1. Introduction
Haberlea rhodopensis Friv. (HR) is a plant of the
Gesneriaceae family with typical lilac flowers that grows in stony areas and is endemic in the Rhodope Mountains of the Thracian regions of Bulgaria and Greece (
Figure S1A).
H. rhodopensis Friv. belongs to a group of resurrection plants able to withstand prolonged drought periods, tolerating desiccation and quickly resuming growth upon rehydration [
1,
2]. Due to its growth in limited places, HR is a protected species in Greece and Bulgaria [
3].
Primary investigations of Dell’Acqua and Schweikert proved skin benefits of HR extracts that are myconoside-rich [
4]. In recent years, HR has been intensively studied in terms of its broad antimigratory and anticancer effects, antioxidant and free radical-scavenging activity, radioprotective and immunostimulatory effects, as well as antibacterial and anti-aging efficacy [
5]. Therefore, the attention of researchers is focused on the investigation of the potential applications of HR in phytotherapy, human and veterinary medicine, and cosmetics [
5,
6]. Extensive investigations showed that different HR extracts may contain high levels of flavonoid antioxidants, including phenolic acids (e.g., ferulic, staric, caffeic,
p-coumaric, sinapinic acid, and others), flavonoid aglycones, and glycosides (such as luteolin, hesperidin, quercetin, myricitin, rutin, and others) [
7,
8,
9]. However, spectroscopic analyses using (U)HPLC-MS showed that two glycosides—myconoside and paucifloside—together with three other hispidulin-flavone C-glycopyranosides, represent the main constituents of different ethanolic
H. rhodopensis Friv. extracts (HREs) [
5]. The three new flavone C-glycosides are hispidulin 8-C-(2-O-syringoyl glucopyranoside), hispidulin 8-C-(6-O-acetyl glucopyranoside), and hispidulin 8-C-(6-O-acetyl-2-O-syringoyl gluco-pyranoside), which were also isolated and reported by another scientific group [
9]. Noteworthy, alcohol extracts from HR were investigated to show antioxidant, antiviral, antibacterial, and antifungal activities [
10]. In addition, the potent antioxidant and hepatoprotective effects of myconoside were recently demonstrated. The chemical structures and IUPAC names of myconoside and paucifloside are presented in
Figure S1B.
Recent experiments show that among the components with biological activity found in HR, myconoside has potent antioxidant and hepatoprotective effects [
11]. Together with the phenolic content, these compounds significantly contribute to the antioxidant properties of HREs [
7]. In particular, ethanolic extracts of HR leaves have been reported to exhibit vital antimicrobial and antioxidant activities, reduce the clastogenic effect of γ-irradiation, and exert in vivo anticlastogenic and antimutagenic potential against the anticancer drug cyclophosphamide [
12,
13,
14,
15].
Phenolic acids accumulated in high amounts in the resurrection plants possess therapeutic properties due to their ability to capture free radicals and decrease oxidative stress [
9]. Berkov and colleagues reported their study on the polar and apolar fractions of methanol HREs by gas chromatography-mass spectrometry and they have identified five free phenolic acids, namely syringic, vanillic, caffeic, dihydrocaffeic, and
p-coumaric [
16]. Furthermore, another study demonstrated that in alcohol HRE, the most abundant phenolic acids were the sinapic, ferulic, caffeic, and p-coumaric acids, as well as at least five other phenolic acids, which, although not so abundant, were still present in the extracts [
7].
HR is a rare and threatened plant and, therefore, its harvesting from nature is prohibited. In vitro cultivation of plants is a promising technology for the sustainable production of bioactive plant metabolites. On this basis, Bulgarian company Innova BM Ltd. developed in vitro cultures of HR with different degrees of differentiation that are used for industrial sources of water–alcoholic extracts [
17]. These extracts are the subject of the current investigation.
In the present study, we aim to (i) evaluate the cytotoxic and antiproliferative effects of two herb extracts of HR on different cancer cell lines, with one normal cell line used as a reference, and (ii) compare the investigated effects with those observed for the reference anticancer, non-selective compound doxorubicin.
2. Materials and Methods
2.1. Haberlea rhodopensis Friv. In Vitro Culture Extracts
Haberlea rhodopensis Friv. in vitro culture extracts were provided by Innova BM Ltd., Bulgaria
https://innovabm.com/ (accessed on 30 January 2020). The extracts were produced by the company’s proprietary technology by ethanol extraction of
Haberlea rhodopensis Friv. in vitro culture biomass [
17]. The myconoside content was 115 mg/g dry extract and 215 mg/g dry extract for E1 and M2 extracts, respectively.
