Inhibitory Effect of Probiotic Metabolites on Seborrheic Dermatitis and Acne-Related Pathogenic Bacteria
Abstract
:1. Introduction
2. Materials and Methods
2.1. Preparation of Pathogenic Bacteria
2.2. Preparation of Probiotic Strains
2.3. Growth Inhibition Test
2.4. Identification of Active Metabolites
3. Results
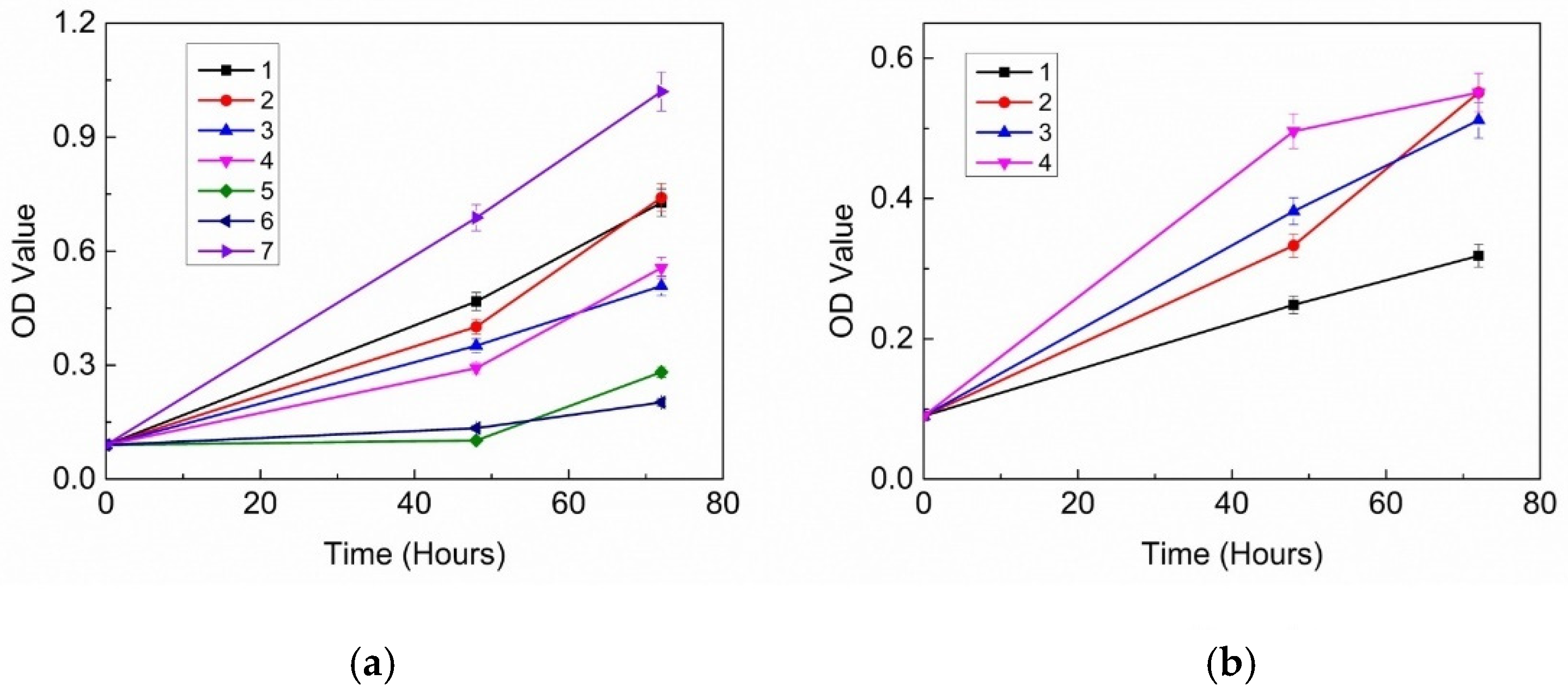
3.1. Inhibitory Effects of 18 Probiotic Strains on Skin Pathogens
3.2. Antibacterial Proteins and Peptides
3.3. Inhibitory Effects of Organic Acids
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Sender, R.; Fuchs, S.; Milo, R. Revised Estimates for the Number of Human and Bacteria Cells in the Body. PLoS Biol. 2016, 14, e1002533. [Google Scholar] [CrossRef] [PubMed]
- Grice, E.A.; Segre, J.A. The skin microbiome. Nat. Rev. Microbiol. 2011, 9, 244. [Google Scholar] [CrossRef] [PubMed]
- Byrd, A.L.; Belkaid, Y.; Segre, J.A. The human skin microbiome. Nat. Rev. Microbiol. 2018, 16, 143–155. [Google Scholar] [CrossRef] [PubMed]
- Elias, P.M.; Choi, E.H. Interactions among stratum corneum defensive functions. Exp. Dermatol. 2005, 14, 719–726. [Google Scholar] [CrossRef]
- Chehoud, C.; Rafail, S.; Tyldsley, A.S.; Seykora, J.T.; Lambris, J.D.; Grice, E.A. Complement modulates the cutaneous microbiome and inflammatory milieu. Proc. Natl. Acad. Sci. USA 2013, 110, 15061–15066. [Google Scholar] [CrossRef]
- Sugimoto, S.; Iwamoto, T.; Takada, K.; Okuda, K.-I.; Tajima, A.; Iwase, T.; Mizunoe, Y. Staphylococcus epidermidis Esp Degrades Specific Proteins Associated with Staphylococcus aureus Biofilm Formation and Host-Pathogen Interaction. J. Bacteriol. 2013, 195, 1645–1655. [Google Scholar] [CrossRef]
- Bomar, L.; Brugger, S.D.; Yost, B.H.; Davies, S.S.; Lemon, K.P. Corynebacterium accolens Releases Antipneumococcal Free Fatty Acids from Human Nostril and Skin Surface Triacylglycerols. mBio 2016, 7, e01725-15. [Google Scholar] [CrossRef]
- Iebba, V.; Totino, V.; Gagliardi, A.; Santangelo, F.; Schippa, S. Eubiosis and Dysbiosis: The Two Sides of the Microbiota. New Microbiol. Off. J. Ital. Soc. Med. Virol. (SIVIM) 2016, 39, 1–12. [Google Scholar]
- Coenye, T.; Peeters, E.; Nelis, H.J. Biofilm formation by Propionibacterium acnes is associated with increased resistance to antimicrobial agents and increased production of putative virulence factors. Res. Microbiol. 2007, 158, 386–392. [Google Scholar] [CrossRef]
- Cogen, A.L.; Nizet, V.; Gallo, R.L. Skin microbiota: A source of disease or defence? Br. J. Dermatol. 2008, 158, 442–455. [Google Scholar] [CrossRef]
