Abstract
The cosmetics industry has experienced rapid growth, driven by consumer demand for products in line with modern lifestyles and technological advances, with greater awareness of the impacts on health and the ecosystem. This review explores the potential use of antibacterial compounds derived from food waste as sustainable alternatives to synthetic preservatives in cosmetic products. Waste obtained from food, including fruit peels, seeds, and plant remnants, is rich in natural bioactives, including polyphenolic compounds and essential oils that exhibit antimicrobial, antioxidant, anti-inflammatory, and soothing features. The integration of these natural ingredients not only improves the shelf-life and safety of cosmetics but also promotes environmentally friendly practices. We discuss the sources and antimicrobial efficacy of these compounds, along with recent technological innovations. This sustainable approach responds to consumer preferences for natural ingredients, reduces food waste, and supports environmental sustainability, ultimately increasing the value and attractiveness of cosmetic products.
1. Introduction
Over the past years, the interest in sustainability and the circular economy has led to a growing attention towards the use of food waste as a valuable asset [1]. Food loss and waste are globally recognized as a big problem due to their high socioeconomic costs [2]. The amount of global waste recorded a marker increase during the COVID-19 pandemic, during which restaurants were forced to remain closed with food seized inside, and the demand for take-out food surged [3].
If a huge worsening of the situation of food wastage was caused by COVID-19, the cosmetics market is one of the sectors that has not been negatively affected by the pandemic, recording an increase in the use and, therefore, in the purchase of cosmetic products [4]. More than makeup products, skin care products have received increasing demand from consumers, who are thus more aware of the importance of the health of their skin, such as that of the face, which has been severely stressed by the massive and unwanted use of masks for individual protection [5]. The cosmetics market tends to grow year by year, so much so that it can be considered the third fastest-growing market. In fact, the value of the worldwide beauty and self-care industry was USD 565 billion in 2022, and researchers predict that it will reach USD 758 billion by 2025 [6].
Recent scientific research has highlighted a potential link between food waste and the cosmetics industry. A notable development in this field is the extraction of antibacterial compounds from food discards, which shows promise for use in cosmetics both as preservatives and as bioactive molecules to treat skin infections, such as cutaneous inflammation, atopic eczema, atopic dermatitis, and acne [7]. Rich in natural bioactives, food waste is an untapped resource that could provide eco-friendly and sustainable alternatives to conventional chemical preservatives in cosmetic products. Furthermore, aside from their cosmetic uses, these discards show great promise for integration into various other sectors, including pharmaceutical, food, textile, farming, and biofuel [8] (Figure 1). The growing concern for human and environmental health related to the use of synthetic preservatives in cosmetics has driven research toward safer and more natural ingredients. Food wastes, such as fruit peels, seeds, and vegetable residues, contain numerous phenolic compounds, flavonoids, and organic acids, known for their antimicrobial properties [9,10,11,12,13,14,15,16,17]. The extraction and use of these compounds not only reduce waste but also improve the sustainability of the entire production cycle. In particular, the cosmetics industry is increasingly recognizing the value of fruit waste, turning it into effective and sustainable ingredients for beauty products, such as apple peels, avocado seeds, and grape peels and seeds that are used for their moisturizing, antioxidant, and anti-aging properties or for their high content of nutrient-rich oils.
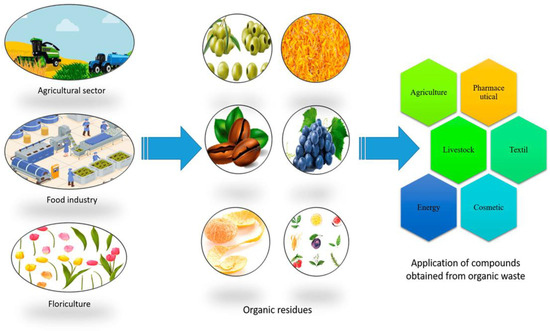
Figure 1.
Applications of bioactives from organic waste (Reproduced by [8]).
2. State-of-the-Art Preservation Strategies
The presence of water and both organic and inorganic compounds in cosmetics, pharmaceuticals, and food products makes them susceptible to the growth and proliferation of microorganisms under certain physicochemical conditions. For this reason, these products need to be effectively and adequately protected against the growth of microorganisms [18]. Microbial contamination can arise during manufacturing (primary contamination) and/or through use and consumption (secondary contamination) [19,20]. Figure 2 provides an overview of the sources, effects, and preventive measures for both primary and secondary contaminations. Furthermore, every possible source of contamination needs to be found and monitored. Cosmetics producers use a variety of tactics to prevent microbial contamination without sacrificing the qualities of their products. Initially, they perform two conservation phases: primary and secondary ones. The primary conservation approach is implemented during the manufacturing process in compliance with good manufacturing procedures (GMPs). GMPs must be followed in full while producing cosmetics, which needs to happen in an absolutely aseptic environment to avoid microbiological contamination [21]. An aseptic environment refers to a space in which microbial contamination is minimized or eliminated entirely, usually through the use of air filtration systems (such as HEPA filters), sterile clothing, and rigorous sanitization procedures [22]. The risk of contamination can be decreased with the use of interventions, including treating wastewater, controlling the microbiological quality of raw materials, disinfecting machinery, and ensuring proper training for personnel [19,23]. The majority of regulatory organizations worldwide have approved and accepted the ISO 22716:2007 certification [24] for cosmetics, especially since the 2008 meeting of the International Cooperation on Cosmetic Regulation (United States, European Union, Canada, and Japan) [21]. The standard ISO 22716:2007 outlines guidelines for the manufacturing, quality assurance, preservation, and shipping of cosmetics. GMPs correspond to that part of quality assurance, which aims to ensure that products are manufactured in such a way as to consistently be of adequate quality for their intended use. They, therefore, have both production and quality control as their objectives [25]. Post-production preservation methods, known as secondary preservation, use chemical, physical, or physicochemical techniques to provide effective protection. Physical preservation involves using primary packaging that acts as a barrier to prevent microbial contamination. Packaging serves two protective functions: (1) preventing contamination during use and (2) stopping the accumulation of contamination during distribution [26]. The design and properties of the primary packaging have a major impact on the likelihood of microbial contamination. Among the properties, the form of the container (such as boxes, jars, bottles, etc.) and the materials used (such as polymers, glass, etc.) are determining factors [27,28].
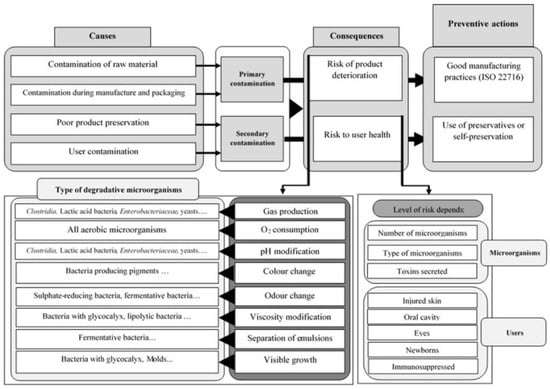
Figure 2.
Sources, impacts, and prevention strategies for cosmetics contamination. (Reproduced by [21]).
The three main physicochemical secondary preservation procedures are pH level regulation, emulsion type management, and water activity monitoring [21]. Water is typically the primary ingredient in cosmetics, but it is also a perfect medium for microbes to proliferate. Some chemicals have the ability to reduce water activity. Salts, polyols (including sorbitol, glycerol, and ethoxydiglycol), protein hydrolysates, amino acids, hydrocolloids (like guar gum and xanthan gum), sodium polyacrylate, glyceryl polyacrylate gel, and sodium chloride are a few examples of these materials. The selection of these substances depends on their properties, potential toxicity, and the nature of the cosmetics [18,23,28,29]. When comparing water-in-oil (W/O) emulsions to oil-in-water (O/W) emulsions, the latter is generally less susceptible to microbial attacks [23]. This effectiveness is primarily due to the continuous oil phase in W/O emulsions, which limits the availability of water necessary for microbial growth. The reduced water activity in the oil phase creates a less favorable environment for microorganisms, thereby reducing the chances of contamination and proliferation [30]. Moreover, the emulsion particle size can significantly enhance the efficiency of cosmetic products containing antimicrobial agents. Smaller droplet sizes, such as those found in nanoemulsions, increase the ratio of the surface area to volume, thus improving the interaction between the antimicrobial agents and microorganisms [31]. This enhanced interaction ensures a more uniform distribution of active ingredients and prolongs the stability of the product. Consequently, nanoemulsions can provide better antimicrobial activity and improve the overall preservation of cosmetic products [31,32]. However, the nature of the oil phase, particularly the type, quantity, and structure, also affects the antibacterial activity [33,34,35]. Finally, maintaining pH balance is essential to lowering bacterial infiltration. The ideal pH range for controlling microorganism growth in cosmetics is between 5 and 8. pH values beyond this interval result in unsafe settings, which increase the rate of growth [23,36]. The antibacterial activity of cationic hair conditioners is partly attributed to their acidic pH (around pH = 4) [18]. Other formulations with an acidic pH, such as antiperspirant treatments with aluminum compounds and salicylic acid (pH between 3.5 and 4.5), can also prevent the growth of bacteria [37]. Secondary chemical preservation methods can be categorized into synthetic and natural preservatives. The EU Cosmetics Directive defines synthetic preservatives as substances designed to primarily or exclusively inhibit the growth of microorganisms in cosmetic products. The selection of the substances for use in cosmetics must adhere to Annex V of the Cosmetics Regulation (Article 14 of the Cosmetics Regulation) [25]. Usually, three criteria define the choice of preservatives (in addition to regulatory requirements): (1) excellent antimicrobial effectiveness, (2) non-toxicity, and (3) compatibility with other components in the cosmetic preparation [38,39]. Presently, the cosmetics industry faces a significant shortage of less toxic preservatives, thanks to regulations frequently revising the permissible usage limits. As a result, there has been a growing focus on discovering new preservatives that are effective and safe. Future alternatives are expected to offer a broad spectrum against microorganisms while possessing a better safety profile. Strong antimicrobial compounds that are low in toxicity, including plant extracts, are viewed as desirable prospective substitutes [21].
3. Food Waste Natural Compounds with Antimicrobial Properties
Compounds derived from food waste are being used by numerous natural cosmetics companies. More and more companies are focused on producing environmentally friendly cosmetics using materials that would otherwise be considered waste, such as seeds, peels, pomace, cortexes, leaves, juicing industry by-products, and stones. Food waste from plants derived from industrial procedures can serve as a valuable source for the cosmetics industry, offering biodegradable, skin-compatible, and environmentally sustainable ingredients. These ingredients include peptides, carbohydrates, triglycerides, fibers, phytochemicals, nutrients, alkaloids, terpenoids, polypeptides, polyphenols, and polyacetylenes that can serve functional or technical purposes, such as oxidative stress reduction, hydration, nutrition, preservation, and maintenance of consistency, as well as conferring anti-aging and volume-enhancing benefits in addition to antimicrobial activity [40,41,42,43]. Furthermore, numerous plant-based derivatives utilized in the food sector are classified as “GRAS”—Generally Recognized as Safe—resulting in their widespread application in cosmetics [43].
The European Regulation [25] allowed the use of only those preservatives included in Annex V of the 7th Amendment of the Cosmetics Directive [44]. Some of the common preservatives listed in Annex V include benzoic and salicylic acid, with their salts, paraben compounds, formaldehyde, hydroxymethylglycine sodium salt, and triclosan (subject to limitations and with reduced use in recent years). However, many other natural substances that have antibacterial properties are not included in this list; among them, essential oils and extracts are examples [21]. Indeed, these substances are not listed in Annex V as preservatives because they are used for their therapeutic effects on the skin, but they may unintentionally help to preserve the formulation. As a result, it is possible to create cosmetics with better dermo-cosmetic qualities and reduce or even eliminate the need for traditional chemical preservatives by carefully selecting these ingredients [44]. Some alternative preservatives are mentioned below.
3.1. Plant-Based Extracts
Stems, flowers, roots, and peels are commonly used to obtain extracts useful to prevent microbial growth, which can cause primary and secondary skin diseases [45,46,47,48] (Table 1). Due to their antibacterial features, these plant extracts could also be used as preservatives [49]. Cosmetics can easily be contaminated by bacteria and fungi during use, with the most common microbes being Staphylococcus aureus, Escherichia coli, and Pseudomonas aeruginosa for bacteria and Candida albicans for fungi [21,43,45].

Table 1.
Antimicrobial potential found in some plant-based extracts from wastes.
