A Randomized, Double-Blind, Placebo-Controlled Pilot Study to Evaluate the Efficacy and Safety of Latanoprost for Eyelash Growth in Aesthetic Medicine
Abstract
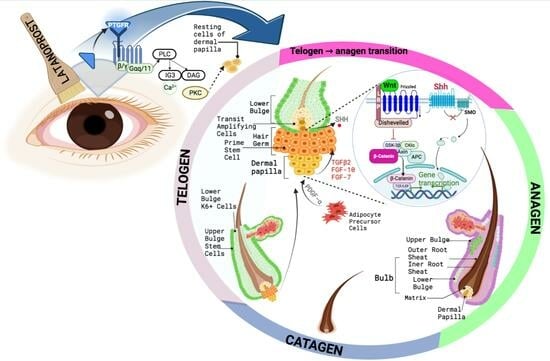
:1. Introduction
2. Materials and Methods
2.1. Materials
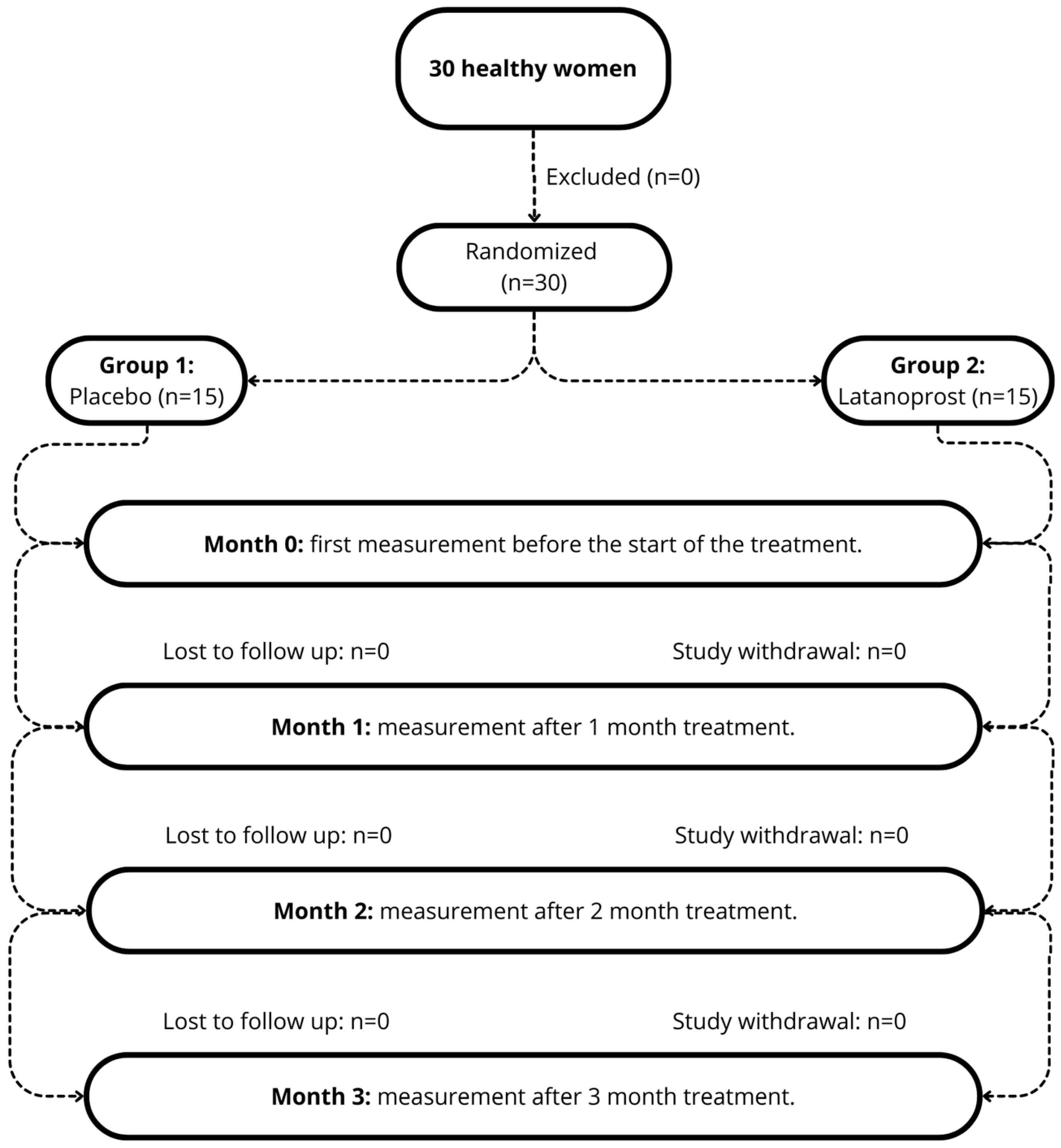
2.2. Study Design
2.3. Participants
2.4. Experimental Procedure
2.5. Measurement of Eyelash Growth
2.6. Measurement of Eyelash Color Change
2.7. Measurement of Intraocular Pressure
2.8. Statistical Analysis
3. Results
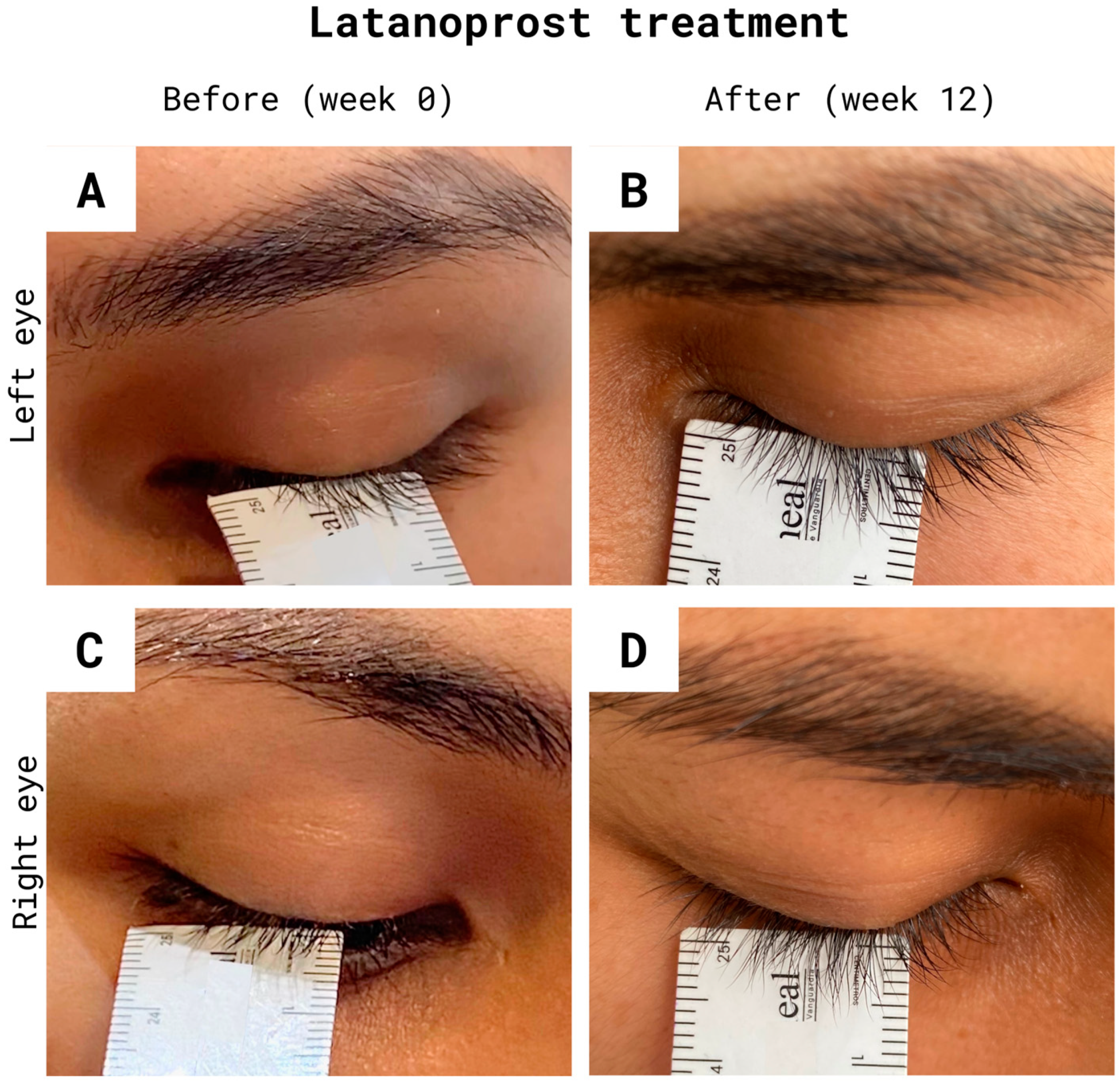
3.1. Eyelash Growth
3.2. Eyelash Color Change
3.3. Intraocular Pressure and Ophthalmic Pathologies
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Montagna, W.; Ford, D.M. Histology and cytochemistry of human skin. 3. The eyelid. Arch. Dermatol. 1969, 100, 328–335. [Google Scholar] [CrossRef] [PubMed]
- Amador, G.J.; Mao, W.; DeMercurio, P.; Montero, C.; Clewis, J.; Alexeev, A.; Hu, D.L. Eyelashes divert airflow to protect the eye. J. R. Soc. Interface 2015, 12, 20141294. [Google Scholar] [CrossRef] [PubMed]
- Marro, M.; Moccozet, L.; Vernez, D. Modeling the protective role of human eyelashes against ultraviolet light exposure. Comput. Biol. Med. 2022, 141, 105135. [Google Scholar] [CrossRef] [PubMed]
- Na, J.I.; Kwon, O.S.; Kim, B.J.; Park, W.S.; Oh, J.K.; Kim, K.H.; Cho, K.H.; Eun, H.C. Ethnic characteristics of eyelashes: A comparative analysis in Asian and Caucasian females. Br. J. Dermatol. 2006, 155, 1170–1176. [Google Scholar] [CrossRef] [PubMed]
- Paus, R.; Burgoa, I.; Platt, C.I.; Griffiths, T.; Poblet, E.; Izeta, A. Biology of the eyelash hair follicle: An enigma in plain sight. Br. J. Dermatol. 2016, 174, 741–752. [Google Scholar] [CrossRef]
- Burgoyne, P.S.; Mahadevaiah, S.K.; Turner, J.M.A. The consequences of asynapsis for mammalian meiosis. Nat. Rev. Genet. 2009, 10, 207–216. [Google Scholar] [CrossRef]
- Hsu, Y.-C.; Li, L.; Fuchs, E. Emerging interactions between skin stem cells and their niches. Nat. Med. 2014, 20, 847–856. [Google Scholar] [CrossRef]
