Current Insights into the Formulation and Delivery of Therapeutic and Cosmeceutical Agents for Aging Skin
Abstract
:1. Introduction
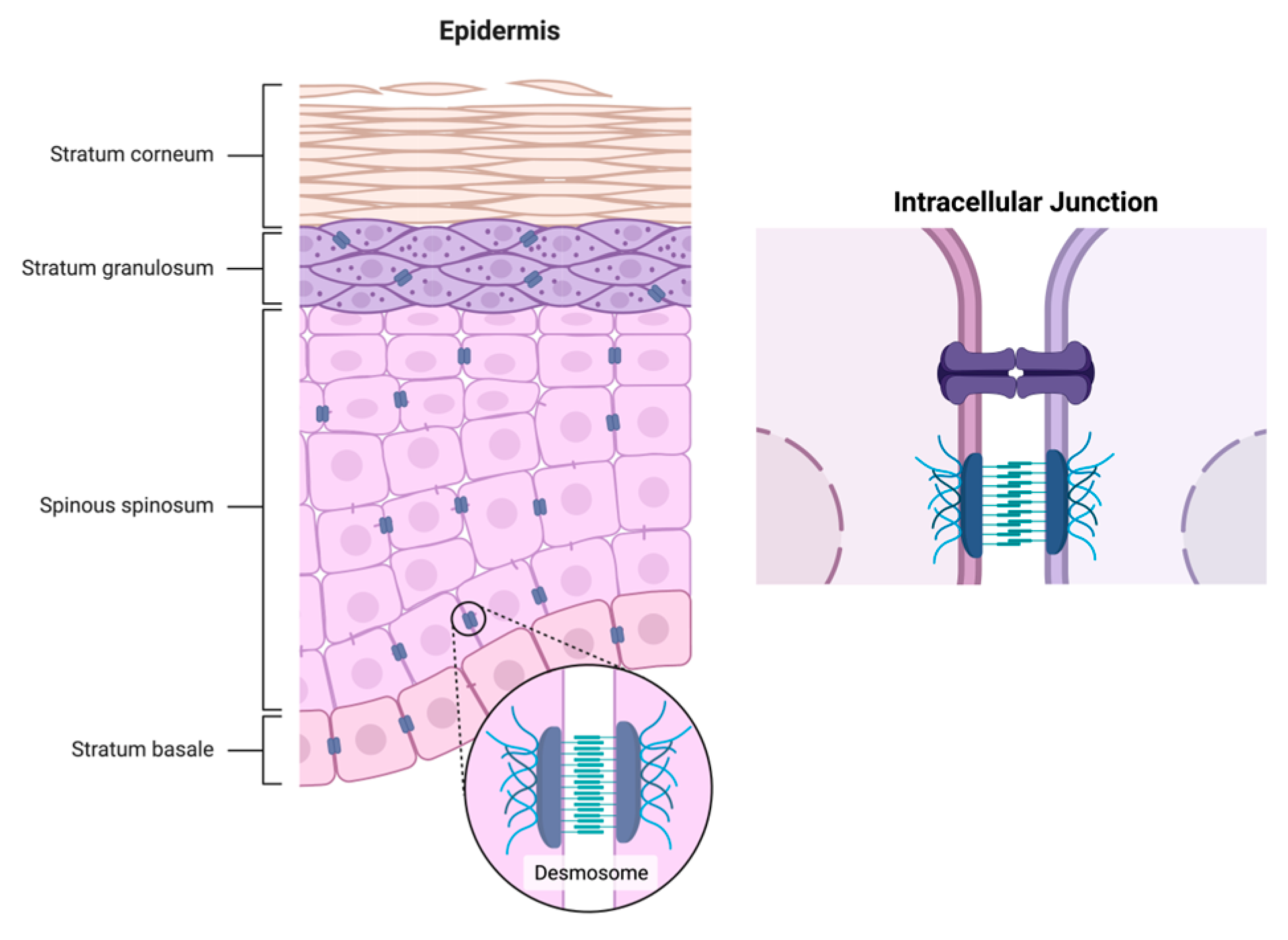
1.1. Physiological Change in Aging Skin
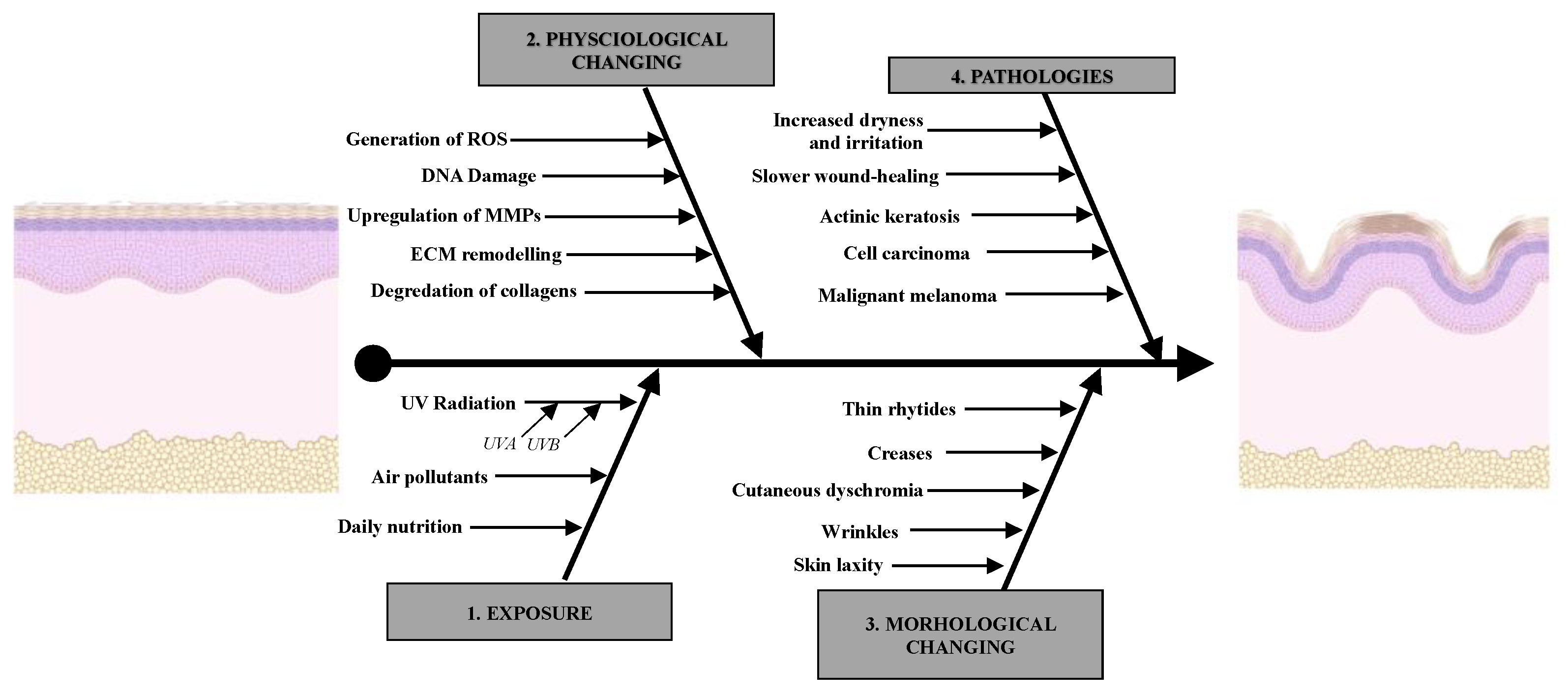
1.2. Primary Factors That Accelerate Aging
2. Mechanisms of Potential Therapeutics and Cosmetic Agents against Aging
2.1. Synthetic and Plant-Derived Products
2.1.1. Synthetic Products
2.1.2. Vitamins
2.1.3. Endogenous Compounds
2.1.4. Carotenoids
2.1.5. Polyphenols
2.2. Peptides, Cell-Derived Products, and Biologics
2.2.1. Peptides, Proteins, and Cell Culture-Derived Extracts
| Brand | Ingredient(s) | Origin | Cosm. Use | Mechanism | Delivery System | Ref. |
|---|---|---|---|---|---|---|
| Iamin | GHK-Cu or copper tripeptide-1 | fragment of the α2-chain of collagen I | anti-aging | enhances Cu2+ uptake, TIMP-1, TIMP-2, and MMP-2; promotes degradation of collagen aggregates; promotes collagen, elastin, proteoglycan, and glycosaminoglycan production; anti-inflammatory and antioxidant | hydrogel alternative: microneedles | [42,78,79,80] |
| Matrixyl | pal-KTTKS, palmitoyl pentapeptide-4, palmitoyl pentapeptide-3, or palmitoyl oligopeptide | procollagen I-derived pentamer (KTTKS) with palmitoyl added to improve permeability | anti-aging | promotes the production of fibronectin, elastin, glycosaminoglycans, and collagen types I, III and VI; stabilizes mRNAs that upregulate TGF-β | O/W emulsion moisturizer | [42,77,81] |
| Preregen | soybean protein, glycine soy protein, and amino acids | soybean seed | anti-aging | inhibits proteinase formation and increases number of dermal papillae | O/W emulsion in Tegocare-45 base | [42,82] |
| Keramino 25 | keratin protein and amino acids | human hair and sheep wool | anti-aging moisturizer | improves elasticity and hydration of the skin and hair | multilamellar vesicle liposomes, 0.9% NaCl | [42,83] |
| Citrix CRS | cell rejuvenation system (L-ascorbic acid, TGF-β1, and Cimicifuga) | recombinant TGF-β1, stem cell extract from Cimicifuga racemosa | anti-aging | profibrotic cytokine modulates angiogenesis, cellular migration and proliferation, neocollagenesis, and degradation of matrix proteins | liposome cream in silicone base | [42,84] |
| Processed skin cell proteins (PSP) | PSP bio-restorative skin cream (mixture of cytokines and growth factors) | human fibroblast cell culture lysate | anti-aging | growth factors promote angiogenesis and cytokines modulate inflammation | cream | [14,40,85] |
| Nouricel-MD TNS | tissue nutrient solution recovery complex (VEGF, PDGF-A, G-CSF, HGF, IL-6, IL-8, and TGF-β1) | human neonatal foreskin fibroblast culture | anti-aging | growth factors promote angiogenesis, and cytokines modulate inflammation and enhance ECM component deposition | oil-free gel | [14,40,42] |
| ReGenica, MRCx | MRCx (VEGF, IL-8, and keratinocyte growth factor, but no TGF-β) | Cytokines and growth factors from conditioned medium of fibroblasts | anti-aging moisturizer | growth factors promote angiogenesis and cytokines modulate inflammation | cream | [40,86] |
| Micro-protein complex (MPC) | GEGK, pal-GHK, and N-octanoyl-carnosine | synthetic | anti-wrinkle | increases ECM collagen, hyaluronan, and fibronectin | cream | [40,76,87] |
| Decorinyl | tripeptide-10 citrulline, or Lys-α-Asp-Ile-citrulline | synthetic decorin-like tetrapeptide | anti-aging | regulates collagen fibrillogenesis, and improves uniformity of and influences diameter and position of collagen fibers | liposomal cream | [42,88] |