Peels and seeds of pomegranates (Punica granatum L.), accounting for approximately 54% of the fruit’s bulk, are usually thrown away as waste once the juice is extracted. For example, since 90% of the pomegranates farmed in the United States are produced in California, about 118,000 tons of unused peels and seeds were produced in this country alone up until 2020 [50]. In fact, these pomegranate wastes represent 43% of the weight and 11% of the weight of the fruit, respectively [64]. The trash that remains after pomegranate juice is extracted includes a number of bioactive and nutritional elements, such as fatty acids, hydrolyzable tannins (including ellagic acid and punicalagin), and flavonoids (like anthocyanins). These elements, which are included in organic pomegranate waste, hold significant potential for valuable applications in cosmetics and improving skin health [50]. Pomegranate peel extract is rich in phenolic compounds (PCs) [65]. Specifically, punicalagin, is abundantly present in the fruit and constitutes its primary ellagitannin [64]. Pomegranate peel contains tannins (punicalagin) and polyphenols (ellagic acid) that promote skin health through the inhibition of tyrosinase and provide anti-inflammatory and antifungal benefits. Through the chelation of carbohydrates and nutrients, these compounds exhibit promising antibacterial activity by making these components unavailable to microorganisms [9,50]. Moreover, some researchers used pomegranate peel powder as a natural antioxidant, replacing synthetic compounds, in an effort to enhance lipid stability against oxidation and preserve the product [51]. Because pomegranate peel contains phenolic components, the peel extract not only exhibited higher sensory ratings but also decreased aerobic bacterial counts [51]. Furthermore, a nanofiber covering for rat excision wounds was successfully made with pomegranate peel powder, honey, and bee venom. This implies that adding honey and pomegranate peel to the antibacterial action against E. coli will have a synergistic impact [66]. In a recent study, Gigliobianco M.R. et al. [10] examined the peel extract of various pomegranate varieties grown in the Marche region, such as “Wonderful”, “Mollar de Elche”, “Parfianka”, and the less studied “G1”. The study analyzed the phenolic compounds, antioxidant capacity, and antimicrobial effects of pomegranate extracts for their use in the cosmetics field. An ultrasonic bath was used for the extraction of phenols from peels. Three extraction cycles were conducted for each sample, employing specific solvents to maximize the recovery of polyphenols. The extracted polyphenols were identified and quantified using ultra-performance liquid chromatography coupled with mass spectrometry (UPLC-ESI-MS/MS). The main phenolic compounds identified included isomers of punicalagin, punicalin, gallic acid, ellagic acid, gallocatechin, and anthocyanins. Results indicated that the peel extracts of the “Mollar de Elche” variety contained the highest concentrations of punicalagin A and B. The total phenolic content (TPC) and antioxidant capacity (AC) of the extracts were detected using the Folin–Ciocalteu colorimetric and spectrophotometric methods, respectively. Peel extracts from the “Mollar de Elche” and “Wonderful” varieties exhibited the highest TPC and AC contents, attributed to the significant presence of punicalagin. The antimicrobial activity of the extracts was assessed, considering E. coli, S. aureus, P. aeruginosa, and various species of Candida. Peel extracts from the “Wonderful” and “G1” varieties showed efficacy against E. coli, showing an average inhibition zone of 12–15 mm. Additionally, the “Wonderful” peel extract reported the best effectiveness against S. aureus, with an inhibition zone diameter of 3 mm via disk diffusion. The “G1” peel extract was tested using a turbidimetric test, demonstrating excellent results against S. aureus and P. aeruginosa. A two-fold dilution of the extract was found to be effective in reducing 97% of the bacteria. All extracts exhibited promising effects against Candida, with inhibition zones ranging from 10 to 16 mm, depending on the concentration and species. The cytocompatibility of the extracts was investigated on HaCaT keratinocyte cells using concentrations ranging from 0.15 to 5.00 mg/mL. No toxic effects were reported for the concentrations used, indicating potential safe use in cosmetic ingredients. The results of this research endorse the use of pomegranate by-products, specifically peels, as sustainable sources of natural antioxidants and antimicrobials for the cosmetic industry. This approach contributes to the valorization of agri-food waste, promoting a circular economy and reducing reliance on synthetic preservatives [10]. Pomegranate extract has another important characteristic, which is the inhibition of the growth of several dermatophyte fungi, including Trichophyton rubrum, Trichophyton mentagrophytes, Microsporum canis, and Microsporum gypseum. These fungi are known to cause dermatophytosis infections of the skin and its appendages. The functional component, punicalagin, plays a key role in preventing dermatophytosis [67]. Additionally, pomegranate peel has demonstrated medicinal and antiviral properties. In a study, pomegranate peel extract was applied to porcine skin ex vivo to evaluate its anti-inflammatory effects. The findings indicated that punicalagin, a constituent of pomegranate peel, permeated the skin and subsequently suppressed the activity of COX-2, an inflammatory enzyme [11]. Furthermore, the same research group formulated a hydrogel incorporating pomegranate peel extract and zinc sulfate as a topical intervention for Herpes simplex virus infection. This hydrogel exhibited both antiviral and anti-inflammatory properties, with punicalagin permeating areas of the skin that are predisposed to infection. Overall, pomegranate extract acts as an antibacterial agent while enhancing the healing effects to improve the strength of a cosmetic product.
Karkadè (Hibiscus sabdariffa L.) is a species belonging to the Malvaceae family that typically thrives in tropical and subtropical regions globally. The leaves, calyxes, seeds, and roots of Hibiscus sabdariffa L. (HsL) are often incorporated into local dishes and traditional therapies worldwide. Calyxes are primarily used to prepare drinks, confections, liqueurs, and baked goods. Some items are manufactured on an industrial scale, while others are crafted using traditional methods on a smaller scale using artisanal practices. In certain production processes, both traditional and industrial, the decoction of dried calyxes for juice extraction results in significant waste generation, posing economic and environmental concerns for producers [52]. However, the polyphenols and other substances contained in the dry calyxes are partially extracted into the drink by the decoction because these decoction residues can retain an appreciable quantity of polyphenols and other useful substances [53]. There are limited published data on the use of HsL residues, such as decocting calyxes to extract polyphenols and dietary fibers from waste [53]. The calyx of this plant, for example, contains several active molecules belonging to different classes of compounds, such as polyphenols, flavonoids, tocopherols, and organic acids (including malate, oxalate, and shikimate). The specific contents of these mixtures can vary based on the extraction solvent employed. Studies have demonstrated that the solvent used to obtain hibiscus extracts possesses a crucial role in defining its antimicrobial effectiveness against E. coli strains [12]. For instance, aqueous hibiscus extracts exhibited moderate antimicrobial activity, creating an inhibition zone with a diameter of 18.33 mm. Notably, these extracts were most effective against L. monocytogenes, showing an inhibition zone of 21 mm. Conversely, methanol extracts of hibiscus reported an inhibition zone of 17.12 mm. Furthermore, hibiscus extracts on liquid media showed high efficacy against multiple bacteria and yeasts belonging to the genera Aspergillus, Fusarium, and Penicillium, according to MIC and MBC values performed [12]. Hibiscus extracts were also tested for their antibacterial efficacy against nine bacterial strains that are commonly linked to foodborne illnesses and human infections. These strains include Salmonella typhimurium, Enterococcus faecalis, Vibrio parahaemolyticus, Listeria monocytogenes, Escherichia coli, and Staphylococcus aureus. Additionally, four yeast strains, Candida albicans, C. tropicalis, C. kefyr, and C. parapsilosis, were also tested. The results indicated a higher effectiveness of these extracts against bacteria compared to yeasts. Using liquid cultures, it was found that water-based extracts had the lowest MIC, while methanol-based extracts showed the lowest MC, with values of 9.375 mg/mL and within the range of 18.75–37.5 mg/mL, respectively [12]. Furthermore, hibiscus extracts have been identified for their tonic, calming, softening, and soothing properties [68], which are ideal characteristics for a cosmetic formulation. Based on this, the scientific community should be more interested in studying the recovery of these substances from the calyx, which, following the juice extraction process, still contain many useful substances and could perform antimicrobial activities comparable or similar to that of the extracts on calyx that have not undergone the decoction process.
Saffron (Crocus sativus L.) is a geophyte plant that blooms in the autumn and belongs to the Iridaceae family. It is renowned for its stigmas, the most valued part of the plant, which contain a wealth of bioactive substances and are utilized in culinary applications, preservation of preparations, and as a raw material for health and cosmetic products [13,56]. Monoterpenoids, phytosterols, phenolic acids, flavonoids, terpenoids, amino acids, minerals, proteins, carbohydrates, and gums are all abundant in this portion of the plant [69]. Crocetin and crocin are the key bioactive molecules known for their numerous therapeutic benefits, primarily attributed to their strong antioxidative effects [56]. However, research on the petals that constitute the primary by-product of spice manufacturing is limited [13], with only 10% of the plant material used and the rest thrown away [57]. In accordance with circular economy concepts, finding effective ways to use saffron flower waste, particularly petals, that may be rich in active components like flavonoids, crocin, anthocyanins, and lutein diesters is crucial [57]. Some researchers proved the antimicrobial efficacy of extracts obtained from saffron petals against both fungi and bacteria, as well as a pronounced antioxidant effect [55,57]. Among them, Belyagoubi et al. [59] used seven bacterial strains and two Candida strains to investigate the antimicrobial properties of saffron stigma and flower extracts. Data recorded from B. subtilis and M. luteus reported flowers’ MIC values significantly lower than stigmas’ MIC values (781.25 and 6250 μg/mL for flowers and 1406.25 and 22,500 μg/mL for stigmas). Moreover, the MBC of the flower extract of B. subtilis and B. cereus was at least twice that recorded against M. luteus (MBC 100,000 μg/mL). Concerning stigma extracts, previous results already reported the resistance of M. luteus and B. subtilis to this treatment not exceeding values of 45,000 μg/mL for MBC values. In another study conducted by Wali et al., three extracts of saffron petals, obtained using solvents having varying polarity, reported promising antimicrobial efficacy against P. aeruginosa and S. aureus [70].
Garlic (Allium sativum L.) belongs to the Amaryllidaceae family. Originating in Asia, it is extensively cultivated in regions such as Egypt, Mexico, China, and Europe [71]. Historically, garlic has been used for both cooking and therapeutic uses due to its antioxidant and antimicrobial properties [72]. Gabriel and colleagues investigated the antimicrobial qualities of ultrasonicated garlic extracts, demonstrating that it was effective against various bacteria (namely S. aureus sub. aureus, S. mutans, P. gingivalis, and E. coli). The presence of amino acids, carboxylic groups, proteins, phenolic compounds, and organosulfur compounds in the examined extracts, according to the researchers, may be responsible for these characteristics [73]. Furthermore, it has been previously reported that water or toluene garlic extracts act well against P. aeruginosa or Shigela flexnerii bacteria [74,75] Variations in the composition of phytochemicals in garlic powder, juice, or extracts obtained using various extractive procedures were noted in the literature review conducted by Bhatwalkar et al. [76]. According to Subramaniam et al., mouthwash formulations containing garlic extract have been found to successfully eradicate S. mutans bacteria in the oral cavity [77]. Chlorhexidine, one of the ingredients in these mouthwashes, has been shown to be effective against Porphyromonas gingivalis, a bacterium often associated with soft tissue inflammation, infection, and compromised stability of dental implants [78]. Interestingly, studies have demonstrated the effectiveness of garlic extracts against this bacteria and their possible use as active ingredients in mouth hygiene products [79]. Owing to their wide range of antimicrobial actions, they can function as organic preservatives for dentistry and cosmetic treatments. Aqueous garlic extracts have been shown by Yadav et al. to be efficacious against S. aureus and E. coli [80]. Notably, they discovered that the 100% concentration against S. aureus and E. coli created the biggest zone of inhibition, measuring 34 mm and 37 mm, respectively. Compared to the overuse of antibiotics or preservatives, the researchers found that raw garlic extract efficiently inhibits bacteria and fungi without creating medication resistance [80]. Consequently, it is worthwhile to investigate the possible use of garlic extracts in the pharmaceutical, medical, and cosmetic industries as natural preservatives that can guard against primary and secondary infections in cosmetics. Since fungal growth is more rapid and obvious compared to bacterial one, fungal infections in the cosmetics sector are a significant issue [81]. Among the fungi that can contaminate cosmetics include Aspergillus, Penicillium, and Candida species, which may have an adverse effect on cosmetic product quality and decrease consumer safety [81]. Dadashi et al. found that shared cosmetic kits had a high level of fungal contamination, affecting 30.0% of eyeliners and 38.5% of powders [82]. Furthermore, Penicillium spp., Aspergillus fumigatus, and C. albicans have been found in cosmetics such as lip pencils, eye pencils, and mascaras, according to Muhammad et al. [83]. The antifungal action of garlic extracts has the potential to replace stronger antifungal drugs in medical and cosmetic sectors, given the sensitivity of products to microbial growth due to water and other nutrients. Pai S. et al. [84] proposed this possible use after demonstrating that concentrated garlic oil and aqueous extracts had either equal or greater inhibitory effects on Aspergillus species than pharmaceutical preparations. Nevertheless, garlic processing yields a significant quantity of peel waste, approximately 25% of the raw material’s weight, amounting to about 2.3–2.9 million tons annually [14]. Finding ways to use this by-product instead of discarding it is crucial to prevent negative impacts on the sustainability and economy of the garlic processing industry. Interestingly, garlic peels are considered to be richer in bioactive compounds compared to the inner parts of fruits and vegetables due to their protective role [15]. Garlic peel extracts (GPEs) have been reported to be rich in phenolic compounds and exhibit antimicrobial effects comparable to those of fresh garlic extracts [85]. Salem et al. [60] confirmed the great presence of phenols in garlic peels, together with a wide amount of other bioactive compounds, including flavonoids, saponins, and alkaloids, conferring notable antioxidant and antibacterial properties. In particular, garlic peel extract has shown high levels of diosgenin, sarsasapogenin, feruloyltyramide, and quercetin. The garlic peel extract has exhibited significant effects against bacteria such as methicillin-resistant Staphylococcus aureus, Acinetobacter baumannii, E. coli, and P. aeruginosa, with MBC of 6.25 ± 0 mg/mL for Acinetobacter baumannii and 12.5 ± 0 mg/mL for the other bacteria under investigation [60]. Considering this, it would be more advantageous to exploit waste by transforming it into a resource rather than using fresh products, which would also generate additional waste requiring disposal. This approach enhances sustainability and maximizes the use of existing resources, reducing the environmental footprint associated with waste management and fresh product consumption. Lavender (Lavandula angustifolia) is a commonly cultivated plant useful for the essential oil industry. The major producers of lavender oil include Bulgaria, Ukraine, France, the United Kingdom, Spain, China, and Morocco. Bulgaria holds the leading position in lavender production, with over 100 tons produced annually [54]. Melissa (Melissa Officinalis) is a popular medicinal herb that has found various applications over the years [55]. The relatively small amounts of essential oil in lavender and in melissa (lower than 1.5% for both fresh species) result in a large amount of solid waste produced yearly [54]. However, both wastes reported quantities of active ingredients useful in carrying out biological activities, including antimicrobial activity [17]. In a study by Vasileva I. et al. [54], the ability of these waste materials to be used as natural preservatives was investigated. Ethanol extracts from lavender and melissa waste, obtained through ultrasound-assisted extraction, revealed high levels of polyphenols and flavonoids, along with abundant terpenoids. Given the rich content of these compounds, characterized by antimicrobial properties, researchers decided to assess the antimicrobial activity of waste extracts. Gram-negative bacteria (E. coli, P. aeruginosa), Gram-positive bacteria (S. aureus, Proteus vulgaris, Enterococcus faecalis, Listeria monocytogenes), yeasts (Candida utilis), and saprophytic microorganisms (Bacillus subtilis, Aspergillus niger, Penicillium chrysogenum) were used to test the antimicrobial activity. All microorganisms showed sensitivity to the lavender extract, with S. aureus being particularly susceptible, demonstrating a MIC at least ten times lower than that of the other microorganisms. The ethanol extract of melissa inhibited the growth of S. aureus, P. aeruginosa, Enterococcus faecalis, and Candida utilis, with greater MICs (over 600 μg/mL). However, both ethanol extracts exhibited antimicrobial activity against the fungus Penicillium chrysogenum (MIC greater than 600 μg/mL) and the bacterium Bacillus subtilis (MIC 60 μg/mL and 600 μg/mL for ethanol extract of lavender and melissa, respectively).
