Antioxidant and Anti-Inflammatory Potential of Brassica oleracea Accelerates Third-Degree Burn Healing in Rats
Abstract
:1. Introduction
2. Materials and Methods
2.1. Extract Preparation, Phytochemical Prospection, and Ointment Preparation
2.2. Animals and Production of the Skin Burn
2.3. Wound Area and Wound Contraction Index
2.4. Experimental Design
2.5. Histological Analysis
2.6. Antioxidant Enzymes
2.7. Markers of Oxidative Stress
2.7.1. Malondialdehyde Assay
2.7.2. Protein Oxidation
2.7.3. Nitric Oxide Production
2.7.4. Hydrogen Peroxide Production
2.8. Statistical Analysis
3. Results
3.1. Phytochemical Prospection and Wound Area
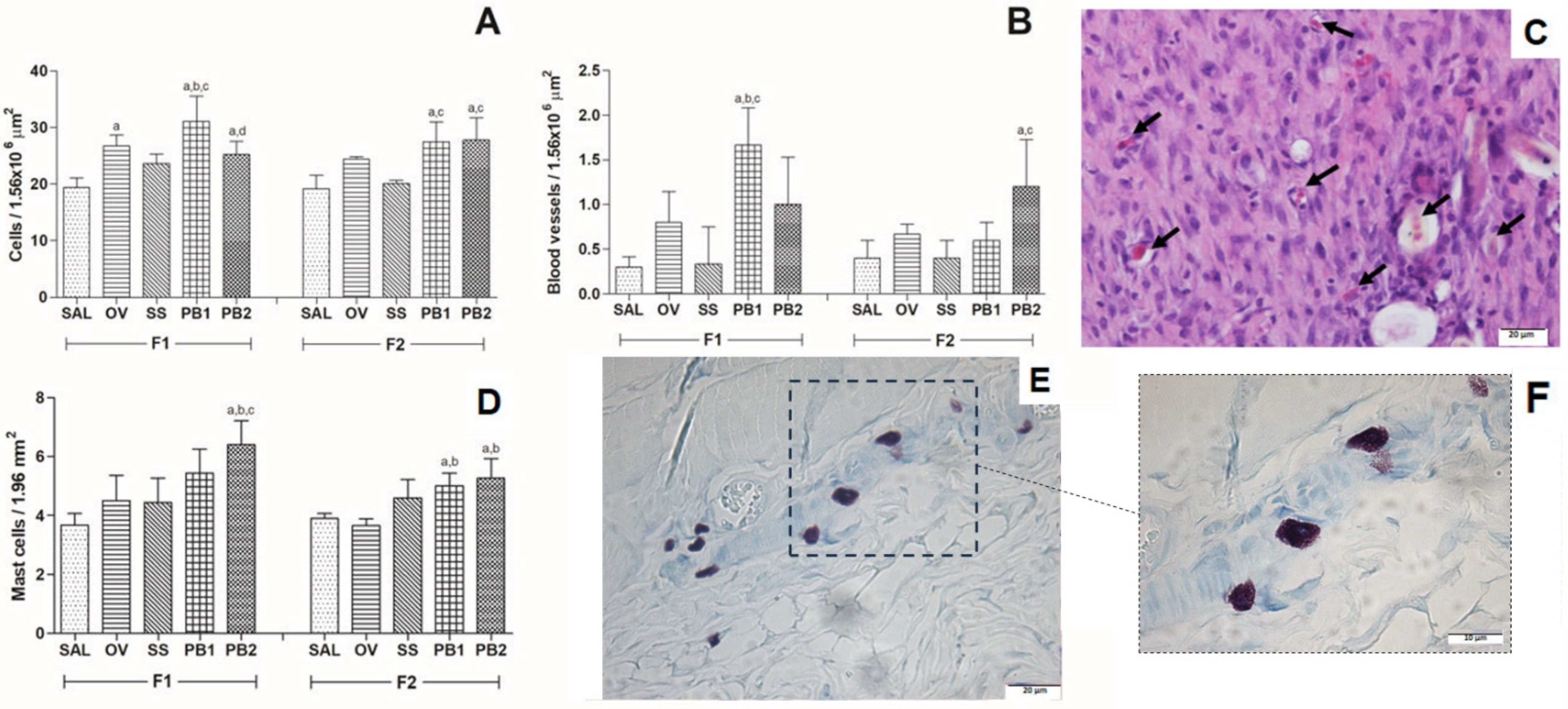
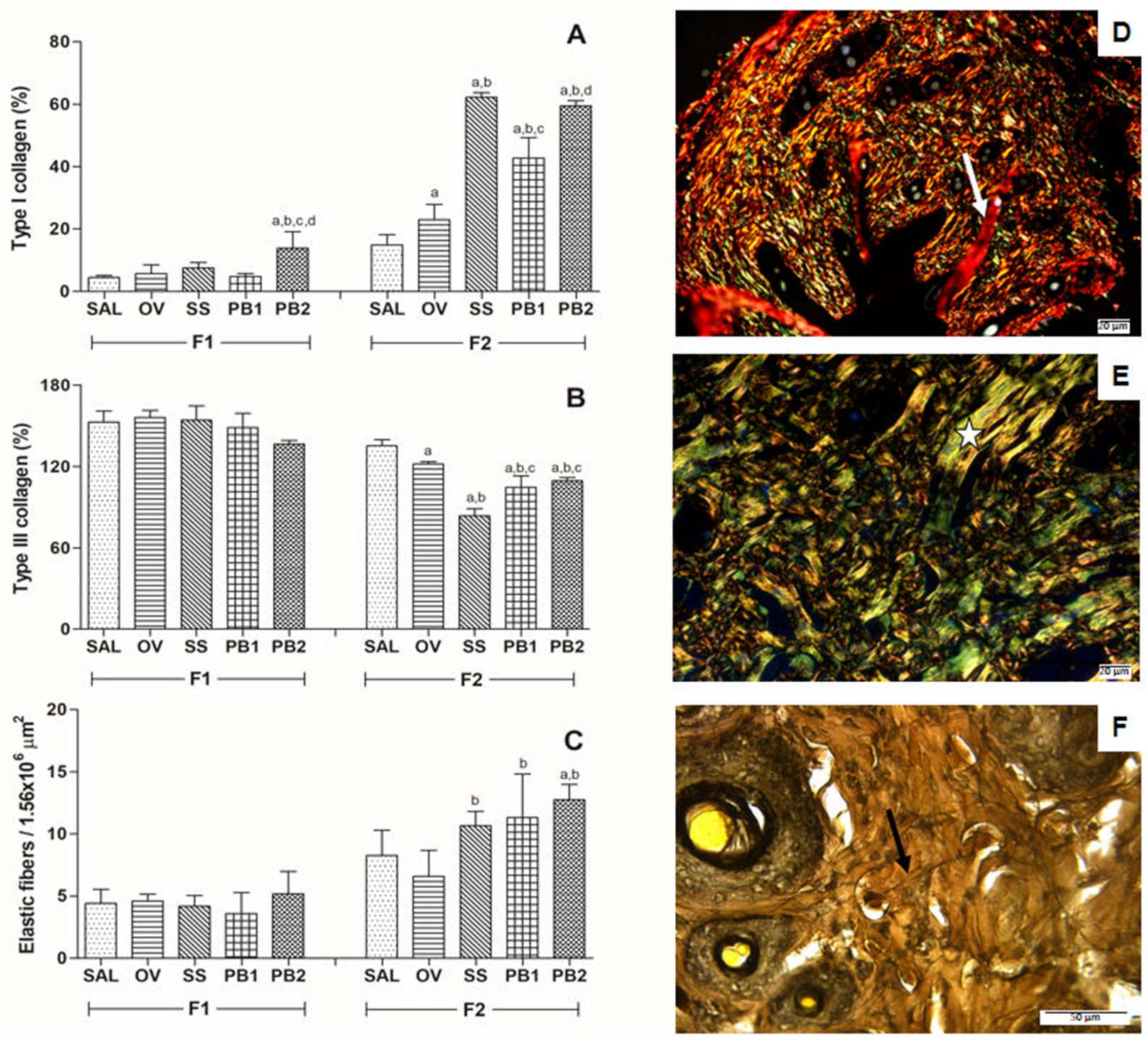
3.2. Histopathological Results
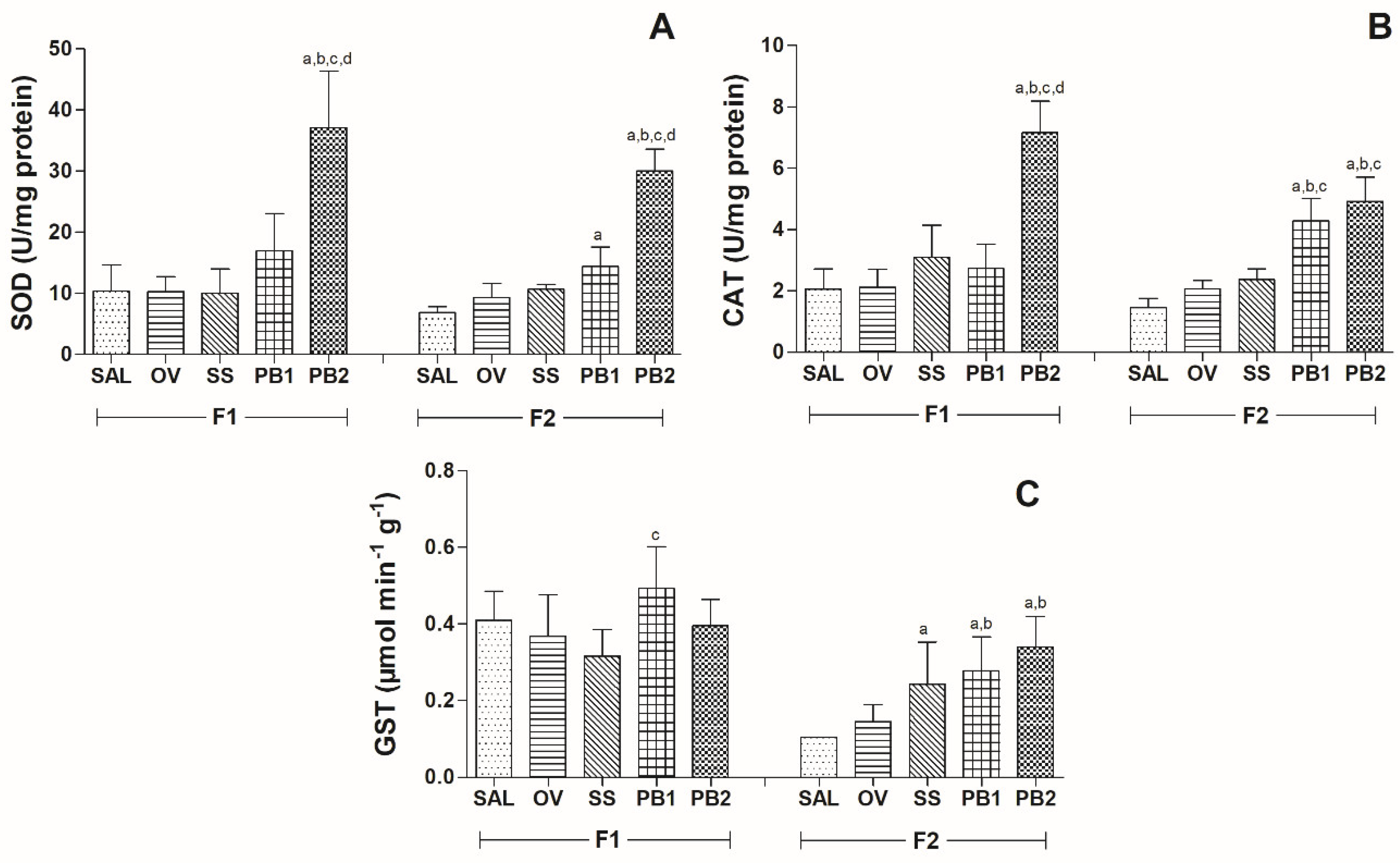
3.3. Antioxidant Enzymes
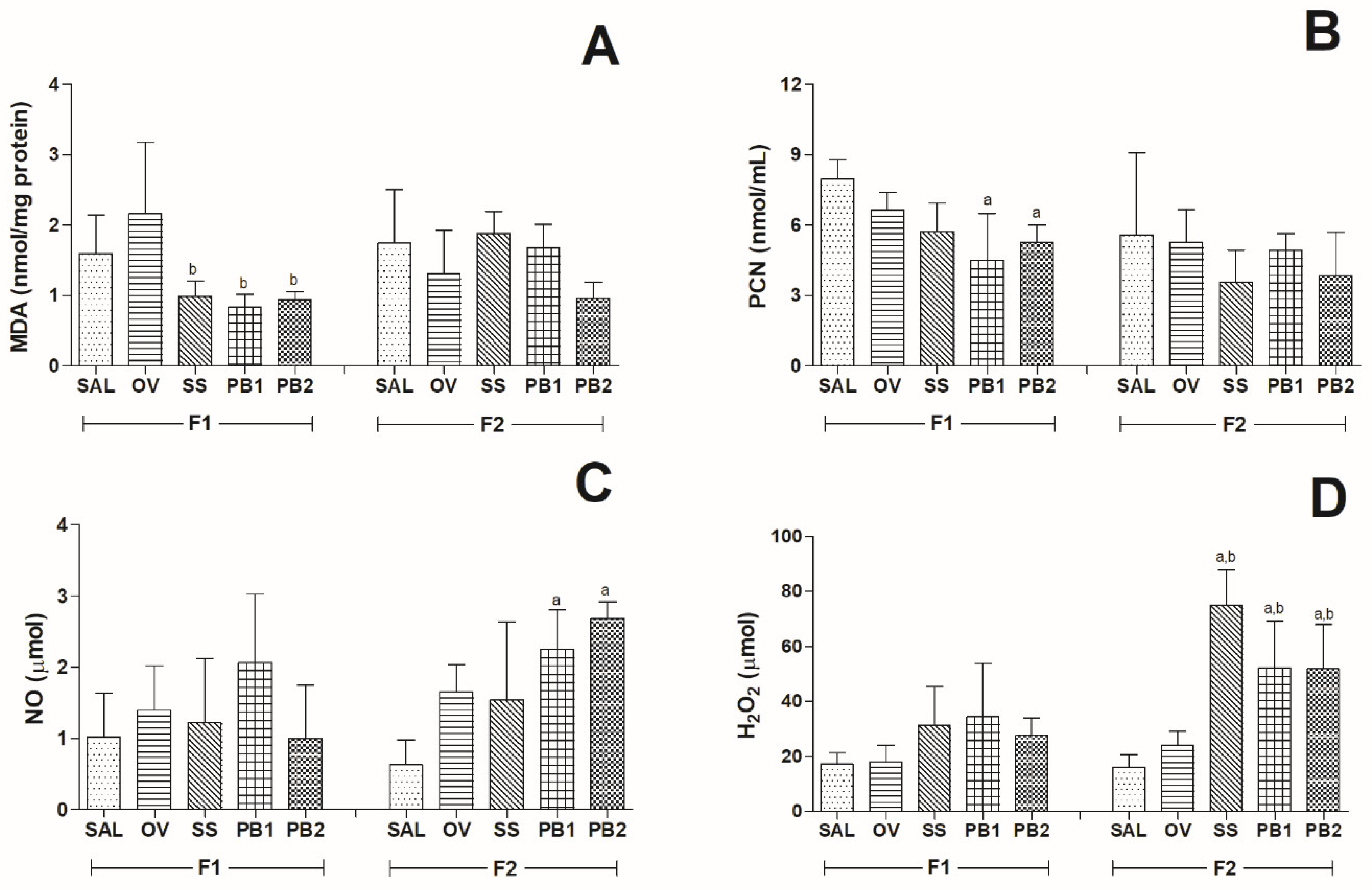
Oxidative Markers
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Ho, J.; Walsh, C.; Yue, D.; Dardik, A.; Cheema, U. Current advancements and strategies in tissue engineering for wound healing: A comprehensive review. Adv. Wound Care 2017, 6, 191–209. [Google Scholar] [CrossRef] [PubMed]
- Basu, P.; Kumar, U.N.; Manjubala, I. Wound healing materials—A perspective for skin tissue engineering. Curr. Sci. 2017, 112, 2392. [Google Scholar] [CrossRef]
- Guo, S.; Dipietro, L.A. Factors affecting wound healing. J. Dent. Res. 2010, 89, 219–229. [Google Scholar] [CrossRef] [PubMed]
- Hamilton, T.J.; Patterson, J.; Williams, R.Y.; Ingram, W.L.; Hodge, J.S.; Abramowicz, S. Management of head and neck burns—A 15-year review. J. Oral Maxillofac. Surg. 2018, 76, 375–379. [Google Scholar] [CrossRef] [PubMed]
- Bahramsoltani, R.; Farzaei, M.H.; Abdolghaffari, A.H.; Rahimi, R.; Samadi, N.; Heidari, M.; Esfandyari, M.; Baeeri, M.; Hassanzadeh, G.; Abdollahi, M.; et al. Evaluation of phytochemicals, antioxidant and burn wound healing activities of Cucurbita moschata Duchesne fruit peel. Iran. J. Basic Med. Sci. 2017, 20, 798–805. [Google Scholar] [PubMed]
- Fekrazad, R.; Nikkerdar, A.; Joharchi, K.; Kalhori, K.A.M. Effect of laser photostimulation on the healing of third-degree burn wounds in rats. Acta Cir. Bras. 2014, 2, 5. [Google Scholar] [CrossRef]
- Singer, A.J.; Taira, B.R.; Anderson, R.; McClain, S.A.; Rosenberg, L. Does pressure matter in creating burns in a porcine model? J. Burn Care Res. 2010, 31, 646–651. [Google Scholar] [CrossRef]
- Ye, H.; De, S. Thermal injury of skin and subcutaneous tissues: A review of experimental approaches and numerical models. Burns 2017, 43, 909–932. [Google Scholar] [CrossRef]
- Takeo, M.; Lee, W.; Ito, M. Wound healing and skin regeneration. Cold Spring Harb. Perspect. Med. 2015, 5, a023267. [Google Scholar] [CrossRef]
- Mulholland, E.J.; Dunne, N.; McCarthy, H.O. MicroRNA as therapeutic targets for chronic wound healing. Mol. Ther. Nucleic Acids 2017, 8, 46–55. [Google Scholar] [CrossRef]
- Sun, B.K.; Siprashvili, Z.; Khavari, P.A. Advances in skin grafting and treatment of cutaneous wounds. Science 2014, 346, 941–944. [Google Scholar] [CrossRef]
- Curtis, B.J.; Hlavin, S.; Brubaker, A.L.; Kovacs, E.J.; Radek, K.A. Episodic binge ethanol exposure impairs murine macrophage infiltration and delays wound closure by promoting defects in early innate immune responses. Alcohol. Clin. Exp. Res. 2014, 38, 1347–1355. [Google Scholar] [CrossRef] [PubMed]
