Abstract
Chios mastic gum (CMG), the resin of Pistacia lentiscus var. Chia, is a product with great ethnopharmacological and economic significance. This study attempts to investigate, for the first time, the activity of CMG, its fractions and isolated compounds against specific enzymes, which play pivotal roles in the degradation of proteins contained in skin connective tissue. Initially, crude CMG was subjected to extraction, fractionation and isolation through different chromatographic techniques to obtain the acidic and neutral fraction of terpenes. Additionally, the characteristic and major active triterpene acids of CMG, masticadienonic and isomasticadienonic acids (MNA, IMNA) were isolated in pure form. All samples were analysed by means of High-Performance Thin-Layer Chromatography (HPTLC) with four distinct development systems to obtain their constituents’ profile. Finally, samples were tested for their ability to inhibit the elastase and collagenase enzymes. According to our findings, for collagenase, a mixture of MNA and IMNA demonstrated the most potent activity with an IC50 value of 31.07 μg/mL, while for elastase CMG’s acidic fraction provided the most promising results with an IC50 value of 17.30 μg/mL. Overall, these results attempt to fill the gap in scientific knowledge about the use of CMG and its constituents in skincare and cosmetic products.
1. Introduction
Skin aging is a complex process influenced by intrinsic and extrinsic factors, impacting the structure and function of the epidermis and dermis. Typically, alterations in skin structure, function, and appearance are more pronounced in photoaged skin compared to chronologically aged skin [1]. Skin exposure to UV radiation significantly contributes to the overproduction of reactive oxygen species (ROS), resulting in elevated levels of oxidative stress in the epidermis and the degradation of the extracellular matrix (ECM) by triggering the production of dermal enzymes such as collagenase (matrix metalloproteinase-1, MMP-1) and elastase. These enzymes break down collagen and elastin, which are essential components of ECM and maintain the skin’s structural integrity and elasticity, helping to maintain a youthful appearance. This degradation manifests in various skin issues, including wrinkles, rough texture, dryness, reduced elasticity, and uneven pigmentation. Consequently, the increased activity of collagenase and elastase accelerates premature skin aging, making them primary targets for testing the efficacy of novel anti-aging treatments [2].
Chios mastic gum (CMG), the resin of Pistacia lentiscus var. Chia, is a product with great ethnopharmacological and economic significance. Both the resin and its essential oil are PDO products of Greece, while cultivation of the mastic tree on the island of Chios has been registered as an intangible heritage of humanity by UNESCO [3]. Based on mastic’s EMA monograph issued in 2015, the resin can be used as a traditional herbal medicinal product with two indications: namely, the treatment of mild dyspeptic disorders and for the treatment of minor skin inflammations and minor wounds [4]. CMG’s application in skin conditions has been known since antiquity. Among the most influential and esteemed physicians of the ancient world, Dioscorides, in the 1st century AD, described mastic as a skincare agent, among its other properties [5]. The Egyptians included the resin in their embalming rituals [6,7,8], while traces of mastic found in an Etruscan ointment of the 1st century BC testify to the widespread use of mastic across the Mediterranean [9]. Nowadays CMG is traded exclusively by the Chios Mastiha Growers Association (CMGA) and a plethora of products containing mastic can be found on the market, including foods and beverages, food supplements, oral hygiene products as well as cosmetics [3].
CMG comprises the natural polymer cis-1,4-poly-β-myrcene, the essential oil (Chios mastic oil—CMO), as well as the terpene fraction which can be further divided into acidic and neutral terpenes [3]. Over the last few decades, several studies have been published concerning the biological activity of the resin in diverse therapeutic targets such as inflammation [10], cardiovascular diseases [11] and metabolic syndrome [12], to name a few. Nevertheless, mastic’s individual constituents have seldom been the focal point of research despite the promising data hinting in this direction [13,14]. This phenomenon can be largely attributed to the resin’s chemical complexity and the presence of the insoluble polymer which often hinders sample handling [3]. Furthermore, the commercial unavailability of these bioactives further complicates the evaluation of their biological and pharmacological properties. However, mastic’s extensive use in diverse applications highlights the need to overcome the challenges in the further exploitation of its constituents in order to unlock their full potential.
Taking the above into account, the current study involves an in vitro evaluation of the biological activity of CMG and its individual components against the elastase and collagenase enzymes, in order to highlight the benefits of their application in cosmetics and skincare products. In this framework, a multi-step extraction, fractionation and purification process was carried out and sample profiling was performed by means of HPTLC, a fast and versatile analytical technique, widely employed in the field of natural products.
2. Materials and Methods
2.1. Materials and Chemicals
Sulfuric acid (puriss. meets analytical specification of Ph. Eur., BP, 95–97%) was purchased from Sigma-Aldrich (St. Louis, MO, USA). Dimethyl sulfoxide and isopropyl alcohol were acquired from Merck (Kenilworth, NJ, USA). Methanol (MeOH) for analysis [≥99.8% (HPLC)] and ethanol absolute (99.8% HPLC grade) were purchased from Fischer Chemical (Pittsburgh, PA, USA). Petroleum ether (RPE, for analysis) and ethanol 96° were supplied by Carlo Erba Reagents (Milan, Italy). Dichloromethane (DCM, reagent grade) was from Scharlau, Barcelona, Spain. Vanillin used for spot visualization (Acros Organics, Fair Lawn, NJ, USA) was 99% pure. Ethyl acetate (EtOAc), n-Hexane, cyclo-hexane, toluene, diisopropyl ether and MeOH used for extraction and HPTLC analysis were of analytical grade (Fisher Scientific, Loughborough, Belgium), while water (H2O) was distilled. For the pH adjustment sodium hydroxide pellets (NaOH- penta CHEMICALS UNITED) and hydrochloric acid (HCl- analytical grade; Fisher Scientific) for extraction and enzymatic assays were used. Acetonitrile (ACN, Avantor Performance Materials, Poland), and H2O (Fisher Scientific) used for preparative high-performance liquid chromatography (HPLC) analysis were of HPLC grade. Food grade CO2 was obtained from Revival A. E. Ursolic acid was purchased from Sigma-Aldrich.
All the required reagents for the enzymatic assays, such as elastase type IV from porcine pancreas, N-succinyl-Ala-Ala-Ala-p-nitroanilide substrate, Trizma-base, elastatinal, collagenase from Clostridium histolyticum released from physiologically active rat pancreatic islets Type V, ≥1 FALGPA units/mg solid, >125 CDU/mg solid, Tris-HCl, MMP-2 substrate (MCA-Pro-Leu-Ala-Nva-DNP-Dap-Ala-Arg-NH2) and phosphoramidon were purchased from Sigma Aldrich. H2O was obtained from a Milli-Q purification system (Merck Millipore, Darmstadt, Germany).
