Cannabinoids for the Treatment of Hair, Scalp, and Skin Disorders: A Systematic Review
Abstract
:1. Introduction
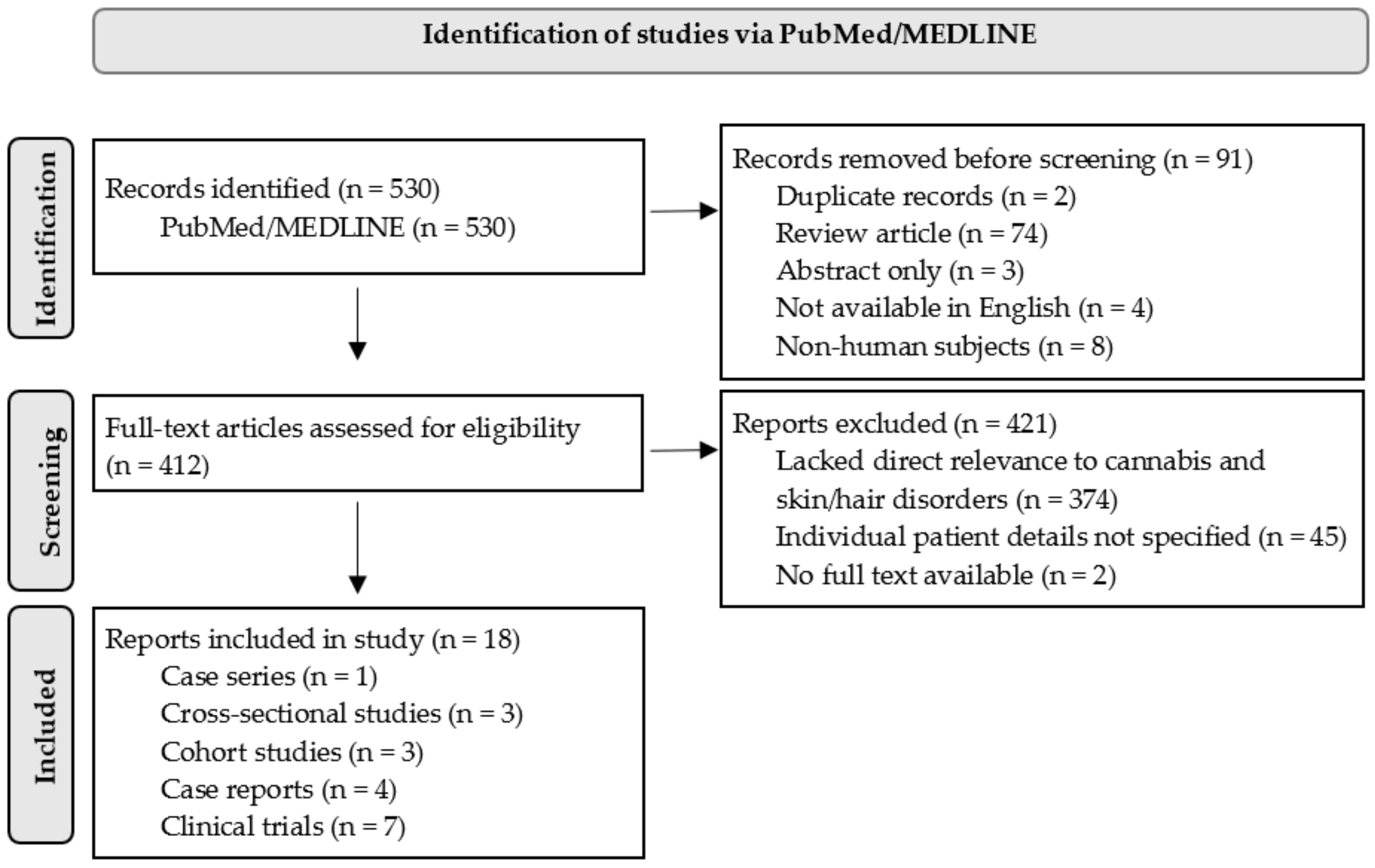
2. Methods
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Robinson, E.; Murphy, E.; Friedman, A. Knowledge, Attitudes, and Perceptions of Cannabinoids in the Dermatology Community. J. Drugs Dermatol. 2018, 17, 1273–1278. [Google Scholar] [PubMed]
- Wang, J.V.; Shah, S.; Albornoz, C.A.; Saedi, N. Consumer interest in topical cannabidiol: An examination of online search trends from 2015 to 2019. Clin. Dermatol. 2021, 39, 1014–1017. [Google Scholar] [CrossRef]
- Yeroushalmi, S.; Nelson, K.; Sparks, A.; Friedman, A. Perceptions and recommendation behaviors of dermatologists for medical cannabis: A pilot survey. Complement. Ther. Med. 2020, 55, 102552. [Google Scholar] [CrossRef] [PubMed]
- Gupta, A.K.; Talukder, M. Cannabinoids for skin diseases and hair regrowth. J. Cosmet. Dermatol. 2021, 20, 2703–2711. [Google Scholar] [CrossRef] [PubMed]
- Werth, V.P.; Hejazi, E.; Pena, S.M.; Haber, J.; Zeidi, M.; Reddy, N.; Okawa, J.; Feng, R.; Bashir, M.M.; Gebre, K.; et al. Safety and Efficacy of Lenabasum, a Cannabinoid Receptor Type 2 Agonist, in Patients with Dermatomyositis with Refractory Skin Disease: A Randomized Clinical Trial. J. Investig. Dermatol. 2022, 142, 2651–2659.e1. [Google Scholar] [CrossRef] [PubMed]
- Zou, S.; Kumar, U. Cannabinoid Receptors and the Endocannabinoid System: Signaling and Function in the Central Nervous System. Int. J. Mol. Sci. 2018, 19, 833. [Google Scholar] [CrossRef]
- Ashton, J.C.; Glass, M. The cannabinoid CB2 receptor as a target for inflammation-dependent neurodegeneration. Curr. Neuropharmacol. 2007, 5, 73–80. [Google Scholar] [CrossRef]
