Characterization and Efficacy of Essential Oil-Based Cosmetic Formulations for Acne-Prone Skin
Abstract
:1. Introduction
2. Materials and Methods
2.1. Formulations
2.2. Raman Spectroscopy
2.3. Formulation Characterization
2.4. Casuistic
2.5. Clinical Scoring of Acne
2.6. Determination of Comedone Amount (Visioscan® VC 98)
2.7. Evaluation of the Size and Cellular Features of Pores and Pilosebaceous Units (Vivascope® 1500 and VivaCam®)
2.8. Statistics
3. Results
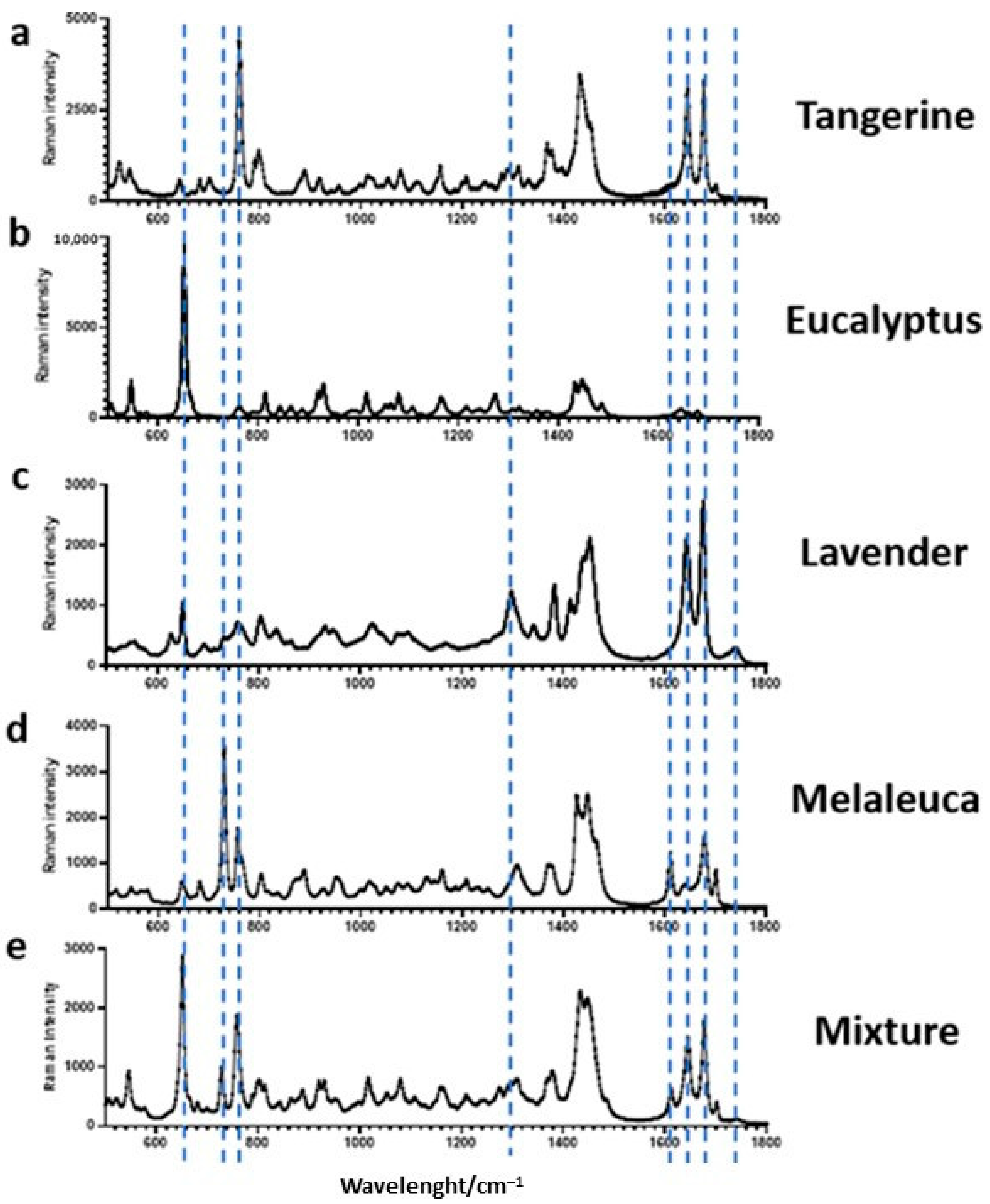
3.1. Characterization of Essential Oils by Raman Spectroscopy
3.2. Nanoemulsion Comparison with Pure Essential Oil
3.3. Formulation Characteristics
3.4. Comedone Count
3.5. Morphological and Structural Analyses of Skin Acne Using RCM Images
3.6. Areas of Pilosebaceous Units
4. Discussion
4.1. Formulation Stability—Raman Spectroscopy of Essential Oils and Nanoemulsion
4.2. Clinical Study
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| 4EO | four essential oils |
| AUC | area under the curve |
| IFRA | International Fragrance Association |
| M.a. | Formulation with Melaleuca alternifolia |
| Nanoem. | nanoemulsion of M.a. |
| RCM | reflectance confocal microscopy |
| SC | stratum corneum |
| SG | stratum granulosum |
| UD | upper dermis |
References
- McCarty, M. Evaluation and management of refractory acne vulgaris in adolescent and adult men. Dermatol. Clin. 2016, 34, 203–206. [Google Scholar] [CrossRef] [PubMed]
- Dagnelie, M.A.; Poinas, A.; Dréno, B. What is new in adult acne for the last 2 years: Focus on acne pathophysiology and treatments. Int. J. Dermatol. 2022, 61, 1205–1212. [Google Scholar] [CrossRef] [PubMed]
- Bagatin, E.; Freitas, T.H.P.D.; Rivitti-Machado, M.C.; Ribeiro, B.M.; Nunes, S.; Rocha, M.A.D.D. Adult female acne: A guide to clinical practice. An. Bras. Dermatol. 2019, 94, 62–75. [Google Scholar] [CrossRef] [PubMed]
- Manfredini, M.; Mazzaglia, G.; Ciardo, S.; Farnetani, F.; Mandel, V.D.; Longo, C.; Zauli, S.; Betolli, V.; Virgili, A.; Pellacani, G. Acne: In vivo morphologic study of lesions and surrounding skin by means of reflectance confocal microscopy. J. Eur. Acad. Dermatol. Venereol. 2015, 29, 933–939. [Google Scholar] [CrossRef]
- Çerman, A.A.; Aktaş, E.; Altunay, İ.K.; Arıcı, J.E.; Tulunay, A.; Ozturk, F.Y. Dietary glycemic factors, insulin resistance, and adiponectin levels in acne vulgaris. J. Am. Acad. Dermatol. 2016, 75, 155–162. [Google Scholar] [CrossRef] [PubMed]
- Dréno, B.; Pécastaings, S.; Corvec, S.; Veraldi, S.; Khammari, A.; Roques, C. Cutibacterium acnes (Propionibacterium acnes) and acne vulgaris: A brief look at the latest updates. J. Eur. Acad. Dermatol. Venereol. 2018, 32, 5–14. [Google Scholar] [CrossRef]