2.2. Chios Mastic Gum Fractionation and Isolation of Terpenes
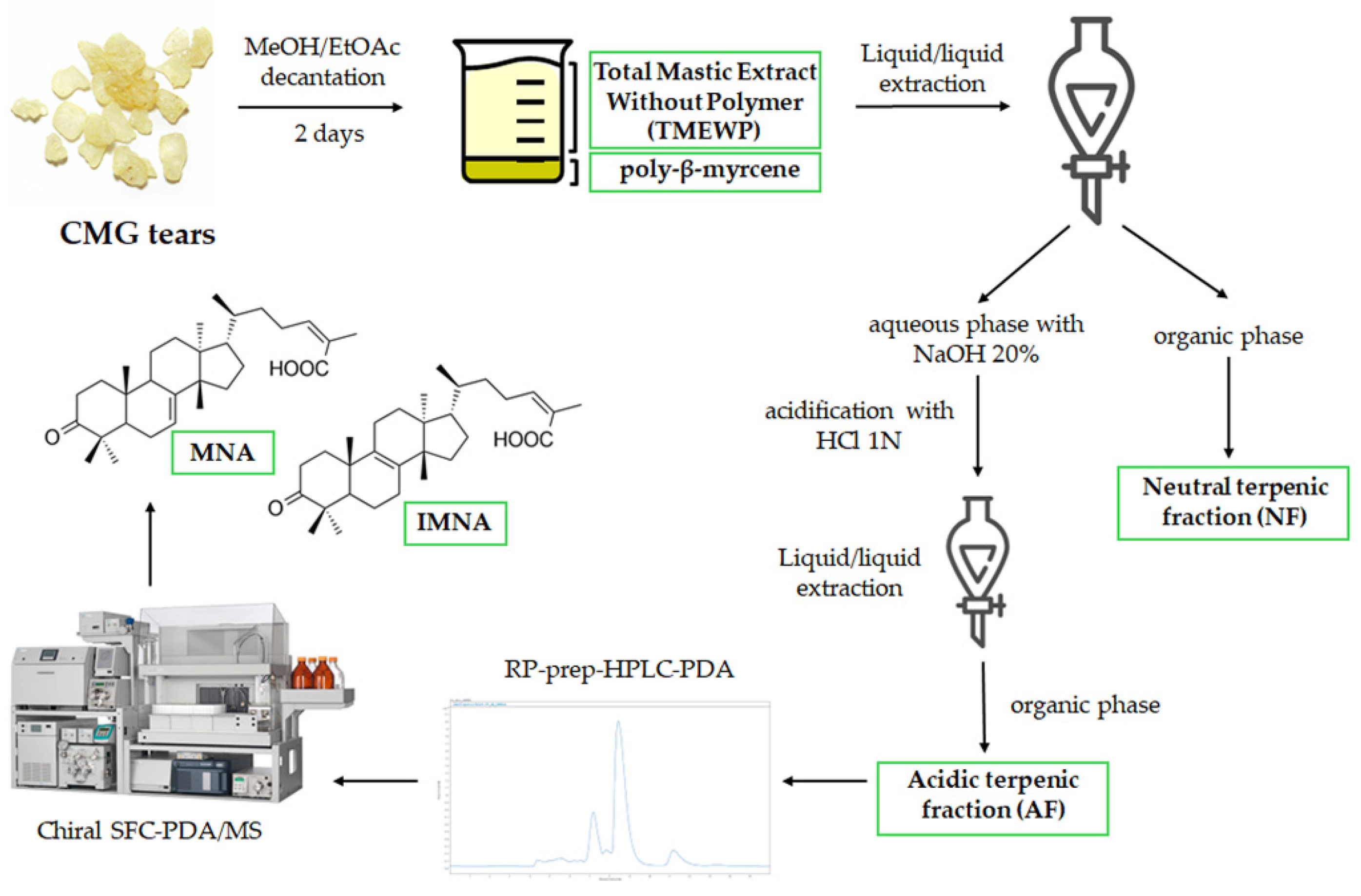
CMG was kindly provided by the Chios Mastiha Growers Association (CMGA). Firstly, a decantation process as described by Paraschos et al., was followed resulting in the Total Mastic Extract Without Polymer (TMEWP) [15]. A total of 500 g of CMG was dissolved in 500 mL of EtOAc and then 1500 mL of MeOH was added. After being left at room temperature for 2 days, the insoluble layer of the polymer, cis-1,4-poly-β-myrcene (150 g), was removed. After filtration and solvent evaporation, 350 g of TMEWP was obtained as white powder. Subsequently, the TMEWP was separated into the acidic (AF) and neutral terpene fractions (NF). In brief, 10 g of TMEWP was partitioned in a separatory funnel between a polar phase (20% NaOH in distilled water/MeOH 1:1 v/v, pH = 11) and a non-polar phase (n-Hexane/EtOAc 8:2 v/v), using 200 mL of each. The non-polar phase was then thrice re-extracted with 100 mL of the polar phase (100 mL each time), and following solvent evaporation, the non-polar extract produced a total of 4.5 g of mastic’s NF. The combined aqueous phase (500 mL in total) was subsequently acidified with 1N HCl until pH = 3 and extracted with 500 mL of EtOAc, which, once evaporated, produced 5.5 g of AF. All solvent evaporations were performed using a rotary evaporator (Buchi) with a water bath at 40 °C.
The isolation of the mixture of MNA and IMNA was performed using preparative Reverse Phase High-Performance Liquid Chromatography (RP-HPLC-PDA) (Laballiance, State College, PA, USA). The system consisted of two Lab Alliance preparative 36 Pumps and an injection valve Rheodyne 7755–027 equipped with a 1 mL loop (Target Analysis, Thessaloniki, Greece) hyphenated to a photo diode array detector (ECOM SN 7007587, Czech Republic). 50 mg of the AF were injected in a reversed-phase column (Fortis, C18, 5 μm, 250 × 21.2 mm) (Fortis Technologies, Cheshire, UK). The prep HPLC system was controlled by ClarityTM ver. 8.2 (DataApex, Prague, Czech Republic). The UV detection wavelength was performed at 210 nm and the desired fraction was collected manually based on UV chromatograms. Elution was carried out with a solvent system consisting of H2O (A) and ACN (B) using a gradient elution with a flow rate of 14 mL/min, which produced 15 mg of MNA/IMNA mixture. The elution method started at 20% B, which increased to 70% B in 3 min, reaching 100% B in 12 min, and was maintained for 13 min before returning to the initial conditions in 4 min for an 8-min re-equilibration (40 min in total).
Pure MNA and IMNA were isolated using Supercritical Fluid Chromatography (SFC) as previously described, with some modifications [14]. Briefly, the instrumentation consisted of a Waters Prep 15 SFC Purification System coupled with a SQ Detector 2 mass spectrometer. The mass analyzer was equipped with a Z-spray ion source operated in ESI negative. For the purification of MNA and IMNA, a ChiralPak IC column (5 μm, 4.6 × 250 mm) was employed. The mobile phase consisted of supercritical CO2 with MeOH/ACN 1:1 v/v as an organic modifier. Flow rate was set at 5 mL/min. Gradient elution started at 15% co-solvent and increased to 20% in 20 min before going back to the initial conditions in 3 min for a 3-min equilibration step. For the MS detection, a SIR function monitoring m/z = 453.40 as well as a full scan function with a mass range of 200 to 800 amu were used simultaneously. Isolated pure compounds were characterized using HRMS and 1D NMR (1H and 13C) and compared with the literature. 1H NMR, 13C NMR spectra were recorded on a Bruker Avance III 600 MHz spectrometer (Bruker Biospin AG, Faellanden, Switzerland) and on a Bruker aV400. For calibration, the solvent’s peak (CDCl3), set at 7.26 ppm for 1H and at 77.23 ppm for 13C, was used for reference. HRMS spectra were obtained on a hybrid Velos Pro Orbitrap Elite Mass Spectrometer (Thermo Scientific, Bremen, Germany). All spectrometric and spectroscopic data are provided in the Supporting Information.
2.3. HPTLC Profiling
HPTLC analyses were performed on a CAMAG (Muttenz, Switzerland) system consisting of an automatic TLC sampler (ATS4), an automatic development chamber (ADC2), a Visualizer 2 Documentation System and a Derivatizer, all controlled by the software platform VisionCats 2.5 (all from CAMAG). Extracts and fractions were prepared at 10 mg/mL in MeOH, while MNA, IMNA and their mixture were prepared at 0.5 mg/mL in the same solvent. Ursolic acid was prepared at three concentrations (0.25, 0.5 and 1.0 mg/mL) in MeOH and used as an intensity marker. For sample application, 20 × 10 cm HPTLC Silica gel 60 F254 glass plates (Merck) were employed. Sample solutions were applied bandwise with the autosampler ATS4 using a syringe of 25 μL (Hamilton, Reno, NV, USA) and a nitrogen aspirator with the following standard settings: tracks with 10 mm bands, 8 mm distance from the lower edge, 20 mm from the left and right edges, 14 mm between the different tracks, and 200 nL/s delivery speed. Four different mobile phases were tested: Development System 1 (DS1) consisted of DCM/MeOH (98:2 v/v), DS2 of cyclohexane/diisopropyl ether/acetic acid (60:40:10 v/v/v); DS3 of cyclohexane/EtOAc (80:20 v/v) and DS4 of toluene/EtOAc (90:10 v/v). In all cases, the application volume was set to 5 μL. The standard settings used in the development chamber were the following: 20 min chamber saturation, 10 min of plate activation (conditioning) at 33% relative humidity using MgCl2 as a desiccant, and 5 min of plate drying. Solvent front was set to 8.5 cm. Plate images were recorded at 254 nm and 366 nm before spraying and at white light after spraying. For spot visualization, the plates were sprayed with vanillin reagent R [i.e., 2 mL of sulfuric acid added to 100 mL of a 10 g/L solution of vanillin in ethanol (96°)] and heated at 100–105 °C for 5 min in the oven.
