Abstract
Environmental pollution is increasingly recognized as a significant contributor to skin and scalp damage. Oral supplementation with a specific blend of four standardized botanical extracts (Rosmarinus officinalis, Lippia citriodora, Olea europaea leaf, and Sophora japonica) has been previously demonstrated to enhance skin health in individuals exposed to high environmental stress. Thus, it might represent a convenient strategy to also improve their scalp health aspect, particularly in subjects with sensitive scalps. To support these effects, a 12-week double-blind, randomized, placebo-controlled trial was performed in 66 women working outdoors in urban areas with high particulate matter (PM) levels and taking 250 mg of the botanical blend daily. Product efficacy was measured as follows: skin antioxidant status (FRAP); skin and scalp moisturization (corneometer), transepidermal water loss (tewameter), and oiliness (sebumeter); skin radiance and colour (spectrophotometer), elasticity and firmness (cutometer) and wrinkle depth (image analysis); and scalp clinical evaluation. Despite constant exposure to increased levels of PM, the tested product positively influenced all monitored parameters compared to both baseline and the placebo-treated group, in as early as 4 weeks. At the end of the study, key improvements included increased skin FRAP (21.9%), moisturization (9.5%), radiance (24.9%) and reduced wrinkle depth (−16.5%), dark spot pigmentation (−26.2%), and skin oiliness (−19.3%). For the scalp, moisturization increased (14.1%), TEWL decreased (−13.8%), and sebum content reduced by 16.2%. Additionally, 71% of subjects with sensitive scalps experienced reduced redness. These findings highlight the extensive benefits of the ingredient, expanding its application beyond conventional skin treatments to also alleviate scalp issues.
1. Introduction
Air pollution is a major problem in recent decades, with a serious toxicological impact on human health. Almost the entire global population (99%) breathes air that exceeds WHO guideline limits, and, in fact, only seven countries meet the WHO annual PM2.5 guideline (annual average of 5 µg/m3 or less) [1]. The WHO estimates that around seven million people die prematurely annually from exposure to polluted air, due to diseases such as stroke, heart disease, lung cancer, chronic obstructive pulmonary diseases and respiratory infections, including pneumonia [1,2].
The skin is the organ that is most exposed to air pollutants. Environmental pollution has been increasingly recognized as a significant factor contributing to skin damage [3,4], and, more recently, to scalp and hair health deterioration [3,5,6].
Experts have found that, in addition to UV radiation, continuous daily exposure to contaminants, such as polycyclic aromatic hydrocarbons (PAHs), volatile organic compounds (VOCs), oxides, particulate matter (PM), ozone (O3), and smoking, impacts the skin on multiple levels: it alters the skin barrier and microflora; increases oxidative stress by boosting reactive oxygen species (ROS) production, lipid peroxidation, and depletes skin antioxidants; activates the aryl hydrocarbon receptor (AhR), which mediates pollutants’ toxic effects; and increases in the inflammatory response, as well as an increase in matrix metalloproteinase (MMP) activation, resulting in collagen breakdown [4,7,8]. These factors can cause dryness, irritation, and the worsening of inflammatory or allergic skin conditions such as atopic dermatitis, eczema [9], psoriasis [10,11] and acne [12,13]. They also lead to accelerated skin aging [14,15] and uneven skin pigmentation [16,17]. The most serious consequence is the increased risk of skin cancer [18,19], as UVA interacts with airborne pollutants synergistically to initiate and promote skin cancer. Moreover, the environmental pollutants generate ROS, which oxidizes epidermal lipids, triggering a series of cellular stress responses that may lead to skin cancer [20].
Regarding scalp health, several studies indicate that individuals living in highly polluted areas presented significantly altered sebum composition, which affects their scalp’s ability to retain moisture and protect against environmental stressors [21]. Pollutants can stimulate sebaceous glands, leading to an overproduction of sebum [22], which leads to an oily scalp and conditions like seborrheic dermatitis, characterized by red, itchy and flaky skin [23]. Indeed, the accumulation of sebum on the scalp can lead to bacterial and parasitic colonization, particularly by fungi Malassezia, which is implicated in the onset of dandruff [23].
The adverse effects of pollution on the scalp inevitably extend to hair health. The scalp serves as the incubatory environment for pre-emergent hair fibres, and its condition directly affects hair growth and quality. Oxidative stress and inflammation induced by pollutants can impair hair follicle function and interfere with the normal keratinization process, potentially leading to hair thinning and loss [24]. Hair follicles are particularly sensitive to oxidative damage, which can disrupt the hair growth cycle, leading to conditions such as telogen effluvium, where hair prematurely enters the shedding phase [8].
In the past, scalp care was mainly limited to anti-dandruff solutions; however, consumers are increasingly aware of the link between hair quality and scalp health. In fact, this trend has made 23% of hair care launches in 2023 claim “scalp” in their product name. Consumers now look for products that help soothe, hydrate, exfoliate, enhance barrier function, and even improve the scalp microbiome to address concerns such as dandruff, itchiness, oiliness, and dryness [25].
Since it is difficult to minimize skin and scalp exposure from air pollutants especially in urban environments, strategies to protect them from air pollution is essential. Topical skincare products protect the skin from air pollution through three primary mechanisms: by forming a film that limits contact with pollutants, favouring their removal, and by incorporating ingredients that reduce oxidative stress [26]. However, these products primarily protect the skin’s outer layers and may not effectively shield deeper skin structures. Ultrafine particles (UFP) and PAHs can penetrate the hypodermis, dermis, and hair follicles, and reach the deep epidermis, especially through indirect systemic distribution via inhalation or ingestion [27,28,29]. Thus, a comprehensive skin and scalp protection strategy should include dietary approaches that replenish the skin with nutrients to counteract the chronic effects of air pollution.
Recent research has explored the potential of botanical-based oral ingredients in counteracting the negative impacts of environmental stress on the skin. Botanical extracts, such as blueberry, green tea, grape, pomegranate, and different marine algae, are known for their antioxidant, anti-inflammatory, and skin-protective properties, offer a promising approach to enhancing skin resilience and appearance [30,31,32,33]. On the other hand, ideally, consuming a diverse range of antioxidants appears to be more effective than a high dosage of a single antioxidant, implying potential complementary or synergistic effects [34,35,36]. Therefore, food supplements containing various phenolic compounds from plants with antioxidant and anti-inflammatory properties could be an effective way to combat the harmful effects of environmental pollution.
Specifically, we have demonstrated, both in vitro and in vivo, the skin protective efficacy of a commercially polyphenol-enriched ingredient (trademark family Zeropollution®, ZP) comprising Lippia citriodora Paláu, Olea europaea L., Rosmarinus officinalis L., and Sophora japonica L. [37,38]. Marked inhibition of inflammation, oxidative stress, and lipid peroxidation was observed both in human keratinocytes and in living human skin explants exposed to pollutants. In addition, the ingredient demonstrated an ability to counteract the pollutant-induced AhR expression in both models [38]. AhR is considered as the major biological pathway of the pollutant stress response, and its activation in the presence of pollutants induces the expression of genes responsible for changes in the barrier function, in melanogenesis and inflammation, in addition to inducing oxidative stress in human skin [39,40].
Moreover, several studies have shown that individual extracts or the main active compounds present in ZP are effective antioxidants and anti-inflammatory agents, with the ability to counteract different environmentally induced skin damage.
The antioxidant and anti-inflammatory effects of olive polyphenols, such as oleuropein and hydroxytyrosol, have been extensively confirmed in the scientific literature [41,42,43]. Several components in olive oil have a direct antioxidant effect on the skin, and it has been suggested to prevent chronic UV-induced skin damage, including melanin production [44,45,46]. Hydroxytyrosol is also effective in reducing the harmful effect of light-mediated skin damage, including UVB [47], UVA [48] and blue light radiation [49].
Additionally, verbascoside, a hydrophilic caffeoyl phenylethanoid glycoside present in Lippia citriodora, offers a wide range of benefits, including antioxidant, anti-inflammatory, photoprotective, whitening, and chelating actions [50,51,52,53,54].
Rosmarinus officinalis is an aromatic plant with a rich history in herbal remedies due to its diverse biological activities including antioxidant, antimicrobial, and anti-inflammatory effects [55,56], as well as skin rejuvenation properties [57]. Studies have shown that rosemary extract exhibits strong antioxidant properties linked to its polyphenol content, notably, carnosic acid [55,58]. A recent study that assessed the impact of spray-dried algae-rosemary particles (RSP) on pollution-induced damage using human biopsies exposed to diesel engine exhaust showed that RSP effectively reduced inflammatory responses in cutaneous tissue, lowering 4-hydroxynonenal protein adducts and active MMP-9 levels, indicating its potential to counteract pollution-induced skin aging/damage [59].
Finally, Sophora japonica, a well-known plant in Chinese herbal traditional medicine, has a multitude of documented biological activities, including antioxidant, antibacterial, anti-allergic, and anti-inflammatory properties [60]. Moreover, the photoprotective and anti-pollution benefits of Sophora japonica and quercetin have also been proven [61,62,63].
It is important that new innovative ingredients coming into the market should be able to substantiate their efficacy with proper studies, and although the beneficial effect of the ZP ingredient on the skin under high environmental stress conditions seems to be proven [37,38], the potential benefit on scalp health remains underexplored. The current study aims to address this gap by investigating the real-world effectiveness of this four-botanical blend ingredient on various physiological parameters of the skin and scalp in 66 women residing in a densely urbanized area with high PM pollution levels. To this end, a 12-week double-blind, randomized, placebo-controlled trial assesses the supplement’s efficacy in improving skin antioxidant status, moisturization, transepidermal water loss (TEWL), oiliness, radiance, elasticity, and firmness, as well as its impact on scalp condition (moisturization, TEWL, and sebum content) and clinical parameters such as redness and sensitivity.
2. Materials and Methods
2.1. Study Design and Ethics Statement
A monocentric, randomized, double-blind, placebo-controlled, parallel-group study was conducted in Milan (Italy) from October 2023 to January 2024. The study took place at Complife Group facilities in Milan in accordance with the World Medical Association’s (WMA) Helsinki Declaration and its amendments. Complife Group is an independent testing laboratory for in vitro and in vivo safety and efficacy assessment of cosmetics, food supplements, and medical devices. Both the study protocol and the informed consent form were approved (ref. no. 2023/12 by 5 October 2023) by the “Comitato Etico Indipendente per le Indagini Cliniche Non Farmacologiche” (Società Scientifica Italiana per le Indagini Cliniche Non Farmacologiche, Genova, Italy). A signed informed consent form was obtained from all the subjects participating in the study before any study-related procedure took place. Subjects were randomly assigned to the test dietary supplement and placebo group in a 1:1 ratio allocation rate. No changes to treatment regimen or methods were necessary after the study initiation.
2.2. Subjects
Subjects were first screened and enrolled from Complife’s volunteer database. Eligible participants were then enrolled in the study by a board-certified dermatologist. During the screening visit, a physical examination was carried out to assess the skin and scalp conditions. Subjects meeting the inclusion criteria were then enrolled and randomized. Inclusion criteria included adult female subjects, aged between 35 and 65 years old, with normal or sensitive skin (1:1 ratio) and normal or sensitive scalp (1:1 ratio), showing slight to moderate wrinkles and dark spots due to photo- or chrono-aging, who spend at least 2 h outdoors every day in an urbanized polluted area. Subjects were of general good health without any alimentary and/or eating disorders (i.e., bulimia, psychogenic eating disorders, etc.). Exclusion criteria included pregnancy or intention to become pregnant, lactation, food intolerances/allergy, pharmacological treatments and/or food supplement intake known to interfere with the test product or influencing metabolism, participation in another dietary supplement study, have a history of irritative reactions to cosmetic, drug, or household products, or inability to comply with the requirements of the study protocol. The study further excluded subjects having applied cosmetic or pharmaceutical products on the face on the day of the study visits. Participants were also given specific guidelines to follow. They were instructed to refrain from using any food supplements or cosmetic products for face and scalp/hair care, were asked to maintain their regular lifestyle and diet (especially fruit and vegetable intake), and were required to use only the provided neutral cleanser, neutral shampoo, and face base cream for the entire study duration.
2.3. Ingredient Information
The test item was a commercially available patented (WO/2019/211501) [64] oral ingredient (trademark family Zeropollution®) supplied by Monteloeder S.L., Miguel Servet 16, Elche, Alicante, Spain. The ingredient is a blend of four polyphenolic botanical extracts: Rosmarinus officinalis leaf extract standardized in diterpenes, Olea europaea leaf extract standardized in oleuropein and hydroxytyrosol, Lippia citriodora leaf extract standardized in verbascoside, and Sophora japonica extract standardized in quercetin.
The four raw materials were extracted using water, ethanol, or a combination of both, and then dried. Before blending, the four herbal extracts were standardized to ensure uniformity and specific concentrations of active compounds. The final blend was sifted to achieve a consistent particle size. Further details are available in Figure S1 of the Supplementary Materials and in the referenced patent.
In total, this blend comprises a minimum content (w/w) of the following phenols: 4.5% diterpenes (mainly a sum of carnosic acid and carnosol); 4.5% oleuropein; 1.5% hydroxytyrosol; 6.8% verbascoside; and flavones such as quercetin minimum 3.7%. These main compounds were identified and quantified by high-performance liquid chromatography with diode array detector (HPLC-DAD) analysis, comparing the retention times and UV spectra of the peaks in the samples with those of pure standards as previously described [37,38]. More details of the method and materials used, as well representative chromatograms, can be seen in Supplementary Materials (Figure S2). In addition to the main actives, other phenolic compounds were identified by HPLC-MS analysis: phenolic acids like chlorogenic acid, caffeic acid, and rosmarinic acid; phenylethanoids such as tyrosol and isoverbascoside, and different flavones, mainly apigenin and luteolin derivatives, and rutin. A table with the actives identified can be seen in Table S1 of the Supplementary Materials.
2.4. Intervention
Each jelly capsule contained 250 mg of Zeropollution® (ZP), 95 mg cellulose microcrystalline, and 98 mg of gelatine. The placebo product was a jelly capsule containing 370 mg of cellulose microcrystalline and 98 mg of gelatine. The active (ZP) and placebo products were in opaque coloured capsules with identical appearances. They were pre-packed in blisters and consecutively numbered for each subject according to the randomization list. After the initial visit, subjects started taking one capsule of the dietary supplement or the placebo product every day for 12 weeks. The product intake was 30 min before or after eating to swallow with a large glass of water.
To standardize the volunteers’ cosmetic habits, two weeks before the baseline, volunteers were supplied with a base face cream without any cosmetic activity, a face cleanser, and a neutral shampoo to be used in substitution of their usual product for the 14-day washout period and for the whole study period (Table S2). Volunteers did not apply the cream and did not use the face cleanser and shampoo in the morning of the day when measurements were performed.
2.5. Randomization and Masking
The research coordinator at Complife Italia Srl randomly assigned eligible subjects to either the active or the placebo product using a restricted randomization list. The list was stratified with a 1:1 allocation using “Efron’s biased coin” algorithm (PASS 11, version 11.0.10 software, PASS, LLC., Kaysville, UT, USA). The allocation sequence was concealed in sequentially numbered, opaque, and sealed envelopes that reported the unblinded treatment allocation based on the subject’s entry number in the study. A masked allocation sequence was then prepared to be used by the staff who delivered the intervention. An independent technician dispensed either the active or the placebo products. To ensure unbiased results, the subjects, investigators, and collaborators were all blinded.
2.6. Primary, Secondary Objectives and Outcome Measures
The primary endpoints related to the efficacy in improving the face skin and scalp conditions in highly polluted environments were the evaluation of face and scalp skin moisturization, transepidermal water loss (TEWL) and sebum content, face skin elasticity, profilometry (wrinkle depth), dark spot intensity, radiance, scalp aspect, and product antioxidant potential. The secondary outcome measures were the investigator safety evaluation and the self-assessment questionnaire.
