Research on the Correlation Between Skin Elasticity Evaluation Parameters and Age
Abstract
1. Introduction
2. Materials and Methods
2.1. Subjects
2.2. Instruments
2.3. Methods
2.4. Statistical Analysis
3. Results
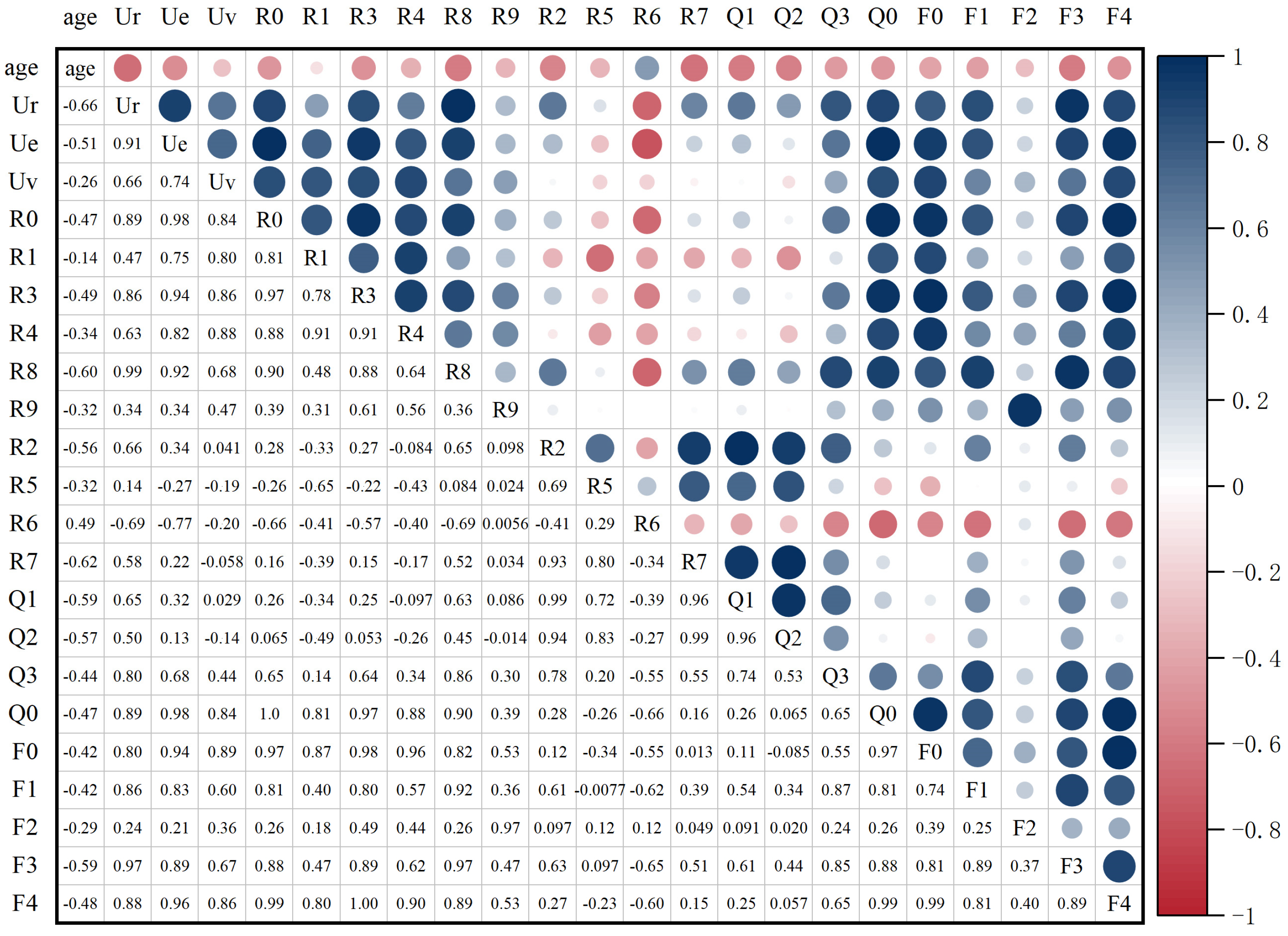
3.1. Correlation Analysis Results
3.2. Top 10 Parameters with the Strongest Correlation with Age
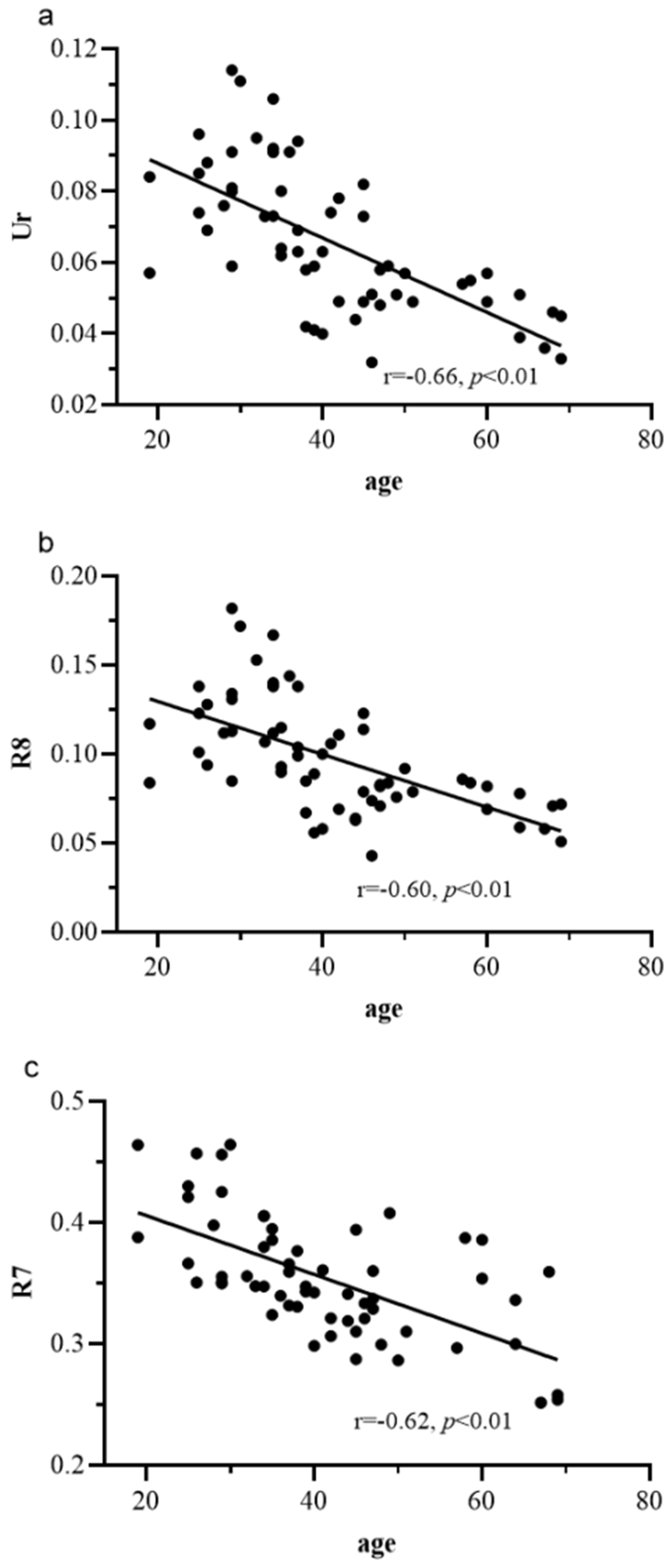
3.3. Scatter Plot Analysis of Age with Ur, R8, and R7
4. Discussion
4.1. Analysis and Discussion of Ur, R7, R8, Q1, Q2, and R2
4.2. Analysis and Discussion of R0, F4, F3, R3, and Ue Parameters
4.3. Analysis and Discussion of R2, R5, R6, and R7 Parameters
4.4. Future Directions
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- George, J.; Sneed, K.; Pathak, Y. The skin aging process and anti-aging strategies. Biomed. J. Sci. Tech. Res. 2022, 42, 33377–33386. [Google Scholar]
- Tobin, D.J. Introduction to skin aging. J. Tissue Viability 2017, 26, 37–46. [Google Scholar] [CrossRef] [PubMed]
- Bonté, F.; Girard, D.; Archambault, J.-C.; Desmoulière, A. Skin Changes During Ageing. In Biochemistry and Cell Biology of Ageing: Part II Clinical Science; Harris, J.R., Korolchuk, V.I., Eds.; Springer: Singapore, 2019; pp. 249–280. [Google Scholar]
- Di Lorenzo, R.; Grumetto, L.; Sacchi, A.; Laneri, S.; Dini, I. Dermocosmetic evaluation of a nutricosmetic formulation based on Curcuma. Phytother. Res. 2023, 37, 1900–1910. [Google Scholar] [CrossRef] [PubMed]
- Imokawa, G.; Ishida, K. Biological Mechanisms Underlying the Ultraviolet Radiation-Induced Formation of Skin Wrinkling and Sagging I: Reduced Skin Elasticity, Highly Associated with Enhanced Dermal Elastase Activity, Triggers Wrinkling and Sagging. Int. J. Mol. Sci. 2015, 16, 7753–7775. [Google Scholar] [CrossRef] [PubMed]
- Addor, F.A.S.A. Beyond photoaging: Additional factors involved in the process of skin aging. Clin. Cosmet. Inv. Derm. 2018, 11, 437–443. [Google Scholar] [CrossRef] [PubMed]