- Motwani, M.P.; Bennett, F.; Norris, P.C.; Maini, A.A.; George, M.J.; Newson, J.; Henderson, A.; Hobbs, A.J.; Tepper, M.; White, B.; et al. Potent Anti-Inflammatory and Pro-Resolving Effects of Anabasum in a Human Model of Self-Resolving Acute Inflammation. Clin. Pharmacol. Ther. 2018, 104, 675–686. [Google Scholar] [CrossRef]
- Baswan, S.M.; Klosner, A.E.; Glynn, K.; Rajgopal, A.; Malik, K.; Yim, S.; Stern, N. Therapeutic Potential of Cannabidiol (CBD) for Skin Health and Disorders. Clin. Cosmet. Investig. Dermatol. 2020, 13, 927–942. [Google Scholar] [CrossRef]
- Solmi, M.; De Toffol, M.; Kim, J.Y.; Choi, M.J.; Stubbs, B.; Thompson, T.; Firth, J.; Miola, A.; Croatto, G.; Baggio, F.; et al. Balancing risks and benefits of cannabis use: Umbrella review of meta-analyses of randomised controlled trials and observational studies. BMJ 2023, 382, e072348. [Google Scholar] [CrossRef]
- Petrosino, S.; Verde, R.; Vaia, M.; Allara, M.; Iuvone, T.; Di Marzo, V. Anti-inflammatory Properties of Cannabidiol, a Nonpsychotropic Cannabinoid, in Experimental Allergic Contact Dermatitis. J. Pharmacol. Exp. Ther. 2018, 365, 652–663. [Google Scholar] [CrossRef]
- Torres-Moreno, M.C.; Papaseit, E.; Torrens, M.; Farre, M. Assessment of Efficacy and Tolerability of Medicinal Cannabinoids in Patients With Multiple Sclerosis: A Systematic Review and Meta-analysis. JAMA Netw. Open 2018, 1, e183485. [Google Scholar] [CrossRef] [PubMed]
- Couch, D.G.; Maudslay, H.; Doleman, B.; Lund, J.N.; O’Sullivan, S.E. The Use of Cannabinoids in Colitis: A Systematic Review and Meta-Analysis. Inflamm. Bowel. Dis. 2018, 24, 680–697. [Google Scholar] [CrossRef]
- Wilkinson, J.D.; Williamson, E.M. Cannabinoids inhibit human keratinocyte proliferation through a non-CB1/CB2 mechanism and have a potential therapeutic value in the treatment of psoriasis. J. Dermatol. Sci. 2007, 45, 87–92. [Google Scholar] [CrossRef]
- State Medical Cannabis Laws: National Conference of State Legislatures. Available online: https://www.ncsl.org/health/state-medical-cannabis-laws (accessed on 1 February 2023).
