Trifluorometyl Phenethyl Mesalazine (TFM) Acts as an Antioxidant and Improves Facial Skin Wrinkles and Whitening
Abstract
:1. Introduction
2. Materials and Methods
2.1. Preparation of TFM
2.2. Radical Scavenging Assay Using 1,1-Diphenyl-2-Picrylhydrazyl (DPPH)
2.3. Cell Culture
2.4. Quantitation of Cellular Melanin
2.5. Cell Viability Assay
2.6. Assay of Procollagen Production
2.7. UV-Induced MMP-1 Production
2.8. Quantification of IL-6 Production
2.9. In Vivo Skin Irritation Test
2.10. Clinical Study Designs for Skin Wrinkle and Whitening Assessment
2.11. Assessment of Skin Wrinkles
2.12. Assessment of Skin Brightness
2.13. Statistical Analysis
3. Results
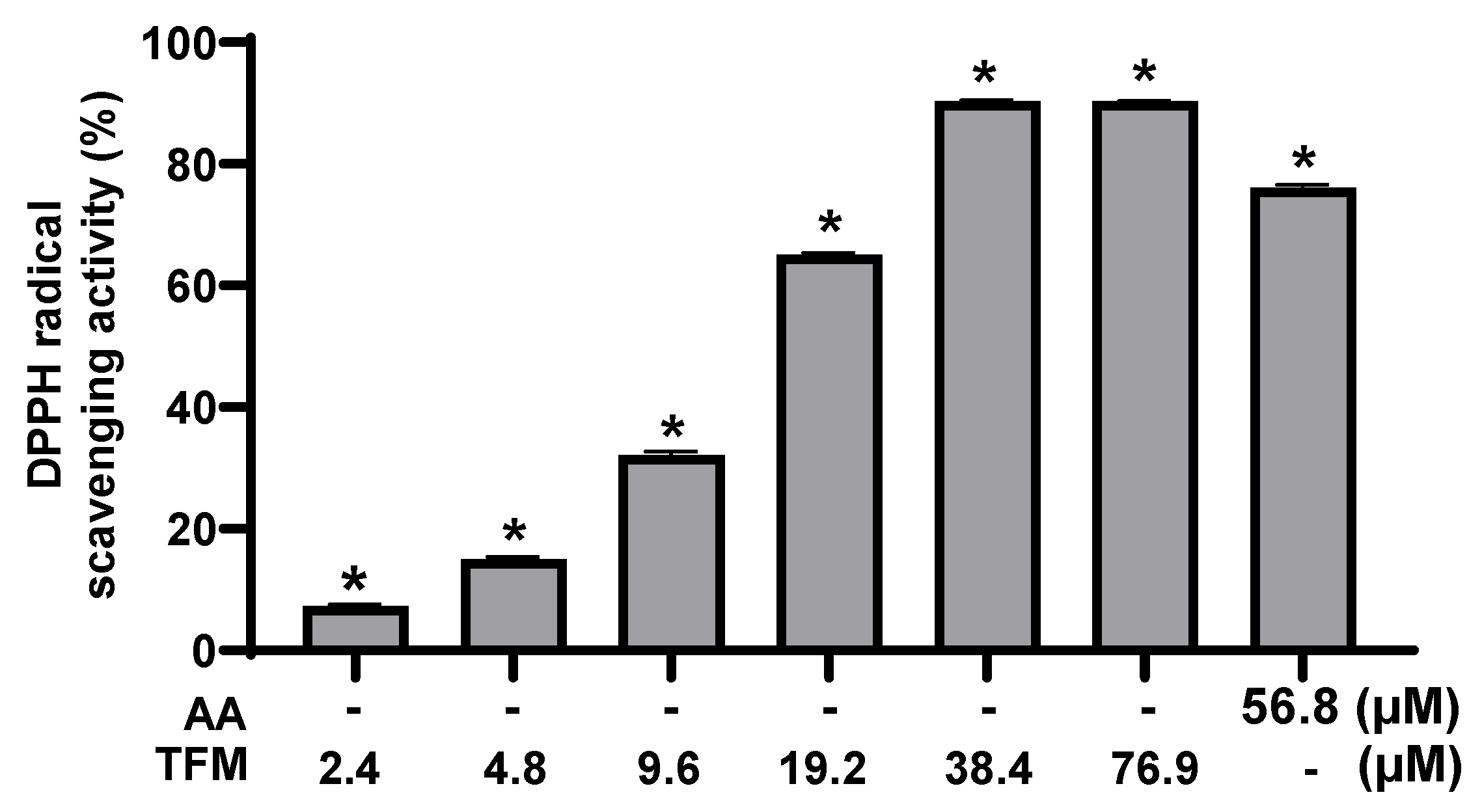
3.1. Antioxidant Capacity of TFM
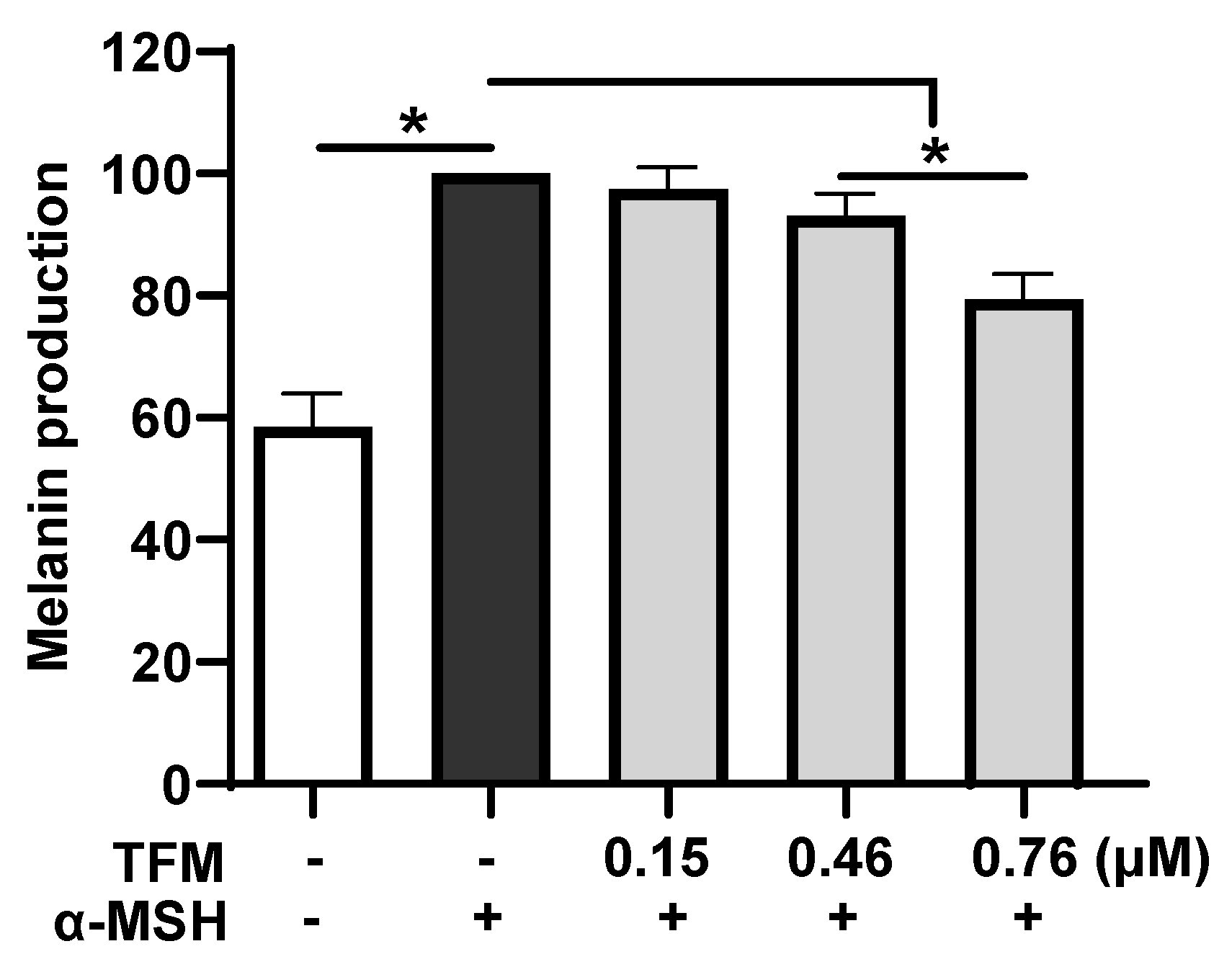
3.2. Effect of TFM on α-MSH-Induced Melanin Production
3.3. Effect of TFM on the UV-Induced Production of MMP-1
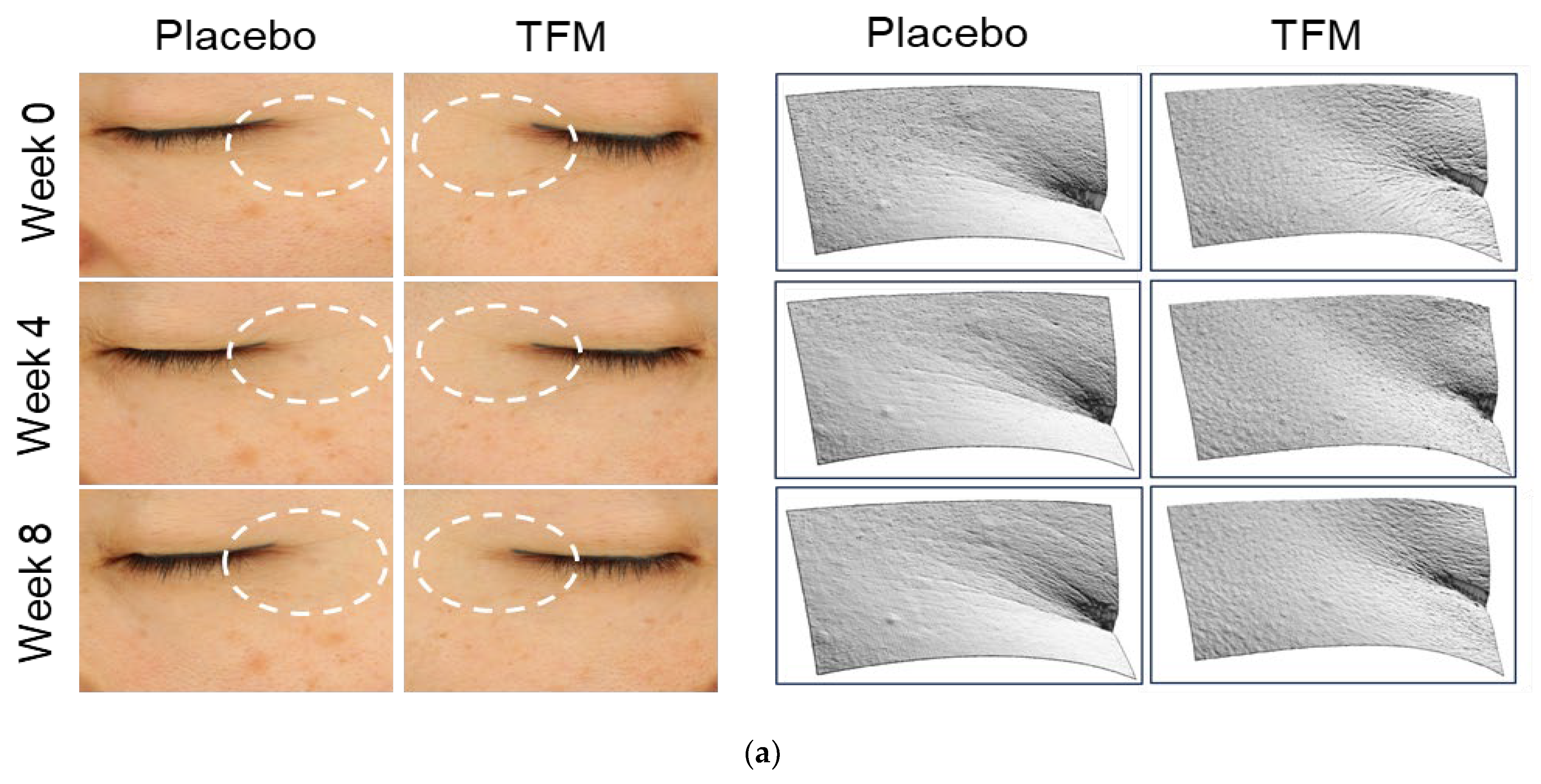
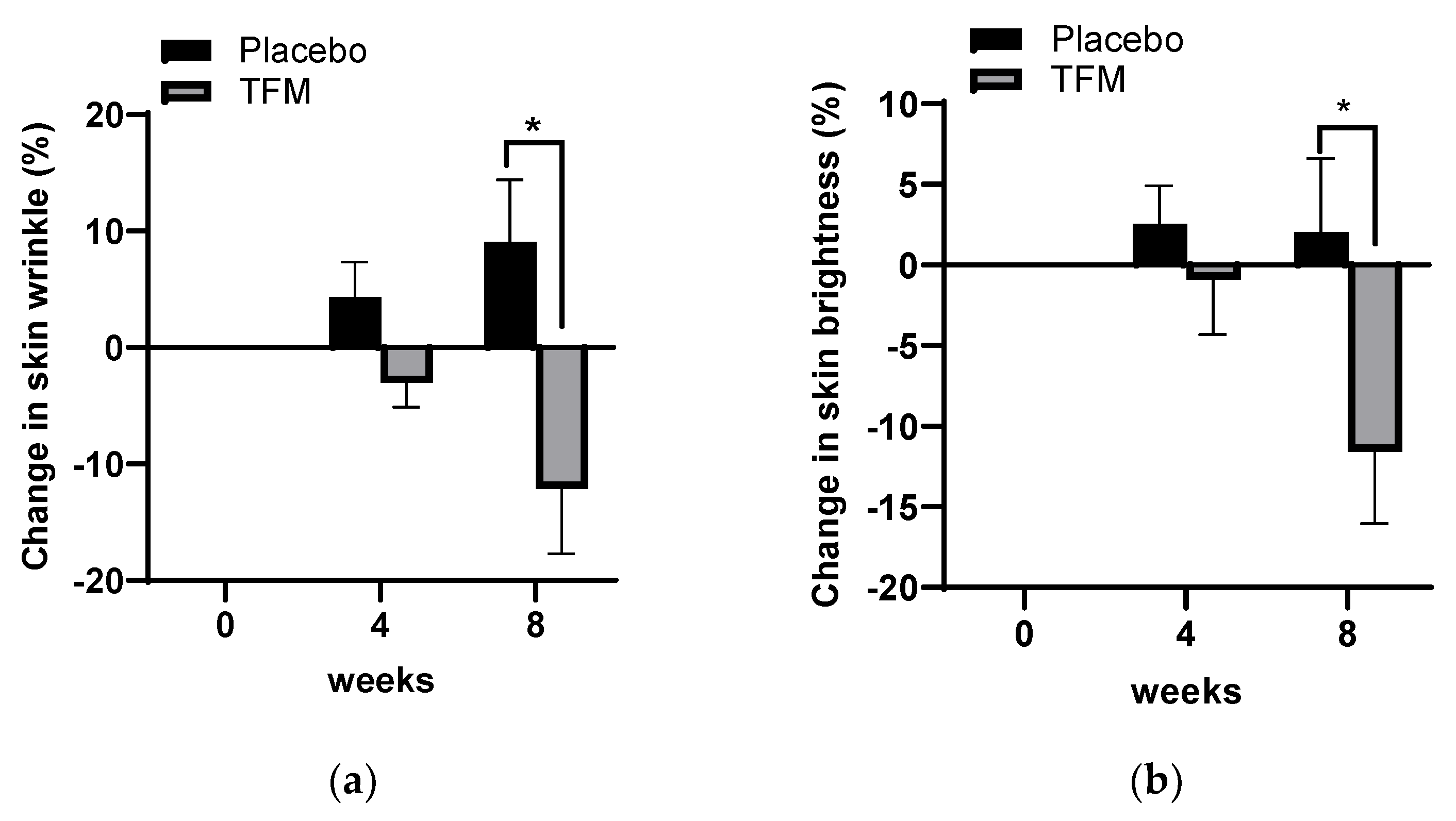
3.4. Clinical Effects of TFM on Skin Wrinkle Changes
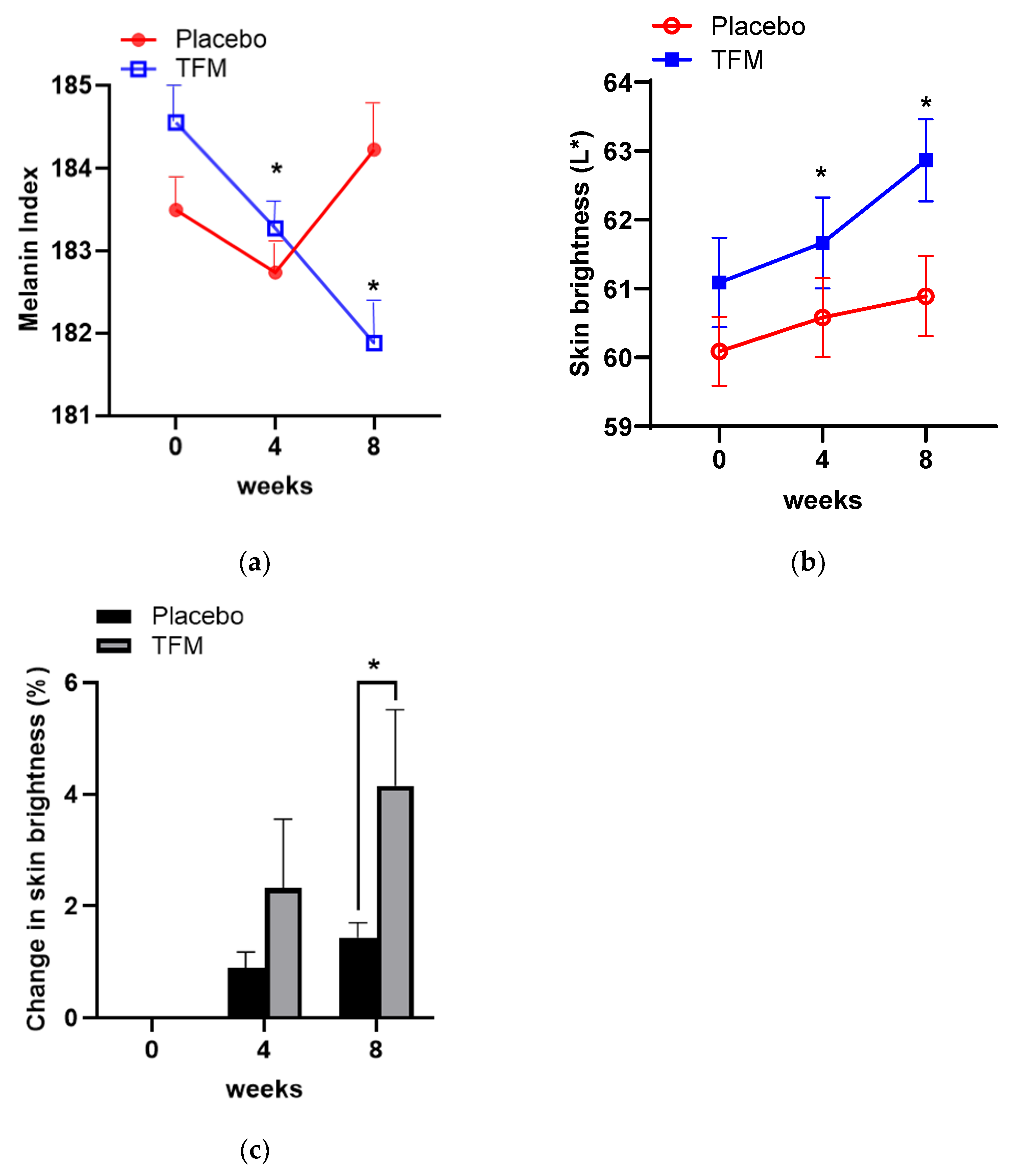
3.5. Effects of TFM on Skin Whitening
3.6. Investigator’s Assessments on Skin Wrinkles and Brightness
3.7. In Vivo Evaluation of Skin Compatibility
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Krutmann, J.; Schikowski, T.; Morita, A.; Berneburg, M. Environmentally-Induced (Extrinsic) Skin Aging: Exposomal Factors and Underlying Mechanisms. J. Investig. Dermatol. 2021, 141, 1096–1103. [Google Scholar] [CrossRef] [PubMed]