2.4. Enzymatic Assays
2.4.1. Collagenase Assay
The inhibition against collagenase enzyme was determined regarding a previously described spectrofluorimetric protocol with slight modifications [16]. Firstly, 50 μL of Tris-HCl buffer solution (50 mM, pH = 7.3), 25 μL of the tested sample (dissolved in Tris-HCl buffer containing < 5% organic solvent), and 25 μL of 30 μg/mL collagenase solution in Tris-HCl buffer were preincubated in a black 96-well microplate for 10 min at 37 °C. Then, 25 μL of 50 μM MMP-2 substrate in the abovementioned buffer was added, and the plate was incubated for an additional 30 min under the same experimental conditions. The fluorescence intensity was measured at an excitation maximum of 320 nm and an emission maximum of 405 nm, at the same temperature, using an Infinite 200 PRO series reader (Tecan, Männedorf, Switzerland). During the incubation periods, light exposure was avoided. Phosphoramidon is a strong metallo-endopeptidase inhibitor, with an IC50 = 6.9 μM, and was used as the positive control, while Tris-HCl buffer containing less than 5% of organic solvent (DMSO or isopropyl alcohol) served as the negative control. All experiments were performed three times.
2.4.2. Elastase Assay
Elastase inhibition was determined by the presence of the UV-Vis absorbing product p-nitroaniline, after the reaction of the elastase enzyme with the substrate N-succinyl-Ala-Ala-Ala-p-nitroanilide, based on a previously described protocol with minimum modifications [16]. In a 96-well microplate, 70 μL Trizma base buffer (50 mM, pH= 7.4), 10 μL of tested sample (dissolved in Trizma base buffer containing <5% organic solvent) and 5 μL of elastase solution (initial concentration 0.90 U/mL, dissolved in Trizma base buffer) were incubated for 10 min at room temperature, avoiding light exposure. Next, 15 μL of the substrate (initial concentration 2 mM, dissolved in Trizma base buffer) was added in every well and the mixtures were incubated for 30 min at 37 °C. The presence of p-nitroaniline was measured spectrophotometrically at 405 nm, at a constant temperature of 37 °C, using the abovementioned reader. Trizma base buffer containing less than 5% of the organic solvent used to dissolve the tested extracts (DMSO or isopropyl alcohol) served as the negative control, while elastatinal, a strong irreversible competitive elastase inhibitor (IC100 = 5 μg/mL, and IC50 = 0.5 μg/mL) served as the positive control. All experiments were performed in triplicate.
2.5. Statistical Analysis
The inhibition percentage of collagenase was calculated with the following formula:
where: FA = FControl − FControl Blank and FB = FSample − FSampleBlank. FControl is the measured fluorescence intensity of the negative control and FSample is the measured fluorescence intensity of the tested sample. Blanks consisted of all the abovementioned reagents except for the enzyme.
Inhibition (%) = (FA − FB)/(FA) × 100
Similarly, the inhibition percentage of elastase was calculated using the following formula:
where: AbsA = AbsControl − AbsControlBlank and AbsB = AbsSample − AbsSampleBlank. AbsControl is the measured absorbance of the negative control and AbsSample is the measured absorbance of the tested sample. Blanks consisted of all the abovementioned components except for the corresponding enzyme.
Inhibition (%) = (AbsA − AbsB)/(AbsA) × 100
GraphPad Prism software 8.0.1 was utilized for all the analyses. The means are represented as mean ± SD (n = 3) for all the biological assays and the occurrence of the statistical differences among the data was evaluated using one-way ANOVA. The IC50 calculation was performed using the four-parameter dose−response curve model.
3. Results and Discussion
3.1. Extraction, Fractionation and Isolation of Triterpenes from CMG
CMG is a particularly challenging substrate due to its resinous nature, which is largely due to its natural polymer cis-1,4-poly-β-myrcene [3]. Thus, most extraction efforts for the recovery of mastic’s compounds often begin with the removal of the insoluble polymer, usually by decantation with an appropriate solvent system. In our case the method proposed by Paraschos et al. was employed with slight modifications [15], whereby the polymer is removed after a two-day decantation with a mixture of MeOH and EtOAc. Subsequently, the TMEWP was further separated into the acidic (AF) and neutral terpene fractions (NF) via a series of liquid/liquid extractions with different polarities and pH conditions. Through this process, the acidic terpenes are first transformed to sodium salts with the addition of NaOH—thus staying in the aqueous/polar phase—while neutral terpenes are partitioned in the organic/non-polar phase. Next, the aqueous solution is re-acidified with HCl and extracted with a non-polar solvent system to obtain the non-ionized forms of the acidic terpenes.
Afterwards, the AF can be utilized for the isolation of mastic’s most characteristic compounds, namely masticadienonic (MNA) and isomasticadienonic acids (IMNA) which have been found to comprise approximately 25–30% of the resin’s total weight [17]. In the current work, the AF was further fractionated by prep-HPLC-PDA in order to obtain a fraction rich in MNA and IMNA. Due to their structural similarity (they differ only by the position of one double bond), the two isomers are often observed to co-elute in reverse phase chromatographic separations, particularly when low resolution (preparative) columns are employed. It is important to note that the two triterpenic acids of CMG are not commercially available, a fact that hinders their further investigation and, most importantly, a thorough assessment of their properties. However, in this case, in order to acquire the two triterpenes in pure form, a chiral chromatography semi-preparative SFC-PDA/MS method developed by our research group was employed, which has proven able to isolate the two compounds in high purity and yield [14]. Compound identification was based on the interpretation of 1D NMR spectra, as well as the compounds’ HRMS and HRMS/MS spectra (Supplementary Information, Figures S1–S6) and comparison with the available literature [14,15]. A schematic workflow of CMG’s extraction and fractionation is presented in Figure 1.
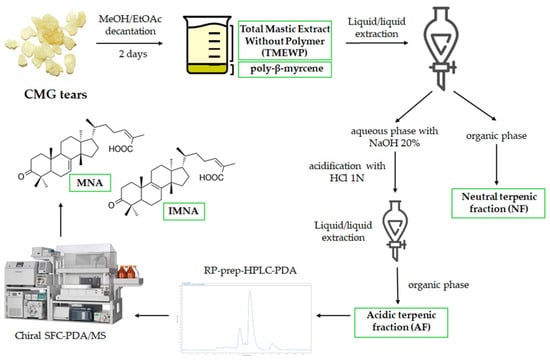
Figure 1.
CMG extraction and fractionation workflow to obtain the different samples used in this study.