- Hopp, D.C.; Belfer, I.; Shurtleff, D. Cannabis (Marijuana) and Cannabinoids: What You Need To Know. National Center for Complementary and Integrative Health: National Institutes of Health. Available online: https://www.nccih.nih.gov/health/cannabis-marijuana-and-cannabinoids-what-you-need-to-know (accessed on 1 February 2023).
- Gao, Y.; Li, Y.; Tan, Y.; Liu, W.; Ouaddi, S.; McCoy, J.; Kovacevic, M.; Situm, M.; Stanimirovic, A.; Li, M.; et al. Novel cannabidiol aspartame combination treatment (JW-100) significantly reduces ISGA score in atopic dermatitis: Results from a randomized double-blinded placebo-controlled interventional study. J. Cosmet. Dermatol. 2022, 21, 1647–1650. [Google Scholar] [CrossRef] [PubMed]
- Maghfour, J.; Rundle, C.W.; Rietcheck, H.R.; Dercon, S.; Lio, P.; Mamo, A.; Runion, T.M.; Fernandez, J.; Kahn, J.; Dellavalle, R.P.; et al. Assessing the effects of topical cannabidiol in patients with atopic dermatitis. Dermatol. Online J. 2021, 27, 15. [Google Scholar] [CrossRef]
- Callaway, J.; Schwab, U.; Harvima, I.; Halonen, P.; Mykkanen, O.; Hyvonen, P.; Jarvinen, T. Efficacy of dietary hempseed oil in patients with atopic dermatitis. J. Dermatol. Treat. 2005, 16, 87–94. [Google Scholar] [CrossRef]
- Puaratanaarunkon, T.; Sittisaksomjai, S.; Sivapornpan, N.; Pongcharoen, P.; Chakkavittumrong, P.; Ingkaninan, K.; Temkitthawon, P.; Promgool, T.; Waranuch, N.; Asawanonda, P. Topical cannabidiol-based treatment for psoriasis: A dual-centre randomized placebo-controlled study. J. Eur. Acad. Dermatol. Venereol. 2022, 36, e718–e720. [Google Scholar] [CrossRef]
- Friedman, A.J.; Momeni, K.; Kogan, M. Topical Cannabinoids for the Management of Psoriasis Vulgaris: Report of a Case and Review of the Literature. J. Drugs Dermatol. 2020, 19, 795. [Google Scholar] [CrossRef]
- Vincenzi, C.; Tosti, A. Efficacy and Tolerability of a Shampoo Containing Broad-Spectrum Cannabidiol in the Treatment of Scalp Inflammation in Patients with Mild to Moderate Scalp Psoriasis or Seborrheic Dermatitis. Ski. Appendage Disord. 2020, 6, 355–361. [Google Scholar] [CrossRef]
- Palmieri, B.; Laurino, C.; Vadala, M. A therapeutic effect of cbd-enriched ointment in inflammatory skin diseases and cutaneous scars. Clin. Ter. 2019, 170, e93–e99. [Google Scholar] [CrossRef] [PubMed]
- Spiera, R.; Hummers, L.; Chung, L.; Frech, T.M.; Domsic, R.; Hsu, V.; Furst, D.E.; Gordon, J.; Mayes, M.; Simms, R.; et al. Safety and Efficacy of Lenabasum in a Phase II, Randomized, Placebo-Controlled Trial in Adults With Systemic Sclerosis. Arthritis Rheumatol. 2020, 72, 1350–1360. [Google Scholar] [CrossRef] [PubMed]
- Grant, J.E.; Odlaug, B.L.; Chamberlain, S.R.; Kim, S.W. Dronabinol, a cannabinoid agonist, reduces hair pulling in trichotillomania: A pilot study. Psychopharmacology 2011, 218, 493–502. [Google Scholar] [CrossRef]
- Grant, J.E.; Valle, S.; Chesivoir, E.; Ehsan, D. Tetrahydrocannabinol fails to reduce hair pulling or skin picking: Results of a double-blind, placebo-controlled study of dronabinol. Int. Clin. Psychopharmacol. 2022, 37, 14–20. [Google Scholar] [CrossRef]
- Han, J.J.; Faletsky, A.; Mostaghimi, A.; Huang, K.P. Cannabis Use among Patients with Alopecia Areata: A Cross-Sectional Survey Study. Int. J. Trichol. 2022, 14, 21–24. [Google Scholar] [CrossRef]
- Schrader, N.H.B.; Gorell, E.S.; Stewart, R.E.; Duipmans, J.C.; Harris, N.; Perez, V.A.; Tang, J.Y.; Wolff, A.P.; Bolling, M.C. Cannabinoid use and effects in patients with epidermolysis bullosa: An international cross-sectional survey study. Orphanet J. Rare Dis. 2021, 16, 377. [Google Scholar] [CrossRef]