- Zhang, S.; Duan, E. Fighting against Skin Aging: The Way from Bench to Bedside. Cell Transplant. 2018, 27, 729–738. [Google Scholar] [CrossRef] [PubMed]
- Hanson, K.M.; Clegg, R.M. Observation and quantification of ultraviolet-induced reactive oxygen species in ex vivo human skin. Photochem. Photobiol. 2002, 76, 57–63. [Google Scholar] [CrossRef] [PubMed]
- Rinnerthaler, M.; Bischof, J.; Streubel, M.K.; Trost, A.; Richter, K. Oxidative stress in aging human skin. Biomolecules 2015, 5, 545–589. [Google Scholar] [CrossRef] [PubMed]
- Pugliese, P.T. The skin’s antioxidant systems. Dermatol. Nurs. 1998, 10, 401–416; quiz 417–418. [Google Scholar]
- Masaki, H. Role of antioxidants in the skin: Anti-aging effects. J. Dermatol. Sci. 2010, 58, 85–90. [Google Scholar] [CrossRef]
- Kang, S.H.; Jeon, Y.D.; Cha, J.Y.; Hwang, S.W.; Lee, H.Y.; Park, M.; Lee, B.R.; Shin, M.K.; Kim, S.J.; Shin, S.M.; et al. Antioxidant and skin-whitening effects of aerial part of Euphorbia supina Raf. Extract. BMC Complement. Altern. Med. 2018, 18, 256. [Google Scholar] [CrossRef]
- Kim, Y.J. Antimelanogenic and antioxidant properties of gallic acid. Biol. Pharm. Bull. 2007, 30, 1052–1055. [Google Scholar] [CrossRef]