- Dapkevicius, I.; Romualdo, V.; Marques, A.C.; Lopes, C.M.; Amaral, M.H. Acne Vulgaris Topical Therapies: Application of Probiotics as a New Prevention Strategy. Cosmetics 2023, 10, 77. [Google Scholar] [CrossRef]
- Dhifi, W.; Bellili, S.; Jazi, S.; Bahloul, N.; Mnif, W. Essential oils’ chemical characterization and investigation of some biological activities: A critical review. Medicines 2016, 3, 25. [Google Scholar] [CrossRef]
- de Andrade, S.F.; Rijo, P.; Rocha, C.; Zhu, L.; Rodrigues, L.M. Characterizing the Mechanism of Action of Essential Oils on Skin Homeostasis—Data from Sonographic Imaging, Epidermal Water Dynamics, and Skin Biomechanics. Cosmetics 2021, 8, 36. [Google Scholar] [CrossRef]
- Lee, S.H.; Chow, P.S.; Yagnik, C.K. Developing Eco-Friendly Skin Care Formulations with Microemulsions of Essential Oil. Cosmetics 2022, 9, 30. [Google Scholar] [CrossRef]
- Guzmán, E.; Lucia, A. Essential oils and their individual components in cosmetic products. Cosmetics 2021, 8, 114. [Google Scholar] [CrossRef]
- Macwan, S.R.; Dabhi, B.K.; Aparnathi, K.D.; Prajapati, J.B. Essential oils of herbs and spices: Their antimicrobial activity and application in preservation of foods. Int. J. Curr. Microbiol. Appl. Sci. 2016, 5, 885–901. [Google Scholar] [CrossRef]
- Nazzaro, F.; Fratianni, F.; Coppola, R.; Feo, V.D. Essential oils and antifungal activity. Pharmaceuticals 2017, 10, 86. [Google Scholar] [CrossRef] [PubMed]
- Pinto, E.P.; Menezes, R.P.; Tavares, W.D.S.; Ferreira, A.M.; de Sousa, F.F.O.; da Silva, G.A.; Zamorra, R.R.M.; Araújo, R.S.; de Souza, T.M. Copaiba essential oil loaded-nanocapsules film as a potential candidate for treating skin disorders: Preparation, characterization, and antibacterial properties. Int. J. Pharm. 2023, 2023, 122608. [Google Scholar] [CrossRef]
- Abelan, U.S.; de Oliveira, A.C.; Cacoci, É.S.P.; Martins, T.E.A.; Giacon, V.M.; Velasco, M.V.R.; Lima, C.R.R.D.C. Potential use of essential oils in cosmetic and dermatological hair products: A review. J. Cosmet. Dermatol. 2022, 21, 1407–1418. [Google Scholar] [CrossRef]
- Sharmeen, J.B.; Mahomoodally, F.M.; Zengin, G.; Maggi, F. Essential oils as natural sources of fragrance compounds for cosmetics and cosmeceuticals. Molecules 2021, 26, 666. [Google Scholar] [CrossRef]
- de Andrade, S.F.; Rocha, C.; Pinheiro, E.J.; Pereira-Leite, C.; Costa, M.D.C.; Monteiro Rodrigues, L. Revealing the protective effect of topically applied cymbopogon citratus essential oil in human skin through a contact model. Cosmetics 2023, 10, 29. [Google Scholar] [CrossRef]
- Sarkic, A.; Stappen, I. Essential oils and their single compounds in cosmetics—A critical review. Cosmetics 2018, 5, 11. [Google Scholar] [CrossRef]