The skin antioxidant capacity was measured in the first layers of the stratum corneum (SC) from the cleaned skin of the cheeks obtained using the skin stripping technique. Skin stripping was performed using Corneofix® foils (Courage+Khazaka Electronic, Köln, Germany) under standard pressure conditions. The first 4 stripped layers were discarded, and strip no. 5 was collected and stored at −80 °C for further analysis. The antioxidant potential was measured via a ferric reducing antioxidant power (FRAP) assay as described by Benzie et al. [65] with minor modifications. Briefly, 100 µL of distilled water and 400 µL of the FRAP reagent (25 mL acetate buffer, 2.5 mL TPTZ, and 2.5 mL FeCl3 6H2O solution) were added to 12-well plates containing the strips. Adhesive foils were placed in the plates with the adhesive side up to ensure proper contact between the reagents and the SC. The samples were incubated at 37 °C with continuous agitation for 30 min, and absorbance was measured at 595 nm. The recorded absorbance was compared to a Fe (II) standard curve of known values. Antioxidant capacity is expressed in µmol Fe (II) per skin sample. Skin moisturization was measured using a Corneometer® CM 825 (Courage + Khazaka electronic GmbH, Cologne, Germany). The Corneometer® method is based on the dielectric constant of water [66]. The measurement was taken at five different points on the right cheek. The selected measurement points delineate the vertices and centre of a quadrangle virtually drawn across the cheek. Data are expressed as corneometric units (c.u.).
The scalp moisturization was measured using a DermaLab-Pin probe® (Cortex Technology, Aalborg, Denmark) device. The device gives information about the hydration state by measuring the skin conductance [67]. The measuring probe contains an array of 8 pins and is designed to facilitate scalp hydration measurements. The measurements were taken at five different points. The hydration value is reported in μS.
Skin and Scalp TEWL were measured using a Tewameter® TM 300 (Courage + Khazaka electronic GmbH, Cologne, Germany). The Tewameter® probe measures, indirectly, the density gradient of water evaporation over the skin surface using two pairs of sensors (temperature and relative humidity) in an ‘open chamber’ configuration mode. The TEWL is reported in g × h−1 × m−2 (evaporation rate). The measurements were taken at the centre of the right cheek and in the scalp area. According to the technical guide, the scale is as follows: 0–10 (very healthy conditions), 10–15 (healthy conditions), 15–25 (normal condition), 25–30 (affected skin), and >30 (critical condition) [68].
The sebum content of the face (in the centre of the forehead) and the scalp skin was measured using a Sebumeter® SM 815 (Courage + Khazaka Electronic, Köln, Germany, Sebumeter®); the measurement is based on ‘greasy spot’ photometry [69]. The transparency (light transmission) of the mat tape is measured by a photocell and allows calculation of the skin sebum content in μg/cm2.
To evaluate the scalp aspect improvement at each timepoint, digital pictures of the scalp (central hair line) were taken by DermoGenius ultra (DermoScan GmbH, Regensburg, Germany). The device allows a reproducible distance and standard light conditions between timepoints. By means of a photographic/clinical scale, the investigator evaluated the improvement of scalp redness (if present) or other visible features of the scalp according to an internal 4-point clinical scale (1—no variation; 2—mild variation; 3—moderate variation; and 4—remarkable variation).
Skin elasticity and firmness were measured using a Cutometer® MPA 580 (Courage + Khazaka electronic GmbH, Cologne, Germany). The skin surface of the face (right cheek) was drawn into the aperture (3 mm) of the probe by a negative pressure (450 mbar) for 3 s and thereafter released for 3 s. The measured parameters were (a) the maximum amplitude of the curve (R0 or skin distensibility), (b) the resistance to the mechanical force versus the ability of the skin to return to its basal state (R2 or gross elasticity), and (c) the elastic portion of the suction curve versus the elastic portion of the relaxation curve (R5 or net elasticity) [69].
The intensity of dark spots and skin radiance were measured using a colorimeter CM 700D (Konica Minolta, Milano, Italy). The L* and b* parameters measured with the colorimeter were then integrated into the individual typology angle formula (ITA° = Arctan [(L* − 50)/b*] × 180/π). Skin radiance was measured by the 8° gloss parameter [70]. The measurement was taken on the cheek.
Wrinkle depth was measured in the periocular area (‘crow’s feet wrinkles’) using a three-dimensional (3D) microtopography imaging system (PrimosCR, Canfield Scientific, Utrecht, The Netherlands). The imaging system projects structured light on a specific surface of the skin with a digital micro-mirror device (DMD, Texas Instruments, Irving, TX, USA) and records the image with a CCD camera. Skin surface microtopography is then reconstructed using temporal phase shift algorithms to generate 3D images [71]. The imaging system has an overlap feature that enables the precise matching of photos taken at different visits. To improve before/after image overlap, the subjects’ position was regulated using a stereotactic device (Canfield Scientific, Inc., Fairfield, NJ, USA).
Except for skin and scalp hydration, which was averaged from five separate measurements, all other parameters were measured once.
The secondary endpoint of the study was the assessment of the visual and self-perceived effect of the product on the skin and product tolerability. The subject-based evaluation of the efficacy of the tested product was performed using a 12-item questionnaire adapted to the investigated product and completed by all study participants. For each item, the answers were recorded on a 4-point grading scale (completely agree, agree, disagree, and completely disagree), and the results were expressed as the percentage of subjects in agreement.
All study procedures were carried out under temperature and humidity-controlled conditions (temperature 22 ± 2 °C and humidity 50 ± 10%).
2.7. Sample Size
The sample size was determined using a 2-sided 5% significance level and a power of 80%. The calculation considered a 20% variation of the primary endpoints due to both inter-individual human variability and measurement technique errors. The PASS 11 statistical software (version 11.0.8 for Windows) was used on a Windows Server 2008 R2 Standard SP1 64-bit edition computer (Microsoft, Redmond, DC, USA) to calculate the sample size.
2.8. Safety
Throughout the study, the investigators monitored the occurrence of adverse events (AEs) based on the subjects’ diary entries and regular check-ins. An AE was defined as any unfavorable and unintended sign, symptom, or disease temporally associated with the use of the test product, whether or not it is considered related to the product. Serious adverse events (SAEs) were considered those resulting in death, life-threatening situations, hospitalization, significant disability, or congenital anomalies. All observed and reported AEs were scored by the investigators as either severe or non-severe, based on their potential relationship to the study treatment. The assessment included the intensity, location, duration, and frequency of each sign, as well as any discomfort or reactions reported by the subjects. In addition to diary entries, subjects had access to the principal investigator via a contact phone number for reporting any intolerance reactions. All severe adverse events were followed up until resolution or stabilization.
2.9. Results Interpretation and Statistical Methods
A total of 66 subjects were enrolled in the study. Efficacy analysis was based on the per protocol (PP) population. Subjects were excluded from the per-protocol population if they miss the one or more evaluation visit or they do not used the product properly during the study period (as referred by the subject themself).
The analysis of safety was based on the intent-to-treat population that is defined as all subjects that have been assigned a subject number and received at least one study treatment.
Demographic variables (sex, age, skin type, phototype, and sensitive skin/scalp) are reported for the PP population. The data are summarized using frequency distributions for categorical/ordinal variables.
Data normality was checked using the Shapiro–Wilk W normality test and data shape. The intra group analysis at different visits vs. T0 was done by the bilateral Student’s test t for paired data. The inter-group statistical analysis (active vs. placebo) was carried out on raw % variations vs. baseline (T0). ANCOVA analysis, using baseline as covariates, was used to determine differences between the active and placebo groups. A p < 0.05 was considered statistically significant. Statistical analysis output was reported as follows: * p < 0.05, ** p < 0.01, *** p < 0.001, and **** p < 0.0001.
For clinical evaluations, the positive effect of the product on the evaluated parameter was confirmed if more than 50% of the subjects register an improvement. Finally, for the self-assessment questionnaires, the performance and the pleasantness of the product must be perceived by at least 60% of the subjects.
3. Results
Throughout the study period, environmental conditions (temperature, humidity, and UV radiation) and air quality (PM10, PM2.5, Sulphur dioxide (SO2), nitrogen dioxide (NO2), O3, and benzene) were recorded.
During the days when the clinical trial was conducted, elevated PM10 and PM2.5 concentrations in the area were registered (Agenzia Regionale per la Protezione dell’ambiente della Lombardia), and the study population was exposed to PM10 air pollutant concentrations above WHO air quality guidelines (>20 μg/m3) during 80% of the study time. The exposure to fine PM (2.5) was worse, surpassing the WHO healthy limits (10 μg/m3) 92% of the time, and above the 25 μg/m3 limit 47% of the time.
The PM10 and PM2.5 average during the period was 34.2 ± 1.4 μg/m3 and 25.0 ± 1.2 μg/m3, respectively. The daily mean value of the major air quality parameters, throughout the study period, can be seen in the Supplementary Materials (Figure S3).
3.1. Participants and Product Tolerability
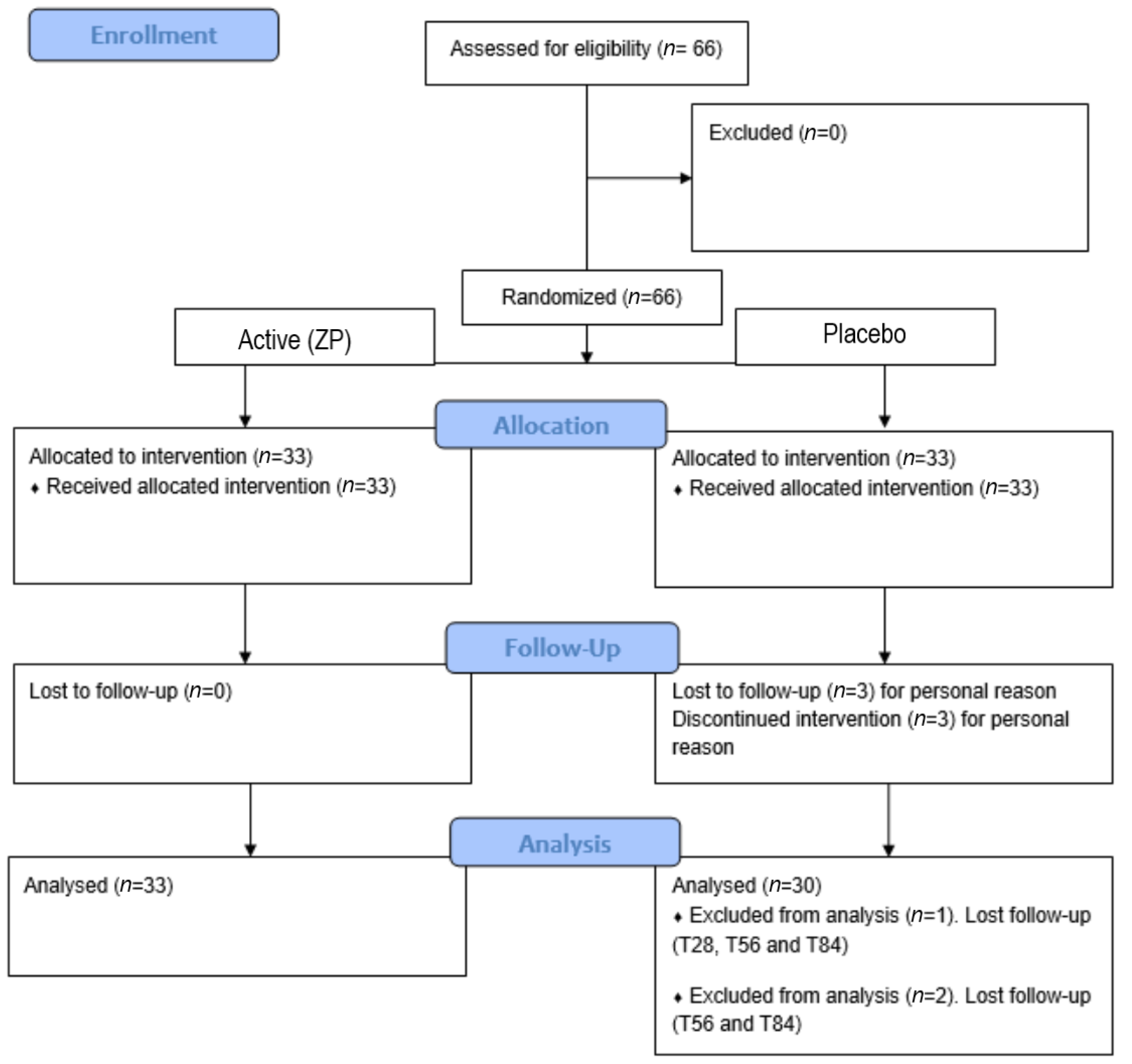
According to inclusion criteria, 66 volunteers were enrolled. Visits to the clinic were performed at baseline and after 28 (T28), 56 (T56), and 84 (T84) days of product intake. A total of 63 subjects completed the study (12 weeks) and respected the fixed timepoints. Three (n = 3) drop-outs in the placebo group, not related to product use, occurred during the study period (one subject was lost to follow-up starting from the T28 visit, and two subjects were lost to follow-up at T56- and T84-day visits). These subjects were not included in the statistical analysis. All subjects followed the study protocol. For detailed information, see the consort flow diagram (Figure 1).
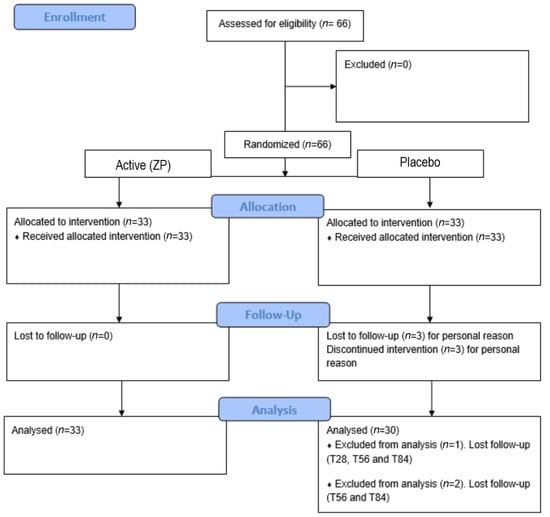
Figure 1.
Participants flow diagram.
Demographic and baseline characteristics (Table 1) were similar across treatment arms, and no differences between groups were observed (p > 0.05) in the active and placebo groups, indicating unbiased randomization and the absence of covariates. The population comprised female subjects aged between 35 and 69 years old, with an average age of 52.4 years old. All subjects were included in the safety analysis data set. Both the active and the placebo products were well-tolerated, and no adverse reactions were reported during the study.

Table 1.
Demographic and baseline characteristics of study participants who finished the study.
3.2. Effects on Facial Skin Conditions
The primary endpoints related to efficacy were measured, using non-invasive skin bioengineering techniques, at baseline and after 28, 56, and 84 days of product intake, except for the antioxidant capacity, which was measured after 28 and 84 days. The following parameters were measured: antioxidant capacity of the skin, skin moisturization, TEWL, skin profilometry, skin elasticity, dark spots staining, and skin radiance.
No statistically significant differences were observed between skin sensitive and non-skin sensitive subjects in any of the endpoints evaluated. A slight but not significant (p = 0.134) improvement was observed regarding skin moisturization in subjects with sensitive skin.