- Chelliah, M.P.; Zinn, Z.; Khuu, P.; Teng, J.M.C. Self-initiated use of topical cannabidiol oil for epidermolysis bullosa. Pediatr. Dermatol. 2018, 35, e224–e227. [Google Scholar] [CrossRef]
- Kaemmerer, T.; Clanner-Engelshofen, B.M.; Lesmeister, T.; French, L.E.; Reinholz, M. Cannabinoids in hyperhidrosis. J. Dermatol. Treat. 2023, 34, 2127308. [Google Scholar] [CrossRef]
- Roh, Y.S.; Sutaria, N.; Biles, N.F.; Kwatra, S.G. Treatment of Chronic Pruritus with Medical Marijuana. JAMA Dermatol. 2021, 157, 879–880. [Google Scholar] [CrossRef]
- Lou, K.; Murphy, S.; Talbot, C. Cannabinoids for the treatment of refractory neuropathic pruritus in amyotrophic lateral sclerosis: A case report. Palliat. Med. 2022, 36, 208–211. [Google Scholar] [CrossRef]
- Mahurin, H.M.; Ware, O.R.; Coolman, T.D.; Stevenson, P.A.; Pergam, S.A.; Shinohara, M.M. Cannabis use among patients with cutaneous lymphoma: A cross-sectional survey. Complement. Ther. Med. 2022, 67, 102830. [Google Scholar] [CrossRef] [PubMed]
- Jugl, S.; Sajdeya, R.; Morris, E.J.; Goodin, A.J.; Brown, J.D. Much Ado about Dosing: The Needs and Challenges of Defining a Standardized Cannabis Unit. Med. Cannabis Cannabinoids 2021, 4, 121–124. [Google Scholar] [CrossRef] [PubMed]
| Condition | Author; Year | Type of Study, Number of Participants, Age (Years), (Sex, Male:Female) | Treatment | Duration of Treatment | Response to Treatment | GRADE Rating |
|---|---|---|---|---|---|---|
| AA | Han; 2022 [27] | Cross-sectional study, n = 1045 Mean age: 47.6 (172 M:870 F: 3 declined to specify) | 689/1045 endorsed use of cannabinoid products, including smoking marijuana or CBD, ingesting marijuana, THC or CBD, inhaling vaporized liquid THC, hash oil, or CBD, and CBD lotions and creams | Varies | 80.4% (n = 287) no change in hair loss, 37.8% (n = 135) no change in discomfort of skin | Very low |
| AD | Gao; 2022 [17] | Randomized clinical trial, n = 57 Age range: 18–65 (not provided) | Randomized to Group 1: JW-100 (Jupiter Wellness, Inc.) (n = 18), topical CBD from hemp with aspartame; Group 2: pure topical CBD from hemp (n = 17); or Group 3: placebo (n = 17), in a 1:1:1 ratio. | Twice daily for 14 days | Efficacy was scored using the Investigator’s Static Global Assessment (ISGA), which grades disease severity based on morphologic appearances on a scale from 0 to 4. JW-100 group demonstrated the most significant reduction in ISGA (1.28, p = 0.042) versus placebo. 50% of patients in the JW-100 group achieved clear or almost clear scores compared with 15% in the placebo group (p = 0.028) No statistically significant improvement in Group 2 compared to placebo (p = 0.727). | Moderate |
| Maghfour; 2021 [18] | Cohort study, n = 14 Mean age: 51.36 (11 M:3 F) | Topical 1% CBD gel and hemp oil containing 1% CBD | 14 days | Reduction in mean EASI score (5.40 at baseline and 3.10 at end of two weeks) (p < 0.005) | Low | |
| Callaway; 2005 [19] | Crossover randomized clinical trial, n = 16 Age range: 25–65 (1 M:15 F) | Oral consumption of 30 mL hempseed oil or olive oil daily | 8 weeks of each treatment with 4-week washout and crossover period | TEWL values decreased from baseline in the hempseed oil group at 8 weeks (p = 0.074), but there was no statistically significant difference between hempseed and olive oil groups at 8 weeks (p = 0.274). Patient reported skin dryness and itchiness improved (p = 0.027 and p = 0.023, respectively) after hempseed oil intervention | Moderate | |
