G.; Chiang, L.; Baek, J.H.; et al. Two-phase delivery using a horse oil and adenosine-loaded dissolving microneedle patch for skin barrier restoration, moisturization, and wrinkle improvement. J. Cosmet. Dermatol. 2019, 18, 936–943. [Google Scholar] [CrossRef]
- Zvezdin, V.; Kasatkina, T.; Kasatkin, I.; Gavrilova, M.; Kazakova, O. Microneedle patch based on dissolving, detachable microneedle technology for improved skin quality of the periorbital region. Part 2: Clinical Evaluation. Int. J. Cosmet. Sci. 2020, 42, 429–435. [Google Scholar] [CrossRef] [PubMed]
- Lim, S.H.; Kathuria, H.; Bin Amir, M.H.; Zhang, X.; Duong, H.T.; Ho, P.C.-L.; Kang, L. High resolution photopolymer for 3D printing of personalised microneedle for transdermal delivery of anti-wrinkle small peptide. J. Control. Release 2021, 329, 907–918. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.; Yang, H.; Kim, M.; Baek, J.H.; Kim, S.J.; An, S.M.; Koh, J.S.; Seo, R.; Jung, H. 4-n-butylresorcinol dissolving microneedle patch for skin depigmentation: A randomized, double-blind, placebo-controlled trial. J. Cosmet. Dermatol. 2016, 15, 16–23. [Google Scholar] [CrossRef] [PubMed]
- Lee, C.; Yang, H.; Kim, S.; Kim, M.; Kang, H.; Kim, N.; An, S.; Koh, J.; Jung, H. Evaluation of the anti-wrinkle effect of an ascorbic acid-loaded dissolving microneedle patch via a double-blind, placebo-controlled clinical study. Int. J. Cosmet. Sci. 2016, 38, 375–381. [Google Scholar] [CrossRef] [PubMed]
- Kapoor, Y.; Milewski, M.; Dick, L.; Zhang, J.; Bothe, J.R.; Gehrt, M.; Manser, K.; Nissley, B.; Petrescu, I.; Johnson, P.; et al. Coated microneedles for transdermal delivery of a potent pharmaceutical peptide. Biomed. Microdevices 2019, 22, 7. [Google Scholar] [CrossRef]
- Lee, A.-R.C. Microneedle-mediated delivery of cosmeceutically relevant nucleoside and peptides in human skin: Challenges and strategies for dermal delivery. J. Pharm. Investig. 2019, 49, 587–601. [Google Scholar] [CrossRef]
- Pinsky, M.A. Efficacy and Safety of an Anti-aging Technology for the Treatment of Facial Wrinkles and Skin Moisturization. J. Clin. Aesthetic Dermatol. 2017, 10, 27–35. [Google Scholar]
- Dillon, C.; Hughes, H.; O’Reilly, N.J.; Allender, C.J.; Barrow, D.A.; McLoughlin, P. Dissolving microneedle based transdermal delivery of therapeutic peptide analogues. Int. J. Pharm. 2019, 565, 9–19. [Google Scholar] [CrossRef]
- Avcil, M.; Akman, G.; Klokkers, J.; Jeong, D.; Çelik, A. Efficacy of bioactive peptides loaded on hyaluronic acid microneedle patches: A monocentric clinical study. J. Cosmet. Dermatol. 2020, 19, 328–337. [Google Scholar] [CrossRef]
- Elahpour, N.; Pahlevanzadeh, F.; Kharaziha, M.; Bakhsheshi-Rad, H.R.; Ramakrishna, S.; Berto, F. 3D printed microneedles for transdermal drug delivery: A brief review of two decades. Int. J. Pharm. 2021, 597, 120301. [Google Scholar] [CrossRef]
- Cárcamo-Martínez, Á.; Mallon, B.; Domínguez-Robles, J.; Vora, L.K.; Anjani, Q.K.; Donnelly, R.F. Hollow microneedles: A perspective in biomedical applications. Int. J. Pharm. 2021, 599, 120455. [Google Scholar] [CrossRef] [PubMed]
- Hoang, H.T.; Moon, J.-Y.; Lee, Y.-C. Natural Antioxidants from Plant Extracts in Skincare Cosmetics: Recent Applications, Challenges and Perspectives. Cosmetics 2021, 8, 106. [Google Scholar] [CrossRef]
- Zhang, S.; Duan, E. Fighting against Skin Aging: The Way from Bench to Bedside. Cell Transplant. 2018, 27, 729–738. [Google Scholar] [CrossRef]
- Taofiq, O.; González-Paramás, A.M.; Barreiro, M.F.; Ferreira, I.C.F.R. Hydroxycinnamic Acids and Their Derivatives: Cosmeceutical Significance, Challenges and Future Perspectives, a Review. Molecules 2017, 22, 281. [Google Scholar] [CrossRef] [PubMed]
- Sapra, P.; Demay, S.; Sapra, S.; Khanna, J.; Mraud, K.; Bonadonna, J. A Single-blind, Split-face, Randomized, Pilot Study Comparing the Effects of Intradermal and Intramuscular Injection of Two Commercially Available Botulinum Toxin A Formulas to Reduce Signs of Facial Aging. J. Clin. Aesthetic Dermatol. 2017, 10, 34–44. [Google Scholar]