3.2. HPTLC Profiling of CMG Constituents
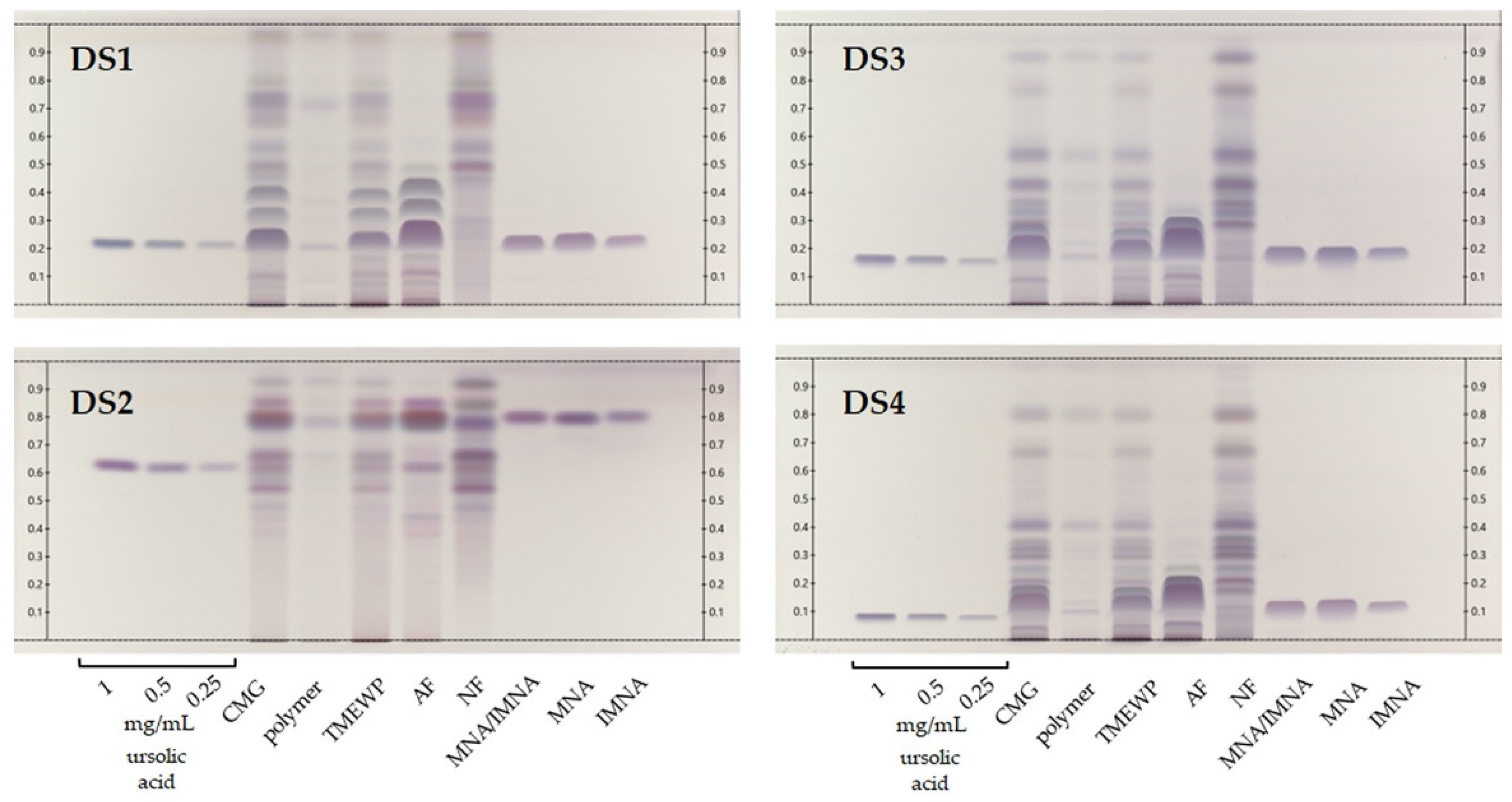
HPTLC is an excellent method for the rapid qualitative screening of plant extracts, fractions and isolated compounds, since it requires minimum solvent consumption and low sample quantities, as well as offering immense versatility in terms of chromatographical and derivatization conditions [18,19]. In one previous publication [20], we compared the method proposed by the European Pharmacopoeia [21] for the quality control of mastic with an in-house method for improved compound resolution. In this study, the in-house method consisting of DCM/MeOH 98:2 (v/v) (DS1) was compared against three additional development systems (DS2-4) that have previously been proposed for samples containing triterpenoids and steroidal compounds [22]. As shown in Figure 2, these were the CMG extract, fractions and isolated compounds, along with three concentrations of ursolic acid reference standard, which was used as an intensity marker [23]. All samples were profiled by means of HPTLC, offering a wide view of the samples’ chemical composition. With the exception of DS2, whose increased polarity appears not to suit the analysis of our samples’ constituents, the other three systems may contribute meaningful information to the analysis of CMG’s products.
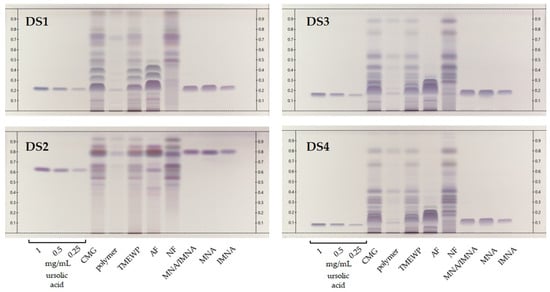
Figure 2.
HPTLC chromatograms of CMG extracts, fractions and isolated compounds. Ursolic acid at three levels of concentration (0.25, 0.5 and 1 mg/mL) was used as intensity marker. DS1: DCM/MeOH (98:2 v/v), DS2: c-Hex/diisopropyl ether/acetic acid (60:40:10 v/v/v); DS3: c-Hex/EtOAc (80:20 v/v) and DS4: toluene/EtOAc (90:10 v/v). Detection at visible light after spraying with sulfuric vanillin reagent.
First, as a general remark, we observe that none of the proposed systems is able to fully distinguish the isomers MNA and IMNA, which in most cases elute as a single dark purple-blue broad band that appears to largely dominate the profiles of both the pure CMG and the TMEWP. Moreover, DS1 offers a significant advantage for the analysis of CMG’s acidic terpenes since it is able to separate several bands that cluster tightly together with the other systems. Among these, we note two light blue-gray bands (DS1, Rf 0.35 and 0.42 respectively) that, according to our previous studies, correspond to the pentacyclic triterpenes and isomers of MNA and IMNA, oleanonic and moronic acids [20]. Additionally, DS1 allows us to observe the successful and satisfactory separation of mastic’s AF and NF that seem to possess quite distinctive profiles.
Moreover, even though DS1 is highly suited for the somewhat more polar, acidic terpenes, DS3 and DS4 offer a detailed view into the NF’s complex chemical composition. Evidently, and despite the fact that mastic’s NF is more diverse in nature compared to the AF, it has not been extensively studied, since to the best of our knowledge, so far only a handful of publications have reported data on its constituents [15,24,25,26,27] and even less on its biological activity [28,29,30]. In fact, NF has been found to contain mainly tirucallol, dammaradienone, 28-norolean-12-en-3-one, oleanonic and oleanolic aldehydes [15]. These compounds present a distinct biological interest since a study by Andreadou et al. demonstrated that NF, along with TMEWP, were able to reduce the infarct size in normal rabbits and reduced total cholesterol levels by 88% and 47% respectively in cholesterol fed animals [13]. Finally, special reference should be made to CMG’s insoluble polymer, cis-1,4-poly-β-myrcene, which was first chemically characterized by Van Den Berg et al. [31] and whose medicinal properties have remained largely unexplored with only a few exceptions [32]. In our HPTLC analysis, the polymer appears as a very dark brown, almost black band at the base of the chromatogram and does not seem prone to elute even with relatively polar development systems (DS2). Nevertheless, in the authors’ opinion, mastic’s insoluble polymer presents promising therapeutic and chemical properties that merit further investigation.
Finally, special reference should be made to the adulteration attempts suffered by CMG and its products, which are commonly observed. These mainly concern blending or replacing authentic mastic resin with inferior quality products such as Pistacia atlantica resin, or other resins from Boswellia and Pinus species, along with other inorganic substances. Unfortunately, these phenomena lead to consumer fraud and/or low quality products. So far, there is no simple method for quality control purposes given the fact that terpenes are difficult to analyze with conventional HPLC methods due to their low absorbance under UV [17]. Thus, an HPTLC methodology like the one proposed herein could be easily employed for the quality assessment not only of crude CMG resin, but also its products.