- Lim, X.; Nusse, R. Wnt signaling in skin development, homeostasis, and disease. Cold Spring Harb. Perspect. Biol. 2013, 5. [Google Scholar] [CrossRef]
- Stenn, K.S.; Paus, R. Controls of hair follicle cycling. Physiol. Rev. 2001, 81, 449–494. [Google Scholar] [CrossRef]
- Lee, A.J.; McCluskey, P. Clinical utility and differential effects of prostaglandin analogs in the management of raised intraocular pressure and ocular hypertension. Clin. Ophthalmol. 2010, 4, 741–764. [Google Scholar] [CrossRef]
- Zhou, L.; Yang, K.; Xu, M.; Andl, T.; Millar, S.E.; Boyce, S.; Zhang, Y. Activating β-catenin signaling in CD133-positive dermal papilla cells increases hair inductivity. FEBS J. 2016, 283, 2823–2835. [Google Scholar] [CrossRef] [PubMed]
- Paus, R.; Foitzik, K. In search of the “hair cycle clock”: A guided tour. Differ. Res. Biol. Divers. 2004, 72, 489–511. [Google Scholar] [CrossRef] [PubMed]
- Mokry, J.; Pisal, R. Development and Maintenance of Epidermal Stem Cells in Skin Adnexa. Int. J. Mol. Sci. 2020, 21, 9736. [Google Scholar] [CrossRef] [PubMed]
- Llamas-Molina, J.M.; Carrero-Castaño, A.; Ruiz-Villaverde, R.; Campos, A. Tissue Engineering and Regeneration of the Human Hair Follicle in Androgenetic Alopecia: Literature Review. Life 2022, 12, 117. [Google Scholar] [CrossRef]
- Plikus, M.V.; Mayer, J.A.; de la Cruz, D.; Baker, R.E.; Maini, P.K.; Maxson, R.; Chuong, C.M. Cyclic dermal BMP signalling regulates stem cell activation during hair regeneration. Nature 2008, 451, 340–344. [Google Scholar] [CrossRef]
- Thibaut, S.; De Becker, E.; Caisey, L.; Baras, D.; Karatas, S.; Jammayrac, O.; Pisella, P.J.; Bernard, B.A. Human eyelash characterization. Br. J. Dermatol. 2010, 162, 304–310. [Google Scholar] [CrossRef]
- Michard, Q.; Commo, S.; Belaidi, J.-P.; Alleaume, A.-M.; Michelet, J.-F.; Daronnat, E.; Eilstein, J.; Duche, D.; Marrot, L.; Bernard, B.A. TRP-2 specifically decreases WM35 cell sensitivity to oxidative stress. Free. Radic. Biol. Med. 2008, 44, 1023–1031. [Google Scholar] [CrossRef] [PubMed]