- Franco, A.C.; Aveleira, C.; Cavadas, C. Skin senescence: Mechanisms and impact on whole-body aging. Trends Mol. Med. 2022, 28, 97–109. [Google Scholar] [CrossRef]
- Rostkowska, E.; Poleszak, E.; Wojciechowska, K.; Dos Santos Szewczyk, K. Dermatological Management of Aged Skin. Cosmetics 2023, 10, 55. [Google Scholar] [CrossRef]
- Mohiuddin, A.K. Skin aging & modern age anti-aging strategies. Int. J. Clin. Dermatol. Res 2019, 7, 209–240. [Google Scholar]
- Sparavigna, A. Role of the extracellular matrix in skin aging and dedicated treatment—State of the art. Plast. Aesthet. Res. 2020, 7, 14. [Google Scholar] [CrossRef]
- Walker, M. Human skin through the ages. Int. J. Pharm. 2022, 622, 121850. [Google Scholar] [CrossRef]
- Dobrev, H. Cutometer®. In Non Invasive Diagnostic Techniques in Clinical Dermatology; Berardesca, E., Maibach, H.I., Wilhelm, K.-P., Eds.; Springer: Berlin/Heidelberg, Germany, 2013; pp. 315–338. [Google Scholar]
- Weickenmeier, J.; Jabareen, M.; Mazza, E. Suction based mechanical characterization of superficial facial soft tissues. J. Biomech. 2015, 48, 4279–4286. [Google Scholar] [CrossRef] [PubMed]
- Kawałkiewicz, W.; Matthews-Kozanecka, M.; Janus-Kubiak, M.; Kubisz, L.; Hojan-Jezierska, D. Instrumental diagnosis of facial skin—A necessity or a pretreatment recommendation in esthetic medicine. J. Cosmet. Dermatol. 2021, 20, 875–883. [Google Scholar] [CrossRef] [PubMed]
- Bonaparte, J.P.; Ellis, D.; Chung, J. The effect of probe to skin contact force on Cutometer MPA 580 measurements. J. Med. Eng. Technol. 2013, 37, 208–212. [Google Scholar] [CrossRef] [PubMed]
- Ohshima, H.; Kinoshita, S.; Oyobikawa, M.; Futagawa, M.; Takiwaki, H.; Ishiko, A.; Kanto, H. Use of Cutometer area parameters in evaluating age-related changes in the skin elasticity of the cheek. Skin Res. Technol. 2013, 19, e238–e242. [Google Scholar] [CrossRef]
- Kim, M.A.; Kim, E.J.; Lee, H.K. Use of SkinFibrometer® to measure skin elasticity and its correlation with Cutometer® and DUB® Skinscanner. Skin Res. Technol. 2018, 24, 466–471. [Google Scholar] [CrossRef]