3.2.1. Antioxidant Capacity of the Skin: FRAP Analysis
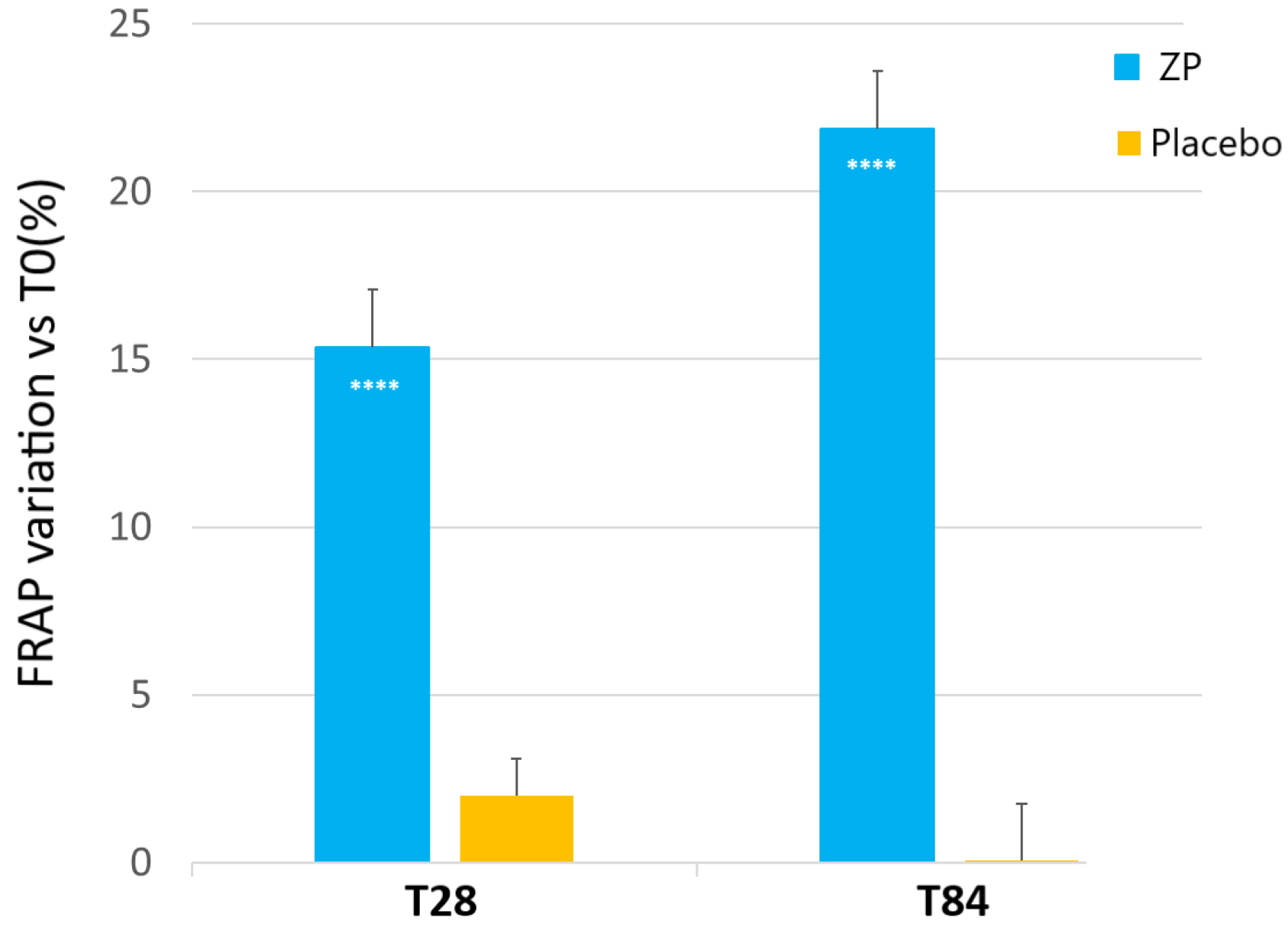
A statistically significant increase of FRAP was observed in the subjects taking the dietary supplement from week 4 onwards (Figure 2). In the active treatment arm, the baseline FRAP (127.88 ± 9.86) was significantly increased by 15.4% (144.30 ± 10.4, p < 0.0001) and 21.9% (154.56 ± 11.54, p < 0.0001) after 28 and 84 days of product intake, respectively. FRAP was unchanged in the placebo-treated subjects throughout the study (p > 0.05). The skin FRAP variation between the active and placebo test products was statistically significant during all the checkpoints (p < 0.0001 at T24 and T84) (Figure 2).
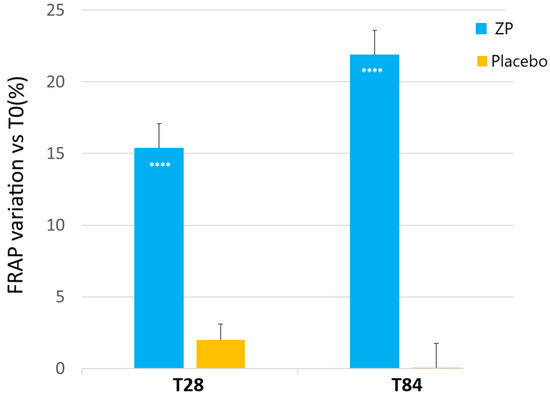
Figure 2.
Change on facial skin FRAP analysis versus baseline after 28 and 84 days in the ZP treatment (blue bars) and placebo (yellow bars) group. Data are means ± SEM. Intergroup (vs. placebo) statistical analysis is reported inside the bars of the histograms. **** p < 0.001.
3.2.2. Skin Moisturization
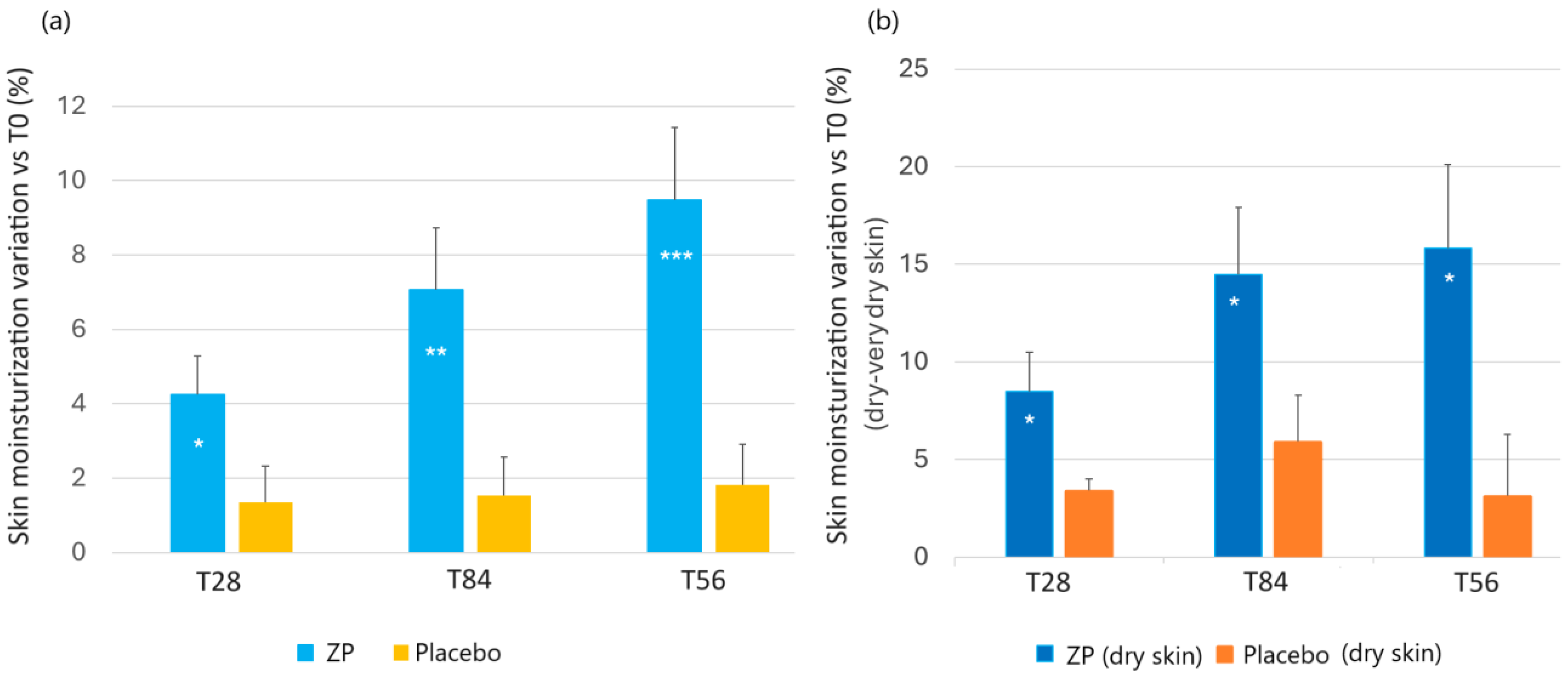
The active product showed a statistically significant improvement in the skin moisturization index at each time point, both compared to baseline (T0) and to the placebo treated group (Figure 3a). In the active treatment arm, the baseline (47.1 ± 1.9) skin moisturization index increased by 4.2% (48.8 ± 1.8, p < 0.001), 7.1% (49.9 ± 1.6, p < 0.001), and 9.5% (51.1 ± 1.6, p < 0.001) after 28, 56, and 84 days of product intake, respectively. No significant variations were seen in the placebo treated group (Figure 3a). The skin moisturization variation between the active and placebo test product was statistically significant at all the checkpoints (p = 0.048 at T28, p = 0.0072 at T56, and p = 0.008 at T84).
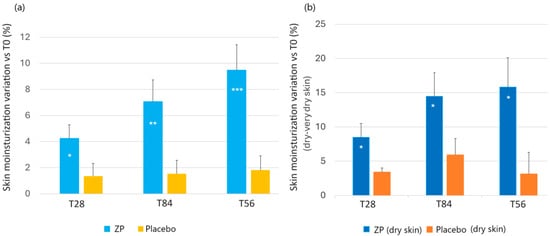
Figure 3.
Change in facial skin moisturisation. (a) Skin moisturisation variation versus baseline after 28, 56, and 84 days in the ZP treatment (blue bars) and placebo (yellow bars) group. (b) Changes in skin hydration versus baseline in subjects in the dry skin type subgroup in the ZP (dark blue bars) and placebo groups (orange bars). Data are means ± SEM. Intergroup (vs. placebo) statistical analysis is reported inside the bars of the histograms as follows: * p < 0.05, ** p < 0.01, and *** p < 0.001.
The moisture-related skin types were determined as follows: dry skin was characterized by corneometer units less than 40 and normal skin higher than 40 c.u. (corneometric units), as previously described [72]. The results showed that the test product had a clear impact on skin type, with drier skin types experiencing higher levels of moisturization. In the active treated group with dry or very dry skin types, skin moisturization significantly increased from the baseline measurement of 34.77 ± 1.12 by 8.8% after one month of treatment (37.70 ± 1.33, p < 0.01). This increase continued to be higher after 8 and 12 weeks, reaching 14.5% (39.81 ± 1.75, p < 0.001) and 15.8% (40.27 ± 1.94, p < 0.001), respectively. The skin moisturization variation between the active and placebo test product was statistically significant at all the checkpoints (p < 0.05). The results are illustrated in Figure 3b.
3.2.3. Transepidermal Water Loss (TEWL)
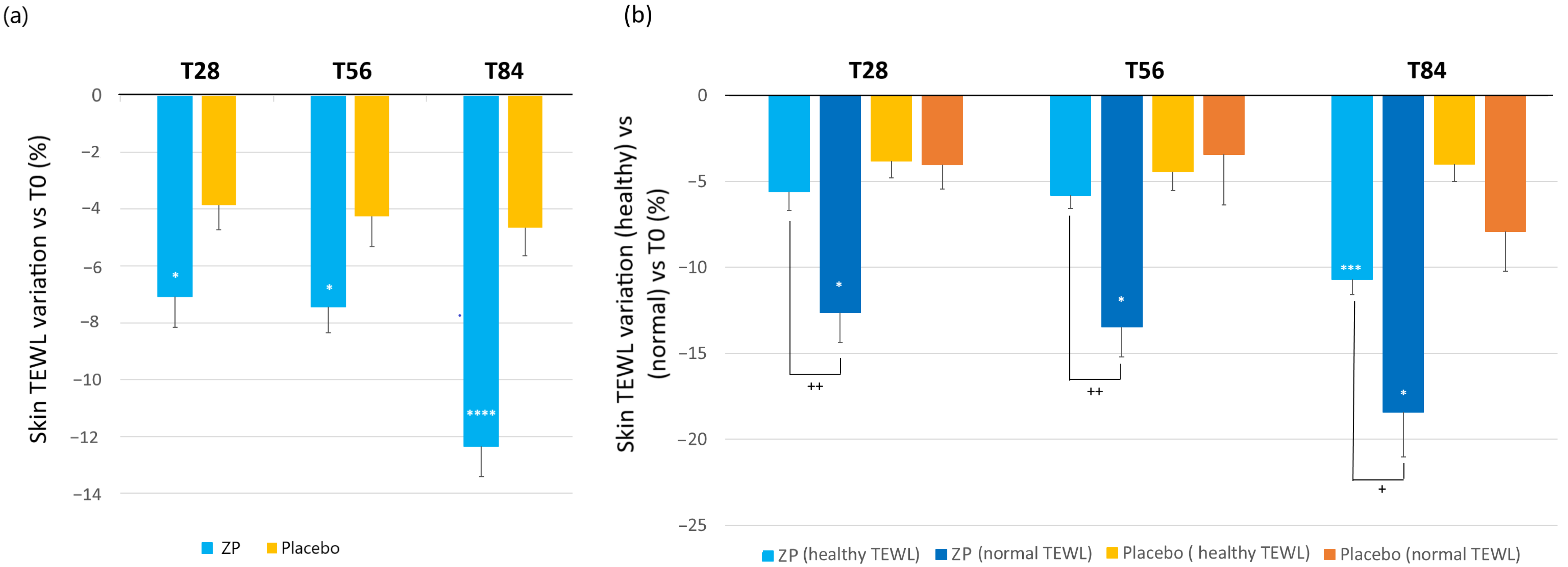
Changes in the epidermal barrier function were evaluated by measuring the TEWL in g × h−1 × m−2. A statistically significant decrease of TEWL was observed from the first month of study in the ZP treatment group (Figure 4). Specifically, in the active treatment arm, TEWL at baseline (13.04 ± 0.44) decreased by 7.1% (12.05 ± 0.36, p < 0.0001), 7.4% (11.99 ± 0.35, p < 0.0001), and 12.3% (11.35 ± 0.35, p < 0.0001) after 28, 56, and 84 days of product intake, respectively. In the placebo treatment arm, TEWL decreased by 3.8% (p = 0.0001), 4.3% (p = 0.0002), and 4.7% (p < 0.0001) after 28, 56, and 84 days of product intake, respectively. The variation between the ingredient and placebo product was statistically significant at all the checkpoints (p = 0.014 at T28, p = 0.027 at T56, and p < 0.0001 at T84) (Figure 4a). The study’s population had TEWL levels within the normal and healthy range, with none of the individuals exceeding the normal threshold of 25. However, when comparing individuals with normal TEWL values (between 15 and 25) to those with optimal TEWL levels (below 15), it was observed that individuals with higher TEWL values showed a more significant improvement in their skin TEWL after using the ZP ingredient. This suggests a potential correlation between initial TEWL levels and the efficacy of ZP treatment. As shown in Figure 4b, the subjects with normal TEWL values had a significant reduction by 12.6%, 13.5%, and 18.4% after 28, 56, and 84 days of product intake, respectively. On the contrary, for people with healthy TEWL (<15), the improvement was significantly lower at the three checkpoints (Figure 4b).
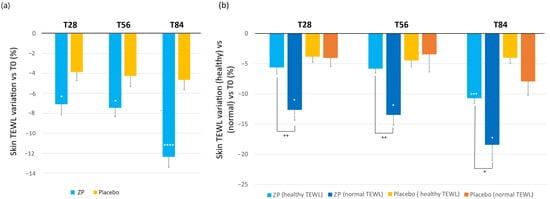
Figure 4.
Change in skin transepidermal water loss (TEWL). (a) TEWL variation a variation versus baseline after 28, 56, and 84 days in the ZP treatment (blue bars) and placebo groups (yellow bar). (b) Changes in TEWL versus baseline in the TEWL subgroups: (TEWL < 15 g·h−1·m−2 (healthy condition)) and TEWL between 15–25 g × h−1 × m−2 (normal condition) both in the ZP treatment (blue and dark blue bars) and placebo groups (blue and orange bars). Data are means ± SEM. Intergroup (vs. placebo) statistical analysis is reported on the bars of the histograms. Statistical analysis is reported as follows: * p < 0.05, *** p < 0.01 and **** p < 0.001. Inter-subgroup statistical analysis is reported with + upon the bars of the histograms as follows: + p < 0.05 and ++ p < 0.01.
3.2.4. Wrinkle Depth
Wrinkle depth in the “crow’s feet” area was significantly decreased in subjects taking the dietary supplement from day 28 onwards. In the active treatment arm, the baseline (311.5 μm) wrinkle depth was significantly decreased by 8%, 12.5% and 16.5%, after 28, 56, and 84 days of product intake, respectively, with no change observed in the placebo treatment arm. The variation between the active and placebo test product was statistically significant at all the checkpoints (p < 0.0001 at T28, T56 and T84) (Table 2).

Table 2.
Effect on wrinkle depth, skin elasticity, luminosity, and dark spot pigmentation.