3.3. Collagenase and Elastase Inhibition
Elastase and collagenase are important enzymes involved in the degradation of proteins contained in skin connective tissue [33,34]. More specifically, elastases are serine proteases that degrade elastin fibers and regulate, along with collagen, the mechanical properties of the skin including strength, tissue remodeling elasticity, and wound healing capacity. Elastin is a major protein in healthy human skin, comprising over 90% of the elastic fibers that provide skin with flexibility. The breakdown of elastin, primarily caused by the enzyme elastase, leads to sagging skin and the formation of fine wrinkles [35].
On the other hand, collagenases are transmembrane zinc endopeptidases that degrade the peptide bonds of collagen, the most abundant component of the extracellular matrix [36]. Collagen is a crucial component for human skin health, providing structural support, elasticity, firmness, and flexibility. Collagenases are enzymes that break down skin collagen, leading to the formation of wrinkles as we age. Under normal physiological conditions, these enzymes accurately regulate the maintenance of skin tissue homeostasis; nonetheless, under oxidative stress or UV radiation, these matrix metalloproteinases (MMPs) are overexpressed, leading to skin disorders such as premature skin aging, inflammation, or, more seriously, degenerative diseases [37,38]. Therefore, compounds capable of inhibiting this enzyme can potentially delay wrinkle formation and the photoaging process [35].
Although research for natural extracts or isolated compounds that could inhibit these enzymes has been extensive and thus of great interest for the cosmetic industry [39], there are limited scientific references regarding the potential dermo-cosmetic applications of Pistacia lentiscus resin and its main constituents, despite the fact that cosmetic products containing mastic can be found on the market. This literature gap may be attributed to mastic’s challenging chemical composition, and particularly to the fact that it is highly insoluble in water, which serves as the base for most enzymatic assays [3]. Consequently, in the current study, following a series of extraction and fractionation steps, we investigated for the first time the elastase and collagenase inhibitory properties of not only the pure Chios mastic resin, but also its individual constituents. To counteract issues with solubility, other authors have proposed the use of alcohols for sample dilution when necessary [40,41]. In our case, particularly for the CMG extract, the polymer fraction and NF, isopropyl alcohol was employed for the initial sample dissolution, which was further diluted with buffer to a concentration below 5% (v/v) in the final sample solution.
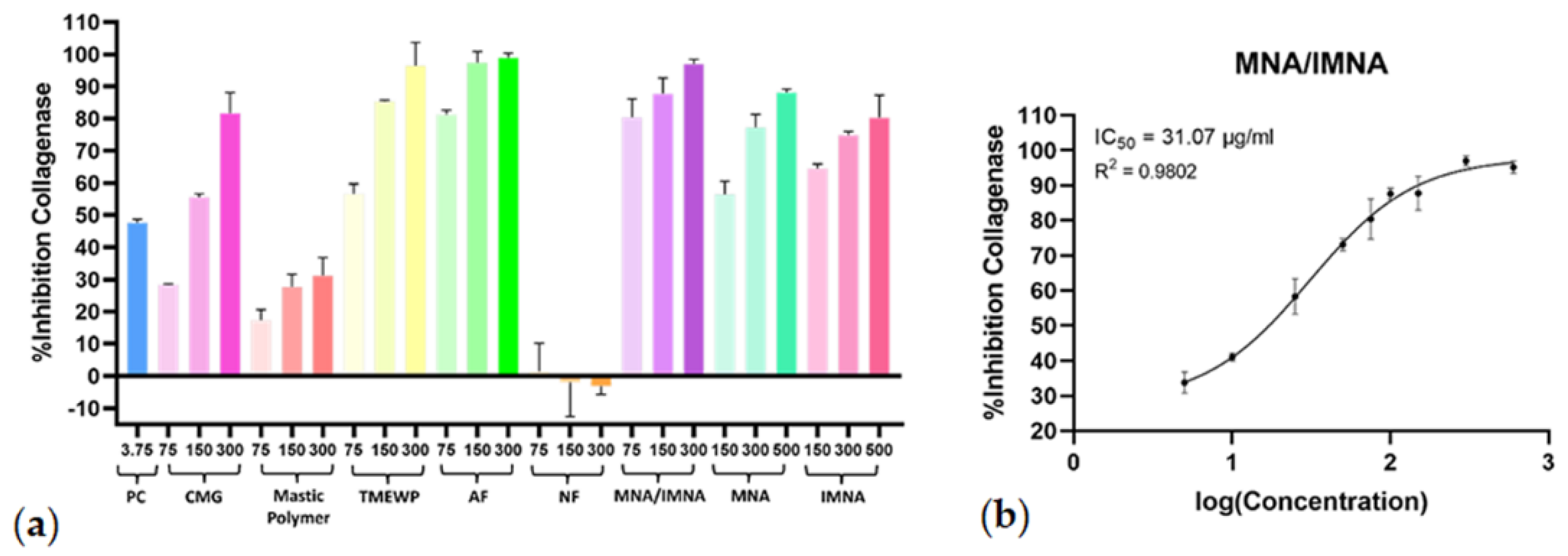
The initial screening results displayed significant collagenase inhibitory effects at concentrations of 75 μg/mL, 150 μg/mL and 300 μg/mL for CMG, TMEWP, AF and the MNA/IMNA mixture, and at 150 μM, 300 μM and 500 μM for the pure compounds. In contrast, the polymer and NF exhibited minimal to no inhibitory effects (Figure 3a). For the CMG products with the most potent activity, further experiments were carried out to calculate dose−response curves (R2 > 0.97) and the half-maximal inhibitory concentration (IC50). The AF exhibited strong anti-collagenase activity (IC50 = 38.52 μg/mL), while the mixture of pure compounds MNA/IMNA was the most potent with an IC50 value of 31.07 μg/mL (Figure 3b); interestingly, the TMEWP extract demonstrated enhanced activity compared to the crude CMG resin. Finally, it is worth mentioning that the MNA/IMNA mixture was more effective than the isolated pure compounds, indicating a possible synergistic effect. What is more, when comparing the two isomers, IMNA showed stronger anti-collagenase activity; a fact which could be attributed to the position of the double bond on the B-ring of the triterpenic scaffold, which greatly influences the compounds’ stereochemistry and therefore their capacity to bond to active sites of enzymes.
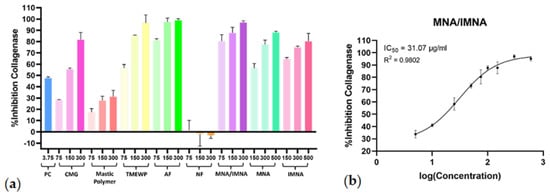
Figure 3.
(a) Collagenase inhibitory activity of CMG, CMG fractions, MNA/IMNA mixture and isolated pure compounds. Bars represent mean ± S.D., n = 3. (b) Dose−response curve of the most potent anti-collagenase sample (MNA/IMNA mixture). PC = positive control.