- Abbas, D.B.; Lavin, C.V.; Fahy, E.J.; Griffin, M.; Guardino, N.; King, M.; Chen, K.; Lorenz, P.H.; Gurtner, G.C.; Longaker, M.T.; et al. Standardizing Dimensionless Cutometer Parameters to Determine In Vivo Elasticity of Human Skin. Adv. Wound Care 2021, 11, 297–310. [Google Scholar] [CrossRef]
- Mueller, B.; Elrod, J.; Distler, O.; Schiestl, C.; Mazza, E. On the Reliability of Suction Measurements for Skin Characterization. J. Biomech. Eng. 2020, 143, 021002. [Google Scholar] [CrossRef]
- Pensalfini, M.; Rotach, M.; Hopf, R.; Bielicki, A.; Santoprete, R.; Mazza, E. How cosmetic tightening products modulate the biomechanics and morphology of human skin. Acta Biomater. 2020, 115, 299–316. [Google Scholar] [CrossRef]
- Ghasemi, E.; Nilforoushzadeh, M.A.; Khani, M.; Amirkhani, M.A.; Nouri, M.; Charipoor, P.; Eftekhari, M.; Izadpanah, S.; Shokri, B. The quantitative investigation of spark plasma on skin parameters with skin elasticity, thickness, density, and biometric characteristics. Sci. Rep. 2023, 13, 7738. [Google Scholar] [CrossRef]
- Charipoor, P.; Nilforoushzadeh, M.A.; Khani, M.; Nouri, M.; Ghasemi, E.; Amirkhani, M.A.; Eftekhari, M.; Shokri, B. The FEDBD plasma’s quantitative investigation of skin parameters Skin elasticity, thickness, density, tissue oxygenation, perfusion, and edema. Heliyon 2024, 10, e23386. [Google Scholar] [CrossRef]
- Langeveld, M.; van de Lande, L.S.; O’ Sullivan, E.; van der Lei, B.; van Dongen, J.A. Skin measurement devices to assess skin quality: A systematic review on reliability and validity. Skin Res. Technol. 2022, 28, 212–224. [Google Scholar] [CrossRef] [PubMed]
- Ahn, S.; Kim, S.; Lee, H.; Moon, S.; Chang, I. Correlation between a Cutometer® and quantitative evaluation using Moire topography in age-related skin elasticity. Skin Res. Technol. 2007, 13, 280–284. [Google Scholar] [CrossRef] [PubMed]