| Progeline | trifluoroacetyl-tripeptide-2 or trifluoroacetyl-Val-Tyr-Val | synthetic | anti-wrinkle | inhibits MMP and decreases the synthesis of progerin, and improves the relation of collagens via the production of proteoglycan | cream | [89] |
| SYN®-AKE | tripeptide-3 or dipeptide diamino butyroyl benzylamide diacetate | Synthetic tripeptide mimicking Waglerin-1 from snake venom | anti-wrinkle | formulated for the treatment of neuromuscular activity; inhibits the muscular activity associated with repeated movement at the neuromuscular junction | Hydrogel = glycerin-based aqueous solution | [89,90,91] |
2.2.2. Biologics and DNA Repair
3. Formulation Approaches and Delivery Strategies—Patents, Papers, and Products
3.1. Common Formulation Approaches for Topical Administration
3.1.1. Emulsions
3.1.2. Vesicular Systems
3.1.3. Particulate Systems
3.1.4. Microneedles
3.2. Common Formulation Delivery Methods Used against Skin Aging
3.2.1. Creams, Gels, and Serums
3.2.2. Sprays and Lotions
3.2.3. Parenteral Preparations
3.3. Limitations on Current Approaches/Agents
3.3.1. Permeation Enhancers
3.3.2. Systemic Delivery
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Hong, J.Y.; Kwon, T.; Kim, J.H.; Lee, B.C.; Kim, B.J. Prospective, preclinical comparison of the performance between radiofrequency microneedling and microneedling alone in reversing photoaged skin. J. Cosmet. Dermatol. 2020, 19, 1105–1109. [Google Scholar] [CrossRef]
- Krutmann, J.; Schikowski, T.; Morita, A.; Berneburg, M. Environmentally-Induced (Extrinsic) Skin Aging: Exposomal Factors and Underlying Mechanisms. J. Investig. Dermatol. 2021, 141, 1096–1103. [Google Scholar] [CrossRef]
- Naylor, E.C.; Watson, R.E.; Sherratt, M.J. Molecular aspects of skin ageing. Maturitas 2011, 69, 249–256. [Google Scholar] [CrossRef] [PubMed]
- Kohl, E.; Steinbauer, J.; Landthaler, M.; Szeimies, R.M. Skin ageing. J. Eur. Acad. Dermatol. Venereol. 2011, 25, 873–884. [Google Scholar] [CrossRef] [PubMed]
- Kammeyer, A.; Luiten, R.M. Oxidation events and skin aging. Ageing Res. Rev. 2015, 21, 16–29. [Google Scholar] [CrossRef] [PubMed]
- Lorencini, M.; Brohem, C.A.; Dieamant, G.C.; Zanchin, N.I.; Maibach, H.I. Active ingredients against human epidermal aging. Ageing Res. Rev. 2014, 15, 100–115. [Google Scholar] [CrossRef]
- Babamiri, K.; Nassab, R. Cosmeceuticals: The Evidence Behind the Retinoids. Aesthetic Surg. J. 2010, 30, 74–77. [Google Scholar] [CrossRef] [Green Version]
- Bradley, E.J.; Griffiths, C.E.; Sherratt, M.J.; Bell, M.; Watson, R.E. Over-the-counter anti-ageing topical agents and their ability to protect and repair photoaged skin. Maturitas 2015, 80, 265–272. [Google Scholar] [CrossRef]
- Vasvani, S.; Kulkarni, P.; Rawtani, D. Hyaluronic acid: A review on its biology, aspects of drug delivery, route of administrations and a special emphasis on its approved marketed products and recent clinical studies. Int. J. Biol. Macromol. 2020, 151, 1012–1029. [Google Scholar] [CrossRef]
- Ariede, M.B.; Candido, T.M.; Jacome, A.L.M.; Velasco, M.V.R.; De Carvalho, J.C.M.; Baby, A.R. Cosmetic attributes of algae—A review. Algal Res. 2017, 25, 483–487. [Google Scholar] [CrossRef]
- Ratnam, D.V.; Ankola, D.; Bhardwaj, V.; Sahana, D.; Kumar, M.R. Role of antioxidants in prophylaxis and therapy: A pharmaceutical perspective. J. Control. Release 2006, 113, 189–207. [Google Scholar] [CrossRef]
- Korkina, L.; De Luca, C.; Pastore, S. Plant polyphenols and human skin: Friends or foes. Ann. New York Acad. Sci. 2012, 1259, 77–86. [Google Scholar] [CrossRef] [PubMed]
