Skin Anti-Aging Effect of Oral Vitamin A Supplementation in Combination with Topical Retinoic Acid Treatment in Comparison with Topical Treatment Alone: A Randomized, Prospective, Assessor-Blinded, Parallel Trial
Abstract
:1. Introduction
1.1. Skin Aging
1.1.1. Oxidative Stress and Skin Aging
1.1.2. Autophagy and Skin Aging
1.2. Topical Retinoids and Skin Aging
1.3. Vitamins and Skin Aging
2. Materials and Methods
2.1. Population and Study Design
2.2. Study Outcomes
2.3. Statistical Analysis
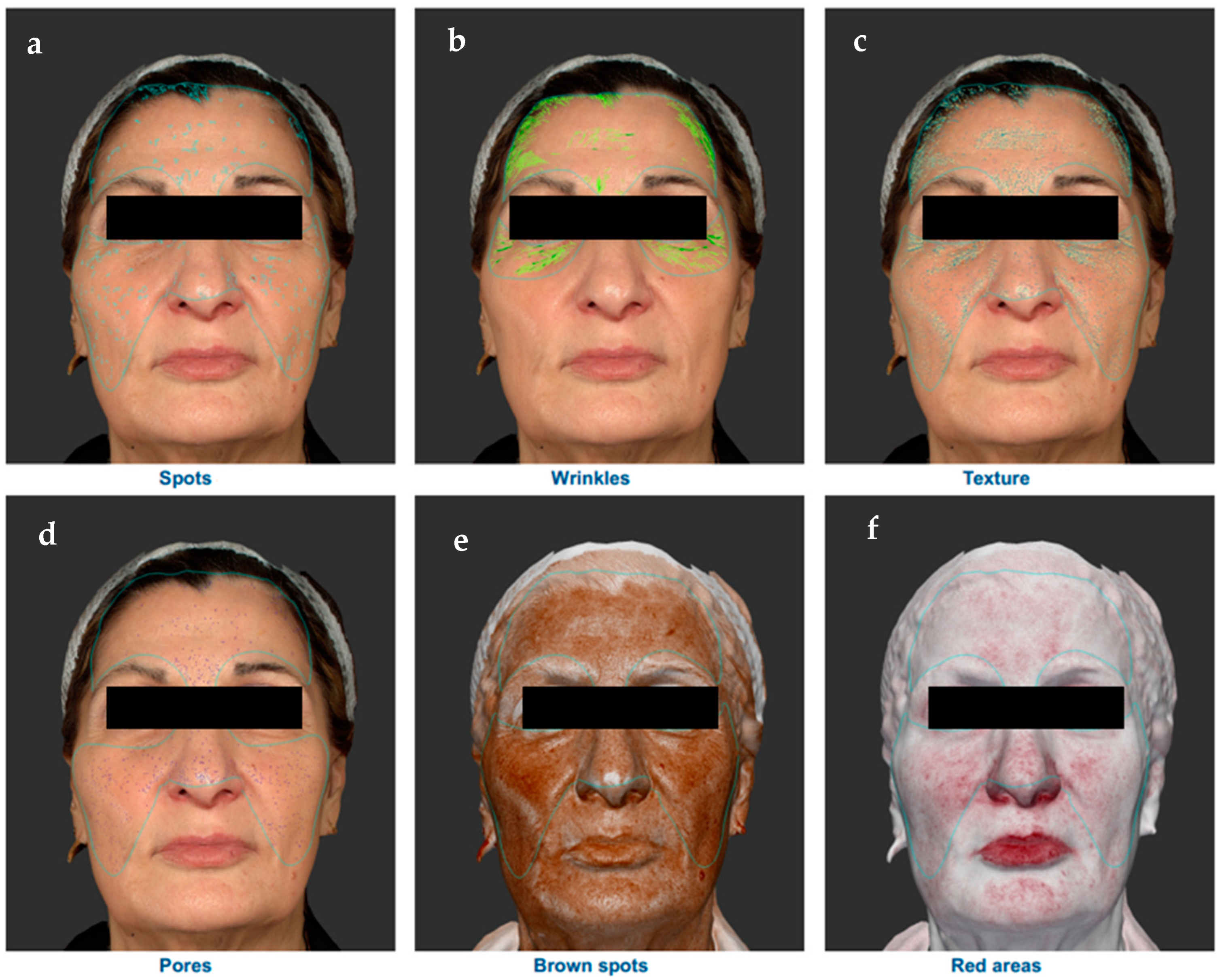
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Farage, M.A.; Miller, K.W.; Elsner, P.; Maibach, H.I. Intrinsic and extrinsic factors in skin ageing: A review. Int. J. Cosmet. Sci. 2008, 30, 87–95. [Google Scholar] [CrossRef]
- Rees, J.L. The Genetics of Sun Sensitivity in Humans. Am. J. Hum. Genet. 2004, 75, 739–751. [Google Scholar] [CrossRef]
- Sachs, D.L.; Fisher, G.; Voorhees, J.J. Skin Ageing. Rook’s Textbook of Dermatology, 9th ed.; Wiley: Hoboken, NJ, USA, 2016; pp. 1–11. [Google Scholar] [CrossRef]
- Fisher, G.J.; Kang, S.; Varani, J.; Bata-Csorgo, Z.; Wan, Y.; Datta, S.; Voorhees, J.J. Mechanisms of Photoaging and Chronological Skin Aging. Arch. Dermatol. 2002, 138, 1462–1470. [Google Scholar] [CrossRef]
- Krutmann, J.; Bouloc, A.; Sore, G.; Bernard, B.A.; Passeron, T. The skin aging exposome. J. Dermatol. Sci. 2017, 85, 152–161. [Google Scholar] [CrossRef]
- Quan, T.; Fisher, G.J. Role of Age-Associated Alterations of the Dermal Extracellular Matrix Microenvironment in Human Skin Aging: A Mini-Review. Gerontology 2015, 61, 427–434. [Google Scholar] [CrossRef]
- Campione, E.; Cosio, T.; Lanna, C.; Mazzilli, S.; Dika, E.; Bianchi, L. Clinical efficacy and reflectance confocal microscopy monitoring in moderate-severe skin aging treated with a polyvinyl gel containing retinoic and glycolic acid: An assessor-blinded 1-month study proof-of-concept trial. J. Cosmet. Dermatol. 2021, 20, 310–315. [Google Scholar] [CrossRef]
- Silva, A.R.; Menezes, P.F.C.; Martinello, T.; Novakovich, G.F.L.; Praes, C.E.O.; Feferman, I.H.S. Antioxidant kinetics of plant-derived substances and extracts. Int. J. Cosmet. Sci. 2010, 32, 73–80. [Google Scholar] [CrossRef]
- Gu, Y.; Han, J.; Jiang, C.; Zhang, Y. Biomarkers, oxidative stress and autophagy in skin aging. Ageing Res. Rev. 2020, 59, 101036. [Google Scholar] [CrossRef]
- Rinnerthaler, M.; Bischof, J.; Streubel, M.; Trost, A.; Richter, K. Oxidative Stress in Aging Human Skin. Biomolecules 2015, 5, 545–589. [Google Scholar] [CrossRef]
- Gragnani, A.; Cornick SMac Chominski, V.; Ribeiro de Noronha, S.M.; Alves Corrêa de Noronha, S.A.; Ferreira, L.M. Review of Major Theories of Skin Aging. Adv. Aging Res. 2014, 3, 265–284. [Google Scholar] [CrossRef]