- Hojan-Jezierska, D.; Matthews-Kozanecka, M.; Kubisz, L. The use of cutometer to evaluate the skin elasticity on the face. J. Face Aesthet. 2022, 5, 18–24. [Google Scholar] [CrossRef]
- Ryu, H.S.; Joo, Y.H.; Kim, S.O.; Park, K.C.; Youn, S.W. Influence of age and regional differences on skin elasticity as measured by the Cutometer®. Skin Res. Technol. 2008, 14, 354–358. [Google Scholar] [CrossRef] [PubMed]
- Rodrigues, L. EEMCO Guidance to the in vivo Assessment of Tensile Functional Properties of the Skin: Part 2: Instrumentation and Test Modes. Skin Pharmacol. Appl. Skin Physiol. 2001, 14, 52–67. [Google Scholar] [CrossRef]
- Berardesca, E.; Maibach, H.I.; Wilhelm, K.-P. (Eds.) Non Invasive Diagnostic Techniques in Clinical Dermatology; Springer: Berlin/Heidelberg, Germany, 2013. [Google Scholar]
- Luebberding, S.; Krueger, N.; Kerscher, M. Mechanical properties of human skin in vivo: A comparative evaluation in 300 men and women. Skin Res. Technol. 2014, 20, 127–135. [Google Scholar] [CrossRef]
- Sandford, E.; Chen, Y.; Hunter, I.; Hillebrand, G.; Jones, L. Capturing skin properties from dynamic mechanical analyses. Skin Res. Technol. 2013, 19, e339–e348. [Google Scholar] [CrossRef]
- Braun, N.; Binder, S.; Grosch, H.; Theek, C.; Ülker, J.; Tronnier, H.; Heinrich, U. Current Data on Effects of Long-Term Missions on the International Space Station on Skin Physiological Parameters. Skin Pharmacol. Phys. 2018, 32, 43–51. [Google Scholar] [CrossRef]
- Kim, J.E.; Chang, S.; Won, C.H.; Kim, C.H.; Park, K.H.; Choi, J.H.; Lee, M.W. Combination Treatment Using Bipolar Radiofrequency-Based Intense Pulsed Light, Infrared Light and Diode Laser Enhanced Clinical Effectiveness and Histological Dermal Remodeling in Asian Photoaged Skin. Dermatol. Surg. 2012, 38, 68–76. [Google Scholar] [CrossRef]
- Kearney, E.M.; Messaraa, C.; Grennan, G.; Koeller, G.; Mavon, A.; Merinville, E. Evaluation of skin firmness by the DynaSKIN, a novel non-contact compression device, and its use in revealing the efficacy of a skincare regimen featuring a novel anti-ageing ingredient, acetyl aspartic acid. Skin Res. Technol. 2017, 23, 155–168. [Google Scholar] [CrossRef]