- Beigvand, H.H.; Razzaghi, M.; Rostami-Nejad, M.; Rezaei-Tavirani, M.; Safari, S.; Rezaei-Tavirani, M.; Mansouri, V.; Heidari, M.H. Assessment of Laser Effects on Skin Rejuvenation. J. Lasers Med. Sci. 2020, 11, 212–219. [Google Scholar] [CrossRef] [PubMed]
- Zasada, M.; Budzisz, E. Retinoids: Active molecules influencing skin structure formation in cosmetic and dermatological treatments. Adv. Dermatol. Allergol. Postępy Dermatol. Alergol. 2019, 36, 392–397. [Google Scholar] [CrossRef]
- Satriyasa, B.K. Botulinum toxin (Botox) A for reducing the appearance of facial wrinkles: A literature review of clinical use and pharmacological aspect. Clin. Cosmet. Investig. Dermatol. 2019, 12, 223–228. [Google Scholar] [CrossRef] [PubMed]
- Rona, C.; Vailati, F.; Berardesca, E. The cosmetic treatment of wrinkles. J. Cosmet. Dermatol. 2004, 3, 26–34. [Google Scholar] [CrossRef]
- Ferreira, M.S.; Magalhães, M.C.; Sousa-Lobo, J.M.; Almeida, I.F. Trending Anti-Aging Peptides. Cosmetics 2020, 7, 91. [Google Scholar] [CrossRef]
- Kim, H.; Kim, N.; Jung, S.; Mun, J.; Kim, J.; Kim, B.; Lee, J.; Ryoo, H.; Jung, H. Improvement in skin wrinkles from the use of photostable retinyl retinoate: A randomized controlled trial. Br. J. Dermatol. 2010, 162, 497–502. [Google Scholar] [CrossRef] [PubMed]
- Xu, T.-H.; Chen, J.Z.S.; Li, Y.-H.; Wu, Y.; Luo, Y.-J.; Gao, X.-H.; Chen, H.-D. Split-face study of topical 23.8% L-ascorbic acid serum in treating photo-aged skin. J. Drugs Dermatol. 2012, 11, 51–56. [Google Scholar] [PubMed]
- Bukhari, S.N.A.; Roswandi, N.L.; Waqas, M.; Habib, H.; Hussain, F.; Khan, S.; Sohail, M.; Ramli, N.A.; Thu, H.E.; Hussain, Z. Hyaluronic acid, a promising skin rejuvenating biomedicine: A review of recent updates and pre-clinical and clinical investigations on cosmetic and nutricosmetic effects. Int. J. Biol. Macromol. 2018, 120, 1682–1695. [Google Scholar] [CrossRef] [PubMed]
- Lee, D.H.; Oh, I.Y.; Koo, K.T.; Suk, J.M.; Jung, S.W.; Park, J.O.; Kim, B.J.; Choi, Y.M. Improvement in skin wrinkles using a preparation containing human growth factors and hyaluronic acid serum. J. Cosmet. Laser Ther. 2015, 17, 20–23. [Google Scholar] [CrossRef]
- Gold, M.H. Use of hyaluronic acid fillers for the treatment of the aging face. Clin. Interv. Aging 2007, 2, 369–376. [Google Scholar] [CrossRef]
- Abella, M. Evaluation of anti-wrinkle efficacy of adenosine-containing products using the FOITS technique. Int. J. Cosmet. Sci. 2006, 28, 447–451. [Google Scholar] [CrossRef]
- Kang, G.; Tu, T.N.T.; Kim, S.; Yang, H.; Jang, M.; Jo, D.; Ryu, J.; Baek, J.; Jung, H. Adenosine-loaded dissolving microneedle patches to improve skin wrinkles, dermal density, elasticity and hydration. Int. J. Cosmet. Sci. 2018, 40, 199–206. [Google Scholar] [CrossRef]
- Lee, C.; Eom, Y.A.; Yang, H.; Jang, M.; Jung, S.U.; Park, Y.O.; Lee, S.E.; Jung, H. Skin Barrier Restoration and Moisturization Using Horse Oil-Loaded Dissolving Microneedle Patches. Ski. Pharmacol. Physiol. 2018, 31, 163–171. [Google Scholar] [CrossRef]
- Tadini, K.A.; Mercurio, D.G.; Campos, P.M.B.G.M. Acetyl hexapeptide-3 in a cosmetic formulation acts on skin mechanical properties—clinical study. Braz. J. Pharm. Sci. 2015, 51, 901–909. [Google Scholar] [CrossRef]
- An, J.H.; Lee, H.J.; Yoon, M.S.; Kim, D.H. Anti-Wrinkle Efficacy of Cross-Linked Hyaluronic Acid-Based Microneedle Patch with Acetyl Hexapeptide-8 and Epidermal Growth Factor on Korean Skin. Ann. Dermatol. 2019, 31, 263–271. [Google Scholar] [CrossRef]
- Park, H.J.; Cho, J.H.; Hong, S.H.; Kim, D.H.; Jung, H.Y.; Kang, I.K.; Cho, Y.J. Whitening and anti-wrinkle activities of ferulic acid isolated from Tetragonia tetragonioides in B16F10 melanoma and CCD-986sk fibroblast cells. J. Nat. Med. 2018, 72, 127–135. [Google Scholar] [CrossRef] [PubMed]
- Lueangarun, S.; Tragulplaingam, P.; Sugkraroek, S.; Tempark, T. The 24-h, 28-day, and 7-day post-moisturizing efficacy of ceramides 1, 3, 6-II containing moisturizing cream compared with hydrophilic cream on skin dryness and barrier disruption in senile xerosis treatment. Dermatol. Ther. 2019, 32, e13090. [Google Scholar] [CrossRef] [PubMed]