- Chen, T.; Hou, H.; Fan, Y.; Wang, S.; Chen, Q.; Si, L.; Li, B. Protective effect of gelatin peptides from pacific cod skin against photoaging by inhibiting the expression of MMPs via MAPK signaling pathway. J. Photochem. Photobiol. B 2016, 165, 34–41. [Google Scholar] [CrossRef] [PubMed]
- Fisher, G.J.; Wang, Z.; Datta, S.C.; Varani, J.; Kang, S.; Voorhees, J.J. Pathophysiology of Premature Skin Aging Induced by Ultraviolet Light. N. Engl. J. Med. 1997, 337, 1419–1429. [Google Scholar] [CrossRef] [PubMed]
- Eckhart, L.; Tschachler, E.; Gruber, F. Autophagic Control of Skin Aging. Front. Cell Dev. Biol. 2019, 7, 143. [Google Scholar] [CrossRef]
- Ma, J.; Teng, Y.; Huang, Y.; Tao, X.; Fan, Y. Autophagy plays an essential role in ultraviolet radiation-driven skin photoaging. Front. Pharmacol. 2022, 13, 864331. [Google Scholar] [CrossRef] [PubMed]
- Wang, M.; Charareh, P.; Lei, X.; Zhong, J.L. Autophagy: Multiple Mechanisms to Protect Skin from Ultraviolet Radiation-Driven Photoaging. Oxid. Med. Cell. Longev. 2019, 2019, 8135985. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.; Park, S.-Y.; Moon, S.; Lee, J.; Kim, S. Autophagy in Human Skin Fibroblasts: Impact of Age. Int. J. Mol. Sci. 2018, 19, 2254. [Google Scholar] [CrossRef]
- Orfali, N.; McKenna, S.L.; Cahill, M.R.; Gudas, L.J.; Mongan, N.P. Retinoid receptor signaling and autophagy in acute promyelocytic leukemia. Exp. Cell Res. 2014, 324, 1–12. [Google Scholar] [CrossRef]
- Rajawat, Y.; Hilioti, Z.; Bossis, I. Autophagy: A target for retinoic acids. Autophagy 2010, 6, 1224–1226. [Google Scholar] [CrossRef]
- VanBuren, C.A.; Everts, H.B. Vitamin A in Skin and Hair: An Update. Nutrients 2022, 14, 2952. [Google Scholar] [CrossRef]
- Masaki, H. Role of antioxidants in the skin: Anti-aging effects. J. Dermatol. Sci. 2010, 58, 85–90. [Google Scholar] [CrossRef]
- Masaki, H.; Okano, Y.; Ochiai, Y.; Obayashi, K.; Akamatsu, H.; Sakurai, H. α-Tocopherol Increases the Intracellular Glutathione Level in HaCaT Keratinocytes. Free Radic. Res. 2002, 36, 705–709. [Google Scholar] [CrossRef] [PubMed]
- Wu, S.; Gao, J.; Dinh, Q.T.; Chen, C.; Fimmel, S. IL-8 production and AP-1 transactivation induced by UVA in human keratinocytes: Roles of d-α-tocopherol. Mol. Immunol. 2008, 45, 2288–2296. [Google Scholar] [CrossRef] [PubMed]
- Ristow, M.; Zarse, K.; Oberbach, A.; Klöting, N.; Birringer, M.; Kiehntopf, M.; Stumvoll, M.; Kahn, C.R.; Blüher, M. Antioxidants prevent health-promoting effects of physical exercise in humans. Proc. Natl. Acad. Sci. USA 2009, 106, 8665–8670. [Google Scholar] [CrossRef] [PubMed]