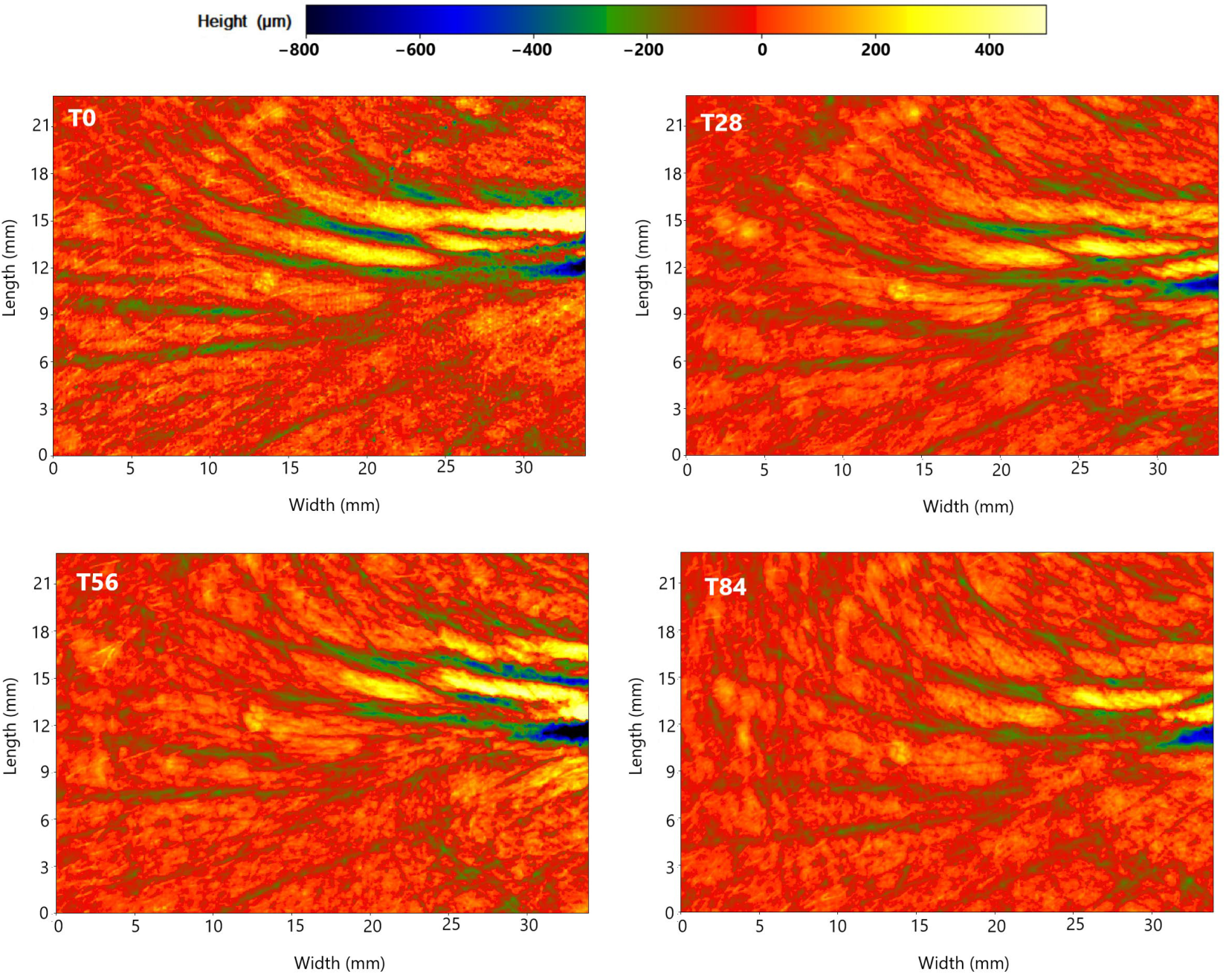
Representative images obtained using PrimosCR are shown in Figure 5. A reduction in periocular wrinkles was observed, as indicated by the change in colour from blue to green. The blue area, which represents wrinkle depth, became less prominent and intense. In the case of this specific volunteer, who is 56 years old and has sensitive skin and scalp, the change in wrinkle depth in the evaluated area showed a reduction of 22.0% at T28, 25.8% at T56, and 26.3% at T84.
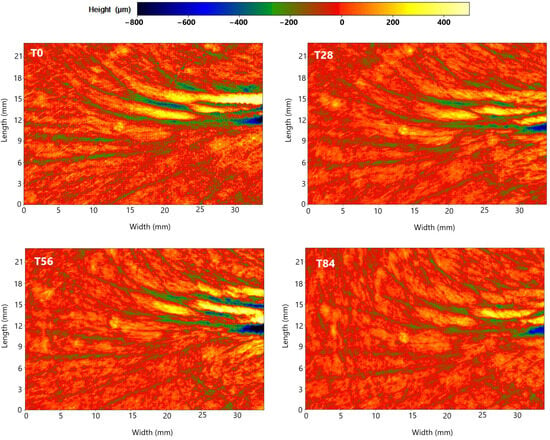
Figure 5.
Periocular wrinkles evaluated with PrimosCR of one the volunteers (volunteer 62) in the ZP treatment group. The skin surface is red in colour value, corresponding approximatively to a height of 0 mm (according to the colour scale present in the upper part of the figure). Green and blue colours represent negative values, indicating the presence of wrinkles. Orange and yellow colours represent values higher than zero.
3.2.5. Skin Elasticity
Results showed that the overall skin firmness and elasticity parameter improved during the study in the treatment group (Table 2).
- Skin distensibility, measured as R0 (mm), significantly decreased in the group that consumed the active product, suggesting an increase in skin firmness. The decrease of skin distensibility was as follows: 4.7%, 7.1%, and 9.4% after 28, 56, and 84 days of product consumption (p < 0.0001 vs. T0 at all the checkpoints). A smaller decrease of 2.6% (p = 0.0013) and 4.6% (p < 0.0001) was also observed in the placebo group after 56 and 84 days, respectively. The variation between the active and placebo groups was statistically significant at all the checkpoints (p = 0.0004 at T28, 0.0005 at T56 and p = 0.0002 at T84)
- The gross elasticity of the skin, including viscous deformation, R2 parameter, was significantly increased in the ZP dietary supplement group. In the ZP treatment arm, the skin gross elasticity increased by 5.5%, 7.1%, and 9.4%, after 28, 56, and 84 days of product intake (p < 0.0001 vs. T0). A minor decrease of 1.6% (p = 0.0002) and 3.2% (p < 0.0001) was also observed in the placebo group after 56 and 84 days, respectively, while no significant change was observed in the placebo group. The variation between the active and placebo test product was statistically significant at all the checkpoints (p = 0.002 at T28, 0.006 at T56, and p = 0.0004 at T84)
- Net elasticity, not including viscous deformation, R5 parameter, significantly increased in the ZP group. Increased R5 may suggest reduced skin ageing. In the active treatment arm, skin net elasticity increased by 5.3%, 6.8%, and 9%, after 28, 56, and 84 days of product intake, respectively (p < 0.0001 vs. T0 at all the checkpoints). Minor improvements were observed in the placebo group after 56 days (2.5%, p = 0.012) and 84 days (3.1%, p = 0.013). The variation between the active and placebo test product was statistically significant at all the checkpoints (p = 0.0004 at T28, p = 0.025 at T56, and p = 0.013 at T84)
3.2.6. Effects on Radiance and Skin Colour
- Intensity of Dark Spots: Individual Typology Angle (ITA°)
Throughout the study, there was a significant decrease in the intensity of pigmentation of dark spots as determined by ITA° measurements (Table 2). In the ZP active treatment arm, the intensity of the dark spots decreased by 19.6%, 23.5%, and 26.2% after 28, 56, and 84 days of product intake, respectively (p < 0.0001 vs. T0 at all the checkpoints). A smaller decrease of 5.9%, 11.1%, and 14.2% after 28, 56, and 84 days of product intake, respectively, was also observed in the placebo group (p < 0.0001 vs. T0 at all the checkpoints). Statistically significant differences between the active ingredient and the placebo group were observed at all checkpoints (p = 0.0009 at T28, p = 0.0004 at T56, and p = 0.0003 at D84).
- Skin Radiance, Gloss Value
The study showed a significant increase in skin gloss, also known as skin radiance (see Table 2). In the group using the ZP active product, skin radiance increased by 13.6%, 21.7%, and 24.9% after 28, 56, and 84 days of product use, respectively (p < 0.0001 vs. T0 at all time points). The placebo group also showed a small increase of 6.5%, 7%, and 9.3% after 28, 56, and 84 days of product use, respectively (p < 0.0001 vs. T0 at all checkpoints). The difference between the active and placebo products was statistically significant at all-time points (p < 0.0001 at checkpoints).
3.2.7. Skin Oiliness
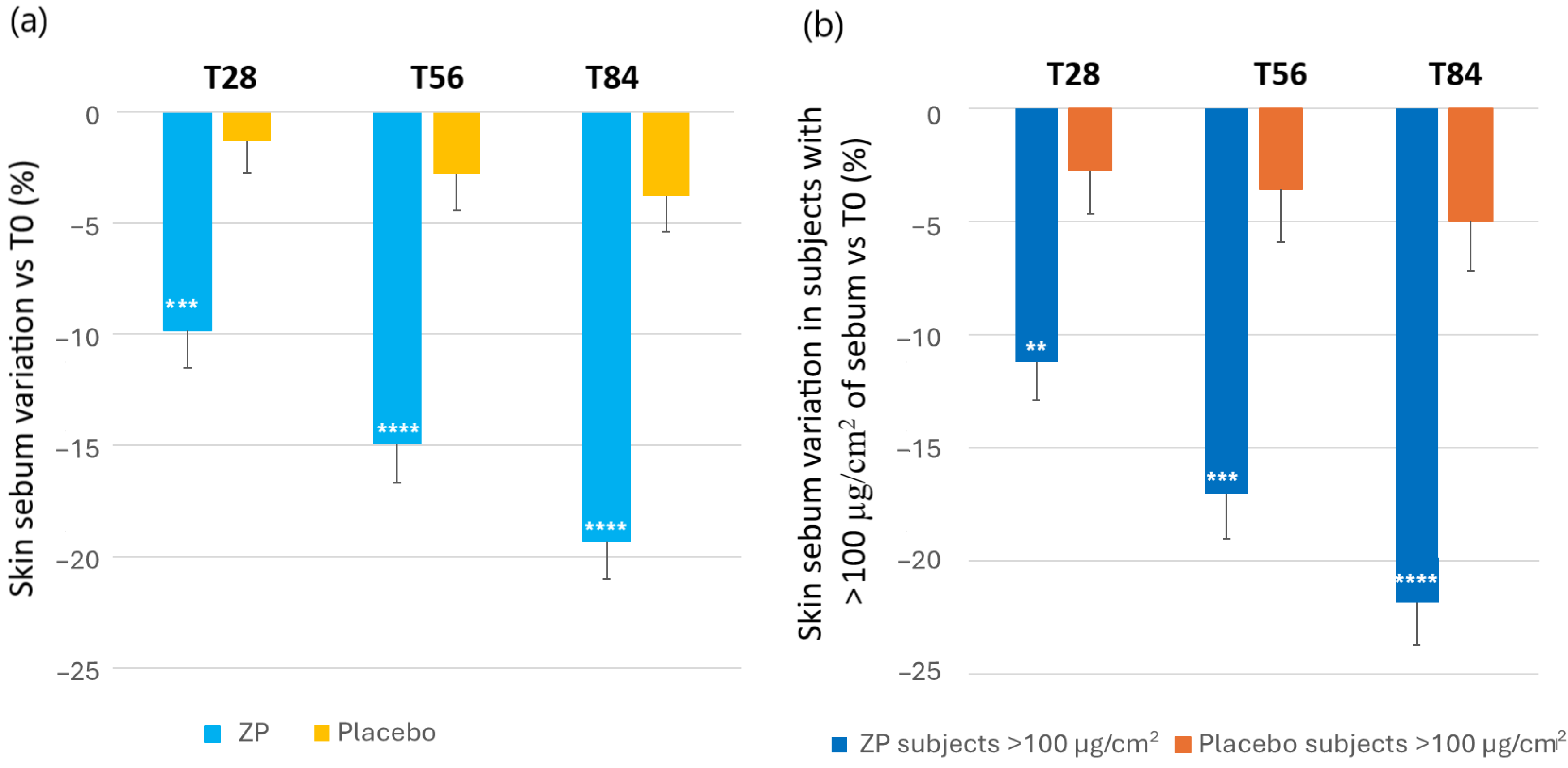
The changes in skin oiliness were evaluated by measuring the skin sebum content in µg/cm2. A statistically significant decrease of sebum was observed from the first month of study in the ZP treatment group (Figure 6). Thus, the baseline sebum (129.7 ± 7.20) decreased by 9.8% (116 ± 6.2, p < 0.0001), 14.9% (109.4 ± 5.9, p < 0.0001) and 19.3% (102.9 ± 5.3, p < 0.0001) after 28, 56, and 84 days of product intake, respectively. A small decrease of 3.8% (p = 0.014) was also observed in the placebo group after 84 days of product intake. The variation between the ingredient and placebo product was statistically significant at all the checkpoints (p = 0.0006 at T28, and p < 0.0001 at T56 and T84) (Figure 6a).
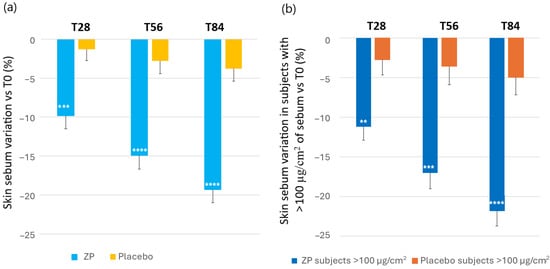
Figure 6.
Change in skin sebum content. (a) Skin sebum variation versus baseline after 28, 56, and 84 days in the ZP treatment (blue bars) and placebo (yellow bars) group. (b) Changes in the skin sebum versus baseline in subjects with >100 µg/cm2 of sebum (oily skin subgroup in the ZP (dark blue bars) and placebo groups (orange bars). Data are means ± SEM. Intergroup (vs. placebo) statistical analysis is reported inside the bars of the histograms as follows: ** p < 0.01, *** p < 0.001 and **** p < 0.0001.
In a detailed analysis, using cut-points of >100 µg/cm2 of sebum to determine individuals with a high oily skin type, the decrease in these subjects within the active treatment group was significantly greater compared to those with a sebum content ≤100 µg/cm2. Accordingly, the baseline sebum (151.59 ± 6.82) decreased by 11.2% (p < 0.0001), 17.0% (p < 0.0001) and 22.8% (p < 0.0001) after 28, 56, and 84 days of product intake, respectively. A decrease of 5.0% (p = 0.037) was also observed in the placebo group after 84 days of product intake. The variation between the active and placebo group among individual with high sebum content was statistically significant at all the checkpoints (p = 0.0043 at T28, p = 0.0005 at T56, and p = 0.0001 atT84) (Figure 6b).
3.3. Effects on Scalp Conditions
The main effectiveness measures for scalp condition were assessed at baseline and after 28, 56, and 84 days of product use. The following parameters were evaluated on the scalp: moisture levels, transepidermal water loss (TEWL), and sebum content. Furthermore, a clinical assessment was conducted (Table 3).

Table 3.
Effect on scalp Moisturization, TEWL and Sebum content.
No statistically significant differences were observed between subjects with sensitive and non-sensitive scalps in any of the endpoints evaluated, except for the scalp aspect, where a significant improvement was observed only in those with sensitive scalps at the first checkpoint
3.3.1. Scalp Moisturization
The group consuming the active product presented a statistically significant improvement in scalp moisturization index during each monitored check, both compared to baseline (p < 0.0001) and to the placebo-treated group (p = 0.0005 at T28, p = 0.0002 at T56, and p < 0.0001 at T56 and T84). In the group that received the active treatment, scalp moisturization increased by 6.4%, 10.3%, and 14.1% after 28, 56, and 84 days of taking the product. On the contrary, no significant variations were seen in the placebo-treated group (Table 3).
Similarly to the skin, an evaluation was conducted based on the baseline hydration level of the scalp. The findings indicated that subjects with a drier scalp (less than 40 µS) experienced greater improvements in moisturization, although no significant differences were observed when compared to the group with higher levels of scalp moisturization.
3.3.2. Scalp TEWL
The active product resulted in a statistically significant improvement in scalp TEWL index at each checkpoint compared to both the baseline (p < 0.0001) and the placebo-treated group (p = 0.018 at T28, p = 0.0036 at T56, and p = 0.0006 at T84). In the active ZP group, TEWL decreased by 7.2%, 10.1%, and 13.8% after 28, 56, and 84 days of using the product. A minor statistically significant decrease in the placebo group was also observed at all the checkpoints (Table 3).