- Bissett, D.L.; Oblong, J.E.; Berge, C.A. Niacinamide: A B Vitamin that Improves Aging Facial Skin Appearance. Dermatol. Surg. 2005, 31, 860–866. [Google Scholar] [CrossRef] [PubMed]
- van der Maaden, K.; Heuts, J.; Camps, M.; Pontier, M.; van Scheltinga, A.T.; Jiskoot, W.; Ossendorp, F.; Bouwstra, J. Hollow microneedle-mediated micro-injections of a liposomal HPV E743–63 synthetic long peptide vaccine for efficient induction of cytotoxic and T-helper responses. J. Control. Release 2018, 269, 347–354. [Google Scholar] [CrossRef]
- Pamornpathomkul, B.; Wongkajornsilp, A.; Laiwattanapaisal, W.; Rojanarata, T.; Opanasopit, P.; Ngawhirunpat, T. A Combined Approach of Hollow Microneedles and Nanocarriers for Skin Immunization with Plasmid DNA Encoding Ovalbumin. Int. J. Nanomed. 2017, 12, 885–898. [Google Scholar] [CrossRef]
- Waghule, T.; Singhvi, G.; Dubey, S.K.; Pandey, M.M.; Gupta, G.; Singh, M.; Dua, K. Microneedles: A smart approach and increasing potential for transdermal drug delivery system. Biomed. Pharmacother. 2019, 109, 1249–1258. [Google Scholar] [CrossRef]
- Guillot, A.J.; Cordeiro, A.S.; Donnelly, R.F.; Montesinos, M.C.; Garrigues, T.M.; Melero, A. Microneedle-Based Delivery: An Overview of Current Applications and Trends. Pharmaceutics 2020, 12, 569. [Google Scholar] [CrossRef]
- Roxhed, N.; Griss, P.; Stemme, G. Membrane-sealed hollow microneedles and related administration schemes for transdermal drug delivery. Biomed. Microdevices 2008, 10, 271–279. [Google Scholar] [CrossRef]
- Burton, S.T.; Frederickson, F.L.; Hansen, K.J.; Simmers, R.P.; Fenn, P.T.; Moeckly, C.S. Hollow Microneedle Array and Method. U.S. Patent US-2011213335-A1, 1 September 2011. Available online: https://pubchem.ncbi.nlm.nih.gov/patent/US-2011213335-A1 (accessed on 22 February 2023).
- Fabbrocini, G.; De Vita, V.; Monfrecola, A.; De Padova, M.P.; Brazzini, B.; Teixeira, F.; Chu, A. Percutaneous collagen induction: An effective and safe treatment for post-acne scarring in different skin phototypes. J. Dermatol. Treat. 2013, 25, 147–152. [Google Scholar] [CrossRef]
- Liu, T.; Chen, M.; Fu, J.; Sun, Y.; Lu, C.; Quan, G.; Pan, X.; Wu, C. Recent advances in microneedles-mediated transdermal delivery of protein and peptide drugs. Acta Pharm. Sin. B 2021, 11, 2326–2343. [Google Scholar] [CrossRef]
- Badran, M.; Kuntsche, J.; Fahr, A. Skin penetration enhancement by a microneedle device (Dermaroller®) in vitro: Dependency on needle size and applied formulation. Eur. J. Pharm. Sci. 2009, 36, 511–523. [Google Scholar] [CrossRef] [PubMed]
- Doddaballapur, S. Microneedling with dermaroller. J. Cutan. Aesthetic Surg. 2009, 2, 110–111. [Google Scholar] [CrossRef] [PubMed]
- Oyunsaikhan, S.; Amarsaikhan, B.; Batbayar, B.; Dungubat, E. Morphometric Study of Facial Wrinkles and Aesthetic Skin as Dermaroller Treatment Combined with Platelet Rich Plasma (PRP). Diagn. Pathol. 2017, 3, 238–2364. [Google Scholar] [CrossRef]
- Subburaj, K.; Thakur, V.; Kumaran, M.S.; Vinay, K.; Srivastava, N.; Parsad, D. A prospective, randomized clinical study to compare the efficacy of recipient site preparation using dermabrasion, cryoblister, and dermaroller in autologous noncultured epidermal cell suspension in stable vitiligo. Dermatol. Ther. 2021, 34, e14683. [Google Scholar] [CrossRef] [PubMed]
- Winter, A.; Lintner, M.; Knezevich, E. V-Go Insulin Delivery System Versus Multiple Daily Insulin Injections for Patients With Uncontrolled Type 2 Diabetes Mellitus. J. Diabetes Sci. Technol. 2015, 9, 1111–1116. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Zhang, H.; Yang, R.; Laffitte, Y.; Schmill, U.; Hu, W.; Kaddoura, M.; Blondeel, E.J.M.; Cui, B. Fabrication of sharp silicon hollow microneedles by deep-reactive ion etching towards minimally invasive diagnostics. Microsyst. Nanoeng. 2019, 5, 41. [Google Scholar] [CrossRef]
- Narayanan, S.P.; Raghavan, S. Solid silicon microneedles for drug delivery applications. Int. J. Adv. Manuf. Technol. 2017, 93, 407–422. [Google Scholar] [CrossRef]
- Jung, J.H.; Jin, S.G. Microneedle for transdermal drug delivery: Current trends and fabrication. J. Pharm. Investig. 2021, 51, 503–517. [Google Scholar] [CrossRef]