- Hyldig, K.; Riis, S.; Pennisi, C.P.; Zachar, V.; Fink, T. Implications of extracellular matrix production by adipose tissue-derived stem cells for development of wound healing therapies. Int. J. Mol. Sci. 2017, 18, 1167. [Google Scholar] [CrossRef] [PubMed]
- Werner, S.; Antsiferova, M. Wound healing: An orchestrated process of cell cycle, adhesion, and signaling. Encycl. Cell Biol. 2016, 3, 216–222. [Google Scholar]
- Chouhan, D.; Chakraborty, B.; Nandi, S.K.; Mandal, B.B. Role of non-mulberry silk fibroin in deposition and regulation of extracellular matrix towards accelerated wound healing. Acta Biomater. 2017, 48, 157–174. [Google Scholar] [CrossRef] [PubMed]
- Docheva, D.; Müller, S.A.; Majewski, M.; Evans, C.H. Biologics for tendon repair. Adv. Drug Deliv. Rev. 2015, 84, 222–239. [Google Scholar] [CrossRef] [PubMed]
- Bankoti, K.; Rameshbabu, A.P.; Datta, S.; Maity, P.P.; Goswami, P.; Datta, P.; Ghosh, S.K.; Mitra, A.; Dhara, S. Accelerated healing of full thickness dermal wounds by macroporous waterborne polyurethane-chitosan hydrogel scaffolds. Mater. Sci. Eng. C 2017, 81, 133–143. [Google Scholar] [CrossRef]
- Wagener, F.A.; Carels, C.E.; Lundvig, D.M.S. Targeting the redox balance in inflammatory skin conditions. Int. J. Mol. Sci. 2013, 14, 9126–9167. [Google Scholar] [CrossRef]
- Schieber, M.; Chandel, N.S. ROS function in redox signaling and oxidative stress. Curr. Biol. 2014, 24, R453–R462. [Google Scholar] [CrossRef]
- Kirici, M.; Turk, C.; Caglayan, C.; Kirici, M. Toxic effects of copper sulfate pentahydrate on antioxidant enzyme activities and lipid peroxidation of freshwater fish capoeta Umbla (Heckel, 1843) tissues. Appl. Ecol. Environ. Res. 2017, 15, 1685–1696. [Google Scholar] [CrossRef]
- Chtourou, Y.; Aouey, B.; Kebieche, M.; Fetoui, H. Protective role of naringin against cisplatin induced oxidative stress, inflammatory response and apoptosis in rat striatum via suppressing ROS-mediated NF-κB and P53 signaling pathways. Chem. Biol. Interact. 2015, 239, 76–86. [Google Scholar] [CrossRef] [PubMed]
- Holmström, K.M.; Finkel, T. Cellular mechanisms and physiological consequences of redox-dependent signalling. Nat. Rev. Mol. Cell Biol. 2014, 15, 411–421. [Google Scholar] [CrossRef] [PubMed]
- Ighodaro, O.M.; Akinloyeb, O.A. First line defence antioxidants-superoxide dismutase (SOD), catalase (CAT) and glutathione peroxidase (GPX): Their fundamental role in the entire antioxidant defence grid. Alexandria J. Med. 2017, 54, 287–293. [Google Scholar] [CrossRef]
- Sarandy, M.M.; Miranda, L.L.; Altoé, L.S.; Novaes, R.D.; Zanuncio, V.V.; Leite, J.P.V.; Gonçalves, R.V. Strychnos pseudoquina modulates the morphological reorganization of the scar tissue of second intention cutaneous wounds in rats. PLoS ONE 2018, 13, e0195786. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Beekman, J.; Hew, J.; Jackson, S.; Issler-Fisher, A.C.; Parungao, R.; Lajevardi, S.S.; Li, Z.; Maitz, P.K. Burn injury: Challenges and advances in burn wound healing, infection, pain and scarring. Adv. Drug Deliv. Rev. 2018, 123, 3–17. [Google Scholar] [CrossRef] [PubMed]