- Knott, A.; Achterberg, V.; Smuda, C.; Mielke, H.; Sperling, G.; Dunckelmann, K.; Vogelsang, A.; Kruger, A.; Schwengler, H.; Behtash, M.; et al. Topical treatment with coenzyme Q10-containing formulas improves skin’s Q10 level and provides antioxidative effects. Biofactors 2015, 41, 383–390. [Google Scholar] [CrossRef]
- Kim, H.H.; Jeong, S.H.; Park, M.Y.; Bhosale, P.B.; Abusaliya, A.; Kim, H.W.; Seong, J.K.; Ahn, M.; Park, K.I.; Kim, G.S. Antioxidant effects of phenolic compounds in through the distillation of Lonicera japonica & Chenpi extract and anti-inflammation on skin keratinocyte. Sci. Rep. 2023, 13, 20883. [Google Scholar] [CrossRef]
- Barbosa, E.; Faintuch, J.; Machado Moreira, E.A.; Goncalves da Silva, V.R.; Lopes Pereima, M.J.; Martins Fagundes, R.L.; Filho, D.W. Supplementation of vitamin E, vitamin C, and zinc attenuates oxidative stress in burned children: A randomized, double-blind, placebo-controlled pilot study. J. Burn Care Res. 2009, 30, 859–866. [Google Scholar] [CrossRef] [PubMed]
- Enescu, C.D.; Bedford, L.M.; Potts, G.; Fahs, F. A review of topical vitamin C derivatives and their efficacy. J. Cosmet. Dermatol. 2022, 21, 2349–2359. [Google Scholar] [CrossRef] [PubMed]
- Davinelli, S.; Bertoglio, J.C.; Polimeni, A.; Scapagnini, G. Cytoprotective Polyphenols Against Chronological Skin Aging and Cutaneous Photodamage. Curr. Pharm. Des. 2018, 24, 99–105. [Google Scholar] [CrossRef]