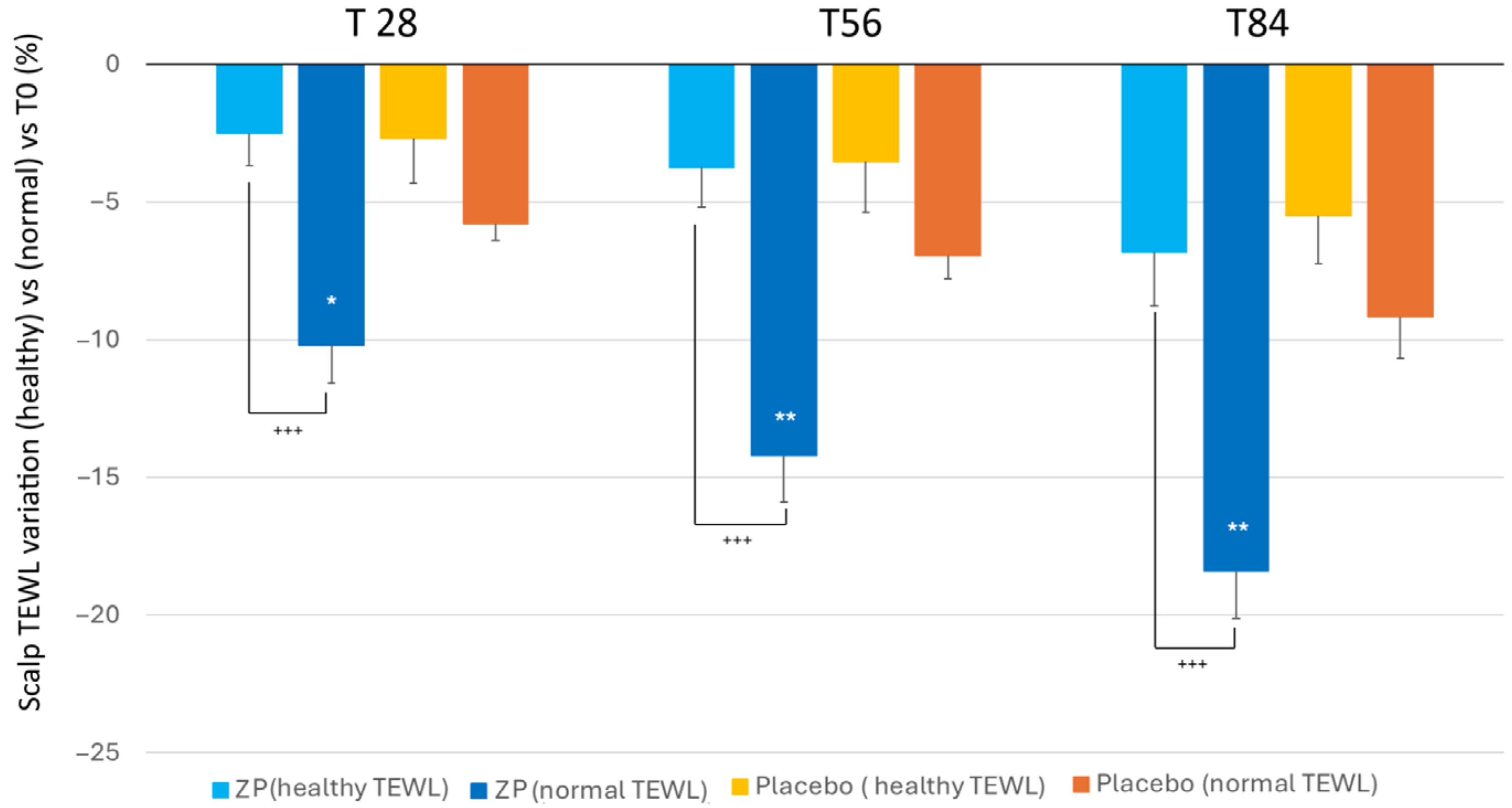
Similar to the skin, the study revealed that individuals with higher baseline levels of transepidermal water loss (TEWL) exhibited a higher improvement in their skin’s water retention after applying the ZP ingredient. Those with normal TEWL values (between 15 and 25 g × h−1 × m−2) recorded a notable reduction in TEWL by 10.2%, 14.2%, and 18.4% after 28, 56, and 84 days, respectively. However, participants with lower initial TEWL levels (<15 g × h−1 × m−2) did not show a significant decrease when compared to the placebo group (Figure 7).
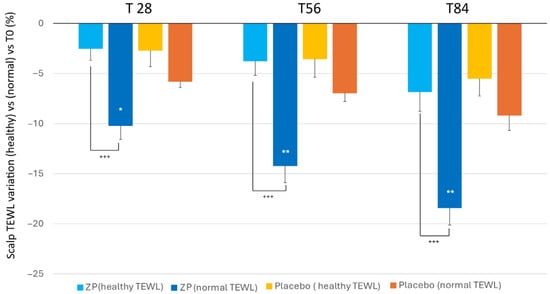
Figure 7.
Changes in TEWL versus baseline in the TEWL subgroups: (TEWL < 15 g·h−1·m−2 (healthy condition)) and TEWL between 15–25 g × h−1 × m−2 (normal condition) both in the ZP treatment (blue and dark blue bars) and placebo groups (blue and orange bars). Data are means ± SEM. Intergroup (vs. placebo) statistical analysis is reported on the bars of the histograms. Statistical analysis is reported as follows: * p < 0.05, ** p < 0.01. Inter-subgroup statistical analysis is reported with + upon the bars of the histograms as follows: +++ p < 0.001.
3.3.3. Scalp Oiliness
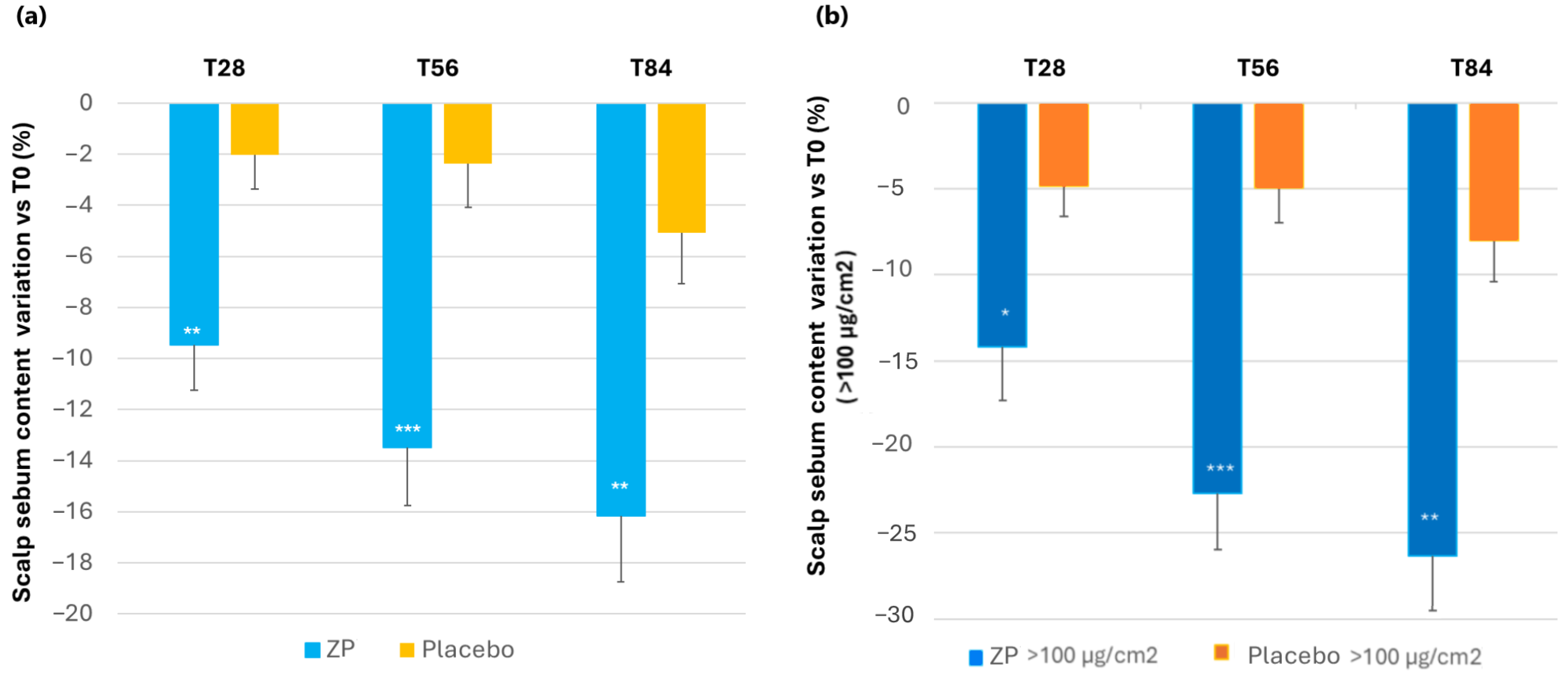
A statistically significant decrease of scalp sebum content was detected in the active ZP group during all the monitored checks, both compared to baseline (p < 0.0001) and the placebo-treated group (p = 0.009 at T28, p = 0.001 at T56, and p = 0.0054 at T84). In the ZP active treatment arm, the sebum content decreased by 9.5%, 13.5%, and 16.2% after 28, 56, and 84 days of product intake, respectively. A small decrease of 5.1% (p = 0.013) was also observed in the placebo group after 84 days of product intake (Table 3 and Figure 8a).
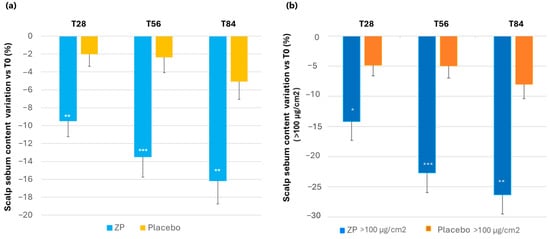
Figure 8.
Change in scalp sebum content. (a) Scalp sebum variation versus baseline after 28, 56, and 84 days in the ZP treatment (blue bars) and placebo (yellow bars) groups. (b) Changes in skin sebum versus baseline in subjects with >100 µg/cm2 of sebum (oily skin subgroup) in the ZP (dark blue bars) and placebo group (orange bars). Data are means ± SEM. Intergroup (vs. placebo) statistical analysis is reported inside the bars of the histograms as follows: * p < 0.05, ** p < 0.01, and *** p < 0.001.
An evaluation based on the baseline sebum content of the scalp was also performed. The results showed that subjects with higher sebum content (above 100 µg/cm2) experienced a greater reduction. Specifically, the baseline sebum level (125.10 ± 7.28) decreased by 14.73% (p < 0.0001), 22.72% (p < 0.0001), and 26.32% (p < 0.0001) after 28, 56, and 84 days of using the product, respectively. Although to a lesser extent, a decrease was also observed in the placebo group. The difference between the active and placebo groups among individuals with high sebum content was statistically significant at all the checkpoints (p = 0.04 at T28, p = 0.0009 at T56, and p = 0.002 at T84) (Figure 8b).
3.3.4. Scalp Appearance
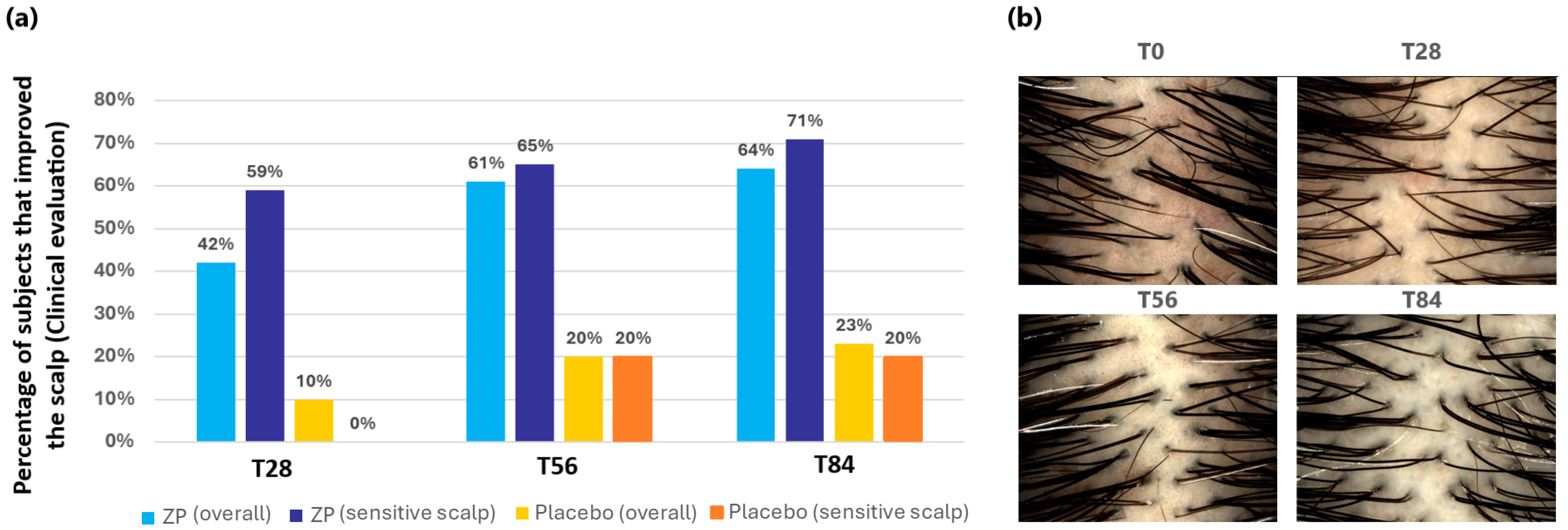
In clinical assessments, the product’s positive impact on the evaluated parameter is considered confirmed if more than 50% of the participants demonstrate improvement. The clinical evaluation results for the scalp indicated a significant enhancement in scalp appearance, with 61% of the participants showing improvement after 56 days and 64% at the end of the study (T84) across the entire group. Furthermore, the product demonstrated a notable reduction in scalp redness, with 59%, 65%, and 71% improvement at T28, T56, and T84, respectively, in subjects with sensitive scalps (Figure 9a). A clear example of the scalp redness reduction with the time of ZP consumption can be seen in the pictures in Figure 9b.
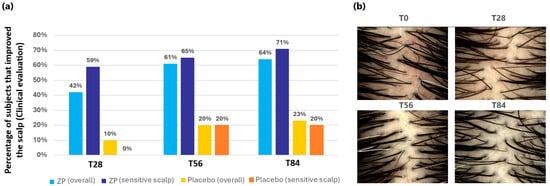
Figure 9.
Scalp aspect improvement. (a) Percentage of subjects that improved their scalp condition in the entire panel and in the sensitive subgroup. (b) Images of the scalp of a volunteer with sensitive scalp in the active treatment group at baseline (T0) and at the different checkpoints. Digital pictures were acquired by means of DermoGenius ultra (DermoScan GmbH, Regensburg, Germany). Starting from T28, scalp redness is less evident.
3.4. Self-Assessment Questionnaire
The subjective and qualitative assessment of the product’s effectiveness revealed that the dietary supplement ingredient had a positive impact on their skin and scalp. The experimental product was scored by volunteers better than the placebo product at all the checkpoints and for all the questionnaire items. Notably, subjects in the active product group reported improvements in response to all questions asked after the first month (more that 60% of subjects gave positive judgement).
After three months of ZP intake, 69.7% of the participants perceived their skin protected from environmental pollution, and 78.8% experienced less discomfort due to pollutant sensitivity. Since 50% of the people in the study had clinically sensitive skin and scalp, the responses were analysed by separating those with sensitive skin and scalp from those without. The results showed that 76.5% and 70.6% of the participants with sensitive skin and scalp, respectively, experienced less discomfort and felt their skin was protected from environmental pollution after only 4 weeks of taking the product. By the end of the study, the percentage had increased even further, with 82.4% of the participants with sensitivity in their skin and scalp reporting less discomfort and a greater sensation of protection. In contrast, the placebo group was less than 60% effective.
In terms of skin health, at the end of the study, 84.8% of the subjects observed their skin complexion appearing healthier. Improvements in skin texture, including smoothness, reduced dilated pores, and fewer pimples, were reported by 90.9% of participants. Furthermore, 78.8% felt their skin was more moisturized, and 75.8% noticed their skin was more homogeneous and had a more even tone. Additionally, 69.7% observed a reduction in the visibility of fine lines and wrinkles, and 75.8% felt their skin looked firmer.
Regarding scalp and hair health, 75.8% of participants noticed an improvement in their scalp condition, and 78.8% reported healthier hair. Among those with sensitive scalps, the percentage increased, with 82.4% acknowledging enhanced scalp and hair health. Additionally, 78.8% found their hair to be more resistance, while 75.8% remarked that their hair looked brighter. More details can be found in Figure S4 in the Supplementary Materials.