- Hashmi, M.U.; Khan, F.; Khalid, N.; Shahid, A.A.; Javed, A.; Alam, T.; Jalal, N.; Hayat, M.Q.; Abbas, S.R.; Janjua, H.A. Hydrogels incorporated with silver nanocolloids prepared from antioxidant rich Aerva javanica as disruptive agents against burn wound infections. Colloids Surf. A Physicochem. Eng. Asp. 2017, 529, 475–486. [Google Scholar] [CrossRef]
- Bahramsoltani, R.; Farzaei, M.H.; Rahimi, R. Medicinal plants and their natural components as future drugs for the treatment of burn wounds: An integrative review. Arch. Dermatol. Res. 2014, 306, 601–617. [Google Scholar] [CrossRef]
- Efferth, T.; Koch, E. Complex interactions between phytochemicals. The multi-target therapeutic concept of phytotherapy. Curr. Drug Targets 2011, 12, 122–132. [Google Scholar] [CrossRef]
- Akter, K.; Barnes, E.C.; Brophy, J.J.; Harrington, D.; Yaegl Community Elders; Vemulpad, S.R.; Jamie, J.F. Phytochemical profile and antibacterial and antioxidant activities of medicinal plants used by aboriginal people of New South Wales, Australia. Evid. Based Complement. Altern. Med. 2016, 2016, 4683059. [Google Scholar] [CrossRef]
- Rekik, D.M.; Ben Khedir, S.; Moalla, K.K.; Kammoun, N.G.; Rebai, T.; Sahnoun, Z. Evaluation of wound healing properties of grape seed, sesame, and fenugreek oils. Evid. Based Complement. Altern. Med. 2016, 2016, 7965689. [Google Scholar]
- Šamec, D.; Pavlović, I.; Salopek-Sondi, B. White cabbage (Brassica oleracea var. capitata f. Alba): Botanical, phytochemical and pharmacological overview. Phytochem. Rev. 2017, 16, 117–135. [Google Scholar] [CrossRef]
- Hallmann, E.; Kazimierczak, R.; Marszałek, K.; Drela, N.; Kiernozek, E.; Toomik, P.; Matt, D.; Luik, A.; Rembiałkowska, E. The nutritive value of organic and conventional white cabbage (Brassica oleracea L. var. capitata) and anti-Apoptotic activity in gastric adenocarcinoma cells of sauerkraut juice produced Therof. J. Agric. Food Chem. 2017, 65, 8171–8183. [Google Scholar] [CrossRef] [PubMed]
- Samuelsen, A.B.; Westereng, B.; Yousif, O.; Holtekjølen, A.K.; Michaelsen, T.E.; Knutsen, S.H. Structural features and complement-fixing activity of pectin from three Brassica oleracea varieties: White cabbage, kale, and red kale. Biomacromolecules 2007, 8, 644–649. [Google Scholar] [CrossRef] [PubMed]
- Vale, A.P.; Cidade, H.; Pinto, M.; Oliveira, M.B.P. Effect of sprouting and light cycle on antioxidant activity of Brassica oleracea varieties. Food Chem. 2014, 165, 379–387. [Google Scholar] [CrossRef] [PubMed]
- De Carvalho, C.A.; da Silva, M.B.; de Oliveira, T.G.; de Matos, J.; da Rosa, M.B. Estudo espectrométrico de diferentes estágios fenológicos da Brassica oleracea var. capitata . Rev. Bras. Farmacogn. 2008, 18, 249–257. [Google Scholar] [CrossRef]
- Prá, V.D.; Dolwitsch, C.B.; Lima, F.O.; De Carvalho, C.A.; Viana, C.; Nascimento, P.C.D.; Da Rosa, M.B. Ultrasound-assisted extraction and biological activities of extracts of Brassica oleracea var. capitata . Food Technol. Biotechnol. 2015, 53, 102–109. [Google Scholar]
- Nugrahedi, P.Y.; Verkerk, R.; Widianarko, B.; Dekker, M. A mechanistic perspective on process-induced changes in glucosinolate content in Brassica vegetables: A review. Crit. Rev. Food Sci. Nutr. 2015, 55, 823–838. [Google Scholar] [CrossRef]