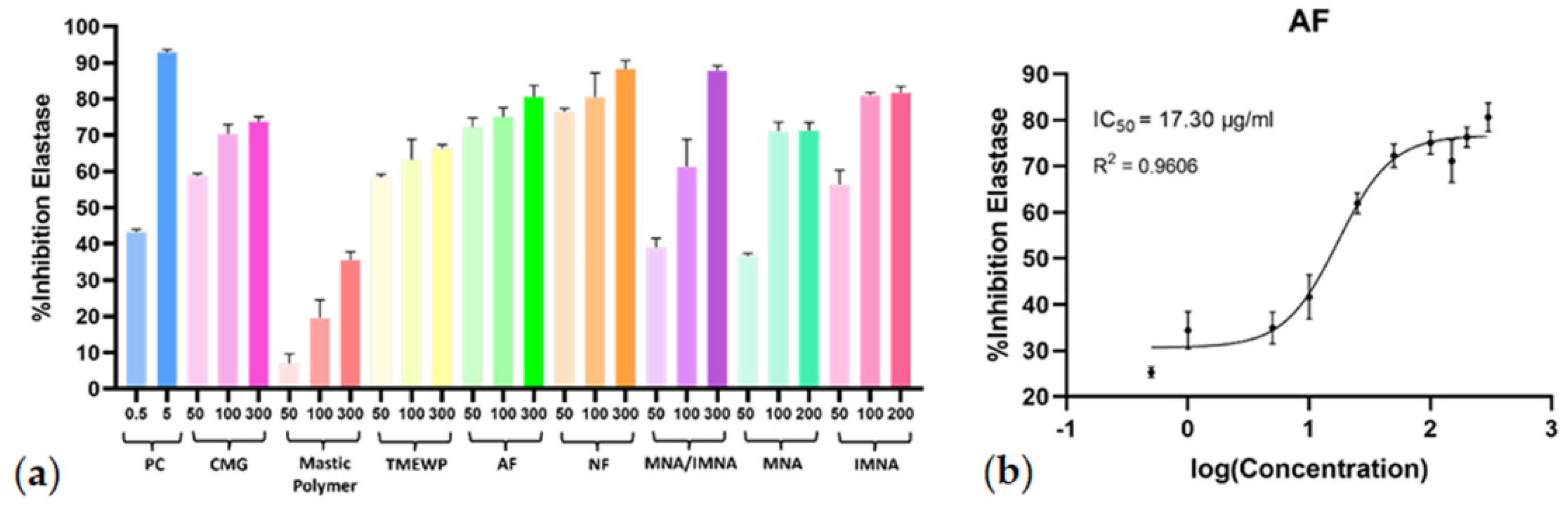
Regarding their capacity to inhibit elastase, all screened samples, except for the polymer fraction, exhibited significant activity, further encouraging their potential usage in the cosmetics industry. During the initial screening, the total CMG extract and its fractions were tested at concentrations of 50 μg/mL, 100 μg/mL, and 300 μg/mL, while the pure compounds were tested at 50 μM, 100 μM, and 200 μM (Figure 4a). The dose−response curves (R2 > 0.96) and the IC50 values were calculated for all samples that demonstrated anti-elastase activity at the initial screening. Further in vitro evaluation revealed the AF as the most potent again, with an IC50 value of 17.30 μg/mL (Figure 4b) while, similar to the collagenase results, CMG and all the fractions (except for the NF) exhibited remarkable inhibitory activity on elastase enzyme with IC50 values < 31 μg/mL. In this case, however, the MNA/IMNA mixture as well as the pure isolated compounds MNA and IMNA showed lower anti-elastase activity. Similarly to the collagenase results, IMNA demonstrated stronger anti-elastase activity compared to MNA.
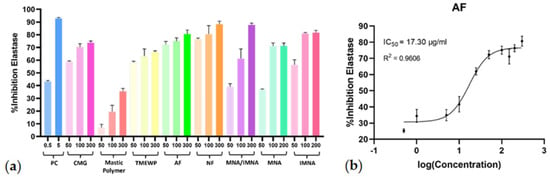
Figure 4.
(a) Elastase inhibitory activity of CMG, CMG fractions, MNA/IMNA mixture and isolated pure compounds. Bars represent mean ± S.D., n = 3. (b) Dose−response curve of the most potent anti-elastase sample (AF). PC = positive control.
Representatives of the Pistacia genus have been employed since antiquity in wound healing and overall skin health, with many modern studies verifying the traditional use of these products through in vitro and in vivo assays [42]. Regarding P. lentiscus resin in particular, as previously mentioned, despite the widespread application of CMG in skincare and cosmetic products, information concerning scientific data of such use remains rather scarce. To the best of our knowledge, there are no reports regarding the effects of CMG products and CMG terpenes on elastase and collagenase enzymes. As demonstrated by the IC50 calculated values (Table 1) and the dose−response curves for the tested samples (Supplementary Information, Figures S7 and S8), this study shows that certain compounds in CMG can inhibit collagenase and elastase, highlighting their potential in cosmeceutical applications for preventing skin photoaging.

Table 1.
IC50 values and standard error (SE) of screened samples for collagenase and elastase enzymes. (ND = Not Determined).
So far, only the anti-elastase and anti-tyrosinase activity of P. lentiscus’ distilled leaves has been investigated, with the extracts standing out as potential cosmeceutical ingredients with promising activity [43]. In a following study, the same group attributed the observed results of a wound healing effect on an animal model to the presence of quercetin-3-O-rhamnoside and myricetin-3-O-rhamnoside [44]. In another interesting study, Chiocchio et al. highlighted the anti-elastase and anti-tyrosinase activity of both leaves and fruits of P. lentiscus, as part of an extensive screening of a hundred plant samples for potential cosmetic applications; in fact both extracts were among the most potent candidates in both bioassays [45]. Furthermore, formulations containing pure CMG resin have shown promising activity using animal models, in skin conditions such as allergic dermatitis [46]. Additionally, other products of CMG such as Chios mastic oil (CMO), have stood out as remarkable cosmetic agents; in fact, CMO and pure compounds of the resinous extract, such as α-pinene and myrcene, have been shown to confer cytoprotective properties under oxidative stress conditions by upregulating a set of genes associated with antioxidant cellular response [47].
4. Conclusions
To sum up, the current study described a detailed extraction and fractionation process for CMG, which resulted in different extracts, fractions as well as isolated compounds, including mastic’s major triterpenic acids, namely MNA and IMNA. All samples were profiled by means of HPTLC with different development systems, and to the best of our knowledge, this is the first published record of the application of HPTLC for the analysis of mastic’s fractions (polymer, AF, NF) and isolated constituents (MNA, IMNA). Evidently, most tested systems offered diverse insight into the samples’ chemical composition; nevertheless, the one containing DCM and MeOH at an analogy of 98:2 (v/v) was most suited for triterpene analysis. Additionally, as far as the authors are aware, this is the first time that CMG and its products are investigated for their anti-elastase and anti-collagenase activities. Based on the current study’s findings, for collagenase, a mixture of MNA and IMNA demonstrated the most potent activity with an IC50 value of 31.07 μg/mL, while for elastase CMG’s acidic fraction provided the most promising results with an IC50 value of 17.30 μg/mL. This is of particular importance for the cosmetics industry, given that it provides a scientific base for the inclusion of mastic in cosmetic applications and justifies the traditional use of this valuable natural product, whose skincare properties have been appreciated by Mediterranean populations ever since antiquity.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/cosmetics11050155/s1, Figure S1: 1H (600 MHz, CDCl3) spectrum of IMNA; Figure S2. 13C (151 MHz, CDCl3) spectrum of IMNA; Figure S3. 1H (600 MHz, CDCl3) spectrum of MNA; Figure S4. 13C (151 MHz, CDCl3) spectrum of MNA; Figure S5. HRMS spectrum of IMNA.; Figure S6. HRMS spectrum of MNA; Figure S7. Dose-response curves for anti-collagenase activity.; Figure S8. Dose-response curves for anti-elastase activity.
Author Contributions
Conceptualization, S.P. and M.H.; methodology, P.S., E.V.M., M.C., A.B. and L.A.; validation, E.V.M., L.A., S.P. and M.H.; formal analysis, P.S., E.V.M., M.C., A.B. and L.A.; investigation, P.S., M.C., A.B. and. L.A.; resources, M.H.; data curation, E.V.M. and L.A.; writing—original draft preparation, P.S., E.V.M., M.C., A.B. and L.A.; writing—review and editing, S.P. and M.H.; visualization, P.S., E.V.M., M.C. and A.B.; supervision, S.P. and M.H.; project administration, M.H.; funding acquisition, S.P. and M.H. All authors have read and agreed to the published version of the manuscript.