When asked if they would recommend the product, 90.9% of the participants responded affirmatively.
4. Discussion
Considering the bibliography collected in the introduction, it is well known that pollution contributes to extrinsic skin aging including pigment irregularities, nasolabial folds, and wrinkles. It is also an aggravating factor in several inflammatory or allergic skin conditions [4]. In addition, recent studies have also correlated the increase of pollution with detrimental scalp and hair health. Exposure to particulate matter (PM) can lead to sensitivity, discomfort, dryness, or oiliness of the scalp. Hair follicles may become weakened, and there is evidence suggesting this could result in hair loss [73]. The hair fibres and scalp provide a sufficient surface area for PM to bind and potentially penetrate the outer cuticle or skin layers. A key mechanism causing PM’s harmful effects in the skin and scalp is the generation of ROS [14,29,74,75,76], which is notably exacerbated when combined with UV irradiation.
To counteract the negative effects of skin and scalp oxidative stress, it is important to consistently introduce a variety of antioxidants [24,77,78], where the combination of these compounds seems to have a stronger effect [34,35,36]. Plant extracts are particularly suitable for this purpose, which are highly effective due to their protective defence mechanisms against ROS. The demand for natural and plant-based “nutricosmetics” has been rising in recent years, driven by growing awareness of their health and beauty benefits. This trend, combined with the increasing need for environmental protection of the skin beyond sunscreens, has led to greater attention on plant-derived anti-pollution strategies. In addition to the established antioxidant and anti-inflammatory benefits of the phenolic compounds found in the investigational ingredient ZP—including hydroxytyrosol, verbascoside, oleuropein, rosemary diterpenes, and quercetin [42,53,58,79,80,81,82]—previous preclinical studies have proven that the above-mentioned ingredient provides a comprehensive protective mechanism for the skin exposed to environmental pollutants by reducing oxidative stress, inflammation, lipid peroxidation, and AhR overactivation [38].
The result of this study provides clear evidence that oral supplementation with the ZP ingredient is beneficial to the skin of subjects exposed to high environmental stress, independent of whether they have sensitive skin or not. Specifically, the results of the present study demonstrated that a daily intake of 250 mg of ZP was enough to produce visible and structural improvements in the skin health and in decreasing the signs of skin ageing in as early as 4 weeks of product intake. Specifically, the skin health benefits of the test product were demonstrated by the increase in skin moisturization, the decrease of TEWL, and a reduction in the oiliness.
The skin barrier integrity is crucial for proper functioning of the skin. Since pollutants highly influence this integrity, the efficacy of ZP in this regard is important to consider [29,76,83]. The results of the study revealed that ZP significantly increased skin moisturization, particularly for individuals with initially dry skin, helping them achieve normal conditions. It also led to a significant reduction in water loss, especially among those subjects with higher basal values. Water loss is a critical indicator of skin barrier function. The decrease suggests that the ingredient not only enhances skin hydration but also fortifies the skin barrier function against water loss, making it more resistant to the dehydrating effects of pollutants. By fortifying the skin barrier, the ingredient helps prevent harmful pollutants from infiltrating the skin, providing additional protection against environmental damage. Prior studies have shown that exposure to oxidative stress can induce epidermal permeability barrier disruption, suggesting that antioxidant compounds could improve barrier function [84]. Thus, the proven antioxidant properties of the tested ZP ingredient [37,38], as well the phenolic compounds present in Lippia citriodora [53,54,81], Rosmarinus officinalis [58], Sophora japonica [82], and Olea europaea [41,45], could favour an improvement in the skin barrier function.
Additionally, the ingredient’s ability to inhibit the overexpression of AhR in the presence of particulate matter (PM) contributes to its effectiveness [38]. Elevated AhR activity, induced by PM, can lead to increased oxidative stress and inflammation, which are associated with the upregulation of cyclooxygenase 2 (COX-2) and prostaglandin E2 (PGE2), and subsequent disruption of the skin barrier through reduced filaggrin levels [85]. This effect is particularly important given the role of filaggrin in the skin’s barrier function. Filaggrin is a structural protein that contributes to the formation of the skin’s outermost layer, the stratum corneum, by binding to keratin fibres and helping to maintain the integrity of the skin barrier. It also plays a key role in the hydration of the skin by breaking down into natural moisturizing factors (NMFs) that help retain moisture [86]. Thus, by inhibiting AhR, the ZP ingredient could help mitigate these inflammatory responses, thereby supporting the maintenance of skin barrier integrity and enhancing the skin’s ability to resist environmental stressors. Future studies could explore the potential of the ZP ingredient in preserving filaggrin levels and elucidate its impact on skin barrier integrity more comprehensively.
Pollution significantly impacts sebum production by inducing oxidative stress, increasing sebum secretion, and promoting inflammation [29,76,83,87]. These effects can exacerbate skin conditions like acne and contribute to overall skin sensitivity and reactivity [13,87]. In the present study, ZP was found to decrease sebum production, particularly in individuals with high oily skin. The observed reduction in sebum production in the study implies that the ingredient assists in regulating sebum levels, thereby reducing the risk of pollution-induced skin issues. This effect is especially beneficial for individuals with oily and acne-prone skin, as it can help maintain a clearer complexion and diminish the likelihood of acne and related conditions. Previous research using the ZP ingredient has also shown a reduction in skin sebum production, although the extent of the decrease was comparatively smaller probably due to the lower baseline sebum levels in the study participants [37]. The more substantial reduction observed in the current study highlights ZP’s enhanced efficacy in managing sebum production, particularly in individuals with higher initial sebum levels. Future research could explore the effectiveness of ZP in individuals with acne-prone skin to determine if it could help reduce oily skin and alleviate their inflammation.
Pollutant exposure has been linked with premature skin ageing, and several studies have explored how pollutants such as PM, ozone, and nitrogen oxides contribute to the development of wrinkles, loss of elasticity, changes in luminosity, and alterations in skin tone [3,15,16,88]. In the present study, an improvement of the skin’s firmness and elasticity as well as a significant reduction in the wrinkle’s depth in the “crow’s feet” area were also observed after only 4 weeks of taking the dietary supplement. Usually, ROS is one of the important factors involved in the formation of wrinkles and loss of elasticity. Oxidative stress induced by ROS causes skin inflammation and consequently activates matrix metalloproteinase (MMP). Activated MMP, in turn, degrades collagen and promotes skin wrinkles and the loss of elasticity and firmness.
The positive effects observed in this study in the subjects taking ZP could be partly due to the capacity of this ingredient to increase the antioxidant capacity of their skin, as was observed by the FRAP measurement. The FRAP analysis evaluates the skin’s ability to counteract ROS that are produced by environmental stressors such as UV radiation, pollution, and smoking [83,89]. By measuring the ability of the skin to counteract ROS, the FRAP analysis can provide valuable information about the skin’s overall health and its potential to maintain its barrier function, which is critical for protecting against external aggressors. Furthermore, the botanical extracts or active compounds present in ZP have been shown to protect extracellular matrix proteins. For instance, verbascoside, the phenylpropanoid present in Lippia citriodora, has been proven to effectively decrease the expression of MMP-1 [90] and to activate the expression of the precursor of MMP-2 in human dermal fibroblasts [91]. Furthermore, oleuropein and hydroxytyrosol from olive extracts reduce the expression of various MMPs involved in skin aging in response to UV radiation, like MMP-2, MMP-9, MMP-13, MMP-1, and MMP-3 [45,48]. Carnosic acid in rosemary also inhibits UVA- and UVB-induced expression of MMP-1, MMP-3, and MMP-9 in human skin cells [92], and quercetin inhibits UVB-induced MMP-1 expression in human keratinocytes [93].
The daily consumption of this botanical blend also provided a more uniform skin complexion. Specifically, the daily consumption of ZP has a progressive brightening effect and significantly lightened the hyperpigmented spots on the cheekbone area. These improvements were statistically significant compared to the placebo group after only four weeks of taking the dietary supplement ingredient. The product’s effect on lightening the dark spots could be correlated with the ability of different active compounds of the ingredient to inhibit melanin production, as has been previously described for both hydroxytyrosol and oleuropein from olive [46,94,95], verbascoside from lemon verbena [52], and quercetin [96].
In this clinical trial, the observed skin benefits were substantiated by the self-assessment questionnaire, as the treatment product was highly rated by volunteers regarding its efficacy in all the questions.
The above findings align with the observations made in a previous clinical study involving this ingredient, where we demonstrated that the intake of ZP also significantly improved the systemic oxidative status, strengthened the skin barrier, improved skin moisturization, and provided anti-aging skin benefits versus the placebo group in both Caucasian and Asian skin-type women exposed to a daily high level of environmental pollution [37].
A recent review showed that, over the past 50 years, more than 20 studies have established a connection between various scalp conditions and negative effects on hair. These include altered cuticular integrity, surface pitting, roughness, and diminished shine. Additionally, the study suggested that normalizing the scalp’s condition can reverse these adverse effects on the hair [97]. Research indicates that oxidative stress from UV rays and pollution significantly impacts scalp and hair health [6,75]. It disrupts the normal keratinization of the hair cuticle, leading to fragile hair that is easily damaged [97]. Individuals with hair loss often have lower antioxidant levels in their scalp and higher lipid peroxidation, suggesting that antioxidant-rich botanical ingredients can benefit both the scalp and hair [98]. Pollutants like diesel exhaust particles and PM induce strong inflammatory responses, leading to chronic inflammation that degrades the scalp’s extracellular matrix, increases susceptibility to infections [59], and can cause scarring alopecia, resulting in permanent hair loss [6].
The current study has demonstrated that daily consumption of the proven antioxidant and anti-inflammatory ZP ingredient enhances scalp health in subjects, regardless of whether they have a sensitive scalp. The study revealed that this botanical blend ingredient helped to re-balance the scalp’s oiliness, improve the scalp’s hydration and scalp barrier, and decrease scalp irritation.
The damage of the scalp barrier leads to higher TEWL, resulting in poor skin hydration with a dry and sensitive scalp. In this study, a positive effect of the ZP product was also seen on the scalp parameters related to the skin’s barrier integrity where the improvement of skin moisturization correlated with TEWL decrease. Moreover, a higher improvement in both TEWL was observed among the individuals with higher baseline values.
Sebum production is a crucial factor in maintaining scalp health by creating a hydrophobic barrier that protects the scalp from external aggressors and keeping the scalp moisturized. However, pollution can disrupt this balance [4,21]. Studies have shown that pollutants can stimulate sebaceous glands [22], leading to oily scalp and conditions like seborrheic dermatitis and dandruff due to bacterial and fungal colonization [23]. In this study, we observed that the botanical blend reduced the presence of sebum on the scalp, particularly in those with very oily scalps. This may lead to improvements in scalp appearance and sensitivity, as well as enhance hair quality, according to the study’s findings.
The exposure to pollution causes redness, irritation, and faster exfoliation of outer layers of the scalp, exposing sensitive inner layers, which in turn causes more sensitivity, leading to further scaling and even psoriasis after prolonged exposure [21,97]. Thus, people with sensitive scalps are particularly susceptible to a worsening of their condition in periods of high environmental pollution. In the present study, the product under investigation improved the appearance of the scalp and reduced redness among individuals with sensitive scalps. Additionally, in the subjective evaluation, study participants with sensitive scalp reported significantly less discomfort. These findings suggest that the oral product not only enhances the overall health and appearance of the scalp but also provides significant relief for individuals with sensitive scalps, particularly in adverse environmental conditions. In this context, the anti-inflammatory properties of the ZP ingredient could play a crucial role. Previous studies have demonstrated that ZP exhibits anti-inflammatory effects in response to pollution-induced stress by inhibiting various pro-inflammatory mediators, such as such as interleukin-6 (IL-6) and interleukin-1 alpha (IL-1α), in human skin explants and keratinocytes exposed to pollutants [38]. Such effects could contribute to the observed improvements in scalp health and comfort, highlighting the potential of ZP as an effective intervention for managing scalp and hair health. In fact, there is evidence that suggests that both IL-1 and IL-6 might be crucial inducers of hair loss [99,100,101].
In this clinical trial, the observed functional and structural changes were substantiated by the self-assessment questionnaire, as the treatment product was highly rated by volunteers regarding its efficacy both as skin and scalp/hair health ingredient. This underscores the product’s potential as an effective dietary supplement to not only improve the appearance and health of the skin but also to manage scalp sensitivity and enhance hair quality, thereby promoting greater adherence and user satisfaction.
The strengths of this study include its randomized, double-blind, placebo-controlled design, the equitable distribution of participants across different skin and scalp conditions, age, and grades of skin ageing, and the relatively long duration of the assessment period. Additional strengths include the control of external variables (e.g., diet, dietary supplement use, sun, or tanning bed exposure, etc.) that could influence the results, as well as the use of a standardized facial cleanser, moisturizer, and shampoo throughout the study. However, it is important to note some limitations, such as the exclusive participation of Caucasian women in the study. Nonetheless, we believe that our findings could be applicable to the broader population since the primary molecular, cellular, and tissue-specific events that contribute to pollutant skin and scalp damage are common across genders and ethnicities. Indeed, in a prior study, we did not see significant differences in any of the parameters evaluated among Caucasian and Asian subjects treated with the studied botanical ingredient [37]. In any case, it would be worthwhile to investigate whether this product would yield similar benefits in other ethnic skin types and in men.
Moreover, considering that each of the individual ingredients of ZP, extracts of Lippia citriodora, Olea europaea, Rosmarinus officinalis, and Sophora japonica, have previously been employed in topical formulations, future research could explore the development of a topical formulation of this product for direct application to the skin and scalp. Such studies could provide further insights into its efficacy and potential benefits in topical skincare treatments.
5. Conclusions
The results clearly demonstrated the benefits of a daily intake of 250 mg of the botanical mixture containing Lippia citriodora, Olea europaea, Rosmarinus officinalis, and Sophora japonica for the skin and scalp of individuals exposed to high environmental stress, irrespective of pre-existing skin and scalp sensitivities. Quantitative measurements revealed significant improvements in several skin parameters, including antioxidant capacity, moisturization, improved radiance, and reduced wrinkle depth and elasticity. Additionally, dark spot pigmentation and skin oiliness was reduced. For the scalp, we observed an increase in moisturization and a reduction in the TEWL and sebum content. Additionally, 71% of subjects with sensitive scalps experienced reduced redness, demonstrating the mixture’s effectiveness in mitigating common scalp issues. Moreover, no adverse reactions were reported by any participants in the study. Thus, we suggest that the oral consumption of this ingredient could be a viable strategy to enhance the skin and scalp health of those living in an urban environment. These results emphasize the wide-ranging benefits of the ingredient, indicating its potential to address scalp issues and broaden its use beyond skin care.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/cosmetics11040139/s1, Figure S1: Flow Chart of Botanical extract Blend Ingredient; Figure S2: Representative high-performance liquid chromatography4,21 (HPLC) chromatograms of standardized compounds in the botanical blend; Figure S3: Air quality parameters. Figure S4: Results of self-assessment questions; Table S1: Compounds Identified by HPLC-MS in the four- botanical blend; Table S2: Cosmetic products ingredient list.
Author Contributions
Conceptualization, N.C. and V.N.; methodology, V.N. and E.C.; formulation design, N.C.; validation, V.N. and E.C.; formal analysis, S.G.; investigation, E.C. and A.P.; data curation, V.N., S.G., A.G. and P.N.; writing—original draft preparation, N.C. and V.N.; writing—review and editing, N.C., J.J., A.G. and V.N.; visualization, V.N., S.G. and A.P.; supervision, V.N. and S.G.; project administration, N.C. and A.G.; funding acquisition, N.C. and J.J. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by Monteloeder S.L.
Institutional Review Board Statement
The study was conducted in accordance with the Declaration of Helsinki. Both the study protocol and the informed consent form were approved (ref. no. 2023/12 by 5 October 2023) by the “Comitato Etico Indipendente per le Indagini Cliniche Non Farmacologiche”.
Informed Consent Statement
Informed consent was obtained from all subjects involved in the study. Written informed consent has been obtained from the patients to publish this paper.
Data Availability Statement
The data presented in this study are available upon request from the corresponding author.
Acknowledgments
The authors would like to express their gratitude to the Complife Italia staff, who contributed to the study and recruited the subjects, for their professionalism and support during the study development.
Conflicts of Interest
N.C., J.J., A.G. and P.N. belong to the Research and Development Department at Monteloeder S.L. This does not alter the authors adherence to all the journal policies on sharing data and materials. The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest. The funders had no role in the design of the study; in the collection, analyses or interpretation of data; in the writing of the manuscript; or in the decision to publish the results.