- Infante, V.H.; Maia Campos, P.M.; Gaspar, L.R.; Darvin, M.E.; Schleusener, J.; Rangel, K.C.; Meinke, M.C.; Lademann, J. Safety and efficacy of combined essential oils for the skin barrier properties: In vitro, ex vivo and clinical studies. Int. J. Cosmet. Sci. 2022, 44, 118–130. [Google Scholar] [CrossRef]
- Vargas Jentzsch, P.; Ciobotă, V. Raman spectroscopy as an analytical tool for analysis of vegetable and essential oils. Flav. Fragr. J. 2014, 29, 287–295. [Google Scholar] [CrossRef]
- Hammer, K.A. Treatment of acne with tea tree oil (melaleuca) products: A review of efficacy, tolerability, and potential modes of action. Int. J. Antimicrob. Ag. 2015, 45, 106–110. [Google Scholar] [CrossRef] [PubMed]
- Carson, C.F.; Hammer, K.A.; Riley, T.V. Melaleuca alternifolia (tea tree) oil: A review of antimicrobial and other medicinal properties. Clin. Microbiol. Rev. 2006, 19, 50–62. [Google Scholar] [CrossRef] [PubMed]
- Infante, V.; Darvin, M.; Silke, L.; Schleusener, J.; Schanzer, S.; Lademann, J.; Meinke, M. Cosmetic Formulations with Melaleuca alternifolia Essential Oil for the Improvement of Photoaged Skin: A Double-Blind, Randomized, Placebo-Controlled Clinical Study. Photochem. Photobiol. 2022, 99, 176–183. [Google Scholar] [CrossRef] [PubMed]
- Bassett, I.B.; Barnetson, R.S.C.; Pannowitz, D.L. A comparative study of tea-tree oil versus benzoylperoxide in the treatment of acne. Med. J. Aust. 1990, 153, 455–458. [Google Scholar] [CrossRef]
- Enshaieh, S.; Jooya, A.; Siadat, A.H.; Iraji, F. The efficacy of 5% topical tea tree oil gel in mild to moderate acne vulgaris: A randomized, double-blind placebo-controlled study. Ind. J. Dermatol. Venereol. Leprol. 2007, 73, 22. [Google Scholar]
- Pazyar, N.; Yaghoobi, R.; Bagherani, N.; Kazerouni, A. A review of applications of tea tree oil in dermatology. Int. J. Dermatol. 2013, 52, 784–790. [Google Scholar] [CrossRef] [PubMed]
- Ademosun, A.O.; Oboh, G.; Olasehinde, T.A.; Adeoyo, O.O. From folk medicine to functional food: A review on the bioactive components and pharmacological properties of citrus peels. Orient. Pharm. Exp. Med. 2018, 18, 9–20. [Google Scholar] [CrossRef]
- Dhakad, A.K.; Pandey, V.V.; Beg, S.; Rawat, J.M.; Singh, A. Biological, medicinal and toxicological significance of Eucalyptus leaf essential oil: A review. J. Sci. Food Agric. 2018, 98, 833–848. [Google Scholar] [CrossRef] [PubMed]
- Kajjari, S.; Joshi, R.S.; Hugar, S.M.; Gokhale, N.; Meharwade, P.; Uppin, C. The Effects of Lavender Essential Oil and Its Clinical Implications in Dentistry: A Review. Int. J. Clin. Ped. Dent. 2022, 15, 385. [Google Scholar] [CrossRef]
- Kairey, L.; Agnew, T.; Bowles, E.J.; Barkla, B.J.; Wardle, J.; Lauche, R. Efficacy and safety of Melaleuca alternifolia (tea tree) oil for human health—A systematic review of randomized controlled trials. Front. Pharmacol. 2023, 14, 1116077. [Google Scholar] [CrossRef]