- Dobrev, H. Use of Cutometer to assess epidermal hydration. Skin Res. Technol. 2000, 6, 239–244. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.H.; Lee, S.J.; Kim, H.J.; Lee, J.H.; Jeong, H.S.; Suh, I.S. Aging-related changes in the mid-face skin elasticity in East Asian women. Arch. Craniofac. Surg. 2019, 20, 158. [Google Scholar] [CrossRef] [PubMed]
- Eklouh-Molinier, C.; Gaydou, V.; Froigneux, E.; Barlier, P.; Couturaud, V.; Manfait, M.; Piot, O. In vivo confocal Raman microspectroscopy of the human skin: Highlighting of spectral markers associated to aging via a research of correlation between Raman and biometric mechanical measurements. Anal. Bioanal. Chem. 2015, 407, 8363–8372. [Google Scholar] [CrossRef] [PubMed]
- Langton, A.K.; Alessi, S.; Hann, M.; Chien, A.L.-L.; Kang, S.; Griffiths, C.E.M.; Watson, R.E.B. Aging in Skin of Color: Disruption to Elastic Fiber Organization Is Detrimental to Skin’s Biomechanical Function. J. Investig. Dermatol. 2019, 139, 779–788. [Google Scholar] [CrossRef] [PubMed]
- Montero-Vilchez, T.; Cuenca-Barrales, C.; Rodriguez-Pozo, J.-A.; Diaz-Calvillo, P.; Tercedor-Sanchez, J.; Martinez-Lopez, A.; Molina-Leyva, A.; Arias-Santiago, S. Epidermal Barrier Function and Skin Homeostasis in Atopic Dermatitis: The Impact of Age. Life 2022, 12, 132. [Google Scholar] [CrossRef]
- Yoshida, K.; Yanagisawa, H. Age dependency of the reduced scattering coefficient and viscoelastic character, and their relationship in Japanese female skin. Biomed. Opt. Express 2024, 15, 4775–4785. [Google Scholar] [CrossRef]
- Silver, F.H.; Seehra, G.P.; Freeman, J.W.; DeVore, D. Viscoelastic properties of young and old human dermis: A proposed molecular mechanism for elastic energy storage in collagen and elastin. J. Appl. Polym. Sci. 2002, 86, 1978–1985. [Google Scholar] [CrossRef]