| DM | Werth; 2022 [5] | Randomized clinical trial, n = 22 (11 received lenabasum, 11 received placebo) Mean age of lenabasum group: 53.1 (12 M:10 F) | Oral lenabasum 20 mg daily for 28 days and then 20 mg twice per day for 56 days or placebo | 113 days | On Day 113, the adjusted mean (SD) change from baseline CDASI activity score was −9.3 (10.99) in the lenabasum group and −3.7 (6.83) in the placebo (p = 0.0382). Treatment with lenabasum resulted in statistically significant reductions from the baseline in IFN-b and IFN-g levels (p = 0.030 and p = 0.048, respectively) | Moderate |
| EB | Schäder; 2021 [28] | Cross-sectional study, n = 71 Age: Not reported (40 M:31 F) | Topical, ingested, inhaled, and sublingual cannabinoid-based medicines containing CBD only (n = 24/118), THC only (n = 18/118), THC/CBD (n = 41/118), and unspecified cannabinoids | Variable (<6 months; >5 years) | Statistically significant reductions in self-reported pain and pruritus (median pain change-score: − 3, p < 0.001; median pruritus change-score: − 3, p < 0.001). Patient-reported improvement in overall EB symptoms (95.8%, 46/48), pain (93.8%, 45/48), pruritus (90.9%, 40/44), skin inflammation (72.3%, 34/47) and wound-healing time (60.4%, 29/48) | Low |
| Chelliah; 2018 [29] | Case series, n = 3, Age range: 6 months to 10 years (2 M:1 F) | Topical CBD oil | Varies | Reported reduction in pain and blistering in 3 patients | Low | |
| Hyperhidrosis | Kaemmerer; 2022 [30] | Case report, n = 1 Age: 28 (1 M) | Topical dronabinol drops 25 mg/mL up to three times daily for one month, inhaled 0.5 g medical cannabis buds (Pedanios 8% THC and 8% CBD) for two weeks, and vaporized 0.5 g medical cannabis buds (Pedanios 20% THC, 1% CBD) for two weeks | 2 months | 55.6% decrease in DLQI score (10-point decrease); 25% increase in EQ-5D-3L score (2-point improvement); 40% increase in EQ VAS score (20-point improvement). HDSS decreased by 80% (2-point decrease). | Very low |
| Pruritus | Roh; 2021 [31] | Case report, n = 1 Age: “60s” (1 F) | Smoking THC or indica flower and sublingual indica flower or tincture form (THC and cannabinol compounded in 1:1 ratio) two nights weekly | 20 months | DLQI score reduction from 17 at baseline to 1 at 20 months. | Very low |
| Lou; 2021 [32] | Case report, n = 1 Age: 60 (1 M) | Oral capsule of 2.43 mg THC/CBD 2.75 mg once to twice daily | 2 weeks | Pruritus score decreased from 7/10 to 3/10 | Very Low | |
| Mahurin; 2022 [33] | Cross-sectional study, n = 119 Mean age: 59 (39 M:65 F: 2 decline to specify) | 60 participants endorsed use of non-specific cannabinoid products (smoking, vaporizing, topical creams/ointments, and oral) | Variable | 25% (6/24) of current users reported using cannabis specifically to treat itch. These users reported moderate improvement in itch (VAS scores for degree of symptom improvement mean of 6.6/10) | Low | |
| Psoriasis | Puaratanaarunkon; 2022 [20] | Split-body randomized controlled trial, n = 51 (108 pairs of target plaques) Mean age: 53 (30 M:21 F) | Topical 2.5% CBD ointment or placebo twice daily on target plaques | 12 weeks | Significantly lower difference in PASI score (p = 0.026); 10% higher grade reduction than placebo | Moderate |
| Friedman; 2020 [21] | Case report, n = 1 Age: 33 (1 M) | THC distillate cream with medium-chain triglyceride oil, THC soap, hair oil with THC distillate dissolved into jojoba oil, 5 mg/mL daily | Continuous use for 14 days; every few days thereafter for maintenance | Patient reported resolution of symptoms after two months | Very low | |
| Scalp psoriasis or SD | Vincenzi; 2020 [22] | Cohort study, n = 50 Mean age: 42.16 (24 M:26 F) | Topical shampoo containing 150 mg CBD/205 mL | 14 days | Severity scores of arborizing vessels, twisted capillaries, and scales reduced from 2.3 to 0.5, 2.6 to 0.8, and 3.6 to 0.6, respectively (all p < 0.0001). Severity scores for erythema and scaling reduced from 5.5 to 1.3 and 7.0 to 1.6, respectively (both p < 0.0001). | Low |