Funding
The authors would like to thank Mibelle Group Biochemistry for partially supporting the study and the European Union and specifically project GreenCosmIn “Green chemistry and biotechnology approaches for the development of nature-based cosmetics” (HORIZON-MSCA-2022-SE-01, proposal number 101131346). Views and opinions expressed are, however, those of the authors only and do not necessarily reflect those of the European Union and the European Research Executive Agency (REA). Neither the European Union nor the granting authority can be held responsible for them.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Dataset available on request from the authors.
Acknowledgments
The authors are thankful to the Chios Mastiha Growers Association (CMGA) for kindly providing the mastic crude resin.
Conflicts of Interest
S.P. belongs to the scientific staff of Mibelle Group Biochemistry and L.A. to the scientific staff of Pharmagnose. The remaining authors declare that the research was conducted in the absence of any commercial relationships that could be construed as potential conflicts of interest.
References
- Lee, H.; Hong, Y.; Kim, M. Structural and functional changes and possible molecular mechanisms in aged skin. Int. J. Mol. Sci. 2021, 22, 12489. [Google Scholar] [CrossRef]
- Yanasan, N.; Wangkananon, W.; Natakankitkul, S.; Kiattisin, K. Nanoemulsions Containing Passiflora quadrangularis L. Fruit Extracts for Cosmetic Application and Skin Efficacy Study. Cosmetics 2024, 11, 57. [Google Scholar] [CrossRef]
- Pachi, V.K.; Mikropoulou, E.V.; Gkiouvetidis, P.; Siafakas, K.; Argyropoulou, A.; Angelis, A.; Mitakou, S.; Halabalaki, M. Traditional uses, phytochemistry and pharmacology of Chios mastic gum (Pistacia lentiscus var. Chia, Anacardiaceae): A review. J. Ethnopharmacol. 2020, 254, 112485. [Google Scholar] [CrossRef] [PubMed]
- European Medicines Agency. Draft European Union Herbal Monograph on Pistacia lentiscus L., Resin (Mastic). Available online: https://www.ema.europa.eu/en/documents/herbal-monograph/draft-european-union-herbal-monograph-pistacia-lentiscus-l-resin-mastic_en.pdf (accessed on 4 September 2024).
- Dioscorides, P. De Materia Medica, English ed.; IBIDIS: Johannesburg, South Africa, 2000; ISBN 9780620234351. [Google Scholar]
- Colombini, M.P.; Modugno, F.; Silvano, F.; Onor, M. Characterization of the balm of an Egyptian mummy from the seventh century B.C. Stud. Conserv. 2000, 45, 19–29. [Google Scholar] [CrossRef]
- Łucejko, J.; Connan, J.; Orsini, S.; Ribechini, E.; Modugno, F. Chemical analyses of Egyptian mummification balms and organic residues from storage jars dated from the Old Kingdom to the Copto-Byzantine period. J. Archaeol. Sci. 2017, 85, 1–12. [Google Scholar] [CrossRef]
- Tchapla, A.; Méjanelle, P.; Bleton, J.; Goursaud, S. Characterisation of embalming materials of a mummy of the ptolemaic era. Comparison with balms from mummies of different eras. J. Sep. Sci. 2004, 27, 217–234. [Google Scholar] [CrossRef]
- Colombini, M.P.; Giachi, G.; Iozzo, M.; Ribechini, E. An Etruscan ointment from Chiusi (Tuscany, Italy): Its chemical characterization. J. Archaeol. Sci. 2009, 36, 1488–1495. [Google Scholar] [CrossRef]
- Triantafyllou, A.; Bikineyeva, A.; Dikalova, A.; Nazarewicz, R.; Lerakis, S.; Dikalov, S. Anti-inflammatory activity of Chios mastic gum is associated with inhibition of TNF-alpha induced oxidative stress. Nutr. J. 2011, 10, 64. [Google Scholar] [CrossRef]
- Kontogiannis, C.; Georgiopoulos, G.; Loukas, K.; Papanagnou, E.D.E.-D.; Pachi, V.K.V.K.; Bakogianni, I.; Laina, A.; Kouzoupis, A.; Karatzi, K.; Trougakos, I.P.I.P.; et al. Chios mastic improves blood pressure haemodynamics in patients with arterial hypertension: Implications for regulation of proteostatic pathways. Eur. J. Prev. Cardiol. 2019, 26, 328–331. [Google Scholar] [CrossRef] [PubMed]
- Georgiadis, I.; Karatzas, T.; Korou, L.M.; Agrogiannis, G.; Vlachos, I.S.; Pantopoulou, A.; Tzanetakou, I.P.; Katsilambros, N.; Perrea, D.N. Evaluation of chios mastic gum on lipid and glucose metabolism in diabetic mice. J. Med. Food 2014, 17, 393–399. [Google Scholar] [CrossRef]
- Andreadou, I.; Mitakou, S.; Paraschos, S.; Efentakis, P.; Magiatis, P.; Kaklamanis, L.; Halabalaki, M.; Skaltsounis, L.; Iliodromitis, E.K. “Pistacia lentiscus L.” reduces the infarct size in normal fed anesthetized rabbits and possess antiatheromatic and hypolipidemic activity in cholesterol fed rabbits. Phytomedicine 2016, 23, 1220–1226. [Google Scholar] [CrossRef] [PubMed]
- Brieudes Eleni, V.; Kallergis, E.; Kaliora, A.C.; Papada, E.; Gkiouvetidis, P.; Angelis, A.; Halabalaki, M.V.M. Development, Validation and Application of a UHPLC-MS Method for the Quantification of Chios Mastic Gum Triterpenoids in Human Plasma. Planta Med 2021, 87, 1101–1109. [Google Scholar] [CrossRef]
- Paraschos, S.; Magiatis, P.; Mitakou, S.; Petraki, K.; Kalliaropoulos, A.; Maragkoudakis, P.; Mentis, A.; Sgouras, D.; Skaltsounis, A.L. In vitro and in vivo activities of chios mastic gum extracts and constituents against Helicobacter pylori. Antimicrob. Agents Chemother. 2007, 51, 551–559. [Google Scholar] [CrossRef] [PubMed]
- Axiotis, E.; Angelis, A.; Antoniadi, L.; Petrakis, E.A.; Skaltsounis, L.A. Phytochemical Analysis and Dermo-Cosmetic Evaluation of Cymbidium sp. (Orchidaceae) Cultivation By-Products. Antioxidants 2022, 11, 101. [Google Scholar] [CrossRef] [PubMed]
- Svingou, D.; Mikropoulou, E.V.; Pachi, V.K.; Smyrnioudis, I.; Halabalaki, M. Chios mastic gum: A validated method towards authentication. J. Food Compos. Anal. 2023, 115, 104997. [Google Scholar] [CrossRef]
- Beteinakis, S.; Papachristodoulou, A.; Mikros, E.; Halabalaki, M. From sample preparation to NMR-based metabolic profiling in food commodities: The case of table olives. Phytochem. Anal. 2022, 33, 83–93. [Google Scholar] [CrossRef]
- Mikropoulou, E.V.; Petrakis, E.A.; Argyropoulou, A.; Halabalaki, M.; Skaltsounis, L.A. Quantification of bioactive lignans in sesame seeds using HPTLC densitometry: Comparative evaluation by HPLC-PDA. Food Chem. 2019, 288, 1–7. [Google Scholar] [CrossRef]
- Pachi, V.K.; Mikropoulou, E.V.; Dimou, S.; Dionysopoulou, M.; Argyropoulou, A.; Diallinas, G.; Halabalaki, M. Chemical Profiling of Pistacia lentiscus var. Chia Resin and Essential Oil: Ageing Markers and Antimicrobial Activity. Processes 2021, 9, 418. [Google Scholar] [CrossRef]
- Mastic monograph. In European Pharmacopoeia 7.0; Council of Europe: Strasbourg, France, 2008; pp. 1177–1178.
- Reich, E. High-Performance Thin-Layer Chromatography for the Analysis of Medicinal Plants; Thieme Publishing Group: New York, NY, USA, 2014; ISBN 9781588904096. [Google Scholar]
- Methods in pharmacognosy. In European Pharmacopoeia 9.0; Council of Europe: Strasbourg, France, 2017; pp. 295–296.