References
- Air Pollution. Available online: https://www.who.int/health-topics/air-pollution (accessed on 18 August 2023).
- Boogaard, H.; Patton, A.P.; Atkinson, R.W.; Brook, J.R.; Chang, H.H.; Crouse, D.L.; Fussell, J.C.; Hoek, G.; Hoffmann, B.; Kappeler, R.; et al. Long-Term Exposure to Traffic-Related Air Pollution and Selected Health Outcomes: A Systematic Review and Meta-Analysis. Environ. Int. 2022, 164, 107262. [Google Scholar] [CrossRef]
- Lefebvre, M.-A.; Pham, D.-M.; Boussouira, B.; Bernard, D.; Camus, C.; Nguyen, Q.-L. Evaluation of the Impact of Urban Pollution on the Quality of Skin: A Multicentre Study in Mexico. Int. J. Cosmet. Sci. 2015, 37, 329–338. [Google Scholar] [CrossRef] [PubMed]
- Mancebo, S.E.; Wang, S.Q. Recognizing the Impact of Ambient Air Pollution on Skin Health. J. Eur. Acad. Dermatol. Venereol. JEADV 2015, 29, 2326–2332. [Google Scholar] [CrossRef] [PubMed]
- Fussell, J.C.; Kelly, F.J. Oxidative Contribution of Air Pollution to Extrinsic Skin Ageing. Free Radic. Biol. Med. 2020, 151, 111–122. [Google Scholar] [CrossRef] [PubMed]
- Rajput, R. Understanding Hair Loss Due to Air Pollution and the Approach to Management. Hair Ther. Transplant. 2015, 5, 2. [Google Scholar] [CrossRef]
- Cotovio, J.; Onno, L.; Justine, P.; Lamure, S.; Catroux, P. Generation of Oxidative Stress in Human Cutaneous Models Following in Vitro Ozone Exposure. Toxicol. In Vitro 2001, 15, 357–362. [Google Scholar] [CrossRef]
- Trüeb, R.M. The Impact of Oxidative Stress on Hair. Int. J. Cosmet. Sci. 2015, 37, 25–30. [Google Scholar] [CrossRef] [PubMed]
- Fadadu, R.P.; Abuabara, K.; Balmes, J.R.; Hanifin, J.M.; Wei, M.L. Air Pollution and Atopic Dermatitis, from Molecular Mechanisms to Population-Level Evidence: A Review. Int. J. Environ. Res. Public Health 2023, 20, 2526. [Google Scholar] [CrossRef] [PubMed]
- Lowe, M.E.; Akhtari, F.S.; Potter, T.A.; Fargo, D.C.; Schmitt, C.P.; Schurman, S.H.; Eccles, K.M.; Motsinger-Reif, A.; Hall, J.E.; Messier, K.P. The Skin Is No Barrier to Mixtures: Air Pollutant Mixtures and Reported Psoriasis or Eczema in the Personalized Environment and Genes Study (PEGS). J. Expo. Sci. Environ. Epidemiol. 2023, 33, 474–481. [Google Scholar] [CrossRef] [PubMed]
- Wu, J.; Chen, H.; Yang, R.; Yu, H.; Shang, S.; Hu, Y. Short-Term Exposure to Ambient Fine Particulate Matter and Psoriasis: A Time-Series Analysis in Beijing, China. Front. Public Health 2022, 10, 1015197. [Google Scholar] [CrossRef]
- El Haddad, C.; Gerbaka, N.-E.; Hallit, S.; Tabet, C. Association between Exposure to Ambient Air Pollution and Occurrence of Inflammatory Acne in the Adult Population. BMC Public Health 2021, 21, 1664. [Google Scholar] [CrossRef] [PubMed]
- Krutmann, J.; Moyal, D.; Liu, W.; Kandahari, S.; Lee, G.-S.; Nopadon, N.; Xiang, L.F.; Seité, S. Pollution and Acne: Is There a Link? Clin. Cosmet. Investig. Dermatol. 2017, 10, 199–204. [Google Scholar] [CrossRef] [PubMed]
- Kim, K.E.; Cho, D.; Park, H.J. Air Pollution and Skin Diseases: Adverse Effects of Airborne Particulate Matter on Various Skin Diseases. Life Sci. 2016, 152, 126–134. [Google Scholar] [CrossRef] [PubMed]
- Schikowski, T.; Hüls, A. Air Pollution and Skin Aging. Curr. Environ. Health Rep. 2020, 7, 58–64. [Google Scholar] [CrossRef] [PubMed]
- Roberts, W.E. Pollution as a Risk Factor for the Development of Melasma and Other Skin Disorders of Facial Hyperpigmentation—Is There a Case to Be Made? J. Drugs Dermatol. JDD 2015, 14, 337–341. [Google Scholar] [PubMed]
- Grether-Beck, S.; Felsner, I.; Brenden, H.; Marini, A.; Jaenicke, T.; Aue, N.; Welss, T.; Uthe, I.; Krutmann, J. Air Pollution-induced Tanning of Human Skin. Br. J. Dermatol. 2021, 185, 1026–1034. [Google Scholar] [CrossRef] [PubMed]
- Baudouin, C.; Charveron, M.; Tarroux, R.; Gall, Y. Environmental Pollutants and Skin Cancer. Cell Biol. Toxicol. 2002, 18, 341–348. [Google Scholar] [CrossRef] [PubMed]
- Siddens, L.K.; Larkin, A.; Krueger, S.K.; Bradfield, C.A.; Waters, K.M.; Tilton, S.C.; Pereira, C.B.; Löhr, C.V.; Arlt, V.M.; Phillips, D.H.; et al. Polycyclic Aromatic Hydrocarbons as Skin Carcinogens: Comparison of Benzo[a]Pyrene, Dibenzo[Def,p]Chrysene and Three Environmental Mixtures in the FVB/N Mouse. Toxicol. Appl. Pharmacol. 2012, 264, 377–386. [Google Scholar] [CrossRef] [PubMed]
- Burke, K.E. Skin Cancer Induced by Pollution-Mediated ROS. In Handbook of Oxidative Stress in Cancer: Mechanistic Aspects; Chakraborti, S., Ray, B.K., Roychowdhury, S., Eds.; Springer: Singapore, 2020; pp. 1–22. [Google Scholar] [CrossRef]
- Vierkötter, A.; Schikowski, T.; Ranft, U.; Sugiri, D.; Matsui, M.; Krämer, U.; Krutmann, J. Airborne Particle Exposure and Extrinsic Skin Aging. J. Investig. Dermatol. 2010, 130, 2719–2726. [Google Scholar] [CrossRef] [PubMed]
- Kwack, M.H.; Ha, N.G.; Lee, W.J. Effects of <10-Μm Particulate Matter on Cultured Human Sebocytes and Outer Root Sheath Cells and Usefulness of Siegesbeckia Herba Extract. Ann. Dermatol. 2022, 34, 163–172. [Google Scholar] [CrossRef] [PubMed]
- Borda, L.J.; Wikramanayake, T.C. Seborrheic Dermatitis and Dandruff: A Comprehensive Review. J. Clin. Investig. Dermatol. 2015, 3, 10. [Google Scholar] [CrossRef]
- Kim, H.; Woo, H.; Shin, S.; Park, D.; Jung, E. The Potential Application of Ecklonia Cava Extract in Scalp Protection. Cosmetics 2020, 7, 9. [Google Scholar] [CrossRef]
- A Global Consumer Journey in Scalp Care. Available online: https://www.personalcaremagazine.com/story/44438/a-global-consumer-journey-in-scalp-care (accessed on 31 May 2024).
- Juliano, C.; Magrini, G.A. Cosmetic Functional Ingredients from Botanical Sources for Anti-Pollution Skincare Products. Cosmetics 2018, 5, 19. [Google Scholar] [CrossRef]
- Marrot, L. Pollution and Sun Exposure: A Deleterious Synergy. Mechanisms and Opportunities for Skin Protection. Curr. Med. Chem. 2018, 25, 5469–5486. [Google Scholar] [CrossRef] [PubMed]
- Parrado, C.; Mercado-Saenz, S.; Perez-Davo, A.; Gilaberte, Y.; Gonzalez, S.; Juarranz, A. Environmental Stressors on Skin Aging. Mechanistic Insights. Front. Pharmacol. 2019, 10, 759. [Google Scholar] [CrossRef] [PubMed]
- Araviiskaia, E.; Berardesca, E.; Bieber, T.; Gontijo, G.; Sanchez Viera, M.; Marrot, L.; Chuberre, B.; Dreno, B. The Impact of Airborne Pollution on Skin. J. Eur. Acad. Dermatol. Venereol. JEADV 2019, 33, 1496–1505. [Google Scholar] [CrossRef] [PubMed]
- Hernandez, D.F.; Cervantes, E.L.; Luna-Vital, D.A.; Mojica, L. Food-Derived Bioactive Compounds with Anti-Aging Potential for Nutricosmetic and Cosmeceutical Products. Crit. Rev. Food Sci. Nutr. 2021, 61, 3740–3755. [Google Scholar] [CrossRef] [PubMed]
- Pérez-Sánchez, A.; Barrajón-Catalán, E.; Herranz-López, M.; Micol, V. Nutraceuticals for Skin Care: A Comprehensive Review of Human Clinical Studies. Nutrients 2018, 10, 403. [Google Scholar] [CrossRef] [PubMed]
- Lucius, K. Botanical Medicine and Phytochemicals in Healthy Aging and Longevity—Part 1. Altern. Complement. Ther. 2020, 26, 31–37. [Google Scholar] [CrossRef]
- Shen, C.-Y.; Jiang, J.-G.; Yang, L.; Wang, D.-W.; Zhu, W. Anti-Ageing Active Ingredients from Herbs and Nutraceuticals Used in Traditional Chinese Medicine: Pharmacological Mechanisms and Implications for Drug Discovery. Br. J. Pharmacol. 2017, 174, 1395–1425. [Google Scholar] [CrossRef]
- Williamson, E.M. Synergy and Other Interactions in Phytomedicines. Phytomed. Int. J. Phytother. Phytopharm. 2001, 8, 401–409. [Google Scholar] [CrossRef] [PubMed]
- Choi, G.-E.; Lee, G.-H.; Hyun, K.-Y. Anti-Oxidant and Anti-Pollution Composition Containing the Extract of Nypa Fruticans Wurmb, Saussurea Neoserrata, Codium Fragile and Enteromorpha Compressa. Biomed. Sci. Lett. 2020, 26, 157–163. [Google Scholar] [CrossRef]
- Junio, H.A.; Sy-Cordero, A.A.; Ettefagh, K.A.; Burns, J.T.; Micko, K.T.; Graf, T.N.; Richter, S.J.; Cannon, R.E.; Oberlies, N.H.; Cech, N.B. Synergy-Directed Fractionation of Botanical Medicines: A Case Study with Goldenseal (Hydrastis canadensis). J. Nat. Prod. 2011, 74, 1621–1629. [Google Scholar] [CrossRef] [PubMed]
- Nobile, V.; Schiano, I.; Peral, A.; Giardina, S.; Spartà, E.; Caturla, N. Antioxidant and Reduced Skin-Ageing Effects of a Polyphenol-Enriched Dietary Supplement in Response to Air Pollution: A Randomized, Double-Blind, Placebo-Controlled Study. Food Nutr. Res. 2021, 65. [Google Scholar] [CrossRef] [PubMed]
- Peno-Mazzarino, L.; Radionov, N.; Merino, M.; González, S.; Mullor, J.L.; Jones, J.; Caturla, N. Protective Potential of a Botanical-Based Supplement Ingredient against the Impact of Environmental Pollution on Cutaneous and Cardiopulmonary Systems: Preclinical Study. Curr. Issues Mol. Biol. 2024, 46, 1530–1555. [Google Scholar] [CrossRef] [PubMed]
- Furue, M.; Takahara, M.; Nakahara, T.; Uchi, H. Role of AhR/ARNT System in Skin Homeostasis. Arch. Dermatol. Res. 2014, 306, 769–779. [Google Scholar] [CrossRef]
- Mistry, N. Guidelines for Formulating Anti-Pollution Products. Cosmetics 2017, 4, 57. [Google Scholar] [CrossRef]
- Bertelli, M.; Kiani, A.K.; Paolacci, S.; Manara, E.; Kurti, D.; Dhuli, K.; Bushati, V.; Miertus, J.; Pangallo, D.; Baglivo, M.; et al. Hydroxytyrosol: A Natural Compound with Promising Pharmacological Activities. J. Biotechnol. 2020, 309, 29–33. [Google Scholar] [CrossRef]
- Pojero, F.; Aiello, A.; Gervasi, F.; Caruso, C.; Ligotti, M.E.; Calabrò, A.; Procopio, A.; Candore, G.; Accardi, G.; Allegra, M. Effects of Oleuropein and Hydroxytyrosol on Inflammatory Mediators: Consequences on Inflammaging. Int. J. Mol. Sci. 2022, 24, 380. [Google Scholar] [CrossRef] [PubMed]
- Nediani, C.; Ruzzolini, J.; Romani, A.; Calorini, L. Oleuropein, a Bioactive Compound from Olea europaea L. as a Potential Preventive and Therapeutic Agent in Non-Communicable Diseases. Antioxidants 2019, 8, 578. [Google Scholar] [CrossRef] [PubMed]
- Ancora, C.; Roma, C.; Vettor, M. Evaluation of Cosmetic Efficacy of Oleoeuropein. In Proceedings of the Symposium on the New Frontiers of Dermocosmetology: Efficacy, Stability and Safety, Rome, Italy, 4–6 November 2004. [Google Scholar]
- Kimura, Y.; Sumiyoshi, M. Olive Leaf Extract and Its Main Component Oleuropein Prevent Chronic Ultraviolet B Radiation-Induced Skin Damage and Carcinogenesis in Hairless Mice. J. Nutr. 2009, 139, 2079–2086. [Google Scholar] [CrossRef] [PubMed]
- Ha, J.Y.; Choi, H.K.; Oh, M.J.; Choi, H.-Y.; Park, C.S.; Shin, H.-S. Photo-protective and Anti-melanogenic Effect from Phenolic Compound of Olive Leaf (Olea europaea L. var. Kalamata) Extracts on the Immortalized Human Keratinocytes and B16F1 Melanoma Cells. Food Sci. Biotechnol. 2009, 18, 1193–1198. [Google Scholar]
- Guo, W.; An, Y.; Jiang, L.; Geng, C.; Zhong, L. The Protective Effects of Hydroxytyrosol against UVB-Induced DNA Damage in HaCaT Cells. Phytother. Res. PTR 2010, 24, 352–359. [Google Scholar] [CrossRef] [PubMed]
- Jeon, S.; Choi, M. Anti-Inflammatory and Anti-Aging Effects of Hydroxytyrosol on Human Dermal Fibroblasts (HDFs). Biomed. Dermatol. 2018, 2, 21. [Google Scholar] [CrossRef]
- Avola, R.; Graziano, A.C.E.; Pannuzzo, G.; Bonina, F.; Cardile, V. Hydroxytyrosol from Olive Fruits Prevents Blue-Light-Induced Damage in Human Keratinocytes and Fibroblasts. J. Cell. Physiol. 2019, 234, 9065–9076. [Google Scholar] [CrossRef] [PubMed]
- Korkina, L.G.; Mikhal’chik, E.; Suprun, M.V.; Pastore, S.; Dal Toso, R. Molecular Mechanisms Underlying Wound Healing and Anti-Inflammatory Properties of Naturally Occurring Biotechnologically Produced Phenylpropanoid Glycosides. Cell. Mol. Biol. Noisy Gd. Fr. 2007, 53, 84–91. [Google Scholar]
- Espinosa-González, A.M.; García-Bores, A.M.; del Carmen Benítez-Flores, J.; Sandoval-Pérez, C.E.; del Rosario González-Valle, M.; Céspedes, C.L.; Avila-Acevedo, J.G. Photoprotective effect of verbascoside from Buddleja cordata in SKH-1 mice exposed to acute and chronic UV-B radiation. Bol. Latinoam. Caribe Plant. Med. Aromáticas 2016, 15, 288–300. [Google Scholar]
- Young-Ok, S.; Seung-Ah, L.; Seung-Ah, L.; So-Soon, K.; Yong-Suk, J.; Jae-Chul, C.; Jeong-Chae, L. Acteoside Inhibits Melanogenesis in B16F10 Cells through ERK Activation and Tyrosinase Down-Regulation. J. Pharm. Pharmacol. 2011, 63, 1309–1319. [Google Scholar]