- He, X.; Sun, J.; Zhuang, J.; Xu, H.; Liu, Y.; Wu, D. Microneedle System for Transdermal Drug and Vaccine Delivery: Devices, Safety, and Prospects. Dose-Response 2019, 17, 1559325819878585. [Google Scholar] [CrossRef]
- Chen, Y.; Chen, B.Z.; Wang, Q.L.; Jin, X.; Guo, X.D. Fabrication of coated polymer microneedles for transdermal drug delivery. J. Control. Release 2017, 265, 14–21. [Google Scholar] [CrossRef]
- Meng, F.; Hasan, A.; Babadaei, M.M.N.; Kani, P.H.; Talaei, A.J.; Sharifi, M.; Cai, T.; Falahati, M.; Cai, Y. Polymeric-based microneedle arrays as potential platforms in the development of drugs delivery systems. J. Adv. Res. 2020, 26, 137–147. [Google Scholar] [CrossRef] [PubMed]
- Yeung, C.; Chen, S.; King, B.; Lin, H.; King, K.; Akhtar, F.; Diaz, G.; Wang, B.; Zhu, J.; Sun, W.; et al. A 3D-printed microfluidic-enabled hollow microneedle architecture for transdermal drug delivery. Biomicrofluidics 2019, 13, 064125. [Google Scholar] [CrossRef] [PubMed]
- Jamaledin, R.; Di Natale, C.; Onesto, V.; Taraghdari, Z.; Zare, E.; Makvandi, P.; Vecchione, R.; Netti, P. Progress in Microneedle-Mediated Protein Delivery. J. Clin. Med. 2020, 9, 542. [Google Scholar] [CrossRef]
- Aksit, A.; Arteaga, D.N.; Arriaga, M.; Wang, X.; Watanabe, H.; Kasza, K.E.; Lalwani, A.K.; Kysar, J.W. In-vitro perforation of the round window membrane via direct 3-D printed microneedles. Biomed. Microdevices 2018, 20, 47. [Google Scholar] [CrossRef] [PubMed]
- Trautmann, A.; Roth, G.-L.; Nujiqi, B.; Walther, T.; Hellmann, R. Towards a versatile point-of-care system combining femtosecond laser generated microfluidic channels and direct laser written microneedle arrays. Microsyst. Nanoeng. 2019, 5, 6. [Google Scholar] [CrossRef]
- Szeto, B.; Aksit, A.; Valentini, C.; Yu, M.; Werth, E.G.; Goeta, S.; Tang, C.; Brown, L.M.; Olson, E.S.; Kysar, J.W.; et al. Novel 3D-printed hollow microneedles facilitate safe, reliable, and informative sampling of perilymph from guinea pigs. Hear. Res. 2021, 400, 108141. [Google Scholar] [CrossRef]
- Mathew, E.; Gilmore, B.F.; Larrañeta, E.; Lamprou, D.A. Antimicrobial 3D Printed Objects in the Fight Against Pandemics. 3D Print. Addit. Manuf. 2021, 8, 79–86. [Google Scholar] [CrossRef]
- Mathew, E.; Pitzanti, G.; dos Santos, A.L.G.; Lamprou, D.A. Optimization of Printing Parameters for Digital Light Processing 3D Printing of Hollow Microneedle Arrays. Pharmaceutics 2021, 13, 1837. [Google Scholar] [CrossRef]
- Yadav, V.; Sharma, P.K.; Murty, U.S.; Mohan, N.H.; Thomas, R.; Dwivedy, S.K.; Banerjee, S. 3D printed hollow microneedles array using stereolithography for efficient transdermal delivery of rifampicin. Int. J. Pharm. 2021, 605, 120815. [Google Scholar] [CrossRef]
- Dabbagh, S.R.; Sarabi, M.R.; Rahbarghazi, R.; Sokullu, E.; Yetisen, A.K.; Tasoglu, S. 3D-printed microneedles in biomedical applications. Iscience 2021, 24, 102012. [Google Scholar] [CrossRef]
- Derakhshandeh, H.; Aghabaglou, F.; McCarthy, A.; Mostafavi, A.; Wiseman, C.; Bonick, Z.; Ghanavati, I.; Harris, S.; Kreikemeier-Bower, C.; Basri, S.M.M.; et al. A Wirelessly Controlled Smart Bandage with 3D-Printed Miniaturized Needle Arrays. Adv. Funct. Mater. 2020, 30, 1905544. [Google Scholar] [CrossRef] [PubMed]
- Vinayakumar, K.B.; Kulkarni, P.G.; Nayak, M.M.; Dinesh, N.S.; Hegde, G.M.; Ramachandra, S.G.; Rajanna, K. A hollow stainless steel microneedle array to deliver insulin to a diabetic rat. J. Micromechanics Microengineering 2016, 26, 065013. [Google Scholar] [CrossRef]
- Nejad, H.R.; Sadeqi, A.; Kiaee, G.; Sonkusale, S. Low-cost and cleanroom-free fabrication of microneedles. Microsyst. Nanoeng. 2018, 4, 17073. [Google Scholar] [CrossRef]
- Norman, J.J.; Choi, S.-O.; Tong, N.T.; Aiyar, A.R.; Patel, S.R.; Prausnitz, M.R.; Allen, M.G. Hollow microneedles for intradermal injection fabricated by sacrificial micromolding and selective electrodeposition. Biomed. Microdevices 2013, 15, 203–210. [Google Scholar] [CrossRef]
- Li, J.; Liu, B.; Zhou, Y.; Chen, Z.; Jiang, L.; Yuan, W.; Liang, L. Fabrication of a Ti porous microneedle array by metal injection molding for transdermal drug delivery. PLoS ONE 2017, 12, e0172043. [Google Scholar] [CrossRef]