- Ristow, M.; Schmeisser, S. Extending life span by increasing oxidative stress. Free Radic. Biol. Med. 2011, 51, 327–336. [Google Scholar] [CrossRef] [PubMed]
- Schagen, S.K.; Zampeli, V.A.; Makrantonaki, E.; Zouboulis, C.C. Discovering the link between nutrition and skin aging. Dermatoendocrinology 2012, 4, 298–307. [Google Scholar] [CrossRef] [PubMed]
- Praça, F.G.; Viegas, J.S.R.; Peh, H.Y.; Garbin, T.N.; Medina, W.S.G.; Bentley, M.V.L.B. Microemulsion co-delivering vitamin A and vitamin E as a new platform for topical treatment of acute skin inflammation. Mater. Sci. Eng. C 2020, 110, 110639. [Google Scholar] [CrossRef]
- Henseler, H. Investigation of the precision of the Visia® complexion analysis camera system in the assessment of skin surface features. GMS Interdiscip Plast. Reconstr. Surg DGPW 2022, 11, Doc08. [Google Scholar] [CrossRef]
- Navarra, T. The Encyclopedia of Vitamins, Minerals and Supplements, 2nd ed.; Facts on File Inc.: New York, NY, USA, 2004. [Google Scholar]
- Fisher, G.J.; Datta, S.; Wang, Z.; Li, X.Y.; Quan, T.; Chung, J.H.; Kang, S.; Voorhees, J.J. c-Jun–dependent inhibition of cutaneous procollagen transcription following ultraviolet irradiation is reversed by all-trans retinoic acid. J. Clin. Investig. 2000, 106, 663–670. [Google Scholar] [CrossRef]
- Lee, S.-J.; Cho, S.-A.; An, S.-S.; Na, Y.-J.; Park, N.-H.; Kim, H.-S.; Lee, C.-W.; Kim, H.-K.; Kim, E.-K.; Jang, Y.-P.; et al. Significantly Inhibits Retinoid-Induced Skin Irritation In Vitro and In Vivo. Evid.-Based Complement. Altern. Med. 2012, 2012, 190370. [Google Scholar] [CrossRef]
- Packer, L.; Weber, S.U.; Thiele, J.J. Sebaceous Gland Secretion is a Major Physiologic Route of Vitamin E Delivery to Skin. J. Investig. Dermatol. 1999, 113, 1006–1010. [Google Scholar] [CrossRef]
- Cartmel, B.; Moon, T.E.; Levine, N. Effects of long-term intake of retinol on selected clinical and laboratory indexes. Am. J. Clin. Nutr. 1999, 69, 937–943. [Google Scholar] [CrossRef] [PubMed]
- Moon, T.E.; Levine, N.; Cartmel, B.; Bangert, J.L.; Rodney, S.; Dong, Q.; Peng, Y.M.; Alberts, D.S. Effect of retinol in preventing squamous cell skin cancer in moderate-risk subjects: A randomized, double-blind, controlled trial. Southwest Skin Cancer Prevention Study Group. Cancer Epidemiol. Biomark. Prev. 1997, 6, 949–956. [Google Scholar]
- Alberts, D.; Ranger-Moore, J.; Einspahr, J.; Saboda, K.; Bozzo, P.; Liu, Y.; Xu, X.-C.; Lotan, R.; Warneke, J.; Salasche, S.; et al. Safety and Efficacy of Dose-Intensive Oral Vitamin A in Subjects with Sun-Damaged Skin. Clin. Cancer Res. 2004, 10, 1875–1880. [Google Scholar] [CrossRef] [PubMed]