Mangifera indica L., widely recognized as mango, is a fruit plant extensively cultivated in tropical regions. It is an extremely sought-after fruit in the global market, but its processing generates substantial waste [61]. Approximately 30–50% of the fruit is discarded, often without treatment, or is used as animal feed. Mango waste comprises peels and seeds, with peels making up 7% to 24% of the fruit’s weight, while seeds constitute 20% to 60% of the fruit’s weight. Furthermore, between 45% and 85% of the weight of the seed is composed of the kernel inside. Mango seeds contain a variety of antioxidant biomolecules, including gallic, caffeic, ellagic, and ferulic acids, as well as carotenoids, tocopherols, and phenolic compounds, including quercetin, anthocyanins, and catechins, varying in their contents as a function of the extraction technique. Mangiferin, a phenolic substance recognized for its exceptional antioxidant qualities, is present in mango peels and leaves. A patent (U.S. Pat. No. US 2011/0135772 A1) has been filed describing its properties against tyrosinase, elastase, and collagenase [62] and its use in the creation of anti-aging cosmetics and dermatological formulations, for example, products acting against cutaneous pigmentation. In addition, in a study conducted by Mutua J.K. et al. [16], the potential against oxidative stress and microbial agents of extracts recovered from mango seeds was evaluated by considering four different varieties. The study showed that mango seed powder possesses high antioxidant effects and high total polyphenol content. The position and variety of the mango seeds affected the overall scavenging performance in moderately significant (p < 0.05) ways. On the other hand, no appreciable variations in total polyphenols were discovered according to cultivar or location. C. albicans, E. coli, and S. aureus were used to check the antimicrobial effects of mango seed extracts. Based on the size of the inhibition zones, the results demonstrated the antibacterial efficacy of all mango seed extracts. The strong antibacterial activity of the extracts was ascribed by the researchers to the presence of certain phytochemicals, such as coumarins, tannins, terpenes, and flavonoids. Furthermore, compared to E. coli, the methanolic extracts in the study showed higher inhibition against the Gram-positive S. aureus bacterium at different doses. This difference can be attributed to the different structures of their cell walls [16]. In addition, in a study by Poomanee et al. [63], the researchers explored the use of hydroethanolic mango seed extract (KMHE) as a cosmetic anti-aging ingredient, including cytotoxicity tests to assess its safety. The experiments were conducted on two cell lines: human BJ fibroblasts and murine RAW 264.7 macrophages. In BJ fibroblasts, KMHE did not show significant cytotoxicity, maintaining cell viability even at high concentrations up to 1000 µg/mL. Additionally, KMHE demonstrated a protective effect against DNA fragmentation induced by hydrogen peroxide (H2O2), an oxidizing agent used to simulate oxidative stress. In RAW 264.7 macrophages, however, KMHE reduced cell viability below 80% at concentrations between 100 and 1000 µg/mL, indicating slight cytotoxicity. Nevertheless, no cytotoxic effects were reported at concentrations ranging from 0.1 to 10 µg/mL. These results suggest that KMHE can be considered safe for cosmetic use, provided it is used at appropriate concentrations.
3.2. Phenolic Compound
Agri-food wastes are significant sources of phytochemicals, encompassing polyphenols, carotenoids, tocopherols, and terpenes (Table 2. These phytochemicals possess antioxidant, therapeutic, nutritional, and antibiotic properties. Their effective use allows for the realization of upgraded formulations, food additives, therapeutic products, and cosmetics [86,87,88,89]. Plant polyphenols, also referred to as phenolic compounds, are organic substances characterized by the presence of multiple phenolic units differing from aromatic rings by the presence of one or more hydroxyl (OH) groups attached [90].

Table 2.
Antimicrobial potential of phenolic compounds found in some agri-food wastes.
Based on their structures, we can distinguish flavonoids and non-flavonoids [107,108] that serve as crucial secondary metabolites in plant physiology, playing a key role in protection against herbivores and pathogens while also offering mechanical support to the vegetal organism [107]. Notably, phenolic compounds naturally present in plants exhibit antimicrobial properties and encompass phenolic acids, flavones, lignans, and tannins [109].
Phenolic molecules can show several functions. Protocatechuic acid (PCA), or 3,4-dihydroxy benzoic acid, for example, has been found to present various health benefits, including antibacterial, hepatoprotective, cardiac, neurological, nephroprotective, antiviral, anti-inflammatory, antidiabetic, anticancer, antiulcer, anti-aging, and antifibrotic properties [110]. PCA is efficient against fungi, bacteria, and Gram-positive and Gram-negative bacteria. Additionally, it cooperates with several antibiotics to combat resistant infections [110,111,112]. Jalali et al. stated that (PCA) exhibits dose-related effects against Cutibacterium acnes and is recommended as a broad-spectrum antiseptic effective against germs linked to skin discomfort from surgery, also derived from drug-resistant microbes [113]. The study by Šeregelj et al. [92] revealed the isolation of multiple bioactive compounds from chili pepper processing waste. These compounds include carotenoids (β-carotene, lutein, zeaxanthin, β-cryptoxanthin), hydroxybenzoic acids (gallic, vanillic acid), hydroxycinnamic acids (sinapic, caffeic, rosmarinic, chlorogenic acid), flavan-3-ols (epicatechin), and flavonols (rutin, quercetin, and myricetin), along with PCA and other D-phytochemicals [92].
Aziz and colleagues investigated how phenolic chemicals inhibited the growth of bacteria, including B. cereus, K.pneumoniae, A. flavus, A. parasiticus, and E. coli. Their research showed that a dose of 0.3 mg/mL of caffeic and protocatechuic acids successfully inhibits the growth of E. coli and K. pneumoniae. Furthermore, similar concentrations (0.5 mg/mL) of p-Hydroxybenzoic, vanillic, caffeic, protocatechuic, and p-coumaric acids, as well as oleuropein and quercetin, showed total suppression of B. cereus growth [91]. At a dosage of 0.4 mg/mL, oleuropein, p-hydroxy benzoic, vanillic, and p-coumaric acids showed inhibitory effects against the development of B. cereus, K. pneumoniae, and E. coli. Moreover, at a dosage of 0.2 mg/mL, vanillic and caffeic acids completely limit the development and aflatoxin generation of A. flavus and A. parasiticus [91]. An illustrative instance of how manufacturing by-products serve as reservoirs for these bioactive compounds [114,115] can be found in a study conducted by Monteiro et al. [116]. In this study, chlorogenic acids were extracted from the leaves of Arabica and Robusta coffee plants. Notably, coffee leaves constitute a significant residual product of coffee bean processing. The research revealed that 5-caffeoylquinic acid was the most prevalent compound in coffee leaves, with greater concentrations identified in arabica than in robusta coffee. This underscores the potential value of using manufacturing waste as a container of bioactive sources [116,117]. As shown in the study, phenolic chemicals can stop bacteria from growing in cosmetics that cause secondary illnesses. The creation of tailored cosmetics, realized to match the specific requirements of each individual customer, represents a relevant industry examined by Kim et al. [81]. The study emphasized the use of heat treatment during production and minimizing the transfer of cosmetics between containers as effective procedures to reduce germs levels and prevent cross-contamination, respectively. Sequencing research has revealed the presence of mold and bacteria, specifically S. epidermidis, B. circulans, B. cereus, and A. versicolor, in customized cosmetic products [81]. It has been demonstrated that phenolic compounds are useful additions to customized cosmetics, acting as safe and natural guardians against the development of dangerous substances. There has been much research conducted on phenolic acids’ antibacterial and antifungal qualities. Vanillic acid was investigated by Qian et al. [97], testing its antibacterial properties against Enterobacter hormaechei (CREH) resistant to carbapenem. According to their findings, vanillic acid was effective against CREH, reporting a MIC value of 0.8 mg/mL. The acid broke down the cell wall, resulting in the cell’s destruction. Vanillic acid also successfully prevented CREH from forming biofilms. The researchers have suggested that this phenolic acid may find application as a disinfectant and preservative. Other phenolic acids that have antibacterial properties are gallic acid and caffeic acid. Gallic acid, caffeic acid, and pyrogallol were found to have antibacterial, antifungal, and antibiotic-modulatory properties by Lima et al. [99]. It was shown that pyrogallol, like gallic acid, increased antibiotic action against S. aureus and optimized the necessary antibiotic concentration needed to eradicate the whole C. albicans population [99]. Other phenolic compounds with antibacterial properties could be useful for this, in addition to phenolic acids, like flavonoids. According to Jaisinghani et al., quercetin has antibacterial effects against E. coli and P. vulgaris at doses of 300 and 400 μg/mL, as well as S. aureus and P. aeruginosa at 20 μg/mL. Furthermore, even at 500 μg/mL, Lactobacillus casei var. shirota and Shigella flexneri showed no discernible reactions [94].
Another example of the presence of these substances in a waste product from the agro-food industry comes from a study by Sharma et al. that investigated the effects of polyphenols derived from fresh peeled and aged onions. The research findings revealed an increasing antibiofilm activity against different microorganisms (E. coli, P. aeruginosa, S. aureus, and B. cereus) in step with the aging of onions. Concurrently, there was a proportional rise in quercetin and total phenolic amounts with the aging of the onion varieties under investigation. These results underscore the presence of these substances in waste products generated by the agro-food industry [93]. As stated by Yang et al., quercetin possesses a wide range of antibacterial activities aimed at nucleic acid synthesis suppression and bacterial cell wall breakdown. Their study elaborated on how quercetin inhibits the growth of various bacteria by interfering with their DNA and RNA synthesis, effectively preventing replication and causing cell death. This broad-spectrum antibacterial effect makes quercetin a potent agent against several pathogenic bacteria [118]. The authors enumerated a number of bacteria found in cosmetic goods that quercetin demonstrated antibacterial activity against. Specifically, quercetin has shown efficacy against S. aureus, E. coli, and P. aeruginosa, which are common contaminants in cosmetic products. These bacteria can cause spoilage and pose health risks to consumers, making their control fundamental to ensure the safety and long-lasting of cosmetic formulations [118]. Orús et al. [119] reported that the chemical exhibited an inhibitory impact on E. coli resistant to drugs and P. aeruginosa resistant to carbapenem. These microorganisms are particularly concerning because they have developed resistance to multiple antibiotics, including carbapenems, which are often used as a last-resort treatment for severe infections. The researchers demonstrated that quercetin could effectively inhibit the growth of these resistant strains, highlighting its potential as a preservative in cosmetic products to combat antibiotic-resistant bacteria [119].