- Wang, Y.; Hata, T.R.; Tong, Y.L.; Kao, M.-S.; Zouboulis, C.C.; Gallo, R.L.; Huang, C.-M. The Anti-Inflammatory Activities of Propionibacterium acnes CAMP Factor-Targeted Acne Vaccines. J. Investig. Dermatol. 2018, 138, 2355–2364. [Google Scholar] [CrossRef] [PubMed]
- Cho, I.; Blaser, M.J. The human microbiome: At the interface of health and disease. Nat. Rev. Genet. 2012, 13, 260–270. [Google Scholar] [CrossRef] [PubMed]
- Byrd, A.L.; Deming, C.; Cassidy, S.K.B.; Harrison, O.J.; Ng, W.-I.; Conlan, S.; Program, N.C.S.; Belkaid, Y.; Segre, J.A.; Kong, H.H. Staphylococcus aureusand Staphylococcus epidermidis strain diversity underlying pediatric atopic dermatitis. Sci. Transl. Med. 2017, 9, eaal4651. [Google Scholar] [CrossRef] [PubMed]
- Lin, Q.; Panchamukhi, A.; Li, P.; Shan, W.; Zhou, H.; Hou, L.; Chen, W. Malassezia and Staphylococcus dominate scalp microbiome for seborrheic dermatitis. Bioprocess Biosyst. Eng. 2021, 44, 965–975. [Google Scholar] [CrossRef]
- DeAngelis, Y.M.; Saunders, C.W.; Johnstone, K.R.; Reeder, N.L.; Coleman, C.G.; Kaczvinsky, J.R.; Gale, C.; Walter, R.; Mekel, M.; Lacey, M.P.; et al. Isolation and Expression of a Malassezia globosa Lipase Gene, LIP1. J. Investig. Dermatol. 2007, 127, 2138–2146. [Google Scholar] [CrossRef]
- Hay, R.J. Malassezia, dandruff and seborrhoeic dermatitis: An overview. Br. J. Dermatol. 2011, 165 (Suppl. S2), 2–8. [Google Scholar] [CrossRef]
- Borda, L.J.; Wikramanayake, T.C. Seborrheic Dermatitis and Dandruff: A Comprehensive Review. J. Clin. Investig. Dermatol. 2015, 3, 10. [Google Scholar]
- Turner, G.A.; Hoptroff, M.; Harding, C.R. Stratum corneum dysfunction in dandruff. Int. J. Cosmet. Sci. 2012, 34, 298–306. [Google Scholar] [CrossRef]
- Kim, S.Y.; Kim, S.H.; Kim, S.N.; Kim, A.-R.; Kim, Y.R.; Kim, M.J.; Park, W.-S.; Lee, J.H.; Jung, W.H.; Lee, Y.W.; et al. Isolation and identification of Malassezia species from Chinese and Korean patients with seborrheic dermatitis and in vitro studies on their bioactivity on sebaceous lipids and IL-8 production. Mycoses 2016, 59, 274–280. [Google Scholar] [CrossRef]
- Sommer, B.; Overy, D.P.; Kerr, R.G. Identification and characterization of lipases from Malassezia restricta, a causative agent of dandruff. FEMS Yeast Res. 2015, 15, fov078. [Google Scholar] [CrossRef]
- Dawson, T.L. Malassezia globosa and restricta: Breakthrough Understanding of the Etiology and Treatment of Dandruff and Seborrheic Dermatitis through Whole-Genome Analysis. J. Investig. Dermatol. Symp. Proc. 2007, 12, 15–19. [Google Scholar] [CrossRef] [PubMed]