- Michard, Q.; Commo, S.; Rocchetti, J.; El Houari, F.; Alleaume, A.M.; Wakamatsu, K.; Ito, S.; Bernard, B.A. TRP-2 expression protects HEK cells from dopamine- and hydroquinone-induced toxicity. Free. Radic. Biol. Med. 2008, 45, 1002–1010. [Google Scholar] [CrossRef]
- Nishimura, E.K. Melanocyte stem cells: A melanocyte reservoir in hair follicles for hair and skin pigmentation. Pigment. Cell Melanoma Res. 2011, 24, 401–410. [Google Scholar] [CrossRef]
- Tobin, D.J. The cell biology of human hair follicle pigmentation. Pigment. Cell Melanoma Res. 2011, 24, 75–88. [Google Scholar] [CrossRef]
- Jones, D. Enhanced Eyelashes: Prescription and Over-the-Counter Options. Aesthetic Plast. Surg. 2011, 35, 116–121. [Google Scholar] [CrossRef] [PubMed]
- Smith, S.; Fagien, S.; Whitcup, S.M.; Ledon, F.; Somogyi, C.; Weng, E.; Beddingfield, F.C., 3rd. Eyelash growth in subjects treated with bimatoprost: A multicenter, randomized, double-masked, vehicle-controlled, parallel-group study. J. Am. Acad. Dermatol. 2012, 66, 801–806. [Google Scholar] [CrossRef] [PubMed]
- Camras, C.B.; Schumer, R.A.; Marsk, A.; Lustgarten, J.S.; Serle, J.B.; Stjernschantz, J.; Bito, L.Z.; Podos, S.M. Intraocular pressure reduction with PhXA34, a new prostaglandin analogue, in patients with ocular hypertension. Arch. Ophthalmol. 1992, 110, 1733–1738. [Google Scholar] [CrossRef] [PubMed]
- Johnstone, M.A. Hypertrichosis and increased pigmentation of eyelashes and adjacent hair in the region of the ipsilateral eyelids of patients treated with unilateral topical latanoprost. Am. J. Ophthalmol. 1997, 124, 544–547. [Google Scholar] [CrossRef]
- Hart, J.; Shafranov, G. Hypertrichosis of vellus hairs of the malar region after unilateral treatment with bimatoprost. Am. J. Ophthalmol. 2004, 137, 756–757. [Google Scholar] [CrossRef] [PubMed]
- Trejo Acuña, J.R.; Rodríguez, M. Hypertrichosis of the Eyelid and Malar area Induced by Latanoprost in a Patient with Glaucoma. A case Report in Dermatologic Office Practice. Dermatol. Cosmética Médica Y Quirúrgica 2020, 18, 229–231. [Google Scholar]
- Tsukahara, S.; Kashiwagi, K. Pharmacological mechanisms, clinical effectiveness, and side-effects of prostaglandin analogues as anti-glaucoma agents. Biomed. Rev. 2002, 2002, 13. [Google Scholar] [CrossRef]