- Shin, J.H.; Lee, Y.A.; Lee, J.K.; Lee, Y.B.; Cho, W.; Im, D.S.; Lee, J.H.; Yun, B.S.; Springer, J.E.; Gwag, B.J. Concurrent blockade of free radical and microsomal prostaglandin E synthase-1-mediated PGE2 production improves safety and efficacy in a mouse model of amyotrophic lateral sclerosis. J. Neurochem. 2012, 122, 952–961. [Google Scholar] [CrossRef]
- Chun, H.; Im, H.; Kang, Y.J.; Kim, Y.; Shin, J.H.; Won, W.; Lim, J.; Ju, Y.; Park, Y.M.; Kim, S.; et al. Severe reactive astrocytes precipitate pathological hallmarks of Alzheimer’s disease via H(2)O(2)(-) production. Nat. Neurosci. 2020, 23, 1555–1566. [Google Scholar] [CrossRef] [PubMed]
- Wang, F.; Tan, J.; Jiang, R.; Li, F.; Zheng, R.; Yu, L.; Luo, L.; Zheng, Y. DPPH Radical Scavenging Activity of New Phenolics from the Fermentation Broth of Mushroom Morehella importuna. Molecules 2023, 28, 4760. [Google Scholar] [CrossRef] [PubMed]
- Baliyan, S.; Mukherjee, R.; Priyadarshini, A.; Vibhuti, A.; Gupta, A.; Pandey, R.P.; Chang, C.M. Determination of Antioxidants by DPPH Radical Scavenging Activity and Quantitative Phytochemical Analysis of Ficus religiosa. Molecules 2022, 27, 1326. [Google Scholar] [CrossRef]
- Baumann, L. How to Use Oral and Topical Cosmeceuticals to Prevent and Treat Skin Aging. Facial Plast. Surg. Clin. North Am. 2018, 26, 407–413. [Google Scholar] [CrossRef]
- Maddodi, N.; Jayanthy, A.; Setaluri, V. Shining light on skin pigmentation: The darker and the brighter side of effects of UV radiation. Photochem. Photobiol. 2012, 88, 1075–1082. [Google Scholar] [CrossRef]