2.2. Preparation of Stock Solutions
Two extracts (indicated throughout as Extract 1 or E1 at 5.0 mg/mL in ddH
2O stock, and myconoside-enriched Extract 2 or M2 at 10.0 mg/mL in ddH
2O stock) were tested for their cytotoxic and antiproliferative effects, respectively, after 24-, 48- and 72-h treatment of three cancer and one non-cancer (reference) cell lines. The stock solutions of both HR extracts were stored at –20 °C in the dark and tempered before use. The experiments were carried out on triple-negative, epithelial human breast adenocarcinoma (MDA-MB-231; ATCC; Manassas, VA, USA), human colorectal adenocarcinoma (HT-29; ATCC, USA), human hepatocellular carcinoma (HepG2; ATCC, USA), and mouse embryonic fibroblasts (3T3/L1; ATCC, USA) cells. Cells were incubated with serial dilutions (0.01–100 µg/mL) of both extracts using a modified MTT assay [
18,
19]. The anticancer, nonselective agent doxorubicin (Key Organics Ltd., Camelford, UK) (100 mM stock in DMSO) was tested in the concentration range of 0.001–100 µM (DMSO concentration ≤ 0.05%
v/
v) and was included in each experiment as a reference substance. For the dissolution of the insoluble formazan, a lysis solution containing N,N-dimethylsulfoxide (DMSO, Sigma-Aldrich, St. Louis, MO, USA) was used. The tests were performed in triplicate (eight replications per test) and the results presented as the mean % of the untreated controls ± SD from three independent experiments (
n = 3).
2.3. Preparation of Cell Cultures
Human breast adenocarcinoma (MDA-MB-231), human colorectal adenocarcinoma (HT-29), human hepatocellular carcinoma (HepG2), and mouse embryonic fibroblasts (3T3/L1, reference non-cancer cells) cells were cultured in Dulbecco’s modified Eagle’s medium (DMEM, Gibco, Vienna, Austria) in the presence of 10% fetal bovine serum (FBS; Gibco, Austria), penicillin (100 U/mL) and streptomycin (0.1 mg/mL) solution (Gibco, USA). All cells were cultivated under an atmosphere of CO2 (5.0%) at 37 °C and passaged by trypsinization with trypsin-EDTA (Greiner, Pleidelsheim, Germany) at a confluence of approximately 80%. The experiments were performed with cells in the exponential phase of growth (at a density of 5000 cells/well) using 96-well flat-bottom plates at a final volume of 100 µL/well. Cells were incubated overnight before the addition of doxorubicin and/or tested extracts.
2.4. Determination of Cell Viability
The cytotoxicity and antiproliferation of doxorubicin (1.0 mM stock solution in DMSO), Extract 1 (E1, 10.0 mg/mL stock solution in ddH
2O), and Extract 2 (M2, 5.0 mg/mL, stock solution in ddH
2O) were assessed in HepG2, MDA-MB-231, and HT-29 cancer cell lines, as well as in 3T3 mouse embryonic fibroblasts cells used as a reference cell line by colorimetric assay, applying 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT) (VWR, Darmstadt, Germany), as previously reported [
20].
The MTT test allows for determining the linear dependency between metabolically active cells (viable cells) and the measured color intensity of the purple-colored formazan solution, which can be quantified by spectrophotometric measuring at a certain wavelength (usually 550 or 570 nm). The obtained information is then used to assess the change in cells, e.g., death and/or proliferation. The loss of intensity of the purple color is directly associated with the loss of viable cells in the presence of a cytotoxic compound (agent). The quantity of in situ-formed formazan product can be determined spectrophotometrically by the measurement of the absorption/optical density (Amax/DO), after solubilization with a lysis solution (usually with DMSO, Sigma-Aldrich, St. Louis, MO, USA). The measured OD/Amax corresponds to the number (in %) of viable cells after a certain incubation period (24, 48 or 72 h) with the tested substance.