- Monaco, P.; Caputo, R.; Palumbo, G.; Mangoni, L. Triterpenes from the galls of Pistacia lentiscus. Phytochemistry 1973, 12, 2534–2537. [Google Scholar] [CrossRef]
- Xynos, N.; Termentzi, A.; Fokialakis, N.; Skaltsounis, L.A.; Aligiannis, N. Supercritical CO2 extraction of mastic gum and chemical characterization of bioactive fractions using LC-HRMS/MS and GC–MS. J. Supercrit. Fluids 2018, 133, 349–356. [Google Scholar] [CrossRef]
- Assimopoulou, A.N.; Papageorgiou, V.P. GC-MS analysis of penta- and tetra-cyclic triterpenes from resins of Pistacia species. Part, I. Pistacia lentiscus var. Chia. Biomed. Chromatogr. 2005, 19, 285–311. [Google Scholar] [CrossRef] [PubMed]
- Assimopoulou, A.N.; Papageorgiou, V.P. GC-MS analysis of penta- and tetra-cyclic triterpenes from resins of Pistacia species. Part II. Pistacia terebinthus var. Chia. Biomed. Chromatogr. 2005, 19, 586–605. [Google Scholar] [CrossRef]
- Athanasiadis, V.; Gortzi, O. Study of Antioxidant and Antimicrobial Activity of Chios Mastic Gum Fractions (Neutral, Acidic) Before and After Encapsulation in Liposomes. J. Food Process. Technol. 2014, 5, 355. [Google Scholar] [CrossRef]
- Karygianni, L.; Cecere, M.; Skaltsounis, A.L.; Argyropoulou, A.; Hellwig, E.; Aligiannis, N.; Wittmer, A.; Al-Ahmad, A. High-level antimicrobial efficacy of representative Mediterranean natural plant extracts against oral microorganisms. Biomed Res. Int. 2014, 2014, 839019. [Google Scholar] [CrossRef] [PubMed]
- Sharifi, M.S.; Hazell, S.L. Fractionation of mastic gum in relation to antimicrobial activity. Pharmaceuticals 2009, 2, 2–10. [Google Scholar] [CrossRef] [PubMed]
- Van Den Berg, K.J.; Van Der Horst, J.; Boon, J.J.; Sudmeijer, O.O. Cis-1,4-poly-β-myrcene; The structure of the polymeric fraction of mastic resin (Pistacia lentiscus L.) elucidated. Tetrahedron Lett. 1998, 39, 2645–2648. [Google Scholar] [CrossRef]
- Lemonakis, N.; Magiatis, P.; Kostomitsopoulos, N.; Skaltsounis, A.L.; Tamvakopoulos, C. Oral administration of chios mastic gum or extracts in mice: Quantification of triterpenic acids by liquid chromatography-tandem mass spectrometry. Planta Med. 2011, 77, 1916–1923. [Google Scholar] [CrossRef]
- Birkedal-Hansen, H.; Moore, W.G.I.; Bodden, M.K.; Windsor, L.J.; Birkedal-Hansen, B.; DeCarlo, A.; Engler, J.A. Matrix Metalloproteinases: A Review. Crit. Rev. Oral Biol. Med. 1993, 4, 197–250. [Google Scholar] [CrossRef]
- Imokawa, G.; Ishida, K. Biological Mechanisms Underlying the Ultraviolet Radiation-Induced Formation of Skin Wrinkling and Sagging I: Reduced Skin Elasticity, Highly Associated with Enhanced Dermal Elastase Activity, Triggers Wrinkling and Sagging. Int. J. Mol. Sci. 2015, 16, 7753–7775. [Google Scholar] [CrossRef]
- Cruz, A.M.; Gonçalves, M.C.; Marques, M.S.; Veiga, F.; Paiva-Santos, A.C.; Pires, P.C. In Vitro Models for Anti-Aging Efficacy Assessment: A Critical Update in Dermocosmetic Research. Cosmetics 2023, 10, 66. [Google Scholar] [CrossRef]
- Jenkins, G. Molecular mechanisms of skin ageing. Mech. Ageing Dev. 2002, 123, 801–810. [Google Scholar] [CrossRef] [PubMed]
- Fisher, G.J.; Wang, Z.; Datta, S.C.; Varani, J.; Kang, S.; Voorhees, J.J. Pathophysiology of Premature Skin Aging Induced by Ultraviolet Light. N. Engl. J. Med. 1997, 337, 1419–1429. [Google Scholar] [CrossRef]
- Kolahreez, D.; Ghasemi-Mobarakeh, L.; Liebner, F.; Alihosseini, F.; Quartinello, F.; Guebitz, G.M.; Ribitsch, D. Approaches to Control and Monitor Protease Levels in Chronic Wounds. Adv. Ther. 2024, 7, 2300396. [Google Scholar] [CrossRef]
- Tundis, R.; Loizzo, M.R.; Bonesi, M.; Menichini, F. Potential Role of Natural Compounds Against Skin Aging. Curr. Med. Chem. 2015, 22, 1515–1538. [Google Scholar] [CrossRef]
- Sakurai, Y.; Inoue, H.; Nishii, W.; Takahashi, T.; IIno, Y.; Yamamoto, M.; Takahashi, K. Purification and Characterization of a Major Collagenase from Streptomyces parvulus. Biosci. Biotechnol. Biochem. 2009, 73, 21–28. [Google Scholar] [CrossRef] [PubMed]
- Lee, K.-K.; Kim, J.-H.; Cho, J.-J.; Choi, J.-D. Inhibitory Effects of 150 Plant Extracts on Elastase Activity, and Their Anti-inflammatory Effects. Int. J. Cosmet. Sci. 1999, 21, 71–82. [Google Scholar] [CrossRef]
- Bozorgi, M.; Iranzad, M.; Ali, A.; Memariani, Z. Dermatological effects of Pistacia species: A systematic review. J. HerbMed Pharmacol. 2024, 13, 28–42. [Google Scholar] [CrossRef]
- Elloumi, W.; Maalej, A.; Ortiz, S.; Michel, S.; Chamkha, M.; Boutefnouchet, S.; Sayadi, S. Pistacia lentiscus L. Distilled Leaves as a Potential Cosmeceutical Ingredient: Phytochemical Characterization, Transdermal Diffusion, and Anti-Elastase and Anti-Tyrosinase Activities. Molecules 2022, 27, 855. [Google Scholar] [CrossRef]
- Elloumi, W.; Mahmoudi, A.; Ortiz, S.; Boutefnouchet, S.; Chamkha, M.; Sayadi, S. Wound healing potential of quercetin-3-O-rhamnoside and myricetin-3-O-rhamnoside isolated from Pistacia lentiscus distilled leaves in rats model. Biomed. Pharmacother. 2022, 146, 855. [Google Scholar] [CrossRef]
- Chiocchio, I.; Mandrone, M.; Sanna, C.; Maxia, A.; Tacchini, M.; Poli, F. Screening of a hundred plant extracts as tyrosinase and elastase inhibitors, two enzymatic targets of cosmetic interest. Ind. Crops Prod. 2018, 122, 498–505. [Google Scholar] [CrossRef]
- Kishimoto, R.; Kato, N.; Koike, M.; Iwashita, N.; Takagi, Y.; Fukuyama, T. Topical treatment with mastic (resin from Pistacia lentiscus) elicits anti-inflammatory and anti-pruritic responses by modulating keratinocyte activation in a mouse model of allergic dermatitis. Phytomedicine 2021, 91, 153679. [Google Scholar] [CrossRef] [PubMed]
- Xanthis, V.; Fitsiou, E.; Voulgaridou, G.P.; Bogadakis, A.; Chlichlia, K.; Galanis, A.; Pappa, A. Antioxidant and cytoprotective potential of the essential oil Pistacia lentiscus var. Chia and its major components myrcene and α-pinene. Antioxidants 2021, 10, 127. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).