- Pittayapruek, P.; Meephansan, J.; Prapapan, O.; Komine, M.; Ohtsuki, M. Role of Matrix Metalloproteinases in Photoaging and Photocarcinogenesis. Int. J. Mol. Sci. 2016, 17, 868. [Google Scholar] [CrossRef]
- Wlaschek, M.; Heinen, G.; Poswig, A.; Schwarz, A.; Krieg, T.; Scharffetter-Kochanek, K. UVA-induced autocrine stimulation of fibroblast-derived collagenase/MMP-1 by interrelated loops of interleukin-1 and interleukin-6. Photochem. Photobiol. 1994, 59, 550–556. [Google Scholar] [CrossRef] [PubMed]
- Kahari, V.M.; Saarialho-Kere, U. Matrix metalloproteinases in skin. Exp. Dermatol. 1997, 6, 199–213. [Google Scholar] [CrossRef] [PubMed]
- Brand, R.M.; Wipf, P.; Durham, A.; Epperly, M.W.; Greenberger, J.S.; Falo, L.D., Jr. Targeting Mitochondrial Oxidative Stress to Mitigate UV-Induced Skin Damage. Front. Pharmacol. 2018, 9, 920. [Google Scholar] [CrossRef] [PubMed]
- Huang, W.S.; Wang, Y.W.; Hung, K.C.; Hsieh, P.S.; Fu, K.Y.; Dai, L.G.; Liou, N.H.; Ma, K.H.; Liu, J.C.; Dai, N.T. High correlation between skin color based on CIELAB color space, epidermal melanocyte ratio, and melanocyte melanin content. PeerJ 2018, 6, e4815. [Google Scholar] [CrossRef] [PubMed]
- Kammeyer, A.; Luiten, R.M. Oxidation events and skin aging. Ageing Res. Rev. 2015, 21, 16–29. [Google Scholar] [CrossRef]
- Pinnell, S.R. Cutaneous photodamage, oxidative stress, and topical antioxidant protection. J. Am. Acad. Dermatol. 2003, 48, 1–19. [Google Scholar] [CrossRef]