2.5. MTT Assay
The MTT assay was performed with slight modifications according to the literature [
18,
20]. Cells were seeded in 96-well culture plates with a density of 5 × 10
3 cells per well. Following adherence, the cells were treated with test extracts (0.01, 0.1, 1.0, 5.0, 10, 25, 50, and 100 µg/mL) and the reference compound doxorubicin (0.01, 0.1, 1.0, 2.5, 5.0, 10, 25, 50, and 100 µM), and further incubated for 24, 48 or 72 h at 37 °C (under 5.0% CO
2 atmosphere). After the respective incubation period, 10 µL MTT solution (5.0 mg/mL) per well was added and cells were incubated for a further 180 min. Then, the medium was removed and a lysing solution containing DMSO was added to each well. The plates were then shaken at room temperature until the completed dissolution of the purple crystalline product (formazan). The quantification of the produced formazans after the reduction of MTT was monitored using a microplate ELISA reader VarioscanTM LUX (Thermo Fisher Scientific Inc., Waltham, MA, USA) at 550 and 570 nm. The determined cytotoxicity/antiproliferation is expressed as percentage cell viability using the following equation:
In this equation, OD sample, OD blank and OD control are the measured absorption of the test, blank, and control sample. The results were presented as the mean % of the untreated controls ± SD from three independent experiments (n = 3).
2.6. Statistical Analysis
The statistical analysis and the representative graphs thereof were carried out using GraphPad Prism 6.0 (GraphPad Software, La Jolla, CA, USA). The respective half maximal inhibitory concentration (IC
50) values were obtained by non-linear regression analysis. The inhibitory curves were built using the log[inhibitor] vs. normalized response—Variable slope equation with least squares fit. Since the measured inhibitory concentration (growth inhibitory activity) of the tested Extract 1 and 2 is given in µg/mL, the IC
50 values of doxorubicin (initially obtained as µM) were further converted into their respective µg/mL units using the following equation:
For further simplification, the inhibitory activity (IC
50 values) of doxorubicin is given in µg/mL in order to better compare the respective inhibitory activity for both tested extracts (see
Table S1). The experimentally estimated IC
50 values are obtained from at least three independent experiments and given with their standard deviation (IC
50 ± SD). For the one-way ANOVA test,
p values ≤ 0.05 were considered as statistically significant.
4. Discussion
The obtained results from all performed MTT tests are summarized in
Figure S2. The reference doxorubicin showed inhibitory activity against all investigated cell lines, the cancer cells MDA-MB-231, HT-29, and HT-29, as well as against reference 3T3 cells. The highest inhibitory activity of doxorubicin was measured against HepG2 and 3T3 cells after 48 and 72 h post-treatment (
Figure S2, left). The strongest antiproliferative effect for doxorubicin was observed after 72 h incubation with the non-cancer cell line 3T3 (at concentrations between 0.5 and 100 µM). Similar antiproliferative effect for doxorubicin is measured in the concentration range of 1.0–100 µm against HepG2 cancer cells. The lowest inhibitory activity of doxorubicin was assessed against HT-29 cancer cells, independently of the incubation time (at 24, 48, or 72 h).
Compared to doxorubicin, both tested HR extracts showed an overall decrease in inhibitory activity against all cancer (MDA-MB-231, HT-29, and HepG2) and non-cancer (3T3) cell lines. The MTT tests indicated that both extracts did not show comparable dose- and time-dependent (after 24, 48 or 72 h post-treatment) inhibition of cell growth to those of doxorubicin. However, it can be seen that Extract 1 is not able to inhibit the growth of cancer cell lines (e.g., MDA-MB-231, HT-29, or HepG2), while its inhibitory activity against the non-cancer 3T3 cells is comparable to that observed for Extract 2. Extract 1 was able to inhibit the growth of 3T3 cells after 48 and 72 h post-treatment at a concentration of 100 µg/mL (
Figure S2, middle). From both extracts, the HR Extract 2 is the most active one. Extract 2 is able to inhibit the growth of all examined cancer cells (e.g., MDA-MB-231, HT-29, or HepG2) after 72 h incubation of the respective cells with the highest-tested concentration of 100 µg/mL (
cf. the red arrow in
Figure S2, right panel). The strongest effect for Extract 2 is determined after 24, 48 and 72 h against the non-cancer 3T3 cells (at the highest-tested concentration of 100 µg/mL). The cytotoxicity and antiproliferative effects of HR Extract 2 against 3T3 cells are almost similar to the effects of Extract 1. In order to obtain the respective IC
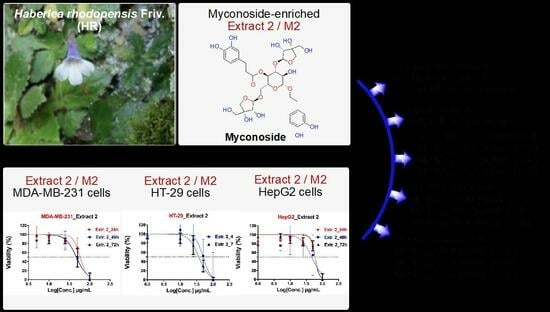
50 values for doxorubicin (in µM), HR Extract 1 and 2 (in µg/mL), dose-dependent non-linear curves were built (
Figure 5).