- Kang, Y.-A.; Na, J.-I.; Choi, H.-R.; Choi, J.-W.; Kang, H.-Y.; Park, K.-C. Novel anti-inflammatory peptides as cosmeceutical peptides. Peptides 2011, 32, 2134–2136. [Google Scholar] [CrossRef] [PubMed]
- Mehta, R.C.; Fitzpatrick, R.E. Endogenous growth factors as cosmeceuticals. Dermatol. Ther. 2007, 20, 350–359. [Google Scholar] [CrossRef] [PubMed]
- Alexander, A.; Dwivedi, S.; Ajazuddin; Giri, T.K.; Saraf, S.; Saraf, S.; Tripathi, D.K. Approaches for breaking the barriers of drug permeation through transdermal drug delivery. J. Control. Release 2012, 164, 26–40. [Google Scholar] [CrossRef] [PubMed]
- Hussain, A.A.; Dakkuri, A. Dosage Form Design: A Physicochemical Approach. In Encyclopedia of Pharmaceutical Science and Technology, 4th ed.; CRC Press: Boca Raton, FL, USA, 2013; pp. 852–872. [Google Scholar]
- Costa, R.; Santos, L. Delivery systems for cosmetics—From manufacturing to the skin of natural antioxidants. Powder Technol. 2017, 322, 402–416. [Google Scholar] [CrossRef]
- Souto, E.B.; Fernandes, A.R.; Martins-Gomes, C.; Coutinho, T.E.; Durazzo, A.; Lucarini, M.; Souto, S.B.; Silva, A.M.; Santini, A. Nanomaterials for Skin Delivery of Cosmeceuticals and Pharmaceuticals. Appl. Sci. 2020, 10, 1594. [Google Scholar] [CrossRef] [Green Version]
- Yaar, M.; Gilchrest, B.A. Photoageing: Mechanism, prevention and therapy. Br. J. Dermatol. 2007, 157, 874–887. [Google Scholar] [CrossRef]
- Luebberding, S.; Krueger, N.; Kerscher, M. Mechanical properties of human skin in vivo: A comparative evaluation in 300 men and women. Ski. Res. Technol. 2014, 20, 127–135. [Google Scholar] [CrossRef]
- Nedelec, B.; Forget, N.J.; Hurtubise, T.; Cimino, S.; De Muszka, F.; Legault, A.; Liu, W.L.; de Oliveira, A.C.M.T.G.; Calva, V.; Correa, J.A. Skin characteristics: Normative data for elasticity, erythema, melanin, and thickness at 16 different anatomical locations. Ski. Res. Technol. 2016, 22, 263–275. [Google Scholar] [CrossRef]
- Kohl, E.; Popp, C.; Zeman, F.; Unger, P.; Koller, M.; Landthaler, M.; Karrer, S.; Szeimies, R. Photodynamic therapy using intense pulsed light for treating actinic keratoses and photoaged skin of the dorsal hands: A randomized placebo-controlled study. Br. J. Dermatol. 2017, 176, 352–362. [Google Scholar] [CrossRef] [PubMed]
- Farage, M.A.; Miller, K.W.; Elsner, P.; Maibach, H.I. Characteristics of the Aging Skin. Adv. Wound Care 2013, 2, 5–10. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- El Domyati, M.; Attia, S.; Saleh, F.; Brown, D. Intrinsic aging vs. photoaging: A comparative histopathological, immunohistochemical, and ultrastructural study of skin. Exp. Dermatol. 2002, 11, 398–405. [Google Scholar] [CrossRef] [PubMed]
- Rabe, J.H.; Mamelak, A.J.; McElgunn, P.J.; Morison, W.L.; Sauder, D.N. Photoaging: Mechanisms and repair. J. Am. Acad. Dermatol. 2006, 55, 1–19. [Google Scholar] [CrossRef] [PubMed]
- Luebberding, S.; Krueger, N.; Kerscher, M. Skin physiology in men and women: In vivo evaluation of 300 people including TEWL, SC hydration, sebum content and skin surface pH. Int. J. Cosmet. Sci. 2013, 35, 477–483. [Google Scholar] [CrossRef] [PubMed]
- Persa, O.D.; Koester, J.; Niessen, C.M. Regulation of Cell Polarity and Tissue Architecture in Epidermal Aging and Cancer. J. Investig. Dermatol. 2021, 141, 1017–1023. [Google Scholar] [CrossRef] [PubMed]
- Jiao, Q.; Yue, L.; Zhi, L.; Qi, Y.; Yang, J.; Zhou, C.; Jia, Y. Studies on stratum corneum metabolism: Function, molecular mechanism and influencing factors. J. Cosmet. Dermatol. 2022, 21, 3256–3264. [Google Scholar] [CrossRef]
- Created with Biorender.com, BioRender. Available online: https://app.biorender.com/biorender-templates (accessed on 20 February 2023).