Di Ming et al. conducted studies to hinder the formation of S. aureus biofilm, causing implant-associated infections without using antibiotics [103]. The authors employed kaempferol as a biofilm-inhibiting agent against S. aureus. It was demonstrated that an application of 64 μg/mL of kaempferol suppressed biofilm growth by 80%. Kaempferol exhibited no antimicrobial action versus S. aureus, according to growth curve experiments and minimum inhibitory concentration measurements. They reported that kaempferol might prevent the initial adhesion in the biofilm development, as well as a reduction in the function of S. aureus sortaseA (SrtA) and the expression of genes linked to adhesion [103]. Kaempferol, alongside other phenolic compounds, is detected in significant quantities in grape waste, as evidenced by a study from Moschona et al. [101]. Grapes, particularly of the Vitis vinifera variety, are cultivated extensively worldwide for the purpose of wine production. The residual waste from grapes contains valuable secondary metabolites, including phenols. Notably, an investigation into the phenolic content of wine wastes (of the Malagouzia and Syrah varieties) was undertaken using high-performance liquid chromatography (HPLC) and electrospray ionization mass spectrometry (ESI/MS). The subsequent encapsulation of the identified phenolic compounds was performed using different polymers. The study revealed that extracts from all grape wastes yielded substantial total phenolic levels (ranging from 13 ± 2.72 to 22 ± 2.69 mg/g) and exhibited great antioxidant effect (67–97%) [101].
The study confirmed that investigating how bacterial contamination and biofilm formation affect the instruments used in the manufacturing of cosmetics is crucial, particularly in light of the rising demand for natural goods that are handmade or customized. Although these molecules present enormous potential, it is important to underline the possible application of naturally occurring plant phenolic compounds as antibacterial agents in cosmetics, and the themes require further investigation. For example, Kumar et al. have shown that kaempferol is a polyphenolic substance that has excellent antibacterial action [120]. However, its significant mass and low water solubility limit its activity. Despite this, the researchers hypothesized that, through inhibition of bacterial porin channels or stimulation of the ATP-dependent efflux pump mechanism, these substances could still effectively suppress bacterial growth [120]. In a different study, Karpiński et al. discovered that apigenin was poorly effective against S. aureus strains (MIC = 500–1000 μg/mL), which were also resistant to the derivatives vitexin and isovitexin (MIC > 1000 μg/mL), while apigenin and luteolin had a MIC of 500 μg/mL against E. coli and P. aeruginosa [106]. Given their potential to replace strong artificial preservatives in cosmetics, it is evident that greater research focus is needed on these materials. The possibility of utilizing naturally sourced phenolic compounds not only represents a sustainable solution for managing agro-industrial waste but could also revolutionize the cosmetic sector by offering safer and more natural alternatives to consumers. Therefore, it is crucial that future studies concentrate on this promising field of application to develop effective and environmentally friendly products.
3.3. Terpenoids
Similar to terpenes, terpenoids are a wide and diverse class of chemical molecules, also known as isoprenoids [107]. These substances are present in every type of living thing and are produced by plants, where they play a pivotal role in a variety of biotic interactions. Terpenoids have numerous essential roles in plants. They contribute to the formation of some carotenoid pigments, as well as the hormones gibberellin and chlorophyll. Furthermore, they are the precursors of steroids and sterols [107]. Terpenoids are the progenitors of tetraterpenoids, also known as carotenoids. Carotenoids are a vast class of pigments found in many plant and animal kingdoms; their colors range from deep red to yellow. At the moment, a large percentage of carotenoids are produced artificially since this is less expensive, but natural sources are being utilized more frequently [121]. Spinach leftovers can be used to extract xanthophylls, whereas orange and red foods are sources of carotene [122]. Mono- and sesquiterpenoids are typically predominant in essential oils (EOs) [107,108]. EOs are plant-based volatile compounds that can be obtained from a variety of plant sections, including fruits and flowers [108]. Their composition consists of a blend of phytochemicals or low-mass plant natural compounds, such as citral, geraniol, eugenol, carvacrol, linalool, citronellal, carvone, limonene, terpinenes, menthol, and menthone [107,108]. They are frequently extracted using a range of techniques from food production waste. In addition to the species, portion of the plant, development stage, cultivation technique, processing method, and storage conditions, the extraction method also affects the content, quality, and characteristics of essential oils [123].
Since EOs have reported several positive effects, the cosmetics industry makes extensive use of their constituent parts. They may have analgesic, antibacterial, diuretic, antioxidant, or anti-inflammatory qualities, in addition to giving products a unique scent [124]. Due to their antibacterial properties, essential oils can be used as preservatives in cosmetics, either as the sole preservative or as an adjunct to other preservatives. Studies have shown that when EOs are mixed with other stabilizers, chelating agents, and preservatives, their antibacterial action increases. EOs must be safe for consumers, toxicity-free, and highly active at low concentrations to be employed as preservatives in cosmetics. They should not have a strong flavor, smell, or color, and their effects should be directed at a wide range of microbes. Almost all kinds of cosmetics can employ EOs as preservatives [124]. Essential oils are predominantly found in another agri-food waste: citrus peels [107,125,126,127,128,129,130,131]. The Rutaceae family of plants, which includes 17 species of citrus plants, is distributed in tropical, subtropical, and temperate climates [132]. This genera includes one of the fruits most consumed globally [133]. Estimates suggest that approximately 124 million tons of citrus fruits are produced per year. Oranges (Citrus sinensis L.) account for 67 million tons, mandarins (Citrus reticulata L.) for 33 million tons, and lemons (Citrus limon L.) and limes (Citrus aurantifolia L.) for 16 million tons [134]. Fresh fruit production generates substantial waste during the entire process, including peels, pulp, and seeds, which are around 50% of the fruit mass. These waste materials can be reused for creating sources of co-products with added value, such as terpenoids [135,136]. It is important to note that citrus peel EOs have been widely studied for their antimicrobial properties. However, further research on the bioactivities of fractions from citrus waste by-products is necessary. Additionally, because certain residues have been valorized but others are still disposed of in landfills, the molecules in the peels cause environmental issues. Therefore, the valorization of these by-products is crucial for protecting the environment in addition to raising the possible economic return [135,136].
Geraci et al. [130] used peels from 12 cultivars of oranges (C. sinensis) to obtain EOs by steam distillation and analyzed their chemical composition. They also carried out a comparison between the cultivars based on their composition in EOs. The identification of the components was evaluated by gas chromatography–mass spectrometry (GC-MS). In the 12 varieties of orange peel essential oils analyzed, a total of 54 compounds were identified. These compounds included hydrocarbons, aldehydes, ketones, alcohols, monoterpene esters, and oxides. Limonene was identified as the predominant component in all EO samples from orange cultivars, with peak area percentages ranging between 73.9% and 97.6%. Additionally, discrete proportions of monoterpenoid alcohols (namely linalool, geraniol, nerol, and α-terpineol) were observed, while the other components were present in trace amounts. Some differences in composition were found among the groups of sweet oranges. All the obtained EOs were tested (2.5–100 mg/mL) on a panel of Gram-positive and Gram-negative strains (S. aureus, L. monocytogenes, P. aeruginosa). The EO of the “Sanguinello di Paternò” cultivar showed a MIC value six times lower than that reported for “Moro Solarino” EO against two strains of L. monocytogenes. The antimicrobial activity detected reinforced the data on the effectiveness of orange peel EOs against pathogens. Contrarily, lower efficacy was observed from EOs derived from the two cultivars, “Sanguinello” and “Moro”, against Gram-negative bacteria, such as S. aureus and P. aeruginosa. These findings are consistent with other research showing the great resistance to citrus EOs of Gram-negative bacteria compared to Gram-positive ones [137,138]. A greater resistance to citrus EOs of S. aureus than L. monocytogenes was also noted [130]. This suggests the potential use of these essential oils in combination with another compound of the same origin, possibly seeking a synergistic effect to overcome these limitations.
Another fruit peel with a high content of bioactive compounds is bergamot (Citrus bergamia Risso et Poiteau) from the Rutaceae family, Citrus genus, which is almost exclusive to the Calabria region, Italy, growing along the southeastern coast in the Reggio area. In a study, Quirino et al. [131] investigated the antibacterial activity of distilled bergamot peel extracts using various methods to determine their efficacy against multidrug-resistant pathogens. Distilled bergamot extracts were prepared and chemically characterized using gas chromatography–mass spectrometry (GC-MS), revealing a composition rich in monoterpenes, such as limonene, linalool, and linalyl acetate, which are known for their antimicrobial properties. The activity of extracts against bacteria and fungi was investigated through broth microdilution assays to find MBC values. Experiments were conducted with serial dilutions of the extracts (from 5% to 0.03% v/v). After incubation at 37 °C for 24 h, the MBC was identified as the lowest dilution at which no microbial growth was observed, indicating the bactericidal efficacy of the extract. The MIC varied depending on the pathogen, with values ranging from 0.125% to 1% (v/v). Kinetic bactericidal profiles were detected using the time-kill assay. Results were presented in terms of log10 CFU/mL, with a bactericidal effect identified as a reduction of 3 log10 CFU/mL from the initial inoculum. Microbial cultures were incubated with the bergamot peel extract, and samples were taken at predefined intervals (0 and 30 min and 1, 2, 4, 6, 8, and 24 h) reported a significant reduction in bacterial growth, confirming the antibacterial efficacy of the extract. Disk diffusion tests demonstrated that distilled bergamot peel extracts produce significant inhibition zones against various pathogens. For instance, the inhibition zones for S. aureus were approximately 15 mm, while for E. coli, they were 18 mm. These results indicate great antibacterial efficacy of the extracts versus both Gram-positive and Gram-negative bacteria. Confocal laser scanning microscopy was utilized to observe the interaction of bergamot extracts with multidrug-resistant bacteria and C. albicans cells. Microscopic preparations of untreated and treated strains, after at least 4 h of incubation, revealed a marked reduction in cell viability, indicating a potent antimicrobial effect. These results suggest that bergamot peel extracts possess significant potential as natural antimicrobial agents, offering a promising therapeutic alternative against multidrug-resistant pathogens [131].
One more fruit whose peels are rich in active compounds is the pomelo (Citrus maxima). This exemplifies how waste materials, which could represent a disposal issue, can be repurposed to provide beneficial solutions. Karakaya et al. [139] evaluated the chemical composition and antimicrobic effect of pomelo (Citrus maxima) EO obtained from the fruit peel. The EO was extracted by hydrodistillation from the flavedo layer of the peel and analyzed using gas chromatography–mass spectrometry (GC-MS), and a total of 62 compounds were found. Monoterpenes comprised 89.36% of the essential oil, predominantly D-limonene, which is almost the entirety of the monoterpenes (76.97%). The EO is notable for its nootkatone content (1.13%). Antimicrobial activity was measured using the broth macrodilution method against S. aureus, B. cereus, E. coli, Saccharomyces cerevisiae, and C. albicans, reporting MIC values of the EO ranging between 2000 and 16,000 μg/mL for all the mentioned microorganisms. These findings suggest that pomelo EO could be utilized as a natural and cost-effective taste enhancer and antimicrobial additive in food, pharmaceutical, and cosmetic fields.
Weng et al. [129] focused on the antimicrobial potential of mandarin (Citrus depressa Hayata) peel essential oil (CD-EO). In their study, EOs were extracted through steam distillation and analyzed using gas chromatography–mass spectrometry (GC-MS). The analysis identified approximately 31 compounds, accounting for 99.98% of the total volatile substances. The principal components were (R)-(+)-limonene (38.97%) and γ-terpinene (24.39%). The antimicrobial activity was assessed against C. albicans, E. coli, P. aeruginosa, and S. aureus. The findings demonstrated that higher concentrations of CD-EO led to more pronounced antimicrobial effects. Using a concentration of 8 mg/mL CD-EO, the authors observed relevant inhibition rates against C. albicans (70.82%), E. coli (68.38%), P. aeruginosa (60.15%), and S. aureus (56.89%). Furthermore, the inhibitory concentration required to suppress 50% of microbial growth (IC50) was determined for each microorganism. The results indicated that CD-EO demonstrated the strongest effects against the following microorganisms: C. albicans > P. aeruginosa > E. coli (IC50 of 7.13 mg/mL) > S. aureus [129].