- Hassini, I.; Baenas, N.; Moreno, D.A.; Carvajal, M.; Boughanmi, N.; Martinez Ballesta, M.D.C. Effects of seed priming, salinity and methyl jasmonate treatment on bioactive composition of Brassica oleracea var. capitata (white and red varieties) sprouts. J. Sci. Food Agric. 2017, 97, 2291–2299. [Google Scholar] [CrossRef]
- Hu, S.-H.; Wang, J.-C.; Kung, H.-F.; Wang, J.-T.; Lee, W.-L.; Yang, Y.-H. Antimicrobial effect of extracts of cruciferous vegetables. Kaohsiung J. Med. Sci. 2004, 20, 591–599. [Google Scholar] [CrossRef]
- Sotelo, T.; Lema, M.; Soengas, P.; Cartea, M.E.; Velasco, P. In vitro activity of glucosinolates and their degradation products against Brassica-Pathogenic bacteria and fungi. Appl. Environ. Microbiol. 2014, 81, 432–440. [Google Scholar] [CrossRef]
- Harborne, J.B. Phytochemical Methods; Chapman and Hall: London, UK, 2002. [Google Scholar]
- Mattosinhos, P.S.; Sarandy, M.M.; Novaes, R.D.; Esposito, D.; Goncalves, R.V. Anti-Inflammatory, Antioxidant, and Skin Regenerative Potential of Secondary Metabolites from Plants of the Brassicaceae Family: A Systematic Review of In Vitro and In Vivo Preclinical Evidence (Biological Activities Brassicaceae Skin Diseases). Antioxidants 2022, 11, 1346. [Google Scholar] [CrossRef] [PubMed]
- Gonçalves, R.V.; Novaes, R.D.; Cupertino, M.C.; Araújo, B.M.; Vilela, E.F.; Machado, A.T.; Leite, J.P.; Matta, S.L. Bathysa cuspidata extract modulates the morphological reorganization of the scar tissue and accelerates skin wound healing in rats: A time-dependent study. Cells Tissues Organs 2014, 199, 266–277. [Google Scholar] [CrossRef]
- Partoazar, A.; Kianvash, N.; Darvishi, M.H.; Nasoohi, S.; Rezayat, S.M.; Bahador, A. Ethosomal curcumin promoted wound healing and reduced bacterial flora in second degree burn in rat. Drug Res. 2016, 66, 660–665. [Google Scholar] [CrossRef] [PubMed]
- Gonçalves, R.; Mezêncio, J.; Benevides, G.; Matta, S.; Neves, C.; Sarandy, M.; Vilela, E. Effect of gallium-arsenide laser, gallium-aluminum-arsenide laser and healing ointment on cutaneous wound healing in Wistar rats. Braz. J. Med. Biol. Res. 2010, 43, 350–355. [Google Scholar] [CrossRef] [PubMed]
- Agren, M.S.; Mertz, P.M.; Franzén, L. A comparative study of three occlusive dressings in the treatment of full-thickness wounds in pigs. J. Am. Acad. Dermatol. 1997, 36, 53–58. [Google Scholar] [CrossRef] [PubMed]
- Altoé, L.S.; Alves, R.S.; Sarandy, M.M.; Morais-Santos, M.; Novaes, R.D.; Gonçalves, R.V. Does antibiotic use accelerate or retard cutaneous repair? A systematic review in animal models. PLoS ONE 2019, 22, e0223511. [Google Scholar] [CrossRef] [PubMed]
- Dolber, P.C.; Spach, M.S. Conventional and confocal fluorescence microscopy of collagen fibers in the heart. J. Histochem. Cytochem. 1993, 41, 465–469. [Google Scholar] [CrossRef] [PubMed]
- Junqueira, L.C.U.; Carneiro, J. Biologia Celular e Molecular, 8th ed.; Guanabara Koogan: Rio de Janeiro, Brazil, 2005. [Google Scholar]
- Churukian, C.J.; Schenk, E.A. A toluidine blue method for demonstrating mast cells. J. Histotechnol. 1981, 4, 85–86. [Google Scholar] [CrossRef]
- Mandarim-de-Lacerda, C.A. Stereological tools in biomedical research. An. Acad. Bras. Cienc. 2003, 75, 469–486. [Google Scholar] [CrossRef]