- Barradas, T.N.; Silva, K.G.D.H. Nanoemulsions of essential oils to improve solubility, stability, and permeability: A review. Environ. Chem. Lett. 2020, 19, 1153–1171. [Google Scholar] [CrossRef]
- Solans, C.; Izquierdo, P.; Nolla, J.; Azemar, N.; Garcia-Celma, M.J. Nano-emulsions. Curr. Opin. Colloid Interface Sci. 2005, 10, 102–110. [Google Scholar] [CrossRef]
- Barradas, T.N.; Senna, J.P.; Cardoso, S.A.; Nicoli, S.; Padula, C.; Santi, P.; Rossi, F.; Holanda e Silva, K.G.; Mansur, C.R.E. Hydrogel-thickened nanoemulsions based on essential oils for topical delivery of psoralen: Permeation and stability studies. Eur. J. Pharm. Biopharm. 2017, 116, 38–50. [Google Scholar] [CrossRef] [PubMed]
- Choe, C.; Schleusener, J.; Lademann, J.; Darvin, M.E. In vivo confocal Raman microscopic determination of depth profiles of the stratum corneum lipid organization influenced by application of various oils. J. Dermatol. Sci. 2017, 87, 183–191. [Google Scholar] [CrossRef] [PubMed]
- Darvin, M.E.; Meinke, M.C.; Sterry, W.; Lademann, J. Optical methods for noninvasive determination of carotenoids in human and animal skin. J. Biomed. Opt. 2013, 18, 061230. [Google Scholar] [CrossRef]
- Mercurio, D.G.; Segura, J.H.; Demets, M.B.A.; Maia Campos, P.M.B.G. Clinical scoring and instrumental analysis to evaluate skin types. Clin. Exp. Dermatol. 2013, 38, 302–309. [Google Scholar] [CrossRef]
- Schulz, H.; Özkan, G.; Baranska, M.; Krüger, H.; Özcan, M. Characterisation of essential oil plants from Turkey by IR and Raman spectroscopy. V. Spectrosc. 2005, 39, 249–256. [Google Scholar] [CrossRef]
- Manfredini, M.; Greco, M.; Farnetani, F.; Mazzaglia, G.; Ciardo, S.; Bettoli, V.; Virgilli, A.; Pellacani, G. In vivo monitoring of topical therapy for acne with reflectance confocal microscopy. Skin Res. Technol. 2017, 23, 36–40. [Google Scholar] [CrossRef]
- Daferera, D.J.; Tarantilis, P.A.; Polissiou, M.G. Characterization of essential oils from Lamiaceae species by Fourier transform Raman spectroscopy. J. Agric. Food Chem. 2002, 50, 5503–5507. [Google Scholar] [CrossRef]
- Hajhashemi, V.; Ghannadi, A.; Sharif, B. Anti-inflammatory and analgesic properties of the leaf extracts and essential oil of Lavandula angustifolia Mill. J. Ethnopharmacol. 2003, 89, 67–71. [Google Scholar] [CrossRef]
- Baranska, M.; Schulz, H.; Reitzenstein, S.; Uhlemann, U.; Strehle, M.A.; Krüger, H.; Quilitzsch, W.; Foley, J.; Popp, J. Vibrational spectroscopic studies to acquire a quality control method of Eucalyptus essential oils. Biopolym. Orig. Res. Biomol. 2005, 78, 237–248. [Google Scholar] [CrossRef] [PubMed]
- Tsuchida, K.; Sakiyama, N. Blue light-induced lipid oxidation and the antioxidant property of hypotaurine: Evaluation via measuring ultraweak photon emission. Photochem. Photobiol. Sci. 2023, 22, 345–356. [Google Scholar] [CrossRef] [PubMed]