In addition to emphasizing the primary agricultural and food wastes, such as citrus peels, from which essential oils rich in antimicrobial terpenoids can be obtained, other forms of waste capable of yielding the same compounds exist. To elucidate this point further, we can cite a study by Salvatori et al. [140], in which the authors evaluated the anti-inflammatory and antimicrobial effects of EOs produced from processing waste of seven types of eucalyptus waste. Australia, New Zealand, and Tasmania are the natural homes of eucalyptus species (Myrtaceae), which are highly valued for their financial contribution to the forestry sector that produces wood and paper [141]. In Brazil, 5.7 million hectares of planted trees are part of eucalyptus plantations [142]. These plants are mostly used in the forestry sector, which supports the economy but also produces millions of tons of waste that is expensive for both the environment and the industry [141]. Leaves and branches of eucalyptus trees are left on the ground since they are mostly used in Brazil to produce timber or pulp for the paper industry [140]. The researchers of this study identified an opportunity in these residues, using them as raw materials in the production of compounds with antimicrobial and anti-inflammatory activities, such as EOs [143]. Studies have previously demonstrated that eucalyptus EOs exhibit antimicrobial activity against both Gram-positive (S. aureus) and Gram-negative (E. coli) bacteria, indicating the possibility of employing the oil as a natural antimicrobial to treat contaminations caused by these two bacteria [144]. These results highlight the need to develop novel compounds with antibacterial potential by suggesting that 1,8-cineole, the primary molecule in eucalyptus, may be in charge of this action [144]. In the study by Salvatori et al. [140], the essential oils were obtained through steam distillation, and their chemical composition was analyzed using gas chromatography–mass spectrometry (GC-MS). The analysis revealed the presence of several terpenoid compounds such as α-pinene, β-pinene, γ-terpinene, Pinocarvone, Caryophyllene, Himbaccol, α-terpineol acetate, Viridiflorol, Aromandendrene, and D-limonene, with some varieties of eucalyptus containing higher concentrations of specific compounds. Interestingly, 1,8-cineole (eucalyptol) was found in all species except one, with the hybrid “Urocam” showing the highest concentration at 82.18%. The effects against bacteria and fungi of the EOs were tested against S. aureus, E. coli, and C. albicans. The agar dilution experiment was utilized to define the EOs’ MIC. The results indicated that all seven oils showed efficacy against S. aureus, E. coli, and C. albicans. In the tests conducted against the two bacteria, the types “E. dunnii” and “Grancam” in particular showed the greatest effectiveness.
4. Limitations and Challenges
Agri-food waste comprises a multifaceted blend of substances, often found in varying concentrations. This intricate composition presents challenges in isolating and purifying the active components, necessitating the application of sophisticated and costly extraction and characterization techniques. The inherent variability in waste components can compromise the quality and quantity of extracted compounds, posing additional complexities in establishing standardized production processes [145].
One important aspect to consider is the safety and effectiveness of these compounds. Although many studies have confirmed the antimicrobial and antioxidant properties of natural extracts, it is vital to conduct additional research to assess their potential toxicity and any possible side effects. This is especially important when considering their use in cosmetic products, where ensuring consumer safety is of utmost importance [146].
The absence of a specific regulatory framework for the employment of innovative natural antibacterial compounds may pose an additional obstacle. The absence of established guidelines can delay the adoption of these compounds, requiring greater efforts by regulatory authorities to establish clear and safe regulations for their use [147]. Additionally, it is important to consider the potential onset of resistance to the component of the extracts, which could compromise their long-term effectiveness and lead to the development of more hostile microbial strains [21].
In numerous instances, the bioactive impact does not stem from a lone phytochemical but rather from the collaborative effect of multiple compounds within an extract. This synergistic collaboration can introduce challenges in standardizing and scaling up production, as the removal or addition of any compound has the potential to modify the effectiveness of the entire extract [148].
Another potential limitation and, consequently, a new challenge can be considered as the microbial resistance induced by the natural extracts obtained from food waste. As with other natural and/or synthetic compounds with antimicrobial activity, the food waste-derived molecules can stimulate the onset of resistance in the treated microorganisms, especially when there is no high specificity of action. The strong point of some substances obtained from waste is their dual function, not only antimicrobial preservative activity on the formulation but also beneficial activities on the skin. This would allow, if included in a cosmetic product, the use of a single ingredient with a dual function and, therefore, would allow to streamline the formulation, reducing the risk of harmful effects on the skin [149].
5. Conclusions
Despite challenges, using natural antimicrobial compounds from agri-food waste in cosmetics offers significant advantages. These phytochemicals serve as viable alternatives to synthetic preservatives and enhance product quality with additional aesthetic and therapeutic benefits. This approach meets growing consumer demand for safe, sustainable products and supports waste reduction and the circular economy. Moreover, many natural compounds possess antioxidant and anti-inflammatory properties, further enriching cosmetic formulations. The adoption of these compounds not only improves consumer safety but also minimizes the environmental impact of the cosmetic industry. To fully harness their potential, an interdisciplinary effort involving research, technological advancement, and regulatory support is essential. With appropriate investment and collaboration, natural compounds could play a fundamental role in the future of sustainable cosmetics.
Author Contributions
Conceptualization, D.P.; writing—original draft preparation, A.S., A.M. and N.d.; writing—review and editing, M.C.C. and D.P.; supervision, D.P. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the Next Generation EU-Italian NRRP, Mission 4, Component 2, Investment 1.5, call for the creation and strengthening of ‘Innovation Ecosystems’, building ‘Territorial R&D Leaders’ (Directorial Decree n. 2021/3277)—project Tech4You—Technologies for climate change adaptation and quality of life improvement, n. ECS0000009. This work reflects only the authors’ views and opinions, and neither the Ministry for University and Research nor the European Commission can be considered responsible for them.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
No new data were created or analyzed in this study.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Usmani, Z.; Sharma, M.; Awasthi, A.K.; Sharma, G.D.; Cysneiros, D.; Nayak, S.C.; Thakur, V.K.; Naidu, R.; Pandey, A.; Gupta, V.K. Minimizing hazardous impact of food waste in a circular economy—Advances in resource recovery through green strategies. J. Hazard. Mater. 2021, 416, 126154. [Google Scholar] [CrossRef]
- Chauhan, C.; Dhir, A.; Akram, M.U.; Salo, J. Food loss and waste in food supply chains. A systematic literature review and framework development approach. J. Clean. Prod. 2021, 295, 126438. [Google Scholar] [CrossRef]
- Morganti, P.; Gao, X.; Vukovic, N.; Gagliardini, A.; Lohani, A.; Morganti, G. Food Loss and Food Waste for Green Cosmetics and Medical Devices for a Cleaner Planet. Cosmetics 2022, 9, 19. [Google Scholar] [CrossRef]
- Ścieszko, E.; Budny, E.; Rotsztejn, H.; Erkiert-Polguj, A. How has the pandemic lockdown changed our daily facial skincare habits? J. Cosmet. Dermatol. 2021, 20, 3722–3726. [Google Scholar] [CrossRef]
- Lee, J.; Kwon, K.H. Changes in the use of cosmetics worldwide due to increased use of masks in the coronavirus disease-19 pandemic. J. Cosmet. Dermatol. 2022, 21, 2708–2712. [Google Scholar] [CrossRef] [PubMed]
- Morganti, P.; Lohani, A.; Gagliardini, A.; Morganti, G.; Coltelli, M.-B. Active Ingredients and Carriers in Nutritional Eco-Cosmetics. Compounds 2023, 3, 122–141. [Google Scholar] [CrossRef]
- Pinto, D.; de la Luz Cádiz-Gurrea, M.; Silva, A.M.; Delerue-Matos, C.; Rodrigues, F. Cosmetics—Food Waste Recovery. In Food Waste Recovery; Elsevier: Amsterdam, The Netherlands, 2021; pp. 503–528. [Google Scholar]
- Parra-Pacheco, B.; Cruz-Moreno, B.A.; Aguirre-Becerra, H.; García-Trejo, J.F.; Feregrino-Pérez, A.A. Bioactive Compounds from Organic Waste. Molecules 2024, 29, 2243. [Google Scholar] [CrossRef]
- Mehdizadeh, T.; Tajik, H.; Langroodi, A.M.; Molaei, R.; Mahmoudian, A. Chitosan-starch film containing pomegranate peel extract and Thymus kotschyanus essential oil can prolong the shelf life of beef. Meat Sci. 2020, 163, 108073. [Google Scholar] [CrossRef]
- Gigliobianco, M.R.; Cortese, M.; Nannini, S.; Di Nicolantonio, L.; Peregrina, D.V.; Lupidi, G.; Vitali, L.A.; Bocchietto, E.; Di Martino, P.; Censi, R. Chemical, Antioxidant, and Antimicrobial Properties of the Peel and Male Flower By-Products of Four Varieties of Punica granatum L. Cultivated in the Marche Region for Their Use in Cosmetic Products. Antioxidants 2022, 11, 768. [Google Scholar] [CrossRef]
- Houston, D.M.; Bugert, J.; Denyer, S.P.; Heard, C.M. Anti-inflammatory activity of Punica granatum L. (Pomegranate) rind extracts applied topically to ex vivo skin. Eur. J. Pharm. Biopharm. 2017, 112, 30–37. [Google Scholar] [CrossRef]
- Hamrita, B.; Emira, N.; Papetti, A.; Badraoui, R.; Bouslama, L.; Ben Tekfa, M.-I.; Hamdi, A.; Patel, M.; Elasbali, A.M.; Adnan, M. Phytochemical Analysis, Antioxidant, Antimicrobial, and Anti-Swarming Properties of Hibiscus sabdariffa L. Calyx Extracts: In Vitro and In Silico Modelling Approaches. Evid. Based Complement. Altern. Med. 2022, 2022, 1252672. [Google Scholar] [CrossRef] [PubMed]
- Primavilla, S.A.-O.; Pagano, C.A.-O.; Roila, R.A.-O.; Branciari, R.A.-O.; Ranucci, D.A.-O.; Valiani, A.; Ricci, M.A.-O.; Perioli, L.A.-O. Antibacterial Activity of Crocus sativus L. Petals Extracts against Foodborne Pathogenic and Spoilage Microorganisms, with a Special Focus on Clostridia. Life 2022, 13, 60. [Google Scholar] [CrossRef]
- Kotenkova, E.; Kupaeva, N. Comparative Antioxidant Study of Onion and Garlic Waste and Bulbs. In Proceedings of the IOP Conference Series: Earth and Environmental Science; IOP Publishing: Bristol, UK, 2019; p. 012031. [Google Scholar]
- Noda, Y.; Asada, C.; Sasaki, C.; Nakamura, Y. Effects of hydrothermal methods such as steam explosion and microwave irradiation on extraction of water-soluble antioxidant materials from garlic husk. Waste Biomass Valoriz. 2019, 10, 3397–3402. [Google Scholar] [CrossRef]
- Mutua, J.K.; Imathiu, S.; Owino, W. Evaluation of the proximate composition, antioxidant potential, and antimicrobial activity of mango seed kernel extracts. Food Sci. Nutr. 2017, 5, 349–357. [Google Scholar] [CrossRef] [PubMed]
- de Elguea-Culebras, G.O.; Sánchez-Vioque, R.; Santana-Méridas, O.; Herraiz-Peñalver, D.; Carmona, M.; Berruga, M.I. In vitro antifungal activity of residues from essential oil industry against Penicillium verrucosum, a common contaminant of ripening cheeses. Lwt 2016, 73, 226–232. [Google Scholar] [CrossRef]
- Kabara, J.J.; Orth, D. Principles for Product Preservation. In Cosmetic Science and Technology Series; Elsevier: Amsterdam, The Netherlands, 1997; pp. 1–14. [Google Scholar]
- Mitsui, T. Preservation of Cosmetics. New Cosmetic Science; Elsevier: Amsterdam, The Netherlands, 1997; pp. 199–208. [Google Scholar]
- Smith, C.; Alexander, B. The relative cytotoxicity of personal care preservative systems in Balb/C 3T3 clone A31 embryonic mouse cells and the effect of selected preservative systems upon the toxicity of a standard rinse-off formulation. Toxicol. In Vitro 2005, 19, 963–969. [Google Scholar] [CrossRef] [PubMed]
- Halla, N.; Fernandes, I.P.; Heleno, S.A.; Costa, P.; Boucherit-Otmani, Z.; Boucherit, K.; Rodrigues, A.E.; Ferreira, I.C.F.R.; Barreiro, M.F. Cosmetics Preservation: A Review on Present Strategies. Molecules 2018, 23, 1571. [Google Scholar] [CrossRef]
- Sandle, T. EU GMP Annex 1: Manufacture of Sterile Medicinal Products; European Commission: Brussels, Belgium, 2022. [Google Scholar]
- Varvaresou, A.; Papageorgiou, S.; Tsirivas, E.; Protopapa, E.; Kintziou, H.; Kefala, V.; Demetzos, C. Self-preserving cosmetics. Int. J. Cosmet. Sci. 2009, 31, 163–175. [Google Scholar] [CrossRef]
- ISO 22716:2007; Cosmetics—Good Manufacturing Practices (GMP)—Guidelines on Good Manufacturing Practices. International Organization for Standardization. Available online: https://www.iso.org/standard/36437.html (accessed on 28 August 2024).