- Law, S.K. Bimatoprost in the treatment of eyelash hypotrichosis. Clin. Ophthalmol. 2010, 4, 349–358. [Google Scholar] [CrossRef]
- Elgin, U.; Batman, A.; Berker, N.; Ilhan, B. The comparison of eyelash lengthening effect of latanoprost therapy in adults and children. Eur. J. Ophthalmol. 2006, 16, 247–250. [Google Scholar] [CrossRef]
- Chiba, T.; Kashiwagi, K.; Ishijima, K.; Furuichi, M.; Kogure, S.; Abe, K.; Chiba, N.; Tsukahara, S. A prospective study of iridial pigmentation and eyelash changes due to ophthalmic treatment with latanoprost. Jpn. J. Ophthalmol. 2004, 48, 141–147. [Google Scholar] [CrossRef]
- Sugimoto, M.; Sugimoto, M.; Uji, Y. Quantitative analysis of eyelash lengthening following topical latanoprost therapy. Can. J. Ophthalmol. 2002, 37, 342–345. [Google Scholar] [CrossRef]
- Morris, C.L.; Stinnett, S.; Woodward, J. The role of bimatoprost eyelash gel in chemotherapy-induced madarosis: An analysis of efficacy and safety. Int. J. Trichology 2011, 3, 84–91. [Google Scholar] [CrossRef] [PubMed]
- Coronel-Pérez, I.M.; Rodríguez-Rey, E.M.; Camacho-Martínez, F.M. Latanoprost in the treatment of eyelash alopecia in alopecia areata universalis. J. Eur. Acad. Dermatol. Venereol. 2010, 24, 481–485. [Google Scholar] [CrossRef] [PubMed]
- Parrish, R.; Palmberg, P.; Group, X.S. Latanoprost, Bimatoprost, and Travoprost in Patients With Elevated Intraocular Pressure: Results of a 12-Week, Masked-Evaluator, Multicenter Study. Investig. Ophthalmol. Vis. Sci. 2003, 44, 100. [Google Scholar]
- Mansberger, S.L.; Cioffi, G.A. Eyelash formation secondary to latanoprost treatment in a patient with alopecia. Arch. Ophthalmol. 2000, 118, 718–719. [Google Scholar] [CrossRef]
- Johnstone, M.A.; Albert, D.M. Prostaglandin-induced hair growth. Surv. Ophthalmol. 2002, 47 (Suppl. 1), S185–S202. [Google Scholar] [CrossRef]
- Aumond, S. L’Infestation Palpébrale et Faciale au Demodex Folliculorum; Université de Montréal: Montreal, QC, Canada, 2018. [Google Scholar]