- Hajem, N.; Chapelle, A.; Bignon, J.; Pinault, A.; Salah-Mohellibi, N.; Lati, E.; Wdzieczak-Bakala, J.; Liu, J.-M. The regulatory role of the tetrapeptide AcSDKP in skin and hair physiology and the prevention of ageing effects in these tissues—A potential cosmetic role. Int. J. Cosmet. Sci. 2013, 35, 286–298. [Google Scholar] [CrossRef]
- López-Otín, C.; Blasco, M.A.; Partridge, L.; Serrano, M.; Kroemer, G. The Hallmarks of Aging. Cell 2013, 153, 1194–1217. [Google Scholar] [CrossRef] [Green Version]
- Velarde, M.C.; Demaria, M. Targeting Senescent Cells: Possible Implications for Delaying Skin Aging: A Mini-Review. Gerontology 2016, 62, 513–518. [Google Scholar] [CrossRef] [Green Version]
- Slominski, A.; Tobin, D.J.; Shibahara, S.; Wortsman, J. Melanin pigmentation in mammalian skin and its hormonal regulation. Physiol. Rev. 2004, 84, 1155–1228. [Google Scholar] [CrossRef]
- Bocheva, G.; Slominski, R.M.; Slominski, A.T. The Impact of Vitamin D on Skin Aging. Int. J. Mol. Sci. 2021, 22, 9097. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.; Lee, K.B.; Hong, A.; Son, Y.H.; Lee, D.H.; Jeong, E.M.; Kim, I. Transglutaminase 2 mediates UVB-induced matrix metalloproteinase-1 expression by inhibiting nuclear p65 degradation in dermal fibroblasts. Exp. Dermatol. 2021, 31, 743–752. [Google Scholar] [CrossRef] [PubMed]
- Quan, T.; Qin, Z.; Xia, W.; Shao, Y.; Voorhees, J.J.; Fisher, G.J. Matrix-Degrading Metalloproteinases in Photoaging. J. Investig. Dermatol. Symp. Proc. 2009, 14, 20–24. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kim, D.J.; Iwasaki, A.; Chien, A.L.; Kang, S. UVB-mediated DNA damage induces matrix metalloproteinases to promote photoaging in an AhR- and SP1-dependent manner. J. Clin. Investig. 2022, 7, e156344. [Google Scholar] [CrossRef]
- Levin, M.; Udi, Y.; Solomonov, I.; Sagi, I. Next generation matrix metalloproteinase inhibitors—Novel strategies bring new prospects. Biochim. Biophys. Acta Mol. Cell Res. 2017, 1864, 1927–1939. [Google Scholar] [CrossRef]
- Ashworth, J.L.; Murphy, G.; Rock, M.J.; Sherratt, M.J.; Shapiro, S.D.; Shuttleworth, C.A.; Kielty, C.M. Fibrillin degradation by matrix metalloproteinases: Implications for connective tissue remodelling. Biochem. J. 1999, 340, 171–181. [Google Scholar] [CrossRef]
- Aldag, C.; Teixeira, D.N.; Leventhal, P.S. Skin rejuvenation using cosmetic products containing growth factors, cytokines, and matrikines: A review of the literature. Clin. Cosmet. Investig. Dermatol. 2016, 9, 411–419. [Google Scholar] [CrossRef] [Green Version]
- Działo, M.; Mierziak, J.; Korzun, U.; Preisner, M.; Szopa, J.; Kulma, A. The Potential of Plant Phenolics in Prevention and Therapy of Skin Disorders. Int. J. Mol. Sci. 2016, 17, 160. [Google Scholar] [CrossRef] [Green Version]
- Gorouhi, F.; Maibach, H.I. Role of topical peptides in preventing or treating aged skin. Int. J. Cosmet. Sci. 2009, 31, 327–345. [Google Scholar] [CrossRef]
- Amar, S.; Minond, D.; Fields, G.B. Clinical Implications of Compounds Designed to Inhibit ECM-Modifying Metalloproteinases. Proteomics 2017, 17, 1600389. [Google Scholar] [CrossRef]
- Stern, R.; Maibach, H.I. Hyaluronan in skin: Aspects of aging and its pharmacologic modulation. Clin. Dermatol. 2008, 26, 106–122. [Google Scholar] [CrossRef] [PubMed]
- Mukherjee, S.; Date, A.; Patravale, V.; Korting, H.C.; Roeder, A.; Weindl, G. Retinoids in the treatment of skin aging: An overview of clinical efficacy and safety. Clin. Interv. Aging 2006, 1, 327–348. [Google Scholar] [CrossRef]
- Davinelli, S.; Nielsen, M.E.; Scapagnini, G. Astaxanthin in Skin Health, Repair, and Disease: A Comprehensive Review. Nutrients 2018, 10, 522. [Google Scholar] [CrossRef] [Green Version]
- Hipkiss, A.R.; Baye, E.; de Courten, B. Carnosine and the processes of ageing. Maturitas 2016, 93, 28–33. [Google Scholar] [CrossRef]
- Alves-Rodrigues, A.; Shao, A. The science behind lutein. Toxicol. Lett. 2004, 150, 57–83. [Google Scholar] [CrossRef] [PubMed]
- Knott, A.; Achterberg, V.; Smuda, C.; Mielke, H.; Sperling, G.; Dunckelmann, K.; Vogelsang, A.; Krüger, A.; Schwengler, H.; Behtash, M.; et al. Topical treatment with coenzyme Q10-containing formulas improves skin’s Q10 level and provides antioxidative effects. Biofactors 2015, 41, 383–390. [Google Scholar] [CrossRef] [PubMed]
- Bhagavan, H.N.; Chopra, R.K. Coenzyme Q10: Absorption, tissue uptake, metabolism and pharmacokinetics. Free. Radic. Res. 2006, 40, 445–453. [Google Scholar] [CrossRef] [PubMed]
- Park, S.-J.; Ahmad, F.; Philp, A.; Baar, K.; Williams, T.; Luo, H.; Ke, H.; Rehmann, H.; Taussig, R.; Brown, A.L.; et al. Resveratrol Ameliorates Aging-Related Metabolic Phenotypes by Inhibiting cAMP Phosphodiesterases. Cell 2012, 148, 421–433. [Google Scholar] [CrossRef] [Green Version]
- Sorsa, T.; Tjäderhane, L.; Konttinen, Y.T.; Lauhio, A.; Salo, T.; Lee, H.; Golub, L.M.; Brown, D.L.; Mäntylä, P. Matrix metalloproteinases: Contribution to pathogenesis, diagnosis and treatment of periodontal inflammation. Ann. Med. 2006, 38, 306–321. [Google Scholar] [CrossRef]