- Parliament, E. Regulation (EC) No 1223/2009 of the European Parliament and of the Council of 30 November 2009 on Cosmetic Products. In Official Journal of the European Union; European Union: Brussels, Belgium, 2009. [Google Scholar]
- Devlieghere, F.; De Loy-Hendrickx, A.; Rademaker, M.; Pipelers, P.; Crozier, A.; De Baets, B.; Joly, L.; Keromen, S. A new protocol for evaluating the efficacy of some dispensing systems of a packaging in the microbial protection of water-based preservative-free cosmetic products. Int. J. Cosmet. Sci. 2015, 37, 627–635. [Google Scholar] [CrossRef]
- Yablonski, J.I.; Mancuso, S.E. Microbial Risks and Eco-Friendly Packaging. Formul. Packag. Mark. Nat. Cosmet. Prod. 2011, 179–211. [Google Scholar] [CrossRef]
- Geis, P.A. Cosmetic Microbiology: A Practical approach, 2nd ed.; CRC Press: Boca Raton, FL, USA, 2006. [Google Scholar]
- Kerdudo, A.; Fontaine-Vive, F.; Dingas, A.; Faure, C.; Fernandez, X. Optimization of cosmetic preservation: Water activity reduction. Int. J. Cosmet. Sci. 2015, 37, 31–40. [Google Scholar] [CrossRef] [PubMed]
- Dao, H.; Lakhani, P.; Police, A.; Kallakunta, V.; Ajjarapu, S.S.; Wu, K.-W.; Ponkshe, P.; Repka, M.A.; Narasimha Murthy, S. Microbial stability of pharmaceutical and cosmetic products. AAPS PharmSciTech 2018, 19. [Google Scholar] [CrossRef] [PubMed]
- Donsì, F.; Ferrari, G. Essential oil nanoemulsions as antimicrobial agents in food. J. Biotechnol. 2016, 233, 106–120. [Google Scholar] [CrossRef] [PubMed]
- Gharenaghadeh, S.; Karimi, N.; Forghani, S.; Nourazarian, M.; Gharehnaghadeh, S.; Jabbari, V.; Khiabani, M.S.; Kafil, H.S. Application of Salvia multicaulis essential oil-containing nanoemulsion against food-borne pathogens. Food Biosci. 2017, 19, 128–133. [Google Scholar] [CrossRef]
- Salvia-Trujillo, L.; Rojas-Graü, A.; Soliva-Fortuny, R.; Martín-Belloso, O. Physicochemical characterization and antimicrobial activity of food-grade emulsions and nanoemulsions incorporating essential oils. Food Hydrocoll. 2015, 43, 547–556. [Google Scholar] [CrossRef]
- Char, C.; Cisternas, L.; Pérez, F.; Guerrero, S. Effect of emulsification on the antimicrobial activity of carvacrol. CyTA J. Food 2016, 14, 186–192. [Google Scholar] [CrossRef]
- Buranasuksombat, U.; Kwon, Y.J.; Turner, M.; Bhandari, B. Influence of emulsion droplet size on antimicrobial properties. Food Sci. Biotechnol. 2011, 20, 793–800. [Google Scholar] [CrossRef]
- Siquet, F.; Devleeschouwer, M.J. Antibacterial Agents and Preservatives. In Handbook of Cosmetic Science and Technology; Paye, M., Barel, A.O., Maibach, H.I., Eds.; CRC Press: Boca Raton, FL, USA, 2009; pp. 223–231. [Google Scholar]
- Lukic, M.; Pantelic, I.; Savic, S. An overview of novel surfactants for formulation of cosmetics with certain emphasis on acidic active substances. Tenside Surfactants Deterg. 2016, 53, 7–19. [Google Scholar] [CrossRef]
- Mccarthy, T.J. Formulated factors affecting the activity of preservatives. In Cosmetic and Drug Preservation: Principles and Practice, 1st ed.; Kabara, J.J., Ed.; Marcel Dekker: New York, NY, USA, 1984; pp. 359–387. [Google Scholar]
- Truchliński, J.; Sembratowicz, I.; Gorzel, M.; Kiełtyka-Dadasiewicz, A. Allergenic potential of cosmetic ingredients. Arch. Physiother. Glob. Res. 2015, 19, 7–15. [Google Scholar] [CrossRef]
- Khang, D.T.; Tien, L.T.T.; Men, T.T.; Thuy, N.P. Potential of Fermented Fruit Peel Liquid in Cosmetics as a Skin Care Agent. Cosmetics 2021, 8, 33. [Google Scholar] [CrossRef]
- Gordobil, O.; Olaizola, P.; Banales, J.M.; Labidi, J. Lignins from Agroindustrial by-Products as Natural Ingredients for Cosmetics: Chemical Structure and In Vitro Sunscreen and Cytotoxic Activities. Molecules 2020, 25, 1131. [Google Scholar] [CrossRef]
- d’Avanzo, N.; Mancuso, A.; Mare, R.; Silletta, A.; Maurotti, S.; Parisi, O.I.; Cristiano, M.C.; Paolino, D. Olive Leaves and Citrus Peels: From Waste to Potential Resource for Cosmetic Products. Cosmetics 2024, 11, 41. [Google Scholar] [CrossRef]
- Nowak, K.; Jabłońska, E.; Ratajczak-Wrona, W. Controversy around parabens: Alternative strategies for preservative use in cosmetics and personal care products. Environ. Res. 2021, 198, 110488. [Google Scholar] [CrossRef] [PubMed]
- Marcelino, J.; Giménez-Arnau, A.M. Impact of Cosmetics and Cleansers in Atopic Dermatitis—How to Advise Patients. Curr. Treat. Options Allergy 2024, 11, 62–76. [Google Scholar] [CrossRef]
- Gonelimali, F.D.; Lin, J.; Miao, W.; Xuan, J.; Charles, F.; Chen, M.; Hatab, S.R. Antimicrobial properties and mechanism of action of some plant extracts against food pathogens and spoilage microorganisms. Front. Microbiol. 2018, 9, 1639. [Google Scholar] [CrossRef]
- Machado, A.; Zamora-Mendoza, L.; Alexis, F.; Álvarez-Suarez, J.M. Use of plant extracts, bee-derived products, and probiotic-related applications to fight multidrug-resistant pathogens in the post-antibiotic era. Future Pharmacol. 2023, 3, 535–567. [Google Scholar] [CrossRef]
- Voon, H.C.; Bhat, R.; Rusul, G. Flower extracts and their essential oils as potential antimicrobial agents for food uses and pharmaceutical applications. Compr. Rev. Food Sci. Food Saf. 2012, 11, 34–55. [Google Scholar] [CrossRef]
- El Khetabi, A.; Lahlali, R.; Ezrari, S.; Radouane, N.; Lyousfi, N.; Banani, H.; Askarne, L.; Tahiri, A.; El Ghadraoui, L.; Belmalha, S.; et al. Role of plant extracts and essential oils in fighting against postharvest fruit pathogens and extending fruit shelf life: A review. Trends Food Sci. Technol. 2022, 120, 402–417. [Google Scholar] [CrossRef]
- Clarke, D.; Tyuftin, A.A.; Cruz-Romero, M.C.; Bolton, D.; Fanning, S.; Pankaj, S.K.; Bueno-Ferrer, C.; Cullen, P.J.; Kerry, J.P. Surface attachment of active antimicrobial coatings onto conventional plastic-based laminates and performance assessment of these materials on the storage life of vacuum packaged beef sub-primals. Food Microbiol. 2017, 62, 196–201. [Google Scholar] [CrossRef]
- Charalampia, D.; Koutelidakis, A. From pomegranate processing by-products to innovative value added functional ingredients and bio-based products with several applications in food sector. BAOJ Biotech 2017, 3, 210. [Google Scholar]
- Shahamirian, M.; Eskandari, M.H.; Niakousari, M.; Esteghlal, S.; Hashemi Gahruie, H.; Mousavi Khaneghah, A. Incorporation of pomegranate rind powder extract and pomegranate juice into frozen burgers: Oxidative stability, sensorial and microbiological characteristics. J. Food Sci. Technol. 2019, 56, 1174–1183. [Google Scholar] [CrossRef] [PubMed]
- Esparza-Merino, R.M.; Macías-Rodríguez, M.E.; Cabrera-Díaz, E.; Valencia-Botín, A.J.; Estrada-Girón, Y. Utilization of by-products of Hibiscus sabdariffa L. as alternative sources for the extraction of high-quality pectin. Food Sci. Biotechnol. 2019, 28, 1003–1011. [Google Scholar] [CrossRef]
- Sáyago-Ayerdi, S.G.; Velázquez-López, C.; Montalvo-González, E.; Goñi, I. By-product from decoction process of Hibiscus sabdariffa L. calyces as a source of polyphenols and dietary fiber. J. Sci. Food Agric. 2014, 94, 898–904. [Google Scholar] [CrossRef] [PubMed]
- Vasileva, I.; Denkova, R.; Chochkov, R.; Teneva, D.; Denkova, Z.; Dessev, T.; Denev, P.; Slavov, A. Effect of lavender (Lavandula angustifolia) and melissa (Melissa officinalis) waste on quality and shelf life of bread. Food Chem. 2018, 253, 13–21. [Google Scholar] [CrossRef]
- Shakeri, A.; Sahebkar, A.; Javadi, B. Melissa officinalis L.–A review of its traditional uses, phytochemistry and pharmacology. J. Ethnopharmacol. 2016, 188, 204–228. [Google Scholar] [CrossRef] [PubMed]
- Lachguer, K.; El Merzougui, S.; Boudadi, I.; Laktib, A.; Ben El Caid, M.; Ramdan, B.; Boubaker, H.; Serghini, M.A. Major Phytochemical Compounds, In Vitro Antioxidant, Antibacterial, and Antifungal Activities of Six Aqueous and Organic Extracts of Crocus sativus L. Flower Waste. Waste Biomass Valoriz. 2023, 14, 1571–1587. [Google Scholar] [CrossRef] [PubMed]
- Zara, S.; Petretto, G.L.; Mannu, A.; Zara, G.; Budroni, M.; Mannazzu, I.; Multineddu, C.; Pintore, G.; Fancello, F. Antimicrobial activity and chemical characterization of a non-polar extract of saffron stamens in food matrix. Foods 2021, 10, 703. [Google Scholar] [CrossRef]
- Jafarı-sales, A.; Pashazadeh, M. Antibacterial effect of methanolic extract of saffron petal (Crocus sativus L.) on some standard gram positive and gram negative pathogenic bacteria in vitro. Curr. Perspect. Med. Aromat. Plants 2020, 3, 1–7. [Google Scholar] [CrossRef]
- Belyagoubi, L.; Loukidi, B.; Belyagoubi-Benhammou, N.; Gismondi, A.; Di Marco, G.; D’Agostino, A.; Canini, A.; Benmahieddine, A.; Rouigueb, K.; Ben Menni, D. Valorization of Algerian saffron: Stigmas and flowers as source of bioactive compounds. Waste Biomass Valoriz. 2021, 12, 6671–6683. [Google Scholar] [CrossRef]
- Salem, M.A.; Mohamed, O.G.; Mosalam, E.M.; Elberri, A.I.; Abdel-Bar, H.M.; Hassan, M.; Al-Karmalawy, A.A.; Tripathi, A.; Ezzat, S.M.; Abo Mansour, H.E. Investigation of the phytochemical composition, antioxidant, antibacterial, anti-osteoarthritis, and wound healing activities of selected vegetable waste. Sci. Rep. 2023, 13, 13034. [Google Scholar] [CrossRef]
- Mwaurah, P.W.; Kumar, S.; Kumar, N.; Panghal, A.; Attkan, A.K.; Singh, V.K.; Garg, M.K. Physicochemical characteristics, bioactive compounds and industrial applications of mango kernel and its products: A review. Compr. Rev. Food Sci. Food Saf. 2020, 19, 2421–2446. [Google Scholar] [CrossRef]
- Rao, G.V.; Mukhopadhyay, T.; Rajesh, G.D. Skin Care Agent and Compositions Thereof. U.S. Patent 20110135772A1, 9 June 2011. [Google Scholar]
- Poomanee, W.; Khunkitti, W.; Chaiyana, W.; Intasai, N.; Lin, W.-C.; Lue, S.-C.; Leelapornpisid, P. Multifunctional biological properties and phytochemical constituents of Mangifera indica L. seed kernel extract for preventing skin aging. Toxicol. Res. 2021, 37, 459–472. [Google Scholar] [CrossRef] [PubMed]
- Ko, K.; Dadmohammadi, Y.; Abbaspourrad, A. Nutritional and Bioactive Components of Pomegranate Waste Used in Food and Cosmetic Applications: A Review. Foods 2021, 10, 657. [Google Scholar] [CrossRef] [PubMed]
- Bertolo, M.R.; Martins, V.C.; Horn, M.M.; Brenelli, L.B.; Plepis, A.M. Rheological and antioxidant properties of chitosan/gelatin-based materials functionalized by pomegranate peel extract. Carbohydr. Polym. 2020, 228, 115386. [Google Scholar] [CrossRef]