- Ruvolo, E.C., Jr.; Stamatas, G.N.; Kollias, N. Skin Viscoelasticity Displays Site- and Age-Dependent Angular Anisotropy. Skin Pharmacol. Phys. 2007, 20, 313–321. [Google Scholar] [CrossRef]
- Park, S. Biochemical, structural and physical changes in aging human skin, and their relationship. Biogerontology 2022, 23, 275–288. [Google Scholar] [CrossRef]
- Oh, B.H.; Kim, K.H.; Chung, K.Y. Skin imaging using ultrasound imaging, optical coherence tomography, confocal microscopy, and two-photon microscopy in cutaneous oncology. Front. Med. 2019, 6, 274. [Google Scholar] [CrossRef]
| Criteria | Details | |
|---|---|---|
| Inclusion Criteria | Exclusion Criteria | |
| ① Willing to participate and provide written informed consent; ② Healthy females aged 18–70 years; ③ No use of anti-inflammatory drugs on the test area in the past two months and no laser treatment in the past three months; ④ No participation in other clinical trials in the past three months; ⑤ Skin in the test area must be free of scars, birthmarks, atrophy, or other conditions that could affect the test results; ⑥ Ability to understand the study requirements and willingness to cooperate in completing all testing procedures. | ① Use of antihistamines in the past week or immunosuppressants in the past month; ② Currently undergoing facial treatment or use of products to improve facial skin condition in the past month; ③ Presence of untreated inflammatory skin diseases; ④ Insulin-dependent diabetes; ⑤ History of cancer chemotherapy in the past six months; ⑥ Immune deficiencies or autoimmune diseases; ⑦ Participation in other clinical trials; ⑧ Pregnant or breastfeeding women. | |
| Parameter Name | Parameter Description | Parameter Significance |
|---|---|---|
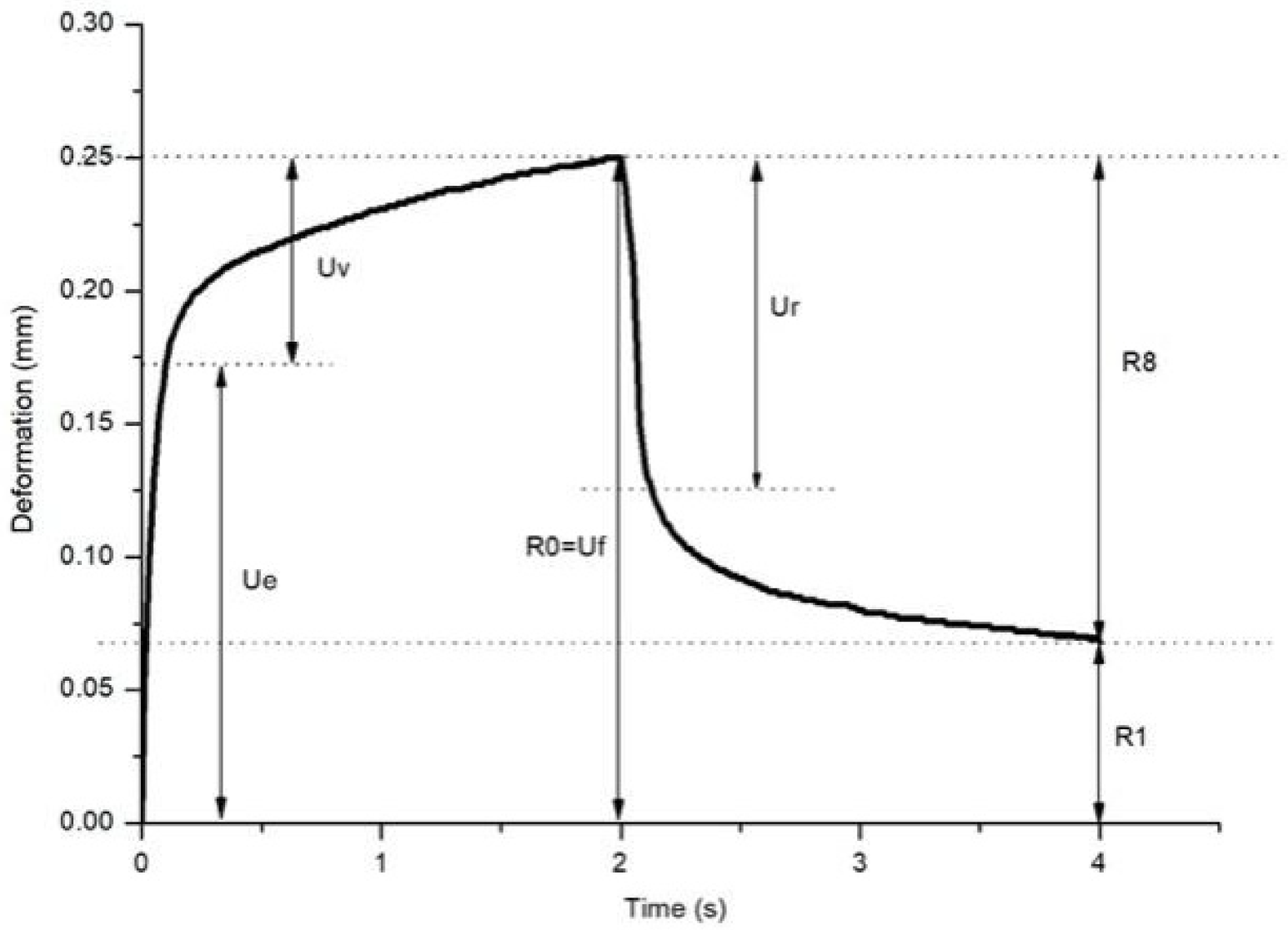
| R0 = Uf | Maximum deformation during the first cycle | Represents the skin’s extensibility. |
| Ue | Instantaneous deformation | Deformation 0.1 s after applying negative pressure, reflecting the skin’s immediate elasticity. |