- Antonio, R.C.; Ceron, C.S.; Rizzi, E.; Coelho, E.B.; Tanus-Santos, J.E.; Gerlach, R.F. Antioxidant effect of doxycycline decreases MMP activity and blood pressure in SHR. Mol. Cell. Biochem. 2013, 386, 99–105. [Google Scholar] [CrossRef] [PubMed]
- Dormán, G.; Cseh, S.; Hajdú, I.; Barna, L.; Kónya, D.; Kupai, K.; Kovács, L.; Ferdinandy, P. Matrix Metalloproteinase Inhibitors: A critical appraisal of design principles and proposed therapeutic utility. Drugs 2010, 70, 949–964. [Google Scholar] [CrossRef] [PubMed]
- Chiarelli, N.; Zoppi, N.; Venturini, M.; Capitanio, D.; Gelfi, C.; Ritelli, M.; Colombi, M. Matrix Metalloproteinases Inhibition by Doxycycline Rescues Extracellular Matrix Organization and Partly Reverts Myofibroblast Differentiation in Hypermobile Ehlers-Danlos Syndrome Dermal Fibroblasts: A Potential Therapeutic Target? Cells 2021, 10, 3236. [Google Scholar] [CrossRef] [PubMed]
- Brown, M.; Jones, S.A. Hyaluronic acid: A unique topical vehicle for the localized delivery of drugs to the skin. J. Eur. Acad. Dermatol. Venereol. 2005, 19, 308–318. [Google Scholar] [CrossRef]
- Humbert, P.G.; Haftek, M.; Creidi, P.; Lapière, C.; Nusgens, B.; Richard, A.; Schmitt, D.; Rougier, A.; Zahouani, H. Topical ascorbic acid on photoaged skin. Clinical, topographical and ultrastructural evaluation: Double-blind study vs. placebo. Exp. Dermatol. 2003, 12, 237–244. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Raschke, T.; Koop, U.; Düsing, H.-J.; Filbry, A.; Sauermann, K.; Jaspers, S.; Wenck, H.; Wittern, K.-P. Topical Activity of Ascorbic Acid: From in vitro Optimization to in vivo Efficacy. Ski. Pharmacol. Physiol. 2004, 17, 200–206. [Google Scholar] [CrossRef]
- Wu, S.; Gao, J.; Dinh, Q.T.; Chen, C.; Fimmel, S. IL-8 production and AP-1 transactivation induced by UVA in human keratinocytes: Roles of d-α-tocopherol. Mol. Immunol. 2008, 45, 2288–2296. [Google Scholar] [CrossRef] [PubMed]
- Žmitek, K.; Pogačnik, T.; Mervic, L.; Žmitek, J.; Pravst, I. The effect of dietary intake of coenzyme Q10 on skin parameters and condition: Results of a randomised, placebo-controlled, double-blind study. BioFactors 2017, 43, 132–140. [Google Scholar] [CrossRef]
- Draelos, Z.D. Nutrition and enhancing youthful-appearing skin. Clin. Dermatol. 2010, 28, 400–408. [Google Scholar] [CrossRef]
- Cao, C.; Xiao, Z.; Wu, Y.; Ge, C. Diet and Skin Aging—From the Perspective of Food Nutrition. Nutrients 2020, 12, 870. [Google Scholar] [CrossRef] [Green Version]
- Hadgraft, J. Passive enhancement strategies in topical and transdermal drug delivery. Int. J. Pharm. 1999, 184, 1–6. [Google Scholar] [CrossRef]
- Chou, H.-Y.; Lee, C.; Pan, J.-L.; Wen, Z.-H.; Huang, S.-H.; Lan, C.-W.J.; Liu, W.-T.; Hour, T.-C.; Hseu, Y.-C.; Hwang, B.H.; et al. Enriched Astaxanthin Extract from Haematococcus pluvialis Augments Growth Factor Secretions to Increase Cell Proliferation and Induces MMP1 Degradation to Enhance Collagen Production in Human Dermal Fibroblasts. Int. J. Mol. Sci. 2016, 17, 955. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bastianetto, S.; Dumont, Y.; Duranton, A.; Vercauteren, F.; Breton, L.; Quirion, R. Protective Action of Resveratrol in Human Skin: Possible Involvement of Specific Receptor Binding Sites. PLoS ONE 2010, 5, e12935. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Matsui, M.S.; Hsia, A.; Miller, J.D.; Hanneman, K.; Scull, H.; Cooper, K.D.; Baron, E. Non-Sunscreen Photoprotection: Antioxidants Add Value to a Sunscreen. J. Investig. Dermatol. Symp. Proc. 2009, 14, 56–59. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chen, J.; Li, Y.; Zhu, Q.; Li, T.; Lu, H.; Wei, N.; Huang, Y.; Shi, R.; Ma, X.; Wang, X.; et al. Anti-skin-aging effect of epigallocatechin gallate by regulating epidermal growth factor receptor pathway on aging mouse model induced by d -Galactose. Mech. Ageing Dev. 2017, 164, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Begum, N.; Prasad, N.R. Apigenin, a dietary antioxidant, modulates gamma radiation-induced oxidative damages in human peripheral blood lymphocytes. Biomed. Prev. Nutr. 2012, 2, 16–24. [Google Scholar] [CrossRef]
- Shen, L.-N.; Zhang, Y.-T.; Wang, Q.; Xu, L.; Feng, N. Enhanced in vitro and in vivo skin deposition of apigenin delivered using ethosomes. Int. J. Pharm. 2014, 460, 280–288. [Google Scholar] [CrossRef]
- Park, C.-H.; Min, S.-Y.; Yu, H.-W.; Kim, K.; Kim, S.; Lee, H.-J.; Kim, J.-H.; Park, Y.-J. Effects of Apigenin on RBL-2H3, RAW264.7, and HaCaT Cells: Anti-Allergic, Anti-Inflammatory, and Skin-Protective Activities. Int. J. Mol. Sci. 2020, 21, 4620. [Google Scholar] [CrossRef]
- Pourzand, C.; Albieri-Borges, A.; Raczek, N.N. Shedding a New Light on Skin Aging, Iron- and Redox-Homeostasis and Emerging Natural Antioxidants. Antioxidants 2022, 11, 471. [Google Scholar] [CrossRef]
- Kimura, Y.; Sumiyoshi, M. Effects of baicalein and wogonin isolated from Scutellaria baicalensis roots on skin damage in acute UVB-irradiated hairless mice. Eur. J. Pharmacol. 2011, 661, 124–132. [Google Scholar] [CrossRef]
- Noordam, R.; Gunn, D.; Tomlin, C.; Maier, A.; Griffiths, T.; Catt, S.; Ogden, S.; Slagboom, P.; Westendorp, R.; Griffiths, C.; et al. Serum insulin-like growth factor 1 and facial ageing: High levels associate with reduced skin wrinkling in a cross-sectional study. Br. J. Dermatol. 2013, 168, 533–538. [Google Scholar] [CrossRef] [PubMed]