- Li, J.; Zhou, Y.; Yang, J.; Ye, R.; Gao, J.; Ren, L.; Liu, B.; Liang, L.; Jiang, L. Fabrication of gradient porous microneedle array by modified hot embossing for transdermal drug delivery. Mater. Sci. Eng. C 2019, 96, 576–582. [Google Scholar] [CrossRef]
- McAllister, D.V.; Wang, P.M.; Davis, S.P.; Park, J.-H.; Canatella, P.J.; Allen, M.G.; Prausnitz, M.R. Microfabricated needles for transdermal delivery of macromolecules and nanoparticles: Fabrication methods and transport studies. Proc. Natl. Acad. Sci. USA 2003, 100, 13755–13760. [Google Scholar] [CrossRef]
- Kim, K.; Park, D.S.; Lu, H.M.; Che, W.; Kim, K.; Lee, J.; Ahn, C.H. A tapered hollow metallic microneedle array using backside exposure of SU-8. J. Micromechanics Microengineering 2004, 14, 597–603. [Google Scholar] [CrossRef]
- Davis, S.; Martanto, W.; Allen, M.; Prausnitz, M. Hollow Metal Microneedles for Insulin Delivery to Diabetic Rats. IEEE Trans. Biomed. Eng. 2005, 52, 909–915. [Google Scholar] [CrossRef]
- Bolton, C.J.W.; Howells, O.; Blayney, G.J.; Eng, P.F.; Birchall, J.C.; Gualeni, B.; Roberts, K.; Ashraf, H.; Guy, O.J. Hollow silicon microneedle fabrication using advanced plasma etch technologies for applications in transdermal drug delivery. Lab Chip 2020, 20, 2788–2795. [Google Scholar] [CrossRef]
- Khanna, P.; Luongo, K.; Strom, J.A.; Bhansali, S. Axial and shear fracture strength evaluation of silicon microneedles. Microsyst. Technol. 2010, 16, 973–978. [Google Scholar] [CrossRef]
- Hu, Z.; Meduri, C.S.; Ingrole, R.S.J.; Gill, H.S.; Kumar, G. Solid and hollow metallic glass microneedles for transdermal drug-delivery. Appl. Phys. Lett. 2020, 116, 203703. [Google Scholar] [CrossRef]
- Mishra, R.; Pramanick, B.; Maiti, T.K.; Bhattacharyya, T.K. Glassy carbon microneedles—New transdermal drug delivery device derived from a scalable C-MEMS process. Microsyst. Nanoeng. 2018, 4, 38. [Google Scholar] [CrossRef] [PubMed]
- Martanto, W.; Moore, J.S.; Kashlan, O.; Kamath, R.; Wang, P.M.; O’Neal, J.M.; Prausnitz, M.R. Microinfusion Using Hollow Microneedles. Pharm. Res. 2006, 23, 104–113. [Google Scholar] [CrossRef] [PubMed]
- Lhernould, M.S.; Deleers, M.; Delchambre, A. Hollow polymer microneedles array resistance and insertion tests. Int. J. Pharm. 2015, 480, 152–157. [Google Scholar] [CrossRef]
- Makvandi, P.; Kirkby, M.; Hutton, A.R.J.; Shabani, M.; Yiu, C.K.Y.; Baghbantaraghdari, Z.; Jamaledin, R.; Carlotti, M.; Mazzolai, B.; Mattoli, V.; et al. Engineering Microneedle Patches for Improved Penetration: Analysis, Skin Models and Factors Affecting Needle Insertion. Nano-Micro Lett. 2021, 13, 93. [Google Scholar] [CrossRef]
- Larrañeta, E.; Lutton, R.E.M.; Woolfson, A.D.; Donnelly, R.F. Microneedle arrays as transdermal and intradermal drug delivery systems: Materials science, manufacture and commercial development. Mater. Sci. Eng. R Rep. 2016, 104, 1–32. [Google Scholar] [CrossRef]
- Esfandyarpour, R.; Esfandyarpour, H.; Javanmard, M.; Harris, J.S.; Davis, R.W. Microneedle biosensor: A method for direct label-free real time protein detection. Sens. Actuators B Chem. 2013, 177, 848–855. [Google Scholar] [CrossRef]
- Blicharz, T.M.; Gong, P.; Bunner, B.M.; Chu, L.L.; Leonard, K.M.; Wakefield, J.A.; Williams, R.E.; Dadgar, M.; Tagliabue, C.A.; El Khaja, R.; et al. Microneedle-based device for the one-step painless collection of capillary blood samples. Nat. Biomed. Eng. 2018, 2, 151–157. [Google Scholar] [CrossRef]
- Zoudani, E.; Soltani, M. A new computational method of modeling and evaluation of dissolving microneedle for drug delivery applications: Extension to theoretical modeling of a novel design of microneedle (array in array) for efficient drug delivery. Eur. J. Pharm. Sci. 2020, 150, 105339. [Google Scholar] [CrossRef]
- Johnson, A.R.; Caudill, C.L.; Tumbleston, J.R.; Bloomquist, C.J.; Moga, K.A.; Ermoshkin, A.; Shirvanyants, D.; Mecham, S.J.; Luft, J.C.; DeSimone, J.M. Single-Step Fabrication of Computationally Designed Microneedles by Continuous Liquid Interface Production. PLoS ONE 2016, 11, e0162518. [Google Scholar] [CrossRef] [PubMed]