- Gonçalves, R.V.; Novaes, R.D.; Sarandy, M.M.; Damasceno, E.M.; da Matta, S.L.; de Gouveia, N.M.; Freitas, M.B.; Espindola, F.S. 5α-Dihydrotestosterone enhances wound healing in diabetic rats. Life Sci. 2016, 152, 67–75. [Google Scholar] [CrossRef]
- Dieterich, S.; Bieligk, U.; Beulich, K.; Hasenfuss, G.; Prestle, J. Gene expression of antioxidative enzymes in the human heart: Increased expression of catalase in the end-stage failing heart. Circulation 2000, 101, 33–39. [Google Scholar] [CrossRef]
- Rosa, D.F.; Sarandy, M.M.; Novaes, R.D.; Freitas, M.B.; Pelúzio, M.D.C.G.; Gonçalves, R.V. High-Fat Diet and Alcohol Intake Promotes Inflammation and Impairs Skin Wound Healing in Wistar Rats. Mediat. Inflamm. 2018, 2018, 4658583. [Google Scholar] [CrossRef] [PubMed]
- Habig, W.H.; Pabst, M.J.; Jakoby, W.B. Glutathione S-transferases. The first enzymatic step in mercapturic acid formation. J. Biol. Chem. 1974, 249, 7130–7139. [Google Scholar] [CrossRef] [PubMed]
- Novaes, R.D.; Santos, E.C.; Fialho, M.D.C.Q.; Gonçalves, W.G.; Sequetto, P.L.; Talvani, A.; Gonçalves, R.V. Nonsteroidal anti-inflammatory is more effective than anti-oxidant therapy in counteracting oxidative/nitrosative stress and heart disease in T. cruzi-infected mice. Parasitology 2017, 144, 904–916. [Google Scholar] [CrossRef] [PubMed]
- Bradford, M.M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem. 1976, 72, 248–254. [Google Scholar] [CrossRef] [PubMed]
- Levine, R.L.; Garland, D.; Oliver, C.N.; Amici, A.; Climent, I.; Lenz, A.G.; Ahn, B.W.; Shaltiel, S.; Stadtman, E.R. Determination of carbonyl content in oxidatively modified proteins. Methods Enzymol. 1990, 186, 464–478. [Google Scholar] [PubMed]
- Tsikas, D. Analysis of nitrite and nitrate in biological fluids by assays based on the Griess reaction: Appraisal of the Griess reaction in the l-arginine/nitric oxide area of research. J. Chromatogr. B 2007, 851, 51–70. [Google Scholar] [CrossRef] [PubMed]
- Oliveira, J.M.; Losano, N.F.; Condessa, S.S.; de Freitas, R.M.P.; Cardoso, S.A.; Freitas, M.B.; de Oliveira, L.L. Exposure to deltamethrin induces oxidative stress and decreases of energy reserve in tissues of the Neotropical fruit-eating bat Artibeus lituratus . Ecotoxicol. Environ. Saf. 2018, 148, 684–692. [Google Scholar] [CrossRef]
- Brassolatti, P.; de Andrade, A.L.M.; Bossini, P.S.; Otterço, A.N.; Parizotto, N.A. Evaluation of the low-level laser therapy application parameters for skin burn treatment in experimental model: A systematic review. Lasers Med. Sci. 2018, 33, 1159–1169. [Google Scholar] [CrossRef]
- Li, M.; Qiu, L.; Hu, W.; Deng, X.; Xu, H.; Cao, Y.; Xiao, Z.; Peng, L.; Johnson, S.; Alexey, L.; et al. Genetically-modified bone mesenchymal stem cells with TGF-β 3 improve wound healing and reduce scar tissue formation in a rabbit model. Exp. Cell Res. 2018, 367, 24–29. [Google Scholar] [CrossRef]
- Silva, K.S.; Santos, E.D.; Benett, C.G.; Laranjeira, L.T.; Eberhardt Neto, E.; Costa, E. Produtividade e desenvolvimento de cultivares de repolho em função de doses de boro. Hortic. Bras. 2012, 30, 520–525. [Google Scholar] [CrossRef]
- Ahmed, M.F.; Rao, A.S.; Ahemad, S.R.; Ibrahim, M. Phytochemical studies and antioxidant activities of Brassica oleracea L. var. capitata. Int. J. Pharm. Pharm. Sci. 2012, 4, 374–378. [Google Scholar]