- Forsberg, E.A.; Botusan, I.R.; Wang, J.; Peters, V.; Ansurudeen, I.; Brismar, K.; Catrina, S.B. Carnosine decreases IGFBP1 production in db/db mice through suppression of HIF-1. J. Endocrinol. 2015, 225, 159–167. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lintner, K. Peptides and proteins. In Cosmetic Dermatology: Products and Procedures; John Wiley & Sons, Inc.: Hoboken, NJ, USA, 2010; pp. 292–301. [Google Scholar] [CrossRef]
- Farwick, M.; Grether-Beck, S.; Marini, A.; Maczkiewitz, U.; Lange, J.; Köhler, T.; Lersch, P.; Falla, T.; Felsner, I.; Brenden, H.; et al. Bioactive tetrapeptide GEKG boosts extracellular matrix formation: In vitro and in vivo molecular and clinical proof. Exp. Dermatol. 2011, 20, 602–604. [Google Scholar] [CrossRef]
- Robinson, L.R.; Fitzgerald, N.C.; Doughty, D.G.; Dawes, N.C.; Berge, C.A.; Bissett, D.L. Topical palmitoyl pentapeptide provides improvement in photoaged human facial skin1. Int. J. Cosmet. Sci. 2005, 27, 155–160. [Google Scholar] [CrossRef]
- Maquart, F.X.; Bellon, G.; Chaqour, B.; Wegrowski, J.; Patt, L.M.; Trachy, R.E.; Monboisse, J.C.; Chastang, F.; Birembaut, P.; Gillery, P. In vivo stimulation of connective tissue accumulation by the tripeptide-copper complex glycyl-L-histidyl-L-lysine-Cu2+ in rat experimental wounds. J. Clin. Investig. 1993, 92, 2368–2376. [Google Scholar] [CrossRef]
- Siméon, A.; Emonard, H.; Hornebeck, W.; Maquart, F. The tripeptide-copper complex glycyl-L-histidyl-L- expression by Þbroblast cultures. Life Sci. 2000, 67, 2257–2265. [Google Scholar] [CrossRef]
- Li, H.; Low, Y.S.J.; Chong, H.P.; Zin, M.T.; Lee, C.-Y.; Li, B.; Leolukman, M.; Kang, L. Microneedle-Mediated Delivery of Copper Peptide Through Skin. Pharm. Res. 2015, 32, 2678–2689. [Google Scholar] [CrossRef] [PubMed]
- Katayama, K.; Armendariz-Borunda, J.; Raghow, R.; Kang, A.; Seyer, J. A pentapeptide from type I procollagen promotes extracellular matrix production. J. Biol. Chem. 1993, 268, 9941–9944. [Google Scholar] [CrossRef]
- Südel, K.M.; Venzke, K.; Mielke, H.; Breitenbach, U.; Mundt, C.; Jaspers, S.; Koop, U.; Sauermann, K.; Knußmann-Hartig, E.; Moll, I.; et al. Novel Aspects of Intrinsic and Extrinsic Aging of Human Skin: Beneficial Effects of Soy Extract. Photochem. Photobiol. 2005, 81, 581. [Google Scholar] [CrossRef]
- Barba, C.; Méndez, S.; Roddick-Lanzilotta, A.; Kelly, R.; Parra, J.L.; Coderch, L. Cosmetic effectiveness of topically applied hydrolysed keratin peptides and lipids derived from wool. Ski. Res. Technol. 2007, 14, 243–248. [Google Scholar] [CrossRef]
- Ehrlich, M.; Rao, J.; Pabby, A.; Goldman, M.P. Improvement in the Appearance of Wrinkles with Topical Transforming Growth Factor beta1 and l-Ascorbic Acid. Dermatol. Surg. 2006, 32, 618–625. [Google Scholar] [CrossRef]
- Gold, M.H.; Sensing, W.; Biron, J.A. A topical regimen improves skin healing and aesthetic outcomes when combined with a radiofrequency microneedling procedure. J. Cosmet. Dermatol. 2019, 18, 1280–1289. [Google Scholar] [CrossRef]
- Sundaram, H.; Gold, M.; Waldorf, H.; Lupo, M.; Nguyen, V.L.; Karnik, J. Pilot, Multicenter, Open-Label Evaluation of Safety, Tolerability and Efficacy of a Novel, Topical Multipotent Growth Factor Formulation for the Periorbital Region. J. Drugs Dermatol. 2015, 14, 1410–1417. [Google Scholar] [PubMed]
- Dreher, F. A Novel Matrikine-Like Micro-Protein Complex (MPC) Technology for Topical Skin Rejuvenation. J. Drugs Dermatol. 2016, 15, 457–464. [Google Scholar] [PubMed]
- Puig, A.; Antón, J.M.G.; Mangues, M. A new decorin-like tetrapeptide for optimal organization of collagen fibres. Int. J. Cosmet. Sci. 2008, 30, 97–104. [Google Scholar] [CrossRef] [PubMed]
- Alcantara, L. Cosmetic composition containing halomonas ferment extract, and use thereof. U.S Patent No. US10,413,501 B2, 17 September 2019. [Google Scholar]
- Trookman, N.S.; Rizer, R.L.; Ford, R.; Ho, E.; Gotz, V. Immediate and long-term clinical benefits of a topical treatment for facial lines and wrinkles. J. Clin. Aesthet. Dermatol. 2009, 2, 38–43. [Google Scholar] [CrossRef] [PubMed]
- Jariwala, N.; Ozols, M.; Bell, M.; Bradley, E.; Gilmore, A.; Debelle, L.; Sherratt, M.J. Matrikines as mediators of tissue remodelling. Adv. Drug Deliv. Rev. 2022, 185, 114240. [Google Scholar] [CrossRef]
- Sampaio, C.; Costa, J.; Ferreira, J.J. Clinical comparability of marketed formulations of botulinum toxin. Mov. Disord. 2004, 19, S129–S136. [Google Scholar] [CrossRef]
- Samizadeh, S.; De Boulle, K. Botulinum neurotoxin formulations: Overcoming the confusion. Clin. Cosmet. Investig. Dermatol. 2018, 11, 273–287. [Google Scholar] [CrossRef] [Green Version]
- Pfaffen, S.; Hemmerle, T.; Weber, M.; Neri, D. Isolation and characterization of human monoclonal antibodies specific to MMP-1A, MMP-2 and MMP-3. Exp. Cell Res. 2010, 316, 836–847. [Google Scholar] [CrossRef]
- Yarosh, D.; Klein, J.; O’Connor, A.; Hawk, J.; Rafal, E.; Wolf, P. Effect of topically applied T4 endonuclease V in liposomes on skin cancer in xeroderma pigmentosum: A randomised study. Lancet 2001, 357, 926–929. [Google Scholar] [CrossRef] [PubMed]