- Maia Campos, P.M.; Melo, M.O.; Mercurio, D. Use of advanced imaging techniques for the characterization of oily skin. Front. Physiol. 2019, 10, 254. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Jiang, Q.D.; Chai, Y.P.; Zhang, H.; Peng, P.; Yang, X.X. Natural terpenes as penetration enhancers for transdermal drug delivery. Molecules 2016, 21, 1709. [Google Scholar] [CrossRef] [PubMed]
- Roberts, C.; Goldstein, E.; Goldstein, B.; Jarman, K.; Paci, K.; Goldstein, A. Men’s attitudes and behaviors about skincare and sunscreen use behaviors. J. Drugs Dermatol. 2020, 20, 88–93. [Google Scholar] [CrossRef]
- Infante, V.H.P.; Bagatin, E.; Maia Campos, P.M. Skin photoaging in young men: A clinical study by skin imaging techniques. Int. J. Cosmet. Sci. 2021, 43, 341–351. [Google Scholar] [CrossRef]
- McKenzie, C.; Rademaker, A.W.; Kundu, R.V. Masculine norms and sunscreen use among adult men in the United States: A cross-sectional study. J. Am. Acad. Dermatol. 2019, 81, 243–249. [Google Scholar] [CrossRef]







| Shape | Absence of Comedones (%) | Edge | Internal Amorphous | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Groups | Regular (%) | Dysmorphic (%) | Thin Edge (%) | Thick Edge (%) | Absent (%) | Present (%) | ||||||||
| T0 | T90 | T0 | T90 | T0 | T90 | T0 | T90 | T0 | T90 | T0 | T90 | T0 | T90 | |
| 4EO | 61 | 67 | 39 | - | - | 33 | 11 | 44 | 89 | 23 | - | 28 | 100 | 50 |
| Nanoem. | 61 | 78 | 39 | - | - | 22 | 33 | 56 | 67 | 22 | - | 17 | 100 | 61 |
| M.a. | 61 | 72 | 30 | 6 | - | 22 | 28 | 56 | 72 | 22 | 6 | 22 | 94 | 56 |
| Placebo | 67 | 56 | 33 | 33 | - | 11 | 17 | 17 | 83 | 72 | 18 | 18 | 82 | 78 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Infante, V.H.P.; Darvin, M.E.; Maia Campos, P.M.B.G. Characterization and Efficacy of Essential Oil-Based Cosmetic Formulations for Acne-Prone Skin. Cosmetics 2023, 10, 158. https://doi.org/10.3390/cosmetics10060158
Infante VHP, Darvin ME, Maia Campos PMBG. Characterization and Efficacy of Essential Oil-Based Cosmetic Formulations for Acne-Prone Skin. Cosmetics. 2023; 10(6):158. https://doi.org/10.3390/cosmetics10060158
Chicago/Turabian StyleInfante, Victor Hugo Pacagnelli, Maxim E. Darvin, and Patrícia M. B. G. Maia Campos. 2023. "Characterization and Efficacy of Essential Oil-Based Cosmetic Formulations for Acne-Prone Skin" Cosmetics 10, no. 6: 158. https://doi.org/10.3390/cosmetics10060158
APA StyleInfante, V. H. P., Darvin, M. E., & Maia Campos, P. M. B. G. (2023). Characterization and Efficacy of Essential Oil-Based Cosmetic Formulations for Acne-Prone Skin. Cosmetics, 10(6), 158. https://doi.org/10.3390/cosmetics10060158