| Ur | Instantaneous retraction | The immediate retraction of the skin after the release of negative pressure. |
| Uv | Delayed deformation | The skin’s deformation during the delayed retraction phase, reflecting the viscous component of the skin. |
| R1 = Uf − Ua | Remaining deformation after the first cycle | The skin’s residual deformation after the first cycle, indicating the skin’s elastic limit. |
| R2 = Ua/Uf | Ratio of total retraction to maximum deformation; total elasticity | The ratio of total retraction to maximum deformation, representing overall skin elasticity. A value closer to 1 indicates better elasticity. |
| R5 = Ur/Ue | Ratio of instantaneous retraction to instantaneous deformation; net elasticity | The ratio of instantaneous retraction to instantaneous deformation, reflecting the skin’s net elasticity. |
| R6 = Uv/Ue | Ratio of delayed deformation to instantaneous deformation | Reflects the ratio of viscous to elastic components under negative pressure. |
| R7 = Ur/Uf | Ratio of instantaneous retraction to maximum deformation; biological elasticity | The ratio of instantaneous retraction to maximum deformation, indicating the skin’s biological elasticity. |
| R8 = Ua | Total retraction after the first cycle | The total retraction of the skin after the first cycle. |
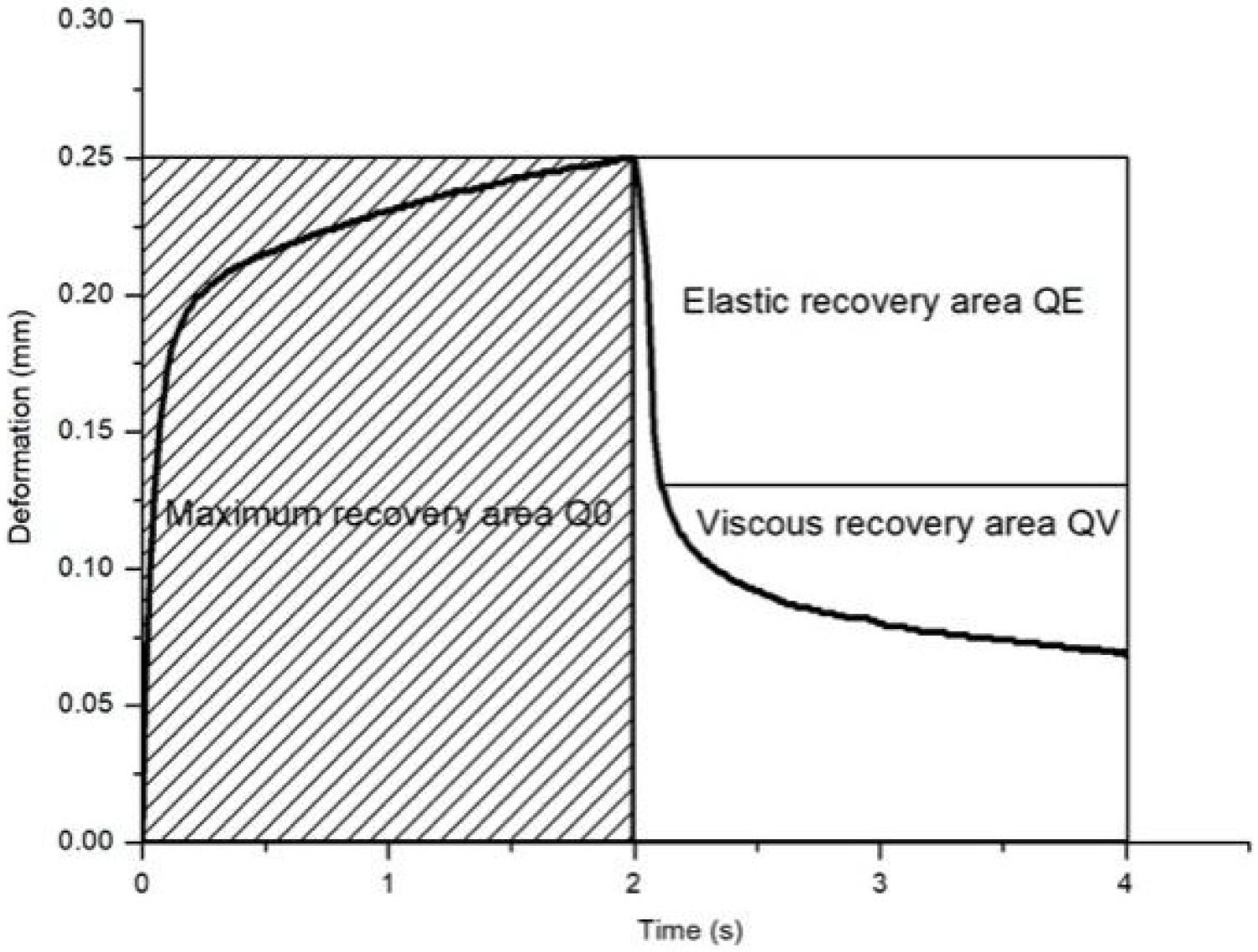
| Q0 | Maximum deformation area | The area under the curve for R0, calculated as Q0 = 200 × R0. |
| Q1 = QE/Q0 | Ratio of the area formed by instantaneous retraction to maximum deformation area | Reflects the immediate elasticity recovery. |
| Q2 = QV/Q0 | Ratio of the area formed by delayed retraction to maximum deformation area | Reflects the delayed elasticity recovery. |
| Q3 = (QE + QV)/Q0 | Ratio of total retraction area to maximum deformation area; overall elasticity | Indicates the overall elasticity of the skin. |
| F0 | The area between the actual curve and the Uf value during the negative pressure phase. | - |