- Müller, R.H.; Radtke, M.; Wissing, S.A. Solid lipid nanoparticles (SLN) and nanostructured lipid carriers (NLC) in cosmetic and dermatological preparations. Adv. Drug Deliv. Rev. 2002, 54, S131–S155. [Google Scholar] [CrossRef] [PubMed]
- Prow, T.W.; Grice, J.E.; Lin, L.L.; Faye, R.; Butler, M.; Becker, W.; Wurm, E.M.T.; Yoong, C.; Robertson, T.A.; Soyer, H.P.; et al. Nanoparticles and microparticles for skin drug delivery. Adv. Drug Deliv. Rev. 2011, 63, 470–491. [Google Scholar] [CrossRef] [PubMed]
- Montenegro, L.; Lai, F.; Offerta, A.; Sarpietro, M.G.; Micicchè, L.; Maccioni, A.M.; Valenti, D.; Fadda, A.M. From nanoemulsions to nanostructured lipid carriers: A relevant development in dermal delivery of drugs and cosmetics. J. Drug Deliv. Sci. Technol. 2016, 32, 100–112. [Google Scholar] [CrossRef]
- Sala, M.; Diab, R.; Elaissari, A.; Fessi, H. Lipid nanocarriers as skin drug delivery systems: Properties, mechanisms of skin interactions and medical applications. Int. J. Pharm. 2018, 535, 1–17. [Google Scholar] [CrossRef]
- Garcês, A.; Amaral, M.; Lobo, J.S.; Silva, A. Formulations based on solid lipid nanoparticles (SLN) and nanostructured lipid carriers (NLC) for cutaneous use: A review. Eur. J. Pharm. Sci. 2018, 112, 159–167. [Google Scholar] [CrossRef]
- Ammala, A. Biodegradable polymers as encapsulation materials for cosmetics and personal care markets. Int. J. Cosmet. Sci. 2012, 35, 113–124. [Google Scholar] [CrossRef]
- Kaur, I.P.; Kapila, M.; Agrawal, R. Role of novel delivery systems in developing topical antioxidants as therapeutics to combat photoageing. Ageing Res. Rev. 2007, 6, 271–288. [Google Scholar] [CrossRef]
- Munin, A.; Edwards-Lévy, F. Encapsulation of natural polyphenolic compounds; a review. Pharmaceutics 2011, 3, 793–829. [Google Scholar] [CrossRef] [Green Version]
- Myriam, M.; Sabatier, M.; Steiling, H.; Williamson, G. Skin bioavailability of dietary vitamin E, carotenoids, polyphenols, vitamin C., zinc and selenium. Br. J. Nutr. 2006, 96, 227–238. [Google Scholar] [CrossRef] [Green Version]
- Tarbox, T.N.; Watts, A.B.; Cui, Z.; Williams, R.O. An update on coating/manufacturing techniques of microneedles. Drug Deliv. Transl. Res. 2018, 8, 1828–1843. [Google Scholar] [CrossRef]
- Bhatnagar, S.; Dave, K.; Venuganti, V.V.K. Microneedles in the clinic. J. Control. Release 2017, 260, 164–182. [Google Scholar] [CrossRef] [PubMed]
- Callender, S.P.; Mathews, J.A.; Kobernyk, K.; Wettig, S.D. Microemulsion utility in pharmaceuticals: Implications for multi-drug delivery. Int. J. Pharm. 2017, 526, 425–442. [Google Scholar] [CrossRef] [PubMed]
- Souto, E.B.; Cano, A.; Martins-Gomes, C.; Coutinho, T.E.; Zielińska, A.; Silva, A.M. Microemulsions and Nanoemulsions in Skin Drug Delivery. Bioengineering 2022, 9, 158. [Google Scholar] [CrossRef]
- McClements, D.J. Nanoemulsions versus microemulsions: Terminology, differences, and similarities. Soft Matter 2012, 8, 1719–1729. [Google Scholar] [CrossRef]
- Tang, S.Y.; Sivakumar, M.; Ng, A.M.-H.; Shridharan, P. Anti-inflammatory and analgesic activity of novel oral aspirin-loaded nanoemulsion and nano multiple emulsion formulations generated using ultrasound cavitation. Int. J. Pharm. 2012, 430, 299–306. [Google Scholar] [CrossRef]
- Anton, N.; Benoit, J.-P.; Saulnier, P. Design and production of nanoparticles formulated from nano-emulsion templates—A review. J. Control. Release 2008, 128, 185–199. [Google Scholar] [CrossRef]
- Ajazuddin; Alexander, A.; Khichariya, A.; Gupta, S.; Patel, R.J.; Giri, T.K.; Tripathi, D.K. Recent expansions in an emergent novel drug delivery technology: Emulgel. J. Control. Release 2013, 171, 122–132. [Google Scholar] [CrossRef]
- Liu, M.; Chen, S.; Zhang, Z.; Li, H.; Sun, G.; Yin, N.; Wen, J. Anti-ageing peptides and proteins for topical applications: A review. Pharm. Dev. Technol. 2022, 27, 108–125. [Google Scholar] [CrossRef]
- Dahiya, S.; Dahiya, R. Potential of Colloidal Carriers for Nanocosmeceutical Applications; Elsevier Inc.: Amsterdam, The Netherlands, 2022. [Google Scholar]
- Akbarzadeh, A.; Rezaei-sadabady, R.; Davaran, S.; Joo, S.W.; Zarghami, N. Liposome: Classification, prepNew aspects of liposomesaration, and applications. Nanoscale Res. Lett. 2013, 8, 102. [Google Scholar] [CrossRef] [Green Version]
- Moghassemi, S.; Hadjizadeh, A. Nano-niosomes as nanoscale drug delivery systems: An illustrated review. J. Control. Release 2014, 185, 22–36. [Google Scholar] [CrossRef]
- Touitou, E.; Dayan, N.; Bergelson, L.; Godin, B.; Eliaz, M. Ethosomes—Novel vesicular carriers for enhanced delivery: Characterization and skin penetration properties. J. Control. Release 2000, 65, 403–418. [Google Scholar] [CrossRef] [PubMed]
- Jain, S.; Jain, P.; Umamaheshwari, R.B.; Jain, N.K. Transfersomes—A Novel Vesicular Carrier for Enhanced Transdermal Delivery: Development, Characterization, and Performance Evaluation. Drug Dev. Ind. Pharm. 2003, 29, 1013–1026. [Google Scholar] [CrossRef]
- Wang, X.; Liu, K.; Fu, S.; Wu, X.; Xiao, L.; Yang, Y.; Zhang, Z.; Lu, Q. Silk Nanocarrier with Tunable Size to Improve Transdermal Capacity for Hydrophilic and Hydrophobic Drugs. ACS Appl. Bio Mater. 2023, 6, 74–82. [Google Scholar] [CrossRef] [PubMed]