- Donnelly, R.F.; Garland, M.J.; Morrow, D.I.; Migalska, K.; Singh, T.R.R.; Majithiya, R.; Woolfson, A.D. Optical coherence tomography is a valuable tool in the study of the effects of microneedle geometry on skin penetration characteristics and in-skin dissolution. J. Control. Release 2010, 147, 333–341. [Google Scholar] [CrossRef] [PubMed]
- Olatunji, O.; Das, D.B.; Garland, M.J.; Belaid, L.; Donnelly, R.F. Influence of Array Interspacing on the Force Required for Successful Microneedle Skin Penetration: Theoretical and Practical Approaches. J. Pharm. Sci. 2013, 102, 1209–1221. [Google Scholar] [CrossRef] [PubMed]
- Rad, Z.F.; Prewett, P.D.; Davies, G.J. High-resolution two-photon polymerization: The most versatile technique for the fabrication of microneedle arrays. Microsyst. Nanoeng. 2021, 7, 71. [Google Scholar] [CrossRef]
- Park, E.; Selvaraj, R.; Kim, Y. High-efficiency photothermal sterilization on PDMS film with Au@CuS yolk-shell nanoparticles. J. Ind. Eng. Chem. 2022, 113, 522–529. [Google Scholar] [CrossRef]
- Celis, P.; Vazquez, E.; Soria-Hernández, C.G.; Bargnani, D.; Rodriguez, C.A.; Ceretti, E.; García-López, E. Evaluation of Ball End Micromilling for Ti6Al4V ELI Microneedles Using a Nanoadditive Under MQL Condition. Int. J. Precis. Eng. Manuf. Technol. 2022, 9, 1231–1246. [Google Scholar] [CrossRef]
- Zhou, P.; Chen, C.; Yue, X.; Zhang, J.; Huang, C.; Zhao, S.; Wu, A.; Li, X.; Qu, Y.; Zhang, C. Strategy for osteoarthritis therapy: Improved the delivery of triptolide using liposome-loaded dissolving microneedle arrays. Int. J. Pharm. 2021, 609, 121211. [Google Scholar] [CrossRef]
- Wang, Z.; Luan, J.; Seth, A.; Liu, L.; You, M.; Gupta, P.; Rathi, P.; Wang, Y.; Cao, S.; Jiang, Q.; et al. Microneedle patch for the ultrasensitive quantification of protein biomarkers in interstitial fluid. Nat. Biomed. Eng. 2021, 5, 64–76. [Google Scholar] [CrossRef]
- Terashima, S.; Tatsukawa, C.; Takahashi, T.; Suzuki, M.; Aoyagi, S. Fabrication of hyaluronic acid hollow microneedle array. Jpn. J. Appl. Phys. 2020, 59, SIIJ03. [Google Scholar] [CrossRef]
- Pahlevaninezhad, H.; Khorasaninejad, M.; Huang, Y.-W.; Shi, Z.; Hariri, L.P.; Adams, D.C.; Ding, V.; Zhu, A.; Qiu, C.-W.; Capasso, F.; et al. Nano-optic endoscope for high-resolution optical coherence tomography in vivo. Nat. Photon 2018, 12, 540–547. [Google Scholar] [CrossRef]
- Santoro, F.; Dasgupta, S.; Schnitker, J.; Auth, T.; Neumann, E.; Panaitov, G.; Gompper, G.; Offenhäusser, A. Interfacing Electrogenic Cells with 3D Nanoelectrodes: Position, Shape, and Size Matter. ACS Nano 2014, 8, 6713–6723. [Google Scholar] [CrossRef] [PubMed]
- Wang, R.; Jiang, X.; Wang, W.; Li, Z. A microneedle electrode array on flexible substrate for long-term EEG monitoring. Sensors Actuators B Chem. 2017, 244, 750–758. [Google Scholar] [CrossRef]
- Wang, M.; Hu, L.; Xu, C. Recent advances in the design of polymeric microneedles for transdermal drug delivery and biosensing. Lab Chip 2017, 17, 1373–1387. [Google Scholar] [CrossRef] [PubMed]
- Yao, G.; Quan, G.; Lin, S.; Peng, T.; Wang, Q.; Ran, H.; Chen, H.; Zhang, Q.; Wang, L.; Pan, X.; et al. Novel dissolving microneedles for enhanced transdermal delivery of levonorgestrel: In vitro and in vivo characterization. Int. J. Pharm. 2017, 534, 378–386. [Google Scholar] [CrossRef]
- Krieger, K.J.; Bertollo, N.; Dangol, M.; Sheridan, J.T.; Lowery, M.M.; O’Cearbhaill, E.D. Simple and customizable method for fabrication of high-aspect ratio microneedle molds using low-cost 3D printing. Microsyst. Nanoeng. 2019, 5, 42. [Google Scholar] [CrossRef]
- Bird, D.; Eker, E.; Ravindra, N.M. 3D Printing of Pharmaceuticals and Transdermal Drug Delivery––An Overview. In 148th Annual Meeting and Exhibition of The Minerals, Metals and Materials Society, TMS 2019; Springer International Publishing: New York, NY, USA, 2019; pp. 1563–1573. [Google Scholar] [CrossRef]
- Pere, C.P.P.; Economidou, S.N.; Lall, G.; Ziraud, C.; Boateng, J.S.; Alexander, B.D.; Lamprou, D.A.; Douroumis, D. 3D printed microneedles for insulin skin delivery. Int. J. Pharm. 2018, 544, 425–432. [Google Scholar] [CrossRef]