- Abou Zekry, S.S.; Abdellatif, A.; Azzazy, H.M.E. Fabrication of pomegranate/honey nanofibers for use as antibacterial wound dressings. Wound Med. 2020, 28, 100181. [Google Scholar] [CrossRef]
- Foss, S.R.; Nakamura, C.V.; Ueda-Nakamura, T.; Cortez, D.A.; Endo, E.H.; Dias Filho, B.P. Antifungal activity of pomegranate peel extract and isolated compound punicalagin against dermatophytes. Ann. Clin. Microbiol. Antimicrob. 2014, 13, 32. [Google Scholar] [CrossRef]
- Islam, M. Food and medicinal values of Roselle (Hibiscus sabdariffa L. Linne Malvaceae) plant parts: A review. Open J. Nutr. Food Sci. 2019, 1, 1003. [Google Scholar]
- Abu-Izneid, T.; Rauf, A.; Khalil, A.A.; Olatunde, A.; Khalid, A.; Alhumaydhi, F.A.; Aljohani, A.S.; Sahab Uddin, M.; Heydari, M.; Khayrullin, M. Nutritional and health beneficial properties of saffron (Crocus sativus L.): A comprehensive review. Crit. Rev. Food Sci. Nutr. 2022, 62, 2683–2706. [Google Scholar] [CrossRef]
- Wali, A.F.; Alchamat, H.A.A.; Hariri, H.K.; Hariri, B.K.; Menezes, G.A.; Zehra, U.; Rehman, M.U.; Ahmad, P. Antioxidant, antimicrobial, antidiabetic and cytotoxic activity of Crocus sativus L. petals. Appl. Sci. 2020, 10, 1519. [Google Scholar] [CrossRef]
- Sirisa-ard, P.; Pholsonklam, K.; Satchachai, A.; Tragoolpua, Y.; Kaewkod, T. Antioxidant, antibacterial activities and cytotoxicity of garlic leaf extract from garlic waste. Nat. Life Sci. Commun. 2023, 22, e2023059. [Google Scholar] [CrossRef]
- Hernández-Montesinos, I.Y.; Carreón-Delgado, D.F.; Ocaranza-Sánchez, E.; Ochoa-Velasco, C.E.; Suárez-Jacobo, Á.; Ramírez-López, C. Garlic (Allium sativum) peel extracts and their potential as antioxidant and antimicrobial agents for food applications: Influence of pretreatment and extraction solvent. Int. J. Food Sci. Technol. 2023, 58, 6794–6805. [Google Scholar] [CrossRef]
- Gabriel, T.; Vestine, A.; Kim, K.D.; Kwon, S.J.; Sivanesan, I.; Chun, S.C. Antibacterial activity of nanoparticles of garlic (Allium sativum) extract against different bacteria such as Streptococcus mutans and Poryphormonas gingivalis. Appl. Sci. 2022, 12, 3491. [Google Scholar] [CrossRef]
- Rasmussen, T.B.; Bjarnsholt, T.; Skindersoe, M.E.; Hentzer, M.; Kristoffersen, P.; Kote, M.; Nielsen, J.; Eberl, L.; Givskov, M. Screening for quorum-sensing inhibitors (QSI) by use of a novel genetic system, the QSI selector. J. Bacteriol. 2005, 187, 1799–1814. [Google Scholar] [CrossRef]
- Rabbani, G.; Albert, M.J.; Rahman, A.H.; Isalm, M.M.; Islam, K.N.; Alam, K. Short-chain fatty acids improve clinical, pathologic, and microbiologic features of experimental shigellosis. J. Infect. Dis. 1999, 179, 390–397. [Google Scholar] [CrossRef] [PubMed]
- Bhatwalkar, S.B.; Mondal, R.; Krishna, S.B.N.; Adam, J.K.; Govender, P.; Anupam, R. Antibacterial properties of organosulfur compounds of garlic (Allium sativum). Front. Microbiol. 2021, 12, 613077. [Google Scholar] [CrossRef] [PubMed]
- Arulselvan, K.; Gayas, Z.; Uday, V. Antibacterial Activity of Garlic Extract against Streptococcus mutans and Lactobacillus acidophilus: An In Vitro Study. J. South Asian Assoc. Pediatr. Dent. 2022, 5, 26–31. [Google Scholar]
- D’Ercole, S.; D’Addazio, G.; Di Lodovico, S.; Traini, T.; Di Giulio, M.; Sinjari, B. Porphyromonas gingivalis load is balanced by 0.20% chlorhexidine gel. A randomized, double-blind, controlled, microbiological and immunohistochemical human study. J. Clin. Med. 2020, 9, 284. [Google Scholar] [CrossRef] [PubMed]
- Ohtani, M.; Nishimura, T. The preventive and therapeutic application of garlic and other plant ingredients in the treatment of periodontal diseases. Exp. Ther. Med. 2020, 19, 1507–1510. [Google Scholar] [CrossRef]
- Yadav, M.; Bohra, R.; Gupta, N. In vitro Determination of Antibacterial Effect of Garlic (Allium sativum) on Staphylococcus aureus and E. coli. Int. J. Curr. Microbiol. Appl. Sci. 2019, 8, 498–506. [Google Scholar] [CrossRef]
- Kim, H.W.; Seok, Y.S.; Cho, T.J.; Rhee, M.S. Risk factors influencing contamination of customized cosmetics made on-the-spot: Evidence from the national pilot project for public health. Sci. Rep. 2020, 10, 1561. [Google Scholar] [CrossRef]
- Dadashi, L.; Dehghanzadeh, R. Investigating incidence of bacterial and fungal contamination in shared cosmetic kits available in the women beauty salons. Health Promot. Perspect. 2016, 6, 159. [Google Scholar] [CrossRef] [PubMed]
- Muhammed, H.J. Bacterial and fungal contamination in three brands of cosmetic marketed in Iraq. Iraqi J. Pharm. Sci. 2011, 20, 38–42. [Google Scholar] [CrossRef]
- Pai, S.; Platt, M. Antifungal effects of Allium sativum (garlic) extract against the Aspergillus species involved in otomycosis. Lett. Appl. Microbiol. 1995, 20, 14–18. [Google Scholar] [CrossRef] [PubMed]
- Min, J.H.; Jeong, J.H.; Park, Y.U.; Lee, J.S.; Lee, S.J.; Chang, W.-B. Quality characteristics of garlic peel according to processing methods. Food Sci. Preserv. 2020, 27, 32–37. [Google Scholar]
- Gong, C.; Singh, A.; Singh, P.; Singh, A. Anaerobic digestion of agri-food wastes for generating biofuels. Indian J. Microbiol. 2021, 61, 427–440. [Google Scholar] [CrossRef]
- Hadj Saadoun, J.; Bertani, G.; Levante, A.; Vezzosi, F.; Ricci, A.; Bernini, V.; Lazzi, C. Fermentation of agri-food waste: A promising route for the production of aroma compounds. Foods 2021, 10, 707. [Google Scholar] [CrossRef]
- Osorio, L.L.D.R.; Flórez-López, E.; Grande-Tovar, C.D. The potential of selected agri-food loss and waste to contribute to a circular economy: Applications in the food, cosmetic and pharmaceutical industries. Molecules 2021, 26, 515. [Google Scholar] [CrossRef]
- Papaioannou, E.H.; Mazzei, R.; Bazzarelli, F.; Piacentini, E.; Giannakopoulos, V.; Roberts, M.R.; Giorno, L. Agri-food industry waste as resource of chemicals: The role of membrane technology in their sustainable recycling. Sustainability 2022, 14, 1483. [Google Scholar] [CrossRef]
- Panda, L.; Duarte-Sierra, A. Recent advancements in enhancing antimicrobial activity of plant-derived polyphenols by biochemical means. Horticulturae 2022, 8, 401. [Google Scholar] [CrossRef]
- Aziz, N.; Farag, S.; Mousa, L.; Abo-Zaid, M. Comparative antibacterial and antifungal effects of some phenolic compounds. Microbios 1998, 93, 43–54. [Google Scholar]
- Šeregelj, V.; Tumbas Šaponjac, V.; Lević, S.; Kalušević, A.; Ćetković, G.; Čanadanović-Brunet, J.; Nedović, V.; Stajčić, S.; Vulić, J.; Vidaković, A. Application of encapsulated natural bioactive compounds from red pepper waste in yogurt. J. Microencapsul. 2019, 36, 704–714. [Google Scholar] [CrossRef]
- Sharma, K.; Mahato, N.; Lee, Y.R. Systematic study on active compounds as antibacterial and antibiofilm agent in aging onions. J. Food Drug Anal. 2018, 26, 518–528. [Google Scholar] [CrossRef] [PubMed]
- Jaisinghani, R.N. Antibacterial properties of quercetin. Microbiol. Res. 2017, 8, 6877. [Google Scholar] [CrossRef]
- Gupta, N.; Poddar, K.; Sarkar, D.; Kumari, N.; Padhan, B.; Sarkar, A. Fruit waste management by pigment production and utilization of residual as bioadsorbent. J. Environ. Manag. 2019, 244, 138–143. [Google Scholar] [CrossRef] [PubMed]
- Piccolo, V.; Maisto, M.; Schiano, E.; Iannuzzo, F.; Keivani, N.; Manuela Rigano, M.; Santini, A.; Novellino, E.; Carlo Tenore, G.; Summa, V. Phytochemical investigation and antioxidant properties of unripe tomato cultivars (Solanum lycopersicum L.). Food Chem. 2024, 438, 137863. [Google Scholar] [CrossRef] [PubMed]
- Qian, W.; Yang, M.; Wang, T.; Sun, Z.; Liu, M.; Zhang, J.; Zeng, Q.; Cai, C.; Li, Y. Antibacterial mechanism of vanillic acid on physiological, morphological, and biofilm properties of carbapenem-resistant Enterobacter hormaechei. J. Food Prot. 2020, 83, 576–583. [Google Scholar] [CrossRef] [PubMed]
- Zdziobek, P.A.-O.X.; Jodłowski, G.A.-O.X.; Strzelec, E.A.-O. Biopreservation and Bioactivation Juice from Waste Broccoli with Lactiplantibacillus plantarum. Molecules 2023, 28, 4594. [Google Scholar] [CrossRef] [PubMed]
- Lima, V.N.; Oliveira-Tintino, C.D.; Santos, E.S.; Morais, L.P.; Tintino, S.R.; Freitas, T.S.; Geraldo, Y.S.; Pereira, R.L.; Cruz, R.P.; Menezes, I.R. Antimicrobial and enhancement of the antibiotic activity by phenolic compounds: Gallic acid, caffeic acid and pyrogallol. Microb. Pathog. 2016, 99, 56–61. [Google Scholar] [CrossRef]
- El-Shahat, M.S.; Rabie, M.A.; Ragab, M.; Siliha, H.I. Changes on physicochemical and rheological properties of biscuits substituted with the peel and alcohol-insoluble solids (AIS) from cactus pear (Opuntia ficus indica). J. Food Sci. Technol. 2019, 56, 3635–3645. [Google Scholar] [CrossRef]
- Moschona, A.; Liakopoulou-Kyriakides, M. Encapsulation of biological active phenolic compounds extracted from wine wastes in alginate-chitosan microbeads. J. Microencapsul. 2018, 35, 229–240. [Google Scholar] [CrossRef]
- Zhang, D.; Nie, S.; Xie, M.; Hu, J. Antioxidant and antibacterial capabilities of phenolic compounds and organic acids from Camellia oleifera cake. Food Sci. Biotechnol. 2020, 29, 17–25. [Google Scholar] [CrossRef] [PubMed]
- Ming, D.; Wang, D.; Cao, F.; Xiang, H.; Mu, D.; Cao, J.; Li, B.; Zhong, L.; Dong, X.; Zhong, X. Kaempferol inhibits the primary attachment phase of biofilm formation in Staphylococcus aureus. Front. Microbiol. 2017, 8, 2263. [Google Scholar] [CrossRef]
- Pereira, M.G.; Maciel, G.M.; Haminiuk, C.W.I.; Bach, F.; Hamerski, F.; de Paula Scheer, A.; Corazza, M.L. Effect of extraction process on composition, antioxidant and antibacterial activity of oil from yellow passion fruit (Passiflora edulis var. Flavicarpa) seeds. Waste Biomass Valoriz. 2019, 10, 2611–2625. [Google Scholar] [CrossRef]
- Budiati, T.; Suryaningsih, W.; Bethiana, T.N. Antimicrobial of tropical fruit and vegetable waste extract for food-borne pathogenic bacteria. Ital. J. Food Saf. 2022, 11, 10510. [Google Scholar] [CrossRef] [PubMed]
- Karpiński, T.; Adamczak, A.; Ożarowski, M. Antibacterial Activity of Apigenin, Luteolin, and Their C-Glucosides. In Proceedings of the 5th International Electronic Conference on Medicinal Chemistry, Online, 1–30 November 2019; pp. 1–9. [Google Scholar]
- Martillanes, S.; Rocha-Pimienta, J.; Delgado-Adámez, J. Agrifood by-products as a source of phytochemical compounds. Descr. Food Sci. 2018, 3, 43–58. [Google Scholar]
- Khare, T.; Anand, U.; Dey, A.; Assaraf, Y.G.; Chen, Z.-S.; Liu, Z.; Kumar, V. Exploring phytochemicals for combating antibiotic resistance in microbial pathogens. Front. Pharmacol. 2021, 12, 720726. [Google Scholar] [CrossRef]