- Scharffetter-Kochanek, K.; Brenneisen, P.; Wenk, J.; Herrmann, G.; Ma, W.; Kuhr, L.; Meewes, C.; Wlaschek, M. Photoaging of the skin from phenotype to mechanisms. Exp. Gerontol. 2000, 35, 307–316. [Google Scholar] [CrossRef]
- Dall’Olmo, L.; Papa, N.; Surdo, N.C.; Marigo, I.; Mocellin, S. Alpha-melanocyte stimulating hormone (alpha-MSH): Biology, clinical relevance and implication in melanoma. J. Transl. Med. 2023, 21, 562. [Google Scholar] [CrossRef]
- Kang, H.Y.; Lee, J.W.; Papaccio, F.; Bellei, B.; Picardo, M. Alterations of the pigmentation system in the aging process. Pigment Cell Melanoma Res. 2021, 34, 800–813. [Google Scholar] [CrossRef]
- Pillaiyar, T.; Namasivayam, V.; Manickam, M.; Jung, S.H. Inhibitors of Melanogenesis: An Updated Review. J. Med. Chem. 2018, 61, 7395–7418. [Google Scholar] [CrossRef]
- Peng, H.Y.; Lin, C.C.; Wang, H.Y.; Shih, Y.; Chou, S.T. The melanogenesis alteration effects of Achillea millefolium L. essential oil and linalyl acetate: Involvement of oxidative stress and the JNK and ERK signaling pathways in melanoma cells. PLoS ONE 2014, 9, e95186. [Google Scholar] [CrossRef] [PubMed]
- Chou, S.T.; Chang, W.L.; Chang, C.T.; Hsu, S.L.; Lin, Y.C.; Shih, Y. Cinnamomum cassia essential oil inhibits alpha-MSH-induced melanin production and oxidative stress in murine B16 melanoma cells. Int. J. Mol. Sci. 2013, 14, 19186–19201. [Google Scholar] [CrossRef] [PubMed]