| Sl no. | Type of MN Used | Active Ingredient | Method | Result | Limitation of the Study | References |
|---|---|---|---|---|---|---|
| 1. | Soluble microneedle patch | HA 16.7% (w/w) | Double-blind clinical trial done for 10 to 12 weeks on 84 Korean females with Crow’s feet and evaluation of other parameters e.g., dermal density, elasticity, and hydration | Effective reduction of epidermal wrinkle after 8 weeks. Other parameters like dermal density, elasticity, and hydration had increased | The clinical test has been done only for Korean skin types; Discontinuation effects were not properly mentioned, giving rise to durability concerns | [57] |
| 2. | Microneedle eye patch | Acetyl hexapeptide-3 (AHP-3) (500 μL of 10 % w/v), Polydimethylsiloxane (PDMS), PrestoBlue® | PMNP implemented increases the delivery of AHP-3 through human cadaver skin of Caucasian female, as compared to FMNP and intact skin to analyze the skin wrinkle reduction. | Efficiently penetrated skin very fast compared to other MNs through the first application; cumulative permeation of AHP-3 across human cadaver skin was approx. ~90× higher compared to intact skin; and ~45× higher than FMNP treated skin | Dose adjustment based on an individual patient is difficult; some allergic reactions can be found in small peptides (rare case); high-cost issues; risky for people with skin cancer | [58] |
| 3. | Dissolvable microneedle | Adenosine incorporated low and high MW HA dissolving MN array | Clinical efficacy and safety tests were executed for 12 weeks long upon 3 Korean females with periorbital wrinkles. Ad-HMN and Ad-LMN, both type of arrays were applied in every three days, in the evening, for 8 weeks. Then, skin wrinkling, dermal density, and elasticity were measured. | Both of the groups showed statistically significant efficacy for most parameters; the adenosine incorporated high MW array had better effect on the mean depth of wrinkles, maximum depth of wider wrinkles, dermal density, and skin elasticity than the adenosine incorporated low MW array | Fabrication of dissolvable MNs with high molecular weight hyaluronic acid is a challenge due to the high viscosity of HMW HA | [54,59,60] |
| 4. | Dissolvable microneedle patch | Horse-oil and Adenosine loaded dissolving microneedle patch (HOS-Ad-DMN patch) | In-vitro analysis was conducted to ensure successful delivery of the active ingredients with a specific composition. Clinical efficacy and safety tests were conducted on the lateral canthus of 20 Korean women to assess and compare the efficacy of HOS-Ad-DMN patches with that of Ad-DMN patches. | Compared with Ad-DMN patches, HOS-Ad-DMN patches significantly improved skin elasticity, hydration, dermal density, and wrinkles | The active compounds for DMNs have been limited to hydrophilic compounds, because most DMNs are made using hydrophilic polymers for the backbone matrix; delivery of lipophilic compounds via DMNs is a challenge because of the difficulty of attaining a homogenous viscous solution for DMN fabrication; the fabrication with this heterogeneous solution produced uneven DMN morphologies and irregular encapsulation of the active compounds | [61] |
| 5. | Dissolving, detachable microneedle technology | Hyaluronic and ferulic acids | 650 micro-needles, which dissolve in 25 min of exposure, were tested on 82 subjects in a randomized split-phase study. Effectiveness was assessed at 6 weeks. | Demonstrated a significant reduction in the average roughness index, with a steady decrease in puffiness of the application area, increased elasticity and reduced severity of epidermal wrinkles | Additional studies of soluble MNs are required to fully assess the amount and distribution area of the injected hyaluronic acid and other active components | [62] |