- Lin, D.; Xiao, M.; Zhao, J.; Li, Z.; Xing, B.; Li, X.; Kong, M.; Li, L.; Zhang, Q.; Liu, Y. An overview of plant phenolic compounds and their importance in human nutrition and management of type 2 diabetes. Molecules 2016, 21, 1374. [Google Scholar] [CrossRef] [PubMed]
- Kakkar, S.; Bais, S. A review on protocatechuic acid and its pharmacological potential. Int. Sch. Res. Not. 2014, 2014, 952943. [Google Scholar] [CrossRef]
- Jayaraman, P.; Sakharkar, M.K.; Lim, C.S.; Tang, T.H.; Sakharkar, K.R. Activity and interactions of antibiotic and phytochemical combinations against Pseudomonas aeruginosa in vitro. Int. J. Biol. Sci. 2010, 6, 556. [Google Scholar] [CrossRef]
- Kuete, V.; Nana, F.; Ngameni, B.; Mbaveng, A.T.; Keumedjio, F.; Ngadjui, B.T. Antimicrobial activity of the crude extract, fractions and compounds from stem bark of Ficus ovata (Moraceae). J. Ethnopharmacol. 2009, 124, 556–561. [Google Scholar] [CrossRef]
- Jalali, O.; Best, M.; Wong, A.; Schaeffer, B.; Bauer, B.; Johnson, L. Protocatechuic acid as a topical antimicrobial for surgical skin antisepsis: Preclinical investigations. JBJS Open Access 2020, 5, e19. [Google Scholar] [CrossRef]
- Chen, X. A review on coffee leaves: Phytochemicals, bioactivities and applications. Crit. Rev. Food Sci. Nutr. 2019, 59, 1008–1025. [Google Scholar] [CrossRef] [PubMed]
- Segheto, L.; Santos, B.C.S.; Werneck, A.F.L.; Vilela, F.M.P.; de Sousa, O.V.; Rodarte, M.P. Antioxidant extracts of coffee leaves and its active ingredient 5-caffeoylquinic acid reduce chemically-induced inflammation in mice. Ind. Crops Prod. 2018, 126, 48–57. [Google Scholar] [CrossRef]
- Monteiro, Â.; Colomban, S.; Azinheira, H.G.; Guerra-Guimarães, L.; Do Céu Silva, M.; Navarini, L.; Resmini, M. Dietary antioxidants in coffee leaves: Impact of botanical origin and maturity on chlorogenic acids and xanthones. Antioxidants 2019, 9, 6. [Google Scholar] [CrossRef] [PubMed]
- Campa, C.; Mondolot, L.; Rakotondravao, A.; Bidel, L.P.; Gargadennec, A.; Couturon, E.; La Fisca, P.; Rakotomalala, J.-J.; Jay-Allemand, C.; Davis, A.P. A survey of mangiferin and hydroxycinnamic acid ester accumulation in coffee (Coffea) leaves: Biological implications and uses. Ann. Bot. 2012, 110, 595–613. [Google Scholar] [CrossRef] [PubMed]
- Yang, D.; Wang, T.; Long, M.; Li, P. Quercetin: Its main pharmacological activity and potential application in clinical medicine. Oxidative Med. Cell. Longev. 2020, 2020, 8825387. [Google Scholar] [CrossRef] [PubMed]
- Orús, P.; Gomez-Perez, L.; Leranoz, S.; Berlanga, M. Increasing antibiotic resistance in preservative-tolerant bacterial strains isolated from cosmetic products. Int. Microbiol. 2015, 18, 51–59. [Google Scholar]
- Santhosh Kumar, Y.; Kulanthaivel, L.; Hikku, G.; Saravanan, R.; Lakshmi, T.; Kirubhanand, C.; Karthikeyan, M.; Vijayalakshmi, P.; Subbaraj, G.K. Improved Antibacterial Activity of Water-Soluble Nanoformulated Kaempferol and Combretastatin Polyphenolic Compounds. Int. J. Polym. Sci. 2021, 2021, 5682182. [Google Scholar] [CrossRef]
- Badui Dergal, S. Química de los Alimentos; Pearson Educación: Mexico City, México, 2016. [Google Scholar]
- de Valle-Prieto, M.B.; Delgado-Adámez, J.; Gil, M.V.; Martillanes, S.; Franco, M.N.; Martín-Vertedor, D. Virgin olive oil enriched with lutein-zeaxanthin from Spinacia oleracea. J. Oleo Sci. 2017, 66, 463–468. [Google Scholar] [CrossRef][Green Version]
- Adeyemi, A.D.; Oluigbo, C.C.; Esan, A.O.; Bello, M.O.; Oladoye, S.O.; Emmanuel, C.P.; Effiong, E. Chemical Composition and Antimicrobial Activity of the Essential oils of 14 known Ficus species—A Concise. Biointerface Res. Appl. Chem 2021, 12, 8003–8034. [Google Scholar]
- Cunha, C.; Ribeiro, H.M.; Rodrigues, M.; Araujo, A.R. Essential oils used in dermocosmetics: Review about its biological activities. J. Cosmet. Dermatol. 2022, 21, 513–529. [Google Scholar] [CrossRef]
- Djenane, D. Chemical profile, antibacterial and antioxidant activity of Algerian citrus essential oils and their application in Sardina pilchardus. Foods 2015, 4, 208–228. [Google Scholar] [CrossRef] [PubMed]
- Farahmandfar, R.; Tirgarian, B.; Dehghan, B.; Nemati, A. Comparison of different drying methods on bitter orange (Citrus aurantium L.) peel waste: Changes in physical (density and color) and essential oil (yield, composition, antioxidant and antibacterial) properties of powders. J. Food Meas. Charact. 2020, 14, 862–875. [Google Scholar] [CrossRef]
- Hou, H.-S.; Bonku, E.M.; Zhai, R.; Zeng, R.; Hou, Y.-L.; Yang, Z.-H.; Quan, C. Extraction of essential oil from Citrus reticulate Blanco peel and its antibacterial activity against Cutibacterium acnes (formerly Propionibacterium acnes). Heliyon 2019, 5, e02947. [Google Scholar] [CrossRef] [PubMed]
- Zema, D.; Calabrò, P.; Folino, A.; Tamburino, V.; Zappia, G.; Zimbone, S. Valorisation of citrus processing waste: A review. Waste Manag. 2018, 80, 252–273. [Google Scholar] [CrossRef] [PubMed]
- Weng, Y.X.; Wang, H.C.; Chu, Y.A.-O.; Wu, Y.Z.; Liao, J.A.; Su, Z.A.-O. Essential oil from Citrus depressa peel exhibits antimicrobial, antioxidant and cancer chemopreventive effects. J. Sci. Food Agric. 2024, 104, 3982–3991. [Google Scholar] [CrossRef]
- Geraci, A.; Di Stefano, V.; Di Martino, E.; Schillaci, D.; Schicchi, R. Essential oil components of orange peels and antimicrobial activity. Nat. Prod. Res. 2017, 31, 653–659. [Google Scholar] [CrossRef]
- Quirino, A.; Giorgi, V.; Palma, E.A.-O.X.; Marascio, N.A.-O.; Morelli, P.A.-O.; Maletta, A.A.-O.X.; Divenuto, F.; De Angelis, G.; Tancrè, V.; Nucera, S.; et al. Citrus bergamia: Kinetics of Antimicrobial Activity on Clinical Isolates. Antibiotics 2022, 11, 361. [Google Scholar] [CrossRef]
- Malik, A.; Najda, A.A.-O.; Bains, A.; Nurzyńska-Wierdak, R.; Chawla, P.A.-O. Characterization of Citrus nobilis Peel Methanolic Extract for Antioxidant, Antimicrobial, and Anti-Inflammatory Activity. Molecules 2021, 26, 4310. [Google Scholar] [CrossRef] [PubMed]
- Satari, B.; Karimi, K. Citrus processing wastes: Environmental impacts, recent advances, and future perspectives in total valorization. Resour. Conserv. Recycl. 2018, 129, 153–167. [Google Scholar] [CrossRef]
- Fresh, F.C.F. Processed Statistical Bulletin 2016; FAO: Rome, Italy, 2017. [Google Scholar]
- Kim, S.Y. Chemical composition and antioxidant activity of crude polysaccharide from citron (Citrus junos Sieb. Ex TANAKA) seed. Prev. Nutr. Food Sci. 2018, 23, 335. [Google Scholar] [CrossRef] [PubMed]
- Farhat, A.; Fabiano-Tixier, A.-S.; El Maataoui, M.; Maingonnat, J.-F.; Romdhane, M.; Chemat, F. Microwave steam diffusion for extraction of essential oil from orange peel: Kinetic data, extract’s global yield and mechanism. Food Chem. 2011, 125, 255–261. [Google Scholar] [CrossRef]
- Fisher, K.; Phillips, C.A. The effect of lemon, orange and bergamot essential oils and their components on the survival of Campylobacter jejuni, Escherichia coli O157, Listeria monocytogenes, Bacillus cereus and Staphylococcus aureus in vitro and in food systems. J. Appl. Microbiol. 2006, 101, 1232–1240. [Google Scholar] [CrossRef]
- Burt, S. Essential oils: Their antibacterial properties and potential applications in foods—A review. Int. J. Food Microbiol. 2004, 94, 223–253. [Google Scholar] [CrossRef]
- Karakaya, H.; Ozturk, F.S.; Koc, T.B.; Yasar, K. Chemical Composition and Antimicrobial Activity of Pummelo (Citrus maxima) Essential Oil Derived from Fruit Peel. J. Essent. Oil Bear. Plants 2022, 25, 524–535. [Google Scholar] [CrossRef]
- Salvatori, E.A.-O.; Morgan, L.A.-O.; Ferrarini, S.A.-O.; Zilli, G.A.-O.; Rosina, A.A.-O.; Almeida, M.A.-O.; Hackbart, H.A.-O.X.; Rezende, R.A.-O.; Albeny-Simões, D.A.-O.X.; Oliveira, J.A.-O.; et al. Anti-Inflammatory and Antimicrobial Effects of Eucalyptus spp. Essential Oils: A Potential Valuable Use for an Industry Byproduct. Evid. Based Complement. Altern. Med. 2023, 2023, 2582698. [Google Scholar] [CrossRef]
- Sembiring, N.; Napitupulu, H.; Sembiring, M.T.; Ishak, A.; Gunawan, H. Fulfilling Eucalyptus Raw Materials for Pulp and Paper Production Plants. In Proceedings of the IOP Conference Series: Earth and Environmental Science, Yogyakarta, Indonesia, 28–29 July 2021; IOP Publishing: Bristol, UK, 2021; p. 012008. [Google Scholar]
- de Amorim, V.d.S.S.; Monteiro, K.M.S.; Sousa, G.O.; Damascena, J.F.; Pereira, J.A.; dos Santos Moraes, W. Os benefícios ambientais do plantio de eucalipto: Revisão de literatura. Res. Soc. Dev. 2021, 10, e318101119604. [Google Scholar] [CrossRef]
- Dhakad, A.K.; Pandey, V.V.; Beg, S.; Rawat, J.M.; Singh, A. Biological, medicinal and toxicological significance of Eucalyptus leaf essential oil: A review. J. Sci. Food Agric. 2018, 98, 833–848. [Google Scholar] [CrossRef]
- Bachir, R.G.; Benali, M. Antibacterial activity of the essential oils from the leaves of Eucalyptus globulus against Escherichia coli and Staphylococcus aureus. Asian Pac. J. Trop. Biomed. 2012, 2, 739–742. [Google Scholar] [CrossRef] [PubMed]
- Manful, M.E.; Ahmed, L.; Barry-Ryan, C. Cosmetic Formulations from Natural Sources: Safety Considerations and Legislative Frameworks in the European Union. Cosmetics 2024, 11, 72. [Google Scholar] [CrossRef]
- Rathee, P.; Sehrawat, R.; Rathee, P.; Khatkar, A.; Akkol, E.K.; Khatkar, S.; Redhu, N.; Türkcanoğlu, G.; Sobarzo-Sánchez, E. Polyphenols: Natural Preservatives with Promising Applications in Food, Cosmetics and Pharma Industries; Problems and Toxicity Associated with Synthetic Preservatives; Impact of Misleading Advertisements—Recent Trends in Preservation and Legislation. Materials 2023, 16, 4793. [Google Scholar] [CrossRef] [PubMed]
- Ribeiro, A.S.; Estanqueiro, M.; Oliveira, M.B.; Sousa Lobo, J.M. Main Benefits and Applicability of Plant Extracts in Skin Care Products. Cosmetics 2015, 2, 48–65. [Google Scholar] [CrossRef]
- Crupi, P.; De Santis, S.; Bavaro, S.L.; Fracassetti, D. New insights and perspectives of polyphenols in nutrition. Front. Nutr. 2023, 10, 1145135. [Google Scholar] [CrossRef] [PubMed]
- Ciubuca, B.-M.; Saviuc, C.-M.; Chifiriuc, M.-C.; Lazar, V. Microbial Resistance to Natural Compounds: Challenges for Developing Novel Alternatives to Antibiotics. Curr. Org. Chem. 2016, 20, 2983–2988. [Google Scholar] [CrossRef][Green Version]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).