- Chin, T.; Lee, X.E.; Ng, P.Y.; Lee, Y.; Dreesen, O. The role of cellular senescence in skin aging and age-related skin pathologies. Front. Physiol. 2023, 14, 1297637. [Google Scholar] [CrossRef]
- Gao, X.H.; Zhang, L.; Wei, H.; Chen, H.D. Efficacy and safety of innovative cosmeceuticals. Clin. Dermatol. 2008, 26, 367–374. [Google Scholar] [CrossRef] [PubMed]
- Scholar, E. Mesalamine. xPharm: The Comprehensive Pharmacology Reference; Elsevier: Amsterdam, The Netherlands, 2009; pp. 1–6. [Google Scholar]
- Kang, S.; Duell, E.A.; Fisher, G.J.; Datta, S.C.; Wang, Z.Q.; Reddy, A.P.; Tavakkol, A.; Yi, J.Y.; Griffiths, C.E.; Elder, J.T.; et al. Application of retinol to human skin in vivo induces epidermal hyperplasia and cellular retinoid binding proteins characteristic of retinoic acid but without measurable retinoic acid levels or irritation. J. Investig. Dermatol. 1995, 105, 549–556. [Google Scholar] [CrossRef]
- Montagnani Marelli, M.; Marzagalli, M.; Moretti, R.M.; Beretta, G.; Casati, L.; Comitato, R.; Gravina, G.L.; Festuccia, C.; Limonta, P. Vitamin E delta-tocotrienol triggers endoplasmic reticulum stress-mediated apoptosis in human melanoma cells. Sci. Rep. 2016, 6, 30502. [Google Scholar] [CrossRef]


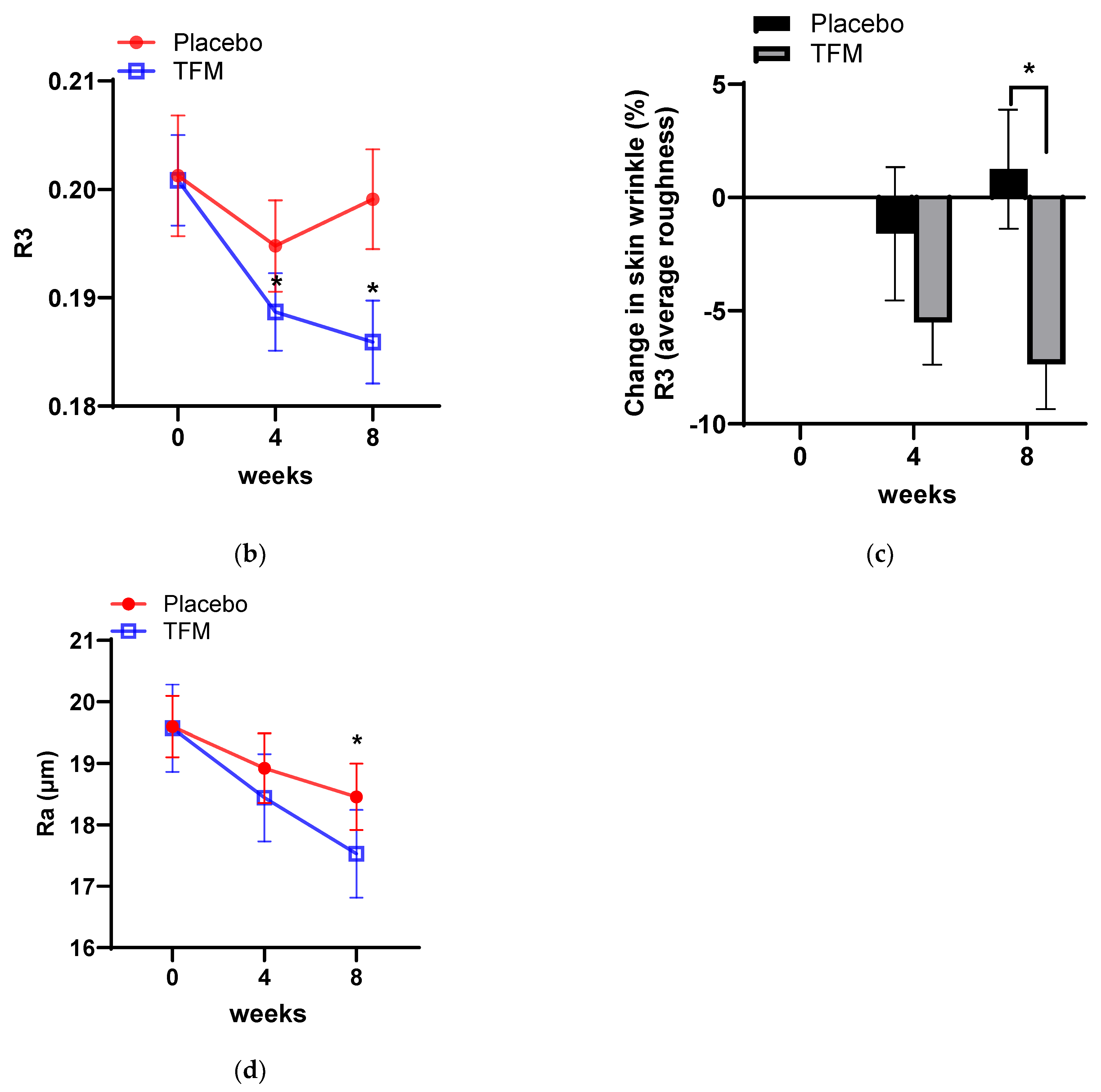
| Evaluation Parameter | Time-Point (week) | TFM-Treated Group | Placebo Group |