The dose-response curves were built after the normalization of transformed values, so that the curves run from 100% (vs. control) down to 0%, depending on cell viability (in % vs. control group) that is measured in MTT assay for each cell line and experiment (e.g., after 24, 48 or 72 h treatment). It can be seen that only one curve was built for Extract 1 since it was evaluated to antiproliferative activity (after 48 and 72 h) only against 3T3 cells. Based on the obtained dose-dependent curves, the inhibitory activity of both tested HR extracts and the reference doxorubicin against three cancer (e.g., MDA-MB-231, HT-29, and HepG2) and one non-cancer cell line (3T3) was determined with higher IC
50 values for 24 h (cytotoxic effects) compared to 48 and 72 h post-treatment, respectively. The IC
50 values (expressed in µg/mL) for both investigated HR extracts and doxorubicin are summarized in
Table 1.
In regard to HR extract E1, the IC
50 values at 48 and 72 h were determined to be equal to 54.0 µg/mL (50% inhibition of cell growth) for 3T3 cells, whereas, for all other cell lines, they could not be estimated. Therefore, the IC
50 values for E1 for all cancer lines are higher than the highest-tested concentration of 100 µg/mL (IC
50 > 100 µg/mL, cf.
Table 1). In contrast, the HR extract M2 inhibits the cell growth of all cancer cell lines by about 50–60% (with the exception of HT-29 cells at 24 h), and by about 40% of 3T3 non-cancer cells. Therefore, the IC
50 values for M2 at 24 h were determined to be higher (approx. 58 µg/mL for MDA-MB-231, approx. 59 µg/mL for HepG2, and approx. 40 µg/mL for 3T3) than those measured at 48 or 72 h post-treatment, showing that M2 exhibits stronger antiproliferative effects than cytotoxic activity (increasing by about 10–15%, on average). Moreover, M2 showed increased cytotoxicity and antiproliferative affects compared to E1, which was not able to inhibit the growth of all cancer cell lines.
The highest cytotoxic effect at 24 h for M2 was determined against 3T3 cells (approx. 40 µg/mL), while the strongest antiproliferative effect was estimated at 72 h post-treatment of HT-29 cancer cells (approx. 37 µg/mL). Overall, it can be concluded that M2 moderately inhibits MDA-MB-231, HT-29, HepG2, and 3T3 growth with IC
50 values in the range of 40–59 µg/mL, whereas Extract 1 (with the exception of 3T3 cells) did not exhibit cytotoxic (at 24 h) or antiproliferative effects (at 48 and 72 h) against all investigated cancer cells (
Table 1). However, the cytotoxicity and antiproliferative activity of M2 are not comparable to the effects observed for the reference doxorubicin. The strongest IC
50 values for doxorubicin were determined to be 0.17 and 0.14 µg/mL against HepG2 cancer cells at 48 or 72 h, respectively (
cf. Table 1). In general, depending on the tested cells, doxorubicin was evaluated to be between 10- and 300-fold more active than M2 at the different evaluated time points (e.g., 24, 48, or 72 h post-treatment).
5. Conclusions
In conclusion, the myconoside-enriched HR plant Extract 2 (M2) showed increased inhibitory activity (cytotoxicity and antiproliferative effects) compared to the HR plant Extract 1. Moreover, the plant Extract 2 showed a significant increase in cytotoxicity (at 24 h) and antiproliferative activity (at 48 and 72 h post-treatment) at its highest-tested concentration of 100 µg/mL compared to plant Extract 1. As obtained herein, both plant Extracts 1 and 2 inhibited the growth of the non-cancer cell line 3T3 at their highest-tested concentration of 100 µg/mL. The reference compound doxorubicin, used in this study as a reference compound, showed strong cytotoxic (at 24 h) and antiproliferative effects (at 48 and 72 h post-treatment) against all tested cell lines (cancer and non-cancer cells) being between 10- and 300-fold more active than the most active plant Extract 2. Since it is considered a skin-protecting cosmetic product, the plant Extract 2 can also be considered for further time- and dose-dependent experiments in order to evaluate its cytotoxicity and antiproliferative effects against a panel of metastatic melanoma cancer cell lines, e.g., SK-MEL-3, SH-4, SK-MEL-24, and other cells.