| F1 | The area between the actual curve and the residual deformation (R1) during the relaxation phase. | - |
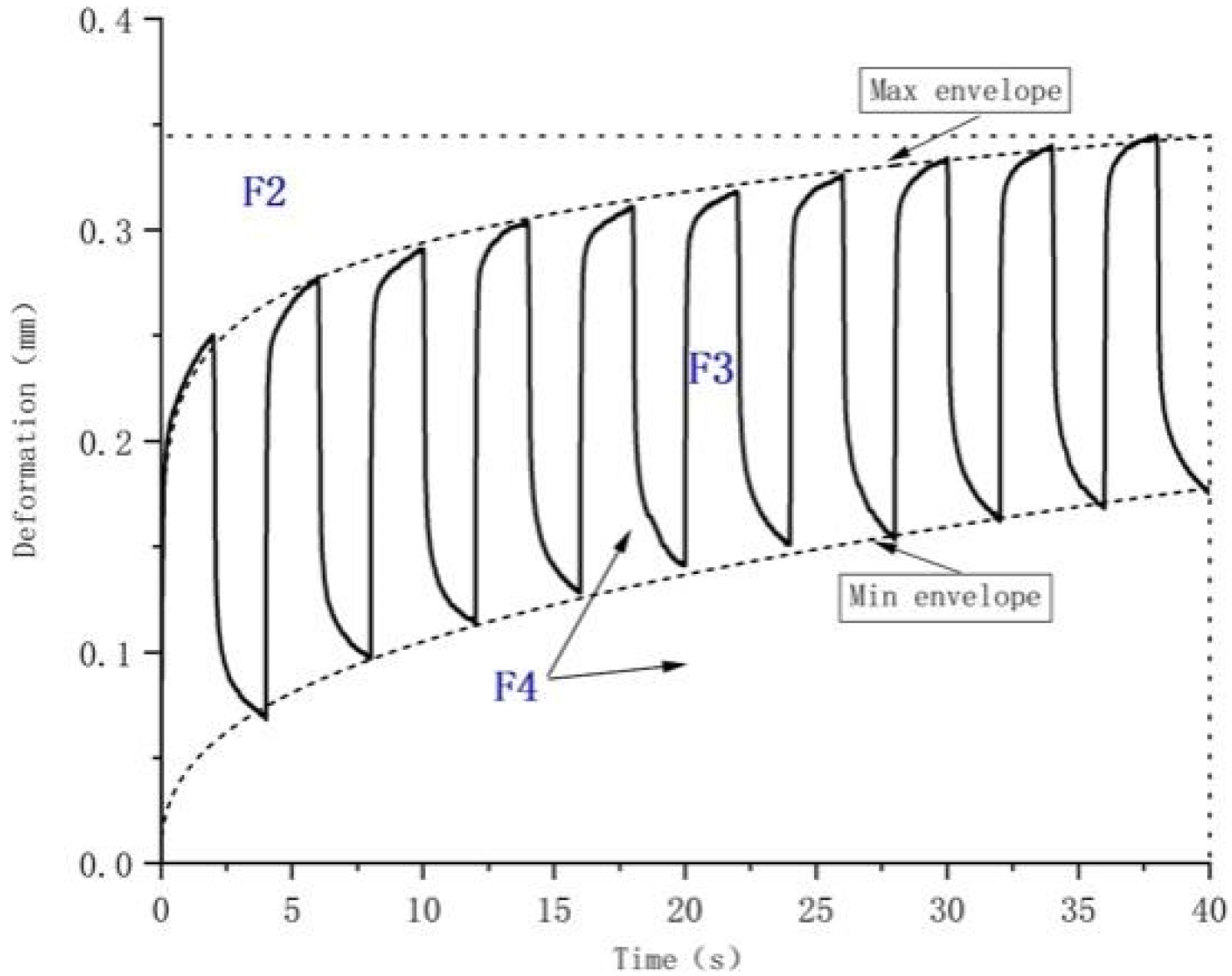
| F2 | The area between the actual curve and R3 after 10 cycles of deformation. | - |
| F3 | The area between the upper and lower curves after 10 cycles of deformation. | - |
| F4 | The total area under the upper curve after 10 cycles of deformation. | - |
| R3 | Maximum deformation after 10 cycles | The maximum deformation of the skin after the 10th cycle. |
| R4 | Residual deformation after 10 cycles | The residual deformation after the 10th cycle. |
| R9 = R3 − R0 | Difference between maximum deformation in the 10th and 1st cycles | Represents the change in maximum deformation between the first and 10th cycles. |
| Parameters | Ur | R7 | R8 | Q1 | F3 | Q2 | R2 | Ue | R3 | R6 |
|---|---|---|---|---|---|---|---|---|---|---|
| Pearson correlation | −0.66 **,1 | −0.62 ** | −0.60 ** | −0.59 ** | −0.59 ** | −0.57 ** | −0.56 ** | −0.51 ** | −0.49 ** | 0.49 ** |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, D.; Yin, S.; Lu, X.; Fu, H.; Gao, H.; Zhang, S. Research on the Correlation Between Skin Elasticity Evaluation Parameters and Age. Cosmetics 2024, 11, 205. https://doi.org/10.3390/cosmetics11060205
Chen D, Yin S, Lu X, Fu H, Gao H, Zhang S. Research on the Correlation Between Skin Elasticity Evaluation Parameters and Age. Cosmetics. 2024; 11(6):205. https://doi.org/10.3390/cosmetics11060205
Chicago/Turabian StyleChen, Dandan, Shipeng Yin, Xuelian Lu, Haokun Fu, Hongqi Gao, and Suning Zhang. 2024. "Research on the Correlation Between Skin Elasticity Evaluation Parameters and Age" Cosmetics 11, no. 6: 205. https://doi.org/10.3390/cosmetics11060205
APA StyleChen, D., Yin, S., Lu, X., Fu, H., Gao, H., & Zhang, S. (2024). Research on the Correlation Between Skin Elasticity Evaluation Parameters and Age. Cosmetics, 11(6), 205. https://doi.org/10.3390/cosmetics11060205