- Fernández-García, E.; Carvajal-Lérida, I.; Jarén-Galán, M.; Garrido-Fernández, J.; Pérez-Gálvez, A.; Hornero-Méndez, D. Carotenoids bioavailability from foods: From plant pigments to efficient biological activities. Food Res. Int. 2012, 46, 438–450. [Google Scholar] [CrossRef]
- Sarandy, M.M.; Novaes, R.D.; da Matta, S.L.P.; Mezencio, J.M.D.S.; da Silva, M.B.; Zanuncio, J.C.; Gonçalves, R.V. Ointment of Brassica oleracea var. capitata matures the extracellular matrix in skin wounds of Wistar rats. Evid. Based Complement. Altern. Med. 2015, 2015, 919342. [Google Scholar] [CrossRef] [PubMed]
- Park, S.; Arasu, M.V.; Jiang, N.; Choi, S.-H.; Lim, Y.P.; Park, J.-T.; Al-Dhabi, N.A.; Kim, S.-J. Metabolite profiling of phenolics, anthocyanins and flavonols in cabbage (Brassica oleracea var. capitata). Ind. Crops Prod. 2014, 60, 8–14. [Google Scholar] [CrossRef]
- Xu, F.; Zheng, Y.; Yang, Z.; Cao, S.; Shao, X.; Wang, H. Domestic cooking methods affect the nutritional quality of red cabbage. Food Chem. 2014, 161, 162–167. [Google Scholar] [CrossRef] [PubMed]
- Senthilkumar, A.; Karuvantevida, N.; Rastrelli, L.; Kurup, S.S.; Cheruth, A.J. Traditional uses, pharmacological efficacy, and phytochemistry of Moringa peregrina (Forssk.) Fiori.—A review. Front. Pharmacol. 2018, 9, 465. [Google Scholar] [CrossRef] [PubMed]
- Wei, M.; Ma, Y.; Liu, Y.; Zhou, Y.; Men, L.; Yue, K.; Pi, Z.; Liu, Z.; Liu, Z. Urinary metabolomics study on the anti-inflammation effects of flavonoids obtained from Glycyrrhiza. J. Chromatogr. B 2018, 1086, 1–10. [Google Scholar] [CrossRef]
- Valenza, A.; Bonfanti, C.; Pasini, M.E.; Bellosta, P. Anthocyanins function as anti-inflammatory agents in a Drosophila model for adipose tissue macrophage infiltration. BioMed Res. Int. 2018, 2018, 6413172. [Google Scholar] [CrossRef]
- Mehrabani, D.; Farjam, M.; Geramizadeh, B.; Tanideh, N.; Amini, M.; Panjehshahin, M.R. The healing effect of curcumin on burn wounds in rat. World J. Plast. Surg. 2015, 4, 29–35. [Google Scholar]
- Das, U.; Behera, S.S.; Pramanik, K. Ethno-herbal-medico in wound repair: An incisive review. Phyther. Res. 2017, 31, 579–590. [Google Scholar] [CrossRef] [PubMed]
- Kurahashi, T.; Fujii, J. Roles of antioxidative enzymes in wound healing. J. Dev. Biol. 2015, 3, 57–70. [Google Scholar] [CrossRef]
- Lai, J.C.-Y.; Lai, H.-Y.; Rao, N.K.; Ng, S.-F. Treatment for diabetic ulcer wounds using a fern tannin optimized hydrogel formulation with antibacterial and antioxidative properties. J. Ethnopharmacol. 2016, 189, 277–289. [Google Scholar] [CrossRef] [PubMed]
- Pringle, N.A.; Koekemoer, T.C.; Holzer, A.; Young, C.; Venables, L.; van de Venter, M. Potential therapeutic benefits of green and fermented rooibos (Aspalathus linearis) in dermal wound healing. Planta Med. 2018, 84, 645–652. [Google Scholar] [CrossRef] [PubMed]
- Lateef, Z.; Stuart, G.; Jones, N.; Mercer, A.; Fleming, S.; Wise, L. The Cutaneous Inflammatory Response to Thermal Burn Injury in a Murine Model. Int. J. Mol. Sci. 2019, 20, 538. [Google Scholar] [CrossRef] [PubMed]
- Wulandari, E.; Jusman, S.W.A.; Moenadjat, Y.; Jusuf, A.A.; Sadikin, M. Expressions of collagen I and III in hypoxic keloid tissue. Kobe J. Med. Sci. 2016, 62, E58–E69. [Google Scholar] [PubMed]