- Nesher, R.; Mimouni, M.; Elnaddaf, H.; Nemet, A.; Kidron, D. Characterization of prostaglandin F2α receptors in human eyelids. Eur. J. Ophthalmol. 2015, 25, 81–84. [Google Scholar] [CrossRef]
- Ocklind, A.; Lake, S.; Wentzel, P.; Nistér, M.; Stjernschantz, J. Localization of the prostaglandin F2 alpha receptor messenger RNA and protein in the cynomolgus monkey eye. Investig. Ophthalmol. Vis. Sci. 1996, 37, 716–726. [Google Scholar]
- Anadol, E.; Kanca, H.; Yar, A.S.; Helvacioğlu, F.; Menevşe, S.; Calgüner, E.; Erdoğan, D. Prostaglandin F receptor expression in intrauterine tissues of pregnant rats. J. Vet. Sci. 2014, 15, 125–131. [Google Scholar] [CrossRef]
- Colombe, L.; Michelet, J.F.; Bernard, B.A. Prostanoid receptors in anagen human hair follicles. Exp. Dermatol. 2008, 17, 63–72. [Google Scholar] [CrossRef]
- Woodward, D.F.; Fairbairn, C.E.; Krauss, A.H.; Lawrence, R.A.; Protzman, C.E. Radioligand binding analysis of receptor subtypes in two FP receptor preparations that exhibit different functional rank orders of potency in response to prostaglandins. J. Pharmacol. Exp. Ther. 1995, 273, 285–287. [Google Scholar] [PubMed]
- Neuschäfer-Rube, F.; Engemaier, E.; Koch, S.; Böer, U.; Püschel, G.P. Identification by site-directed mutagenesis of amino acids contributing to ligand-binding specificity or signal transduction properties of the human FP prostanoid receptor. Biochem. J. 2003, 371, 443–449. [Google Scholar] [CrossRef] [PubMed]
- Sharif, N.A.; Odani-Kawabata, N.; Lu, F.; Pinchuk, L. FP and EP2 prostanoid receptor agonist drugs and aqueous humor outflow devices for treating ocular hypertension and glaucoma. Exp. Eye Res. 2023, 229, 109415. [Google Scholar] [CrossRef] [PubMed]
- Holló, G. The side effects of the prostaglandin analogues. Expert. Opin. Drug Saf. 2007, 6, 45–52. [Google Scholar] [CrossRef]
- Fagien, S.; Walt, J.G.; Carruthers, J.; Cox, S.E.; Wirta, D.; Weng, E.; Beddingfield, F.C., III. Patient-Reported Outcomes of Bimatoprost for Eyelash Growth: Results From a Randomized, Double-Masked, Vehicle-Controlled, Parallel-Group Study. Aesthetic Surg. J. 2013, 33, 789–798. [Google Scholar] [CrossRef]