- Aldawood, F.K.; Andar, A.; Desai, S. A Comprehensive Review of Microneedles: Types, Materials, Processes, Characterizations and Applications. Polymers 2021, 13, 2815. [Google Scholar] [CrossRef] [PubMed]
- Aich, K.; Singh, T.; Dang, S. Advances in microneedle-based transdermal delivery for drugs and peptides. Drug Deliv. Transl. Res. 2022, 12, 1556–1568. [Google Scholar] [CrossRef]
- Goffin, V.; Henry, F.; Piérard-Franchimont, C.; Piérard, G.E. Topical Retinol and the Stratum corneum Response to an Environmental Threat. Ski. Pharmacol. Physiol. 1997, 10, 85–89. [Google Scholar] [CrossRef]
- Kaci, M.; Belhaffef, A.; Meziane, S.; Dostert, G.; Menu, P.; Velot, E.; Desobry, S.; Arab-Tehrany, E. Nanoemulsions and topical creams for the safe and effective delivery of lipophilic antioxidant coenzyme Q10. Colloids Surf. B Biointerfaces 2018, 167, 165–175. [Google Scholar] [CrossRef]
- Pardeike, J.; Hommoss, A.; Müller, R.H. Lipid nanoparticles (SLN, NLC) in cosmetic and pharmaceutical dermal products. Int. J. Pharm. 2009, 366, 170–184. [Google Scholar] [CrossRef]
- Stefanov, S.R.; Andonova, V.Y. Lipid Nanoparticulate Drug Delivery Systems: Recent Advances in the Treatment of Skin Disorders. Pharmaceuticals 2021, 14, 1083. [Google Scholar] [CrossRef]
- Correa, L.; Meirelles, G.D.C.; Balestrin, L.; de Souza, P.O.; Moreira, J.C.F.; Schuh, R.S.; Bidone, J.; von Poser, G.L.; Teixeira, H.F. In vitro protective effect of topical nanoemulgels containing Brazilian red propolis benzophenones against UV-induced skin damage. Photochem. Photobiol. Sci. 2020, 19, 1460–1469. [Google Scholar] [CrossRef]
- Jeon, H.S.; Seo, J.E.; Kim, M.S.; Kang, M.H.; Oh, D.H.; Jeon, S.O.; Jeong, S.H.; Choi, Y.W.; Lee, S. A retinyl palmitate-loaded solid lipid nanoparticle system: Effect of surface modification with dicetyl phosphate on skin permeation in vitro and anti-wrinkle effect in vivo. Int. J. Pharm. 2013, 452, 311–320. [Google Scholar] [CrossRef]
- Souto, E.B.; Jäger, E.; Jäger, A.; Štěpánek, P.; Cano, A.; Viseras, C.; Barbosa, R.D.M.; Chorilli, M.; Zielińska, A.; Severino, P.; et al. Lipid Nanomaterials for Targeted Delivery of Dermocosmetic Ingredients: Advances in Photoprotection and Skin Anti-Aging. Nanomaterials 2022, 12, 377. [Google Scholar] [CrossRef] [PubMed]
- Piccioni, A.; Fargnoli, M.C.; Schoinas, S.; Suppa, M.; Frascione, P.; Ginebri, A.; Chimenti, S.; Peris, K. Efficacy and tolerability of 5-aminolevulinic acid 0.5% liposomal spray and intense pulsed light in wrinkle reduction of photodamaged skin. J. Dermatol. Treat. 2011, 22, 247–253. [Google Scholar] [CrossRef] [PubMed]
- Han, F.; Luo, D.; Qu, W.; Chen, D.; Hong, Y.; Sheng, J.; Yang, X.; Liu, W. Nanoliposomes codelivering bioactive peptides produce enhanced anti-aging effect in human skin. J. Drug Deliv. Sci. Technol. 2020, 57, 101693. [Google Scholar] [CrossRef]
- Bonaparte, J.P.; Ellis, D.; Quinn, J.G.; Rabski, J.; Hutton, B. A Comparative Assessment of Three Formulations of Botulinum Toxin Type A for Facial Rhytides. Plast. Reconstr. Surg. 2016, 137, 1125–1140. [Google Scholar] [CrossRef] [PubMed]
- Trommer, H.; Neubert, R.H.H. Overcoming the Stratum Corneum: The Modulation of Skin Penetration. Ski. Pharmacol. Physiol. 2006, 19, 106–121. [Google Scholar] [CrossRef]
- Lane, A.M.E.; Santos, P.; Watkinson, A.C.; Hadgraft, J. Transdermal and Topical Drug Delivery; John Wiley & Sons, Inc.: Hoboken, NJ, USA, 2012. [Google Scholar]
- Moser, K.; Kriwet, K.; Naik, A.; Kalia, Y.N.; Guy, R.H. Passive skin penetration enhancement and its quantification in vitro. Eur. J. Pharm. Biopharm. 2001, 52, 103–112. [Google Scholar] [CrossRef]
- Larrañeta, E.; Lutton, R.E.M.; Woolfson, A.D.; Donnelly, R.F. Microneedle arrays as transdermal and intradermal drug delivery systems: Materials science, manufacture and commercial development. Mater. Sci. Eng. R Rep. 2016, 104, 1–32. [Google Scholar] [CrossRef] [Green Version]
- Levin, E.C.; Gupta, R.; Shinkai, K. Duration of Remission of Topical Psoriasis Therapies. Psoriasis Forum 2013, 19, 22–33. [Google Scholar] [CrossRef]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Altay Benetti, A.; Tarbox, T.; Benetti, C. Current Insights into the Formulation and Delivery of Therapeutic and Cosmeceutical Agents for Aging Skin. Cosmetics 2023, 10, 54. https://doi.org/10.3390/cosmetics10020054
Altay Benetti A, Tarbox T, Benetti C. Current Insights into the Formulation and Delivery of Therapeutic and Cosmeceutical Agents for Aging Skin. Cosmetics. 2023; 10(2):54. https://doi.org/10.3390/cosmetics10020054
Chicago/Turabian StyleAltay Benetti, Ayça, Tamara Tarbox, and Camillo Benetti. 2023. "Current Insights into the Formulation and Delivery of Therapeutic and Cosmeceutical Agents for Aging Skin" Cosmetics 10, no. 2: 54. https://doi.org/10.3390/cosmetics10020054
APA StyleAltay Benetti, A., Tarbox, T., & Benetti, C. (2023). Current Insights into the Formulation and Delivery of Therapeutic and Cosmeceutical Agents for Aging Skin. Cosmetics, 10(2), 54. https://doi.org/10.3390/cosmetics10020054