| Psoriasis, AD, and resulting scars | Palmieri; 2019 [23] | Cohort study, n = 20 Age range: 20–80 (6 M:14 F) | Topical CBD-enriched ointment (hemptouch organic skin care) twice daily | 3 months | Improvement in PASI score (p < 0.001). Hydration increased (p < 0.01), elasticity improved (p < 0.001), and TEWL improved (p < 0.001) | Low |
| SSc | Spiera; 2020 [24] | Randomized clinical trial, n = 42 (27 received lenabasum, 15 received placebo) Mean age of lenabasum group: 49 (10 M:32 F) | Oral lenabasum 5 mg once daily, 20 mg once daily, or 20 mg twice daily for 4 weeks, followed by 20 mg twice daily for 8 weeks vs. placebo (microcrystalline cellulose) | 16 weeks | Median CRISS score significantly improved in lenabasum group compared to placebo at Week 16 (p = 0.04 by one-sided MMRM analysis and p = 0.07 by two-sided MMRM analysis) | Moderate |
| TTM | Grant; 2011 [25] | Open-label clinical trial, n = 14 Mean age: 33.3 (0 M:14 F) | All patients were started on oral dronabinol (dose ranging from 2.5 to 15 mg/day). No control group | 12 weeks | MGH-HPS scores decreased from a mean of 16.5 ± 4.4 at baseline to 8.7 ± 5.5 at Week 12 (p = 0.001) | Low |
| TTM and skin-picking disorder | Grant; 2022 [26] | Randomized clinical trial, n = 50 (trichotillomania n = 34; skin picking disorder n = 16) Age: 33.04 (6 M:19 F) | Oral dronabinol (5–15 mg/day) (n = 25) vs. placebo (n = 25) | 10 weeks | No statistically significant change in outcomes, as measured by the clinician-rated National Institute of Mental Health scale for hair pulling or skin picking | Moderate |
| Types of Cannabinoid Used | Route of Administration | Number of Patients | Conditions Investigated (Number of Patients Treated) |
|---|---|---|---|
| CBD only | Topical | 173 | Atopic dermatitis (n = 49) [17,18], Epidermolysis bullosa (n = 3) [29], Psoriasis (n = 71) [20,23], Scalp psoriasis and seborrheic dermatitis (n = 50) [22] |
| Dronabinol | Oral | 39 | Trichotillomania (n = 39) [25,26] |
| Hempseed oil | Oral | 16 | Atopic dermatitis (n = 16) [19] |
| Lenabasum | Oral | 38 | Dermatomyositis (n = 11) [5], Diffuse cutaneous systemic sclerosis (n = 27) [24] |
| THC and Cannabis indica flower | Smoking | 1 | Pruritus (n = 1) [31] |
| THC only | Topical | 1 | Psoriasis (n = 1) [21] |
| THC/CBD combination | Oral | 1 | Pruritus (n = 1) [32] |
| Non-specific | Topical, ingested, inhaled, sublingual | 821 | Alopecia areata (n = 689) [27], Epidermolysis bullosa (n = 71) [28], Hyperhidrosis (n = 1) [30], Pruritus secondary to cutaneous lymphoma (n = 60) [33] |
| Total | 1090 | ||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Popp, M.; Latta, S.; Nguyen, B.; Vincenzi, C.; Tosti, A. Cannabinoids for the Treatment of Hair, Scalp, and Skin Disorders: A Systematic Review. Cosmetics 2023, 10, 129. https://doi.org/10.3390/cosmetics10050129
Popp M, Latta S, Nguyen B, Vincenzi C, Tosti A. Cannabinoids for the Treatment of Hair, Scalp, and Skin Disorders: A Systematic Review. Cosmetics. 2023; 10(5):129. https://doi.org/10.3390/cosmetics10050129
Chicago/Turabian StylePopp, Meagan, Steven Latta, Betty Nguyen, Colombina Vincenzi, and Antonella Tosti. 2023. "Cannabinoids for the Treatment of Hair, Scalp, and Skin Disorders: A Systematic Review" Cosmetics 10, no. 5: 129. https://doi.org/10.3390/cosmetics10050129
APA StylePopp, M., Latta, S., Nguyen, B., Vincenzi, C., & Tosti, A. (2023). Cannabinoids for the Treatment of Hair, Scalp, and Skin Disorders: A Systematic Review. Cosmetics, 10(5), 129. https://doi.org/10.3390/cosmetics10050129