- Chen, J.; Gao, K.; Liu, S.; Wang, S.; Elango, J.; Bao, B.; Dong, J.; Liu, N.; Wu, W. Fish collagen surgical compress repairing characteristics on wound healing process in vivo. Mar. Drugs 2019, 17, 33. [Google Scholar] [CrossRef]
- Stipcevic, T.; Piljac, J.; Berghe, D.V. Effect of different flavonoids on collagen synthesis in human fibroblasts. Plant Foods Hum. Nutr. 2006, 61, 29–34. [Google Scholar] [CrossRef]
- Faggio, C.; Sureda, A.; Morabito, S.; Sanches-Silva, A.; Mocan, A.; Nabavi, S.M. Flavonoids and platelet aggregation: A brief review. Eur. J. Pharmacol. 2017, 807, 91–101. [Google Scholar] [CrossRef]
- Corrêa, F.R.S.; Schanuel, F.S.; Moura-Nunes, N.; Monte-Alto-Costa, A.; Daleprane, J.B. Brazilian red propolis improves cutaneous wound healing suppressing inflammation-associated transcription factor NFκB. Biomed. Pharmacother. 2017, 86, 162–171. [Google Scholar] [CrossRef]
- Ayala, A.; Muñoz, M.F.; Argüelles, S. Lipid peroxidation: Production, metabolism, and signaling mechanisms of malondialdehyde and 4-hydroxy-2-nonenal. Oxid. Med. Cell. Longev. 2014, 2014, 360438. [Google Scholar] [CrossRef]
- Pisoschi, A.; Pop, A. The role of antioxidants in the chemistry of oxidative stress: A review. Eur. J. Med. Chem. 2015, 97, 55–74. [Google Scholar] [CrossRef] [PubMed]
- Bollineni, R.C.; Fedorova, M.; Blüher, M.; Hoffmann, R. Carbonylated plasma proteins as potential biomarkers of obesity induced type 2 diabetes mellitus. J. Proteome Res. 2014, 13, 5081–5093. [Google Scholar] [CrossRef] [PubMed]
- Manoto, S.L.; Maepa, M.J.; Motaung, S.K. Medical ozone therapy as a potential treatment modality for regeneration of damaged articular cartilage in osteoarthritis. Saudi J. Biol. Sci. 2018, 25, 672–679. [Google Scholar] [CrossRef] [PubMed]
- Türedi, S.; Yuluğ, E.; Alver, A.; Bodur, A.; İnce, İ. A morphological and biochemical evaluation of the effects of quercetin on experimental sciatic nerve damage in rats. Exp. Ther. Med. 2018, 15, 3215–3224. [Google Scholar] [PubMed]
- Iuchi, Y.; Roy, D.; Okada, F.; Kibe, N.; Tsunoda, S.; Suzuki, S.; Takahashi, M.; Yokoyama, H.; Yoshitake, J.; Kondo, S.; et al. Spontaneous skin damage and delayed wound healing in SOD1-deficient mice. Mol. Cell. Biochem. 2010, 341, 181–194. [Google Scholar] [CrossRef] [PubMed]
- Sarandy, M.M.; Novaes, R.D.; Xavier, A.A.; Vital, C.E.; Leite, J.P.V.; Melo, F.C.S.A.; Gonçalves, R.V. Hydroethanolic extract of Strychnos pseudoquina accelerates skin wound healing by modulating the oxidative status and microstructural reorganization of scar tissue in experimental type I diabetes. BioMed Res. Int. 2017, 2017, 9538351. [Google Scholar] [CrossRef] [PubMed]
- Al-Roujayee, A.S. Naringenin improves the healing process of thermally-induced skin damage in rats. J. Int. Med. Res. 2017, 45, 570–582. [Google Scholar] [CrossRef] [PubMed]
- Bryan, N.S. Functional nitric oxide nutrition to combat cardiovascular disease. Curr. Atheroscler. Rep. 2018, 20, 21. [Google Scholar] [CrossRef]
- Magenta, A.; Greco, S.; Capogrossi, M.C.; Gaetano, C.; Martelli, F. Nitric oxide, oxidative stress, and p66Shc interplay in diabetic endothelial dysfunction. BioMed Res. Int. 2014, 2014, 193095. [Google Scholar] [CrossRef]
- Pierini, D.; Bryan, N.S. Nitric oxide availability as a marker of oxidative stress. Methods Mol. Biol. 2015, 1208, 63–71. [Google Scholar]