- Vertuani, S.; Beghelli, E.; Scalambra, E.; Malisardi, G.; Copetti, S.; Toso, R.D.; Baldisserotto, A.; Manfredini, S. Activity and Stability Studies of Verbascoside, a Novel Antioxidant, in Dermo-Cosmetic and Pharmaceutical Topical Formulations. Molecules 2011, 16, 7068–7080. [Google Scholar] [CrossRef] [PubMed]
- Chiou, W.F.; Lin, L.C.; Chen, C.F. Acteoside Protects Endothelial Cells against Free Radical-Induced Oxidative Stress†. J. Pharm. Pharmacol. 2004, 56, 743–748. [Google Scholar] [CrossRef] [PubMed]
- Nieto, G.; Ros, G.; Castillo, J. Antioxidant and Antimicrobial Properties of Rosemary (Rosmarinus officinalis L.): A Review. Medicines 2018, 5, 98. [Google Scholar] [CrossRef] [PubMed]
- Andrade, J.M.; Faustino, C.; Garcia, C.; Ladeiras, D.; Reis, C.P.; Rijo, P. Rosmarinus officinalis L.: An Update Review of Its Phytochemistry and Biological Activity. Future Sci. OA 2018, 4, FSO283. [Google Scholar] [CrossRef] [PubMed]
- Li Pomi, F.; Papa, V.; Borgia, F.; Vaccaro, M.; Allegra, A.; Cicero, N.; Gangemi, S. Rosmarinus Officinalis and Skin: Antioxidant Activity and Possible Therapeutical Role in Cutaneous Diseases. Antioxidants 2023, 12, 680. [Google Scholar] [CrossRef] [PubMed]
- Loussouarn, M.; Krieger-Liszkay, A.; Svilar, L.; Bily, A.; Birtić, S.; Havaux, M. Carnosic Acid and Carnosol, Two Major Antioxidants of Rosemary, Act through Different Mechanisms. Plant Physiol. 2017, 175, 1381–1394. [Google Scholar] [CrossRef]
- Hoskin, R.; Pambianchi, E.; Pecorelli, A.; Grace, M.; Therrien, J.-P.; Valacchi, G.; Lila, M.A. Novel Spray Dried Algae-Rosemary Particles Attenuate Pollution-Induced Skin Damage. Molecules 2021, 26, 3781. [Google Scholar] [CrossRef]
- He, X.; Bai, Y.; Zhao, Z.; Wang, X.; Fang, J.; Huang, L.; Zeng, M.; Zhang, Q.; Zhang, Y.; Zheng, X. Local and Traditional Uses, Phytochemistry, and Pharmacology of Sophora japonica L.: A Review. J. Ethnopharmacol. 2016, 187, 160–182. [Google Scholar] [CrossRef] [PubMed]
- Jin, X.; Su, R.; Li, R.; Song, L.; Chen, M.; Cheng, L.; Li, Z. Amelioration of Particulate Matter-Induced Oxidative Damage by Vitamin c and Quercetin in Human Bronchial Epithelial Cells. Chemosphere 2016, 144, 459–466. [Google Scholar] [CrossRef]
- Kim, M.; Son, D.; Shin, S.; Park, D.; Byun, S.; Jung, E. Protective Effects of Camellia Japonica Flower Extract against Urban Air Pollutants. BMC Complement. Altern. Med. 2019, 19, 30. [Google Scholar] [CrossRef] [PubMed]
- Durga, M.; Vijayakumar, M.; Priya, K.; Vidhya Kanagarajan, S.; Banu, B.B.; Abraham, V.S.M.; Devasena, T.; Abdelaziz, M.A.; Mohideen, A.P.; Bahakim, N.O.; et al. Effects of Quercetin on Ultrafine Petrol Exhaust Nanoparticles Induced DNA Damage, Oxidative Stress and Inflammation in Different Sections of Rat Brain. J. King Saud Univ. Sci. 2022, 34, 101813. [Google Scholar] [CrossRef]
- Caturla, N.C.; Clement, A.P. Composición de Extractos Vegetales con Flavonoides para Paliar los Múltiples Efectos de la Contaminación del Aire sobre la Piel. WO2019211501A1, 7 November 2019. [Google Scholar]
- Benzie, I.F.; Strain, J.J. The Ferric Reducing Ability of Plasma (FRAP) as a Measure of “Antioxidant Power”: The FRAP Assay. Anal. Biochem. 1996, 239, 70–76. [Google Scholar] [CrossRef] [PubMed]
- Voegeli, R.; Cherel, M.; Schoop, R.; Rawlings, A.V. A Comprehensive Comparison of Facial Skin Hydration Based on Capacitance and Conductance Measurements in Chinese Women. Int. J. Cosmet. Sci. 2022, 44, 703–718. [Google Scholar] [CrossRef] [PubMed]
- Schweiger, D.; Baufeld, C.; Drescher, P.; Oltrogge, B.; Höpfner, S.; Mess, A.; Lüttke, J.; Rippke, F.; Filbry, A.; Max, H. Efficacy of a New Tonic Containing Urea, Lactate, Polidocanol, and Glycyrrhiza Inflata Root Extract in the Treatment of a Dry, Itchy, and Subclinically Inflamed Scalp. Skin Pharmacol. Physiol. 2013, 26, 108–118. [Google Scholar] [CrossRef] [PubMed]
- Seol, J.E.; Cho, G.J.; Jang, S.H.; Ahn, S.W.; Hong, S.M.; Park, S.H.; Kim, H. Effect of Amount of Daily Water Intake and Use of Moisturizer on Skin Barrier Function in Healthy Female Participants. Ann. Dermatol. 2024, 36, 145–150. [Google Scholar] [CrossRef] [PubMed]
- Robic, J.; Lata, W.; Nkengne, A.; Bigouret, A.; Vie, K. The Impact of Air Pollution on the Facial Skin of Caucasian Women Using Real-Life Pollutant Exposure Measurements. Skin Res. Technol. 2024, 30, e13669. [Google Scholar] [CrossRef] [PubMed]
- da Cruz, L.B.; Girasol, C.E.; Coltro, P.S.; de Jesus Guirro, R.R.; Bachmann, L. Optical Properties of Human Skin Phototypes and Their Correlation with Individual Angle Typology. Photobiomodulation Photomed. Laser Surg. 2023, 41, 175–181. [Google Scholar] [CrossRef] [PubMed]
- Nobile, V.; Spartà, E.; Zanoletti, V.; Sandolo, F.; Cestone, E. Efficacy Evaluation of a Cosmetic Product for the Periocular Area in Caucasian Women Aged Over 40 Years Old. Clin. Cosmet. Investig. Dermatol. 2022, 15, 2933–2941. [Google Scholar] [CrossRef] [PubMed]
- Constantin, M.-M.; Poenaru, E.; Poenaru, C.; Constantin, T. Skin Hydration Assessment through Modern Non-Invasive Bioengineering Technologies. Mædica 2014, 9, 33–38. [Google Scholar] [PubMed]
- Jun, M.S.; Kwack, M.H.; Kim, M.K.; Kim, J.C.; Sung, Y.K. Particulate Matters Induce Apoptosis in Human Hair Follicular Keratinocytes. Ann. Dermatol. 2020, 32, 388–394. [Google Scholar] [CrossRef] [PubMed]
- Trüeb, R.M. Oxidative Stress and Its Impact on Skin, Scalp and Hair. Int. J. Cosmet. Sci. 2021, 43, S9–S13. [Google Scholar] [CrossRef] [PubMed]
- Trüeb, R.M.; Henry, J.P.; Davis, M.G.; Schwartz, J.R. Scalp Condition Impacts Hair Growth and Retention via Oxidative Stress. Int. J. Trichol. 2018, 10, 262–270. [Google Scholar] [CrossRef] [PubMed]
- Dijkhoff, I.M.; Drasler, B.; Karakocak, B.B.; Petri-Fink, A.; Valacchi, G.; Eeman, M.; Rothen-Rutishauser, B. Impact of Airborne Particulate Matter on Skin: A Systematic Review from Epidemiology to in Vitro Studies. Part. Fibre Toxicol. 2020, 17, 35. [Google Scholar] [CrossRef] [PubMed]
- Davis, M.G.; Piliang, M.P.; Bergfeld, W.F.; Caterino, T.L.; Fisher, B.K.; Sacha, J.P.; Carr, G.J.; Moulton, L.T.; Whittenbarger, D.J.; Schwartz, J.R. Scalp Application of Antioxidants Improves Scalp Condition and Reduces Hair Shedding in a 24-Week Randomized, Double-Blind, Placebo-Controlled Clinical Trial. Int. J. Cosmet. Sci. 2021, 43, S14–S25. [Google Scholar] [CrossRef] [PubMed]
- Boo, Y.C. Can Plant Phenolic Compounds Protect the Skin from Airborne Particulate Matter? Antioxidants 2019, 8, 379. [Google Scholar] [CrossRef] [PubMed]
- Ahn, H.I.; Jang, H.-J.; Kwon, O.-K.; Kim, J.-H.; Oh, J.-H.; Kim, S.-H.; Oh, S.-R.; Han, S.-B.; Ahn, K.-S.; Park, J.-W. Quercetin Attenuates the Production of Pro-Inflammatory Cytokines in H292 Human Lung Epithelial Cells Infected with Pseudomonas Aeruginosa by Modulating ExoS Production. J. Microbiol. Biotechnol. 2023, 33, 430–440. [Google Scholar] [CrossRef] [PubMed]
- Visioli, F.; Galli, C.; Plasmati, E.; Viappiani, S.; Hernandez, A.; Colombo, C.; Sala, A. Olive Phenol Hydroxytyrosol Prevents Passive Smoking-Induced Oxidative Stress. Circulation 2000, 102, 2169–2171. [Google Scholar] [CrossRef] [PubMed]
- Xiao, Y.; Ren, Q.; Wu, L. The Pharmacokinetic Property and Pharmacological Activity of Acteoside: A Review. Biomed. Pharmacother. 2022, 153, 113296. [Google Scholar] [CrossRef]
- Beken, B.; Serttas, R.; Yazicioglu, M.; Turkekul, K.; Erdogan, S. Quercetin Improves Inflammation, Oxidative Stress, and Impaired Wound Healing in Atopic Dermatitis Model of Human Keratinocytes. Pediatr. Allergy Immunol. Pulmonol. 2020, 33, 69–79. [Google Scholar] [CrossRef] [PubMed]
- Rembiesa, J.; Ruzgas, T.; Engblom, J.; Holefors, A. The Impact of Pollution on Skin and Proper Efficacy Testing for Anti-Pollution Claims. Cosmetics 2018, 5, 4. [Google Scholar] [CrossRef]
- Thiele, J.J. Oxidative Targets in the Stratum Corneum. A New Basis for Antioxidative Strategies. Skin Pharmacol. Appl. Skin Physiol. 2001, 14, 87–91. [Google Scholar] [CrossRef] [PubMed]
- Lee, C.-W.; Lin, Z.-C.; Hu, S.C.-S.; Chiang, Y.-C.; Hsu, L.-F.; Lin, Y.-C.; Lee, I.-T.; Tsai, M.-H.; Fang, J.-Y. Urban Particulate Matter Down-Regulates Filaggrin via COX2 Expression/PGE2 Production Leading to Skin Barrier Dysfunction. Sci. Rep. 2016, 6, 27995. [Google Scholar] [CrossRef]
- Armengot-Carbo, M.; Hernández-Martín, Á.; Torrelo, A. The Role of Filaggrin in the Skin Barrier and Disease Development. Actas Dermo-Sifiliográficas Engl. Ed. 2015, 106, 86–95. [Google Scholar] [CrossRef] [PubMed]
- Moyal, D.; Seite, S. Effect of Air Pollution on Sebum Rate and Acne: How to Manage Acneic Skin in a Polluted Environment. SKIN J. Cutan. Med. 2017, 1, s47. [Google Scholar] [CrossRef]
- Passeron, T.; Krutmann, J.; Andersen, M.; Katta, R.; Zouboulis, C. Clinical and Biological Impact of the Exposome on the Skin. J. Eur. Acad. Dermatol. Venereol. 2020, 34, 4–25. [Google Scholar] [CrossRef]
- Ribet, V.; Nobile, V.; Rossi, A.B. In Situ Antioxidant Activity of a Dermo-cosmetic Product: A Randomized Controlled Clinical Study. Exp. Dermatol. 2019, 28, 1219–1226. [Google Scholar] [CrossRef] [PubMed]
- Gao, W.; Zheng, S.; Hwang, E.; Yi, T.-H.; Wang, Y.-S. Effects of Phenylethanol Glycosides from Orobanche Cernua Loefling on UVB-Induced Skin Photodamage: A Comparative Study. Photochem. Photobiol. Sci. Off. J. Eur. Photochem. Assoc. Eur. Soc. Photobiol. 2021, 20, 599–614. [Google Scholar] [CrossRef] [PubMed]
- Si, N.; Kanazawa, H.; Okuyama, K.; Imada, K.; Wang, H.; Yang, J.; Zhao, H.; Bian, B.; Ito, A.; Sato, T. Involvement of Catechols in Acteoside in the Activation of Promatrix Metalloproteinase-2 and Membrane Type-1-Matrix Metalloproteinase Expression via a Phosphatidylinositol-3-Kinase Pathway in Human Dermal Fibroblasts. Biol. Pharm. Bull. 2018, 41, 1530–1536. [Google Scholar] [CrossRef] [PubMed]
- Park, M.; Han, J.; Lee, C.S.; Heung Soo, B.; Lim, K.-M.; Ha, H. Carnosic Acid, a Phenolic Diterpene from Rosemary, Prevents UV-Induced Expression of Matrix Metalloproteinases in Human Skin Fibroblasts and Keratinocytes. Exp. Dermatol. 2013, 22, 336–341. [Google Scholar] [CrossRef] [PubMed]
- Jnawali, H.N.; Lee, E.; Shin, A.; Park, Y.G.; Kim, Y. Effect of Quercetin in the UV-Irradiated Human Keratinocyte HaCaT Cells and A Model of Its Binding To P38 MAPK. Bull. Korean Chem. Soc. 2014, 35, 2787–2790. [Google Scholar] [CrossRef]
- Elkattan, A.; Gohar, A.; Amer, M.; Naeem, Z.; Ashour, A.; Shimizu, K. Melanin Synthesis Inhibitors from Olea Europeae. Rec. Nat. Prod. 2019, 14, 139–143. [Google Scholar] [CrossRef]
- D’Angelo, S.; Ingrosso, D.; Migliardi, V.; Sorrentino, A.; Donnarumma, G.; Baroni, A.; Masella, L.; Antonietta Tufano, M.; Zappia, M.; Galletti, P. Hydroxytyrosol, a Natural Antioxidant from Olive Oil, Prevents Protein Damage Induced by Long-Wave Ultraviolet Radiation in Melanoma Cells. Free Radic. Biol. Med. 2005, 38, 908–919. [Google Scholar] [CrossRef] [PubMed]
- Fan, M.; Zhang, G.; Hu, X.; Xu, X.; Gong, D. Quercetin as a Tyrosinase Inhibitor: Inhibitory Activity, Conformational Change and Mechanism. Food Res. Int. 2017, 100, 226–233. [Google Scholar] [CrossRef] [PubMed]
- Tosti, A.; Schwartz, J.R. Role of Scalp Health in Achieving Optimal Hair Growth and Retention. Int. J. Cosmet. Sci. 2021, 43, S1–S8. [Google Scholar] [CrossRef] [PubMed]
- Beoy, L.A.; Woei, W.J.; Hay, Y.K. Effects of Tocotrienol Supplementation on Hair Growth in Human Volunteers. Trop. Life Sci. Res. 2010, 21, 91–99. [Google Scholar] [PubMed]
- Hoffmann, R.; Happle, R. Does Interleukin-1 Induce Hair Loss? Dermatology 1995, 191, 273–275. [Google Scholar] [CrossRef] [PubMed]
- Muhammad, S.W.; Pirzado, M.S. To Evaluate the Role of Interleukin-6 in Alopecia Areata. J. Pak. Med. Assoc. 2024, 74, 930–933. [Google Scholar] [CrossRef] [PubMed]
- Yu, M.; Kissling, S.; Freyschmidt-Paul, P.; Hoffmann, R.; Shapiro, J.; McElwee, K.J. Interleukin-6 Cytokine Family Member Oncostatin M Is a Hair-Follicle-Expressed Factor with Hair Growth Inhibitory Properties. Exp. Dermatol. 2008, 17, 12–19. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).