| 6. | 3D printed personalised microneedle | Acetyl-hexapeptide 3 (AHP-3), polyethylene glycol diacrylate (PEGDA) and vinyl pyrrolidone (VP) | AHP-3 loaded MNs patches were loaded with polyethylene glycol diacrylate (PEGDA) and vinyl pyrrolidone (VP) and tested on Human cadaver dermatomed skin of a 30-year-old, Chinese Male. | Significant absorbance rate observed with vinyl pyrrolidone (VP) showing highest mechanical rate among other resins; the resin polymers and the 3D printing process showed good biocompatibility on human skin | Study conducted on one subject only; microneedle patches may not be of sufficient flexibility to account for the minor indentations or variations of the skin | [63] |
| Reagent Used in Formulation | Nature | Function | References |
|---|---|---|---|
| Retinyl retinoate | Lipophilic | Photostable; have lower toxicity and greater skin rejuvenation than retinol; effective in treating periorbital wrinkles | [82] |
| Ascorbic acid | Hydrophilic | Acts as an antioxidant; effective in treating photo-aging | [83] |
| Hyaluronic acid | Hydrophilic polysaccharide | Increases skin moisture and reduces the appearance of fine lines and wrinkles by improving collagen and elastin stimulation | [84,85,86] |
| Adenosine | Amino acid | Effective in treating crow’s feet and frown lines by skin density, elasticity and hydration | [87,88] |
| Horse oil | Lipophilic | Restores stratum corneum and imparts skin-moisturizing effects | [89] |
| Acetyl-hexapeptide 3 (AHP-3) | Small peptide | Decreases the anisotropy of skin to help in treating skin wrinkles | [90] |
| Epidermal growth factor | Small water-soluble polypeptide | Effective in treating periorbital wrinkles | [85,91] |
| Ferulic acid | Hydrophilic | Antioxidant that helps to maintain the skin’s smooth morphology by reducing the development of fine lines, spots, and wrinkles | [92] |
| Ceramide | Lipid | Restores skin moisture, balances skin pH, reduces skin wrinkles and Trans Epidermal Water loss (TEWL) | [93] |
| Niacinamide | Vitamin B3 | Improves skin elasticity, reduces skin wrinkles and fine lines, decreases hyperpigmentation and skin sallowness, minimizes pore size, and eases skin inflammation | [94] |
| Material Group | Material Subtype | Fabrication Technique | References |
|---|---|---|---|
| Silicon | Monocrystalline, Polycrystalline silicon | Etching, lithography, deep reactive ion etching (DRIE) | [108,132,133] |
| Metal | Stainless steel, titanium, palladium, palladium-cobalt alloys, nickel | Laser cutting, laser ablation, etching, electropolishing, lithography, microstereolithogra phy, deep reactive ion | [124] |
| Nickel | SU-8 UV-LIGA Moulding, Nickel electrocoating and Electropolishing | [64] | |
| Pt-Metallic glasses | Thermoplastic Drawing | [134] | |
| Carbon | Glassy carbon | Conventional Micro-electromechanical system (CMEMS) process | [135] |
| Borosilicate | Fire-polished borosilicate glass | Pulling pipettes | [136] |
| Miscellaneous | Polycarbonate | Injection micromolding | [137] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Islam, H.; Poly, T.S.; Tisha, Z.T.; Rahman, S.; Naveed, A.I.J.; Ahmed, A.; Ahmed, S.N.; Hassan, J.; Uddin, M.J.; Das, D.B. 3D Printed Hollow Microneedles for Treating Skin Wrinkles Using Different Anti-Wrinkle Agents: A Possible Futuristic Approach. Cosmetics 2023, 10, 41. https://doi.org/10.3390/cosmetics10020041
Islam H, Poly TS, Tisha ZT, Rahman S, Naveed AIJ, Ahmed A, Ahmed SN, Hassan J, Uddin MJ, Das DB. 3D Printed Hollow Microneedles for Treating Skin Wrinkles Using Different Anti-Wrinkle Agents: A Possible Futuristic Approach. Cosmetics. 2023; 10(2):41. https://doi.org/10.3390/cosmetics10020041
Chicago/Turabian StyleIslam, Humayra, Taslima Sultana Poly, Zarin Tasnim Tisha, Samia Rahman, Ahmed Issa Jahangir Naveed, Alifa Ahmed, Saraf Nawar Ahmed, Jasmin Hassan, Md. Jasim Uddin, and Diganta B. Das. 2023. "3D Printed Hollow Microneedles for Treating Skin Wrinkles Using Different Anti-Wrinkle Agents: A Possible Futuristic Approach" Cosmetics 10, no. 2: 41. https://doi.org/10.3390/cosmetics10020041
APA StyleIslam, H., Poly, T. S., Tisha, Z. T., Rahman, S., Naveed, A. I. J., Ahmed, A., Ahmed, S. N., Hassan, J., Uddin, M. J., & Das, D. B. (2023). 3D Printed Hollow Microneedles for Treating Skin Wrinkles Using Different Anti-Wrinkle Agents: A Possible Futuristic Approach. Cosmetics, 10(2), 41. https://doi.org/10.3390/cosmetics10020041