| Criteria | Description |
|---|---|
| Healthy women between 18–50 years | |
| Inclusion | Any amount of eyelash prominence |
| Any iris color | |
| Intraocular pressure between 10–20 mmHg | |
| Exclusion | Diseases such as hypertension, diabetes mellitus, obesity, cardiovascular and respiratory disease, cancer, psychiatric, neurological disorders, mental illness, abuse of substances |
| Eye diseases such as cataracts, active keratitis, history of herpetic keratitis, iritis, uveitis, glaucoma, intraocular hypertension. Dermatological conditions such as alopecia, atopic or contact dermatitis, vitiligo | |
| No eyelash growth treatment in the last 6 months | |
| No eyelash extension | |
| No history of tattoos on the upper and/or lower eyelids | |
| Patients allergic to analogues of prostaglandins |
| Eyelash | Groups | Growth Measurements | Group Comparison |
|---|---|---|---|
| Left eyelash | Placebo (n = 15) | Month 0 vs. Month 1 | Placebo vs. latanoprost * |
| Month 0 vs. Month 2 | |||
| Month 0 vs. Month 3 | |||
| Month 1 vs. Month 2 | |||
| Month 1 vs. Month 3 | |||
| Month 2 vs. Month 3 | |||
| Latanoprost (n = 15) | Month 0 vs. Month 1 * (+24%) | ||
| Month 0 vs. Month 2 * (+34%) | |||
| Month 0 vs. Month 3 * (+43%) | |||
| Month 1 vs. Month 2 * (+10%) | |||
| Month 1 vs. Month 3 * (+16%) | |||
| Month 2 vs. Month 3 (+6%) | |||
| Right eyelash | Placebo (n = 15) | Month 0 vs. Month 1 | Placebo vs. latanoprost * |
| Month 0 vs. Month 2 | |||
| Month 0 vs. Month 3 | |||
| Month 1 vs. Month 2 | |||
| Month 1 vs. Month 3 | |||
| Month 2 vs. Month 3 | |||
| Latanoprost (n = 15) | Month 0 vs. Month 1 * (+23%) | ||
| Month 0 vs. Month 2 * (+38%) | |||
| Month 0 vs. Month 3 * (+46%) | |||
| Month 1 vs. Month 2 * (+12%) | |||
| Month 1 vs. Month 3 * (+18%) | |||
| Month 2 vs. Month 3 (+5%) |
| Eyelash | Groups | Color Change | Group Comparison |
|---|---|---|---|
| Left eyelash | Placebo (n = 15) | Month 0 vs. Month 1 | Placebo vs. latanoprost * |
| Month 0 vs. Month 2 | |||
| Month 0 vs. Month 3 | |||