- Morgado-Carrasco, D.; Gil-Lianes, J.; Jourdain, E.; Piquero-Casals, J. Oral Supplementation and Systemic Drugs for Skin Aging: A Narrative Review. Actas Dermosifiliogr. 2023, 114, T114–T124. [Google Scholar] [CrossRef]





| Group A Mean ± SD (% Variation) | Group B Mean ± SD (% Variation) | |||||
|---|---|---|---|---|---|---|
| V0— Baseline | V1— 6 Weeks | V2— 12 Weeks | V0— Baseline | V1— 6 Weeks | V2— 12 Weeks | |
| Elasticity | 2.4 ± 0.8 | 2.0 ± 0.6 (−16%) | 1.8 ± 0.6 ** (−25%) | 2.7 ± 0.9 | 2.2 ± 0.7 * (−21%) | 1.9 ± 0.7 ** (−32%) |
| Wrinkle | 2.4 ± 0.9 | 2.2 ± 0.9 (−7%) | 2.1 ± 0.9 (−14%) | 2.8 ± 0.8 | 2.7 ± 0.7 (−5%) | 2.4 ± 0.7 (−13%) |
| Roughness | 2.3 ± 0.8 | 1.8 ± 0.6 (−20%) | 1.5 ± 0.8 *** (−33%) | 2.4 ± 0.9 | 1.8 ± 0.9 (−23%) | 1.5 ± 0.8 *** (−37%) |
| Pigmentation | 2.5 ± 1.0 | 2.2 ± 1.0 (−11%) | 1.8 ± 0.9 * (−26%) | 2.6 ± 1.0 | 2.2 ± 0.8 (−16%) | 1.8 ± 1.0 * (−29%) |
| Erythema | 1.5 ± 1.1 | 1.5 ± 1.0 (−2%) | 1.4 ± 0.9 (−7%) | 1.5 ± 0.9 | 1.5 ± 1.0 (+2%) | 1.2 ± 0.9 (−18%) |
| Skin pores | 1.7 ± 0.9 | 1.4 ± 1.0 (−16%) | 1.3 ± 1.0 (−22%) | 1.8 ± 0.9 | 1.5 ± 1.0 (−18%) | 1.2 ± 0.9 * (−36%) |
| SAGS | 12.8 ± 4.0 | 11.2 ± 4.0 (−13%) | 10.0 ± 4.0 * (−22%) | 13.8 ± 4.6 | 11.9 ± 4.2 (−14%) | 10.0 ± 3.9 ** (−27%) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Milani, M.; Colombo, F., on behalf of the To-Re Trial Study Group. Skin Anti-Aging Effect of Oral Vitamin A Supplementation in Combination with Topical Retinoic Acid Treatment in Comparison with Topical Treatment Alone: A Randomized, Prospective, Assessor-Blinded, Parallel Trial. Cosmetics 2023, 10, 144. https://doi.org/10.3390/cosmetics10050144
Milani M, Colombo F on behalf of the To-Re Trial Study Group. Skin Anti-Aging Effect of Oral Vitamin A Supplementation in Combination with Topical Retinoic Acid Treatment in Comparison with Topical Treatment Alone: A Randomized, Prospective, Assessor-Blinded, Parallel Trial. Cosmetics. 2023; 10(5):144. https://doi.org/10.3390/cosmetics10050144
Chicago/Turabian StyleMilani, Massimo, and Francesca Colombo on behalf of the To-Re Trial Study Group. 2023. "Skin Anti-Aging Effect of Oral Vitamin A Supplementation in Combination with Topical Retinoic Acid Treatment in Comparison with Topical Treatment Alone: A Randomized, Prospective, Assessor-Blinded, Parallel Trial" Cosmetics 10, no. 5: 144. https://doi.org/10.3390/cosmetics10050144
APA StyleMilani, M., & Colombo, F., on behalf of the To-Re Trial Study Group. (2023). Skin Anti-Aging Effect of Oral Vitamin A Supplementation in Combination with Topical Retinoic Acid Treatment in Comparison with Topical Treatment Alone: A Randomized, Prospective, Assessor-Blinded, Parallel Trial. Cosmetics, 10(5), 144. https://doi.org/10.3390/cosmetics10050144