- Kim, A.; Lang, T.; Xue, M.; Wijewardana, A.; Jackson, C.; Vandervord, J. The Role of Th-17 Cells and γδ T-Cells in Modulating the Systemic Inflammatory Response to Severe Burn Injury. Int. J. Mol. Sci. 2017, 18, 758. [Google Scholar] [CrossRef] [PubMed]
- Mittal, M.; Siddiqui, M.R.; Tran, K.; Reddy, S.P.; Malik, A.B. Reactive Oxygen Species in inflammation and tissue injury. Antioxid. Redox Signal. 2014, 20, 1126–1167. [Google Scholar] [CrossRef] [PubMed]
- Cordeiro, J.V.; Jacinto, A. The role of transcription-independent damage signals in the initiation of epithelial wound healing. Nat. Rev. Mol. Cell Biol. 2013, 14, 249–262. [Google Scholar] [CrossRef] [PubMed]
- Sofo, A.; Scopa, A.; Nuzzaci, M.; Vitti, A. Ascorbate peroxidase and catalase activities and their genetic regulation in plants subjected to drought and salinity stresses. Int. J. Mol. Sci. 2015, 161, 1356–13578. [Google Scholar] [CrossRef] [PubMed]
- Sharma, P.; Jha, A.B.; Dubey, R.S.; Pessarakli, M. Reactive oxygen species, oxidative damage, and antioxidative defense mechanism in plants under stressful conditions. J. Bot. 2012, 2012, 217037. [Google Scholar] [CrossRef]
- Sies, H. Role of metabolic H2O2 generation. J. Biol. Chem. 2014, 289, 8735–8741. [Google Scholar] [CrossRef] [PubMed]
| SAL | OV | SS | PB1 | PB2 | ||
|---|---|---|---|---|---|---|
| F0 | Area | 156.6 ± 20.1 | 161.6 ± 29.4 | 141.3 ± 15.7 | 126.9 ± 13.3 | 134.9 ± 13.5 |
| RWC | 0.0 ± 0.0 | 0.0 ± 0.0 | 0.0 ± 0.0 | 0.0 ± 0.0 | 0.0 ± 0.0 | |
| F1 | Area | 153.1 ± 33.4 | 178.4 ± 18.8 | 138.4 ± 32.3 | 87.4 ± 19.7 * | 97.7 ± 22.4 * |
| RWC | 10.3 ± 6.9 | 3.3 ± 20.6 | 2.4 ± 18.4 | 31.4 ± 10.8 | 27.8 ± 13.3 | |
| F2 | Area | 142.2 ± 30.0 | 155.4 ± 34.7 | 133.9 ± 50.4 | 67.6 ± 20.9 * | 66.4 ± 21.4 * |
| RWC | 15.9 ± 14.3 | 10.4 ± 19.8 | 28.1 ± 18.0 | 47.2 ± 12.2 | 51.4 ± 12.5 * |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Miranda, L.L.; Sarandy, M.M.; Altoé, L.S.; Bastos, D.S.S.; Melo, F.C.S.A.; Novaes, R.D.; Esposito, D.A.; Gonçalves, R.V. Antioxidant and Anti-Inflammatory Potential of Brassica oleracea Accelerates Third-Degree Burn Healing in Rats. Cosmetics 2024, 11, 27. https://doi.org/10.3390/cosmetics11010027
Miranda LL, Sarandy MM, Altoé LS, Bastos DSS, Melo FCSA, Novaes RD, Esposito DA, Gonçalves RV. Antioxidant and Anti-Inflammatory Potential of Brassica oleracea Accelerates Third-Degree Burn Healing in Rats. Cosmetics. 2024; 11(1):27. https://doi.org/10.3390/cosmetics11010027
Chicago/Turabian StyleMiranda, Lyvia Lopes, Mariáurea Matias Sarandy, Luciana Schulthais Altoé, Daniel Silva Sena Bastos, Fabiana Cristina Silveira Alves Melo, Rômulo Dias Novaes, Debora Araújo Esposito, and Reggiani Vilela Gonçalves. 2024. "Antioxidant and Anti-Inflammatory Potential of Brassica oleracea Accelerates Third-Degree Burn Healing in Rats" Cosmetics 11, no. 1: 27. https://doi.org/10.3390/cosmetics11010027
APA StyleMiranda, L. L., Sarandy, M. M., Altoé, L. S., Bastos, D. S. S., Melo, F. C. S. A., Novaes, R. D., Esposito, D. A., & Gonçalves, R. V. (2024). Antioxidant and Anti-Inflammatory Potential of Brassica oleracea Accelerates Third-Degree Burn Healing in Rats. Cosmetics, 11(1), 27. https://doi.org/10.3390/cosmetics11010027