| Month 1 vs. Month 2 | |||
| Month 1 vs. Month 3 | |||
| Month 2 vs. Month 3 | |||
| Latanoprost (n = 15) | Month 0 vs. Month 1 (+10%) | ||
| Month 0 vs. Month 2 * (+23%) | |||
| Month 0 vs. Month 3 (+15%) | |||
| Month 1 vs. Month 2 (+5%) | |||
| Month 1 vs. Month 3 * (+15%) | |||
| Month 2 vs. Month 3 (+7%) | |||
| Right eyelash | Placebo (n = 15) | Month 0 vs. Month 1 | Placebo vs. latanoprost * |
| Month 0 vs. Month 2 | |||
| Month 0 vs. Month 3 | |||
| Month 1 vs. Month 2 | |||
| Month 1 vs. Month 3 | |||
| Month 2 vs. Month 3 | |||
| Latanoprost (n = 15) | Month 0 vs. Month 1 (+10%) | ||
| Month 0 vs. Month 2 * (+23%) | |||
| Month 0 vs. Month 3 (+15%) | |||
| Month 1 vs. Month 2 (+5%) | |||
| Month 1 vs. Month 3 * (+15%) | |||
| Month 2 vs. Month 3 (+7%) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Espinoza-Silva, J.I.; Macias-Nevarez, E.; Scheckhuber, C.Q.; Tienda-Vázquez, M.A. A Randomized, Double-Blind, Placebo-Controlled Pilot Study to Evaluate the Efficacy and Safety of Latanoprost for Eyelash Growth in Aesthetic Medicine. Cosmetics 2023, 10, 136. https://doi.org/10.3390/cosmetics10050136
Espinoza-Silva JI, Macias-Nevarez E, Scheckhuber CQ, Tienda-Vázquez MA. A Randomized, Double-Blind, Placebo-Controlled Pilot Study to Evaluate the Efficacy and Safety of Latanoprost for Eyelash Growth in Aesthetic Medicine. Cosmetics. 2023; 10(5):136. https://doi.org/10.3390/cosmetics10050136
Chicago/Turabian StyleEspinoza-Silva, Janette Ivone, Ernesto Macias-Nevarez, Christian Quintus Scheckhuber, and Mario Adrián Tienda-Vázquez. 2023. "A Randomized, Double-Blind, Placebo-Controlled Pilot Study to Evaluate the Efficacy and Safety of Latanoprost for Eyelash Growth in Aesthetic Medicine" Cosmetics 10, no. 5: 136. https://doi.org/10.3390/cosmetics10050136
APA StyleEspinoza-Silva, J. I., Macias-Nevarez, E., Scheckhuber, C. Q., & Tienda-Vázquez, M. A. (2023). A Randomized, Double-Blind, Placebo-Controlled Pilot Study to Evaluate the Efficacy and Safety of Latanoprost for Eyelash Growth in Aesthetic Medicine. Cosmetics, 10(5), 136. https://doi.org/10.3390/cosmetics10050136