|---|---|---|---|
| Mean | Mean | ||
| R1 | 0 | 0.385 ± 0.060 | 0.361 ± 0.046 |
| 4 | 0.353 ± 0.054 * | 0.370 ± 0.052 | |
| 8 | 0.328 ± 0.051 *# | 0.388 ± 0.049 * | |
| R2 | 0 | 0.257 ± 0.027 | 0.250 ± 0.035 |
| 4 | 0.243 ± 0.023 * | 0.251 ± 0.024 | |
| 8 | 0.237 ± 0.030 *# | 0.256 ± 0.030 | |
| R4 | 0 | 0.200 ± 0.041 | 0.193 ± 0.034 |
| 4 | 0.195 ± 0.044 | 0.200 ± 0.036 | |
| 8 | 0.177 ± 0.043 # | 0.251 ± 0.035 | |
| R5 | 0 | 0.058 ± 0.019 | 0.053 ± 0.012 |
| 4 | 0.054 ± 0.017 | 0.055 ± 0.013 | |
| 8 | 0.047 ± 0.012 *# | 0.062 ± 0.015 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Won, S.; Lim, J.M.; Lee, J.H.; Oh, Y.J.; Shin, J.-H.; Gwag, B.J.; Choi, E.-J. Trifluorometyl Phenethyl Mesalazine (TFM) Acts as an Antioxidant and Improves Facial Skin Wrinkles and Whitening. Cosmetics 2024, 11, 158. https://doi.org/10.3390/cosmetics11050158
Won S, Lim JM, Lee JH, Oh YJ, Shin J-H, Gwag BJ, Choi E-J. Trifluorometyl Phenethyl Mesalazine (TFM) Acts as an Antioxidant and Improves Facial Skin Wrinkles and Whitening. Cosmetics. 2024; 11(5):158. https://doi.org/10.3390/cosmetics11050158
Chicago/Turabian StyleWon, Sojung, Jane Melissa Lim, Jin Hwan Lee, Young J. Oh, Jin-Hee Shin, Byoung Joo Gwag, and Eui-Ju Choi. 2024. "Trifluorometyl Phenethyl Mesalazine (TFM) Acts as an Antioxidant and Improves Facial Skin Wrinkles and Whitening" Cosmetics 11, no. 5: 158. https://doi.org/10.3390/cosmetics11050158