- Tanaka, A.; Cho, O.; Saito, C.; Saito, M.; Tsuboi, R.; Sugita, T. Comprehensive pyrosequencing analysis of the bacterial microbiota of the skin of patients with seborrheic dermatitis. Microbiol. Immunol. 2016, 60, 521–526. [Google Scholar] [CrossRef] [PubMed]
- Fuchs-Tarlovsky, V.; Marquez-Barba, M.F.; Sriram, K. Probiotics in dermatologic practice. Nutrition 2016, 32, 289–295. [Google Scholar] [CrossRef] [PubMed]
- Katz, L.; Baltz, R.H. Natural product discovery: Past, present, and future. J. Ind. Microbiol. Biotechnol. 2016, 43, 155–176. [Google Scholar] [CrossRef]
- Al-Ghazzewi, F.H.; Tester, R.F. Effect of konjac glucomannan hydrolysates and probiotics on the growth of the skin bacterium Propionibacterium acnes in vitro. Int. J. Cosmet. Sci. 2010, 32, 139–142. [Google Scholar] [CrossRef]
- Guéniche, A.; Cathelineau, A.C.; Bastien, P.; Esdaile, J.; Martin, R.; Queille Roussel, C.; Breton, L. Vitreoscilla filiformis biomass improves seborrheic dermatitis. J. Eur. Acad. Dermatol. Venereol. 2008, 22, 1014–1015. [Google Scholar] [CrossRef]
- Guéniche, A.; Hennino, A.; Goujon, C.; Dahel, K.; Bastien, P.; Martin, R.; Jourdain, R.; Breton, L.; Gueniche, A.; Hennino, A.; et al. Improvement of atopic dermatitis skin symptoms by Vitreoscilla filiformis bacterial extract. Eur. J. Dermatol. 2006, 16, 380–384. [Google Scholar]
- Lay, C.L.; Coton, E.; Blay, G.L.; Chobert, J.M.; Haertlé, T.; Choiset, Y.; Long, N.V.; Meslet-Cladière, L.; Mounier, J. Identification and quantification of antifungal compounds produced by lactic acid bacteria and propionibacteria. Int. J. Food Microbiol. 2016, 239, 79–85. [Google Scholar] [CrossRef]
- Qiao, N.; Yu, L.; Zhang, C.; Wei, C.; Zhao, J.; Zhang, H.; Tian, F.; Zhai, Q.; Chen, W. A comparison of the inhibitory activities of Lactobacillus and Bifidobacterium against Penicillium expansum and an analysis of potential antifungal metabolites. FEMS Microbiol. Lett. 2020, 367, fnaa130. [Google Scholar] [CrossRef]
- Kang, K.H.; Shin, H.J.; Park, Y.H.; Lee, T.S. Studies on the antibacterial substances produced by lactic acid bacteria: Purification and some properties of antibacterial substance ’Bifilong’ produced by B. longum. Korean J. Dairy Sci. 1989, 1, 204–216. [Google Scholar]
- Angelescu, I.R.; Grosu-Tudor, S.S.; Cojoc, L.R.; Maria, G.M.; Chirițoiu, G.N.; Munteanu, C.V.A.; Zamfir, M. Isolation, characterization, and mode of action of a class III bacteriocin produced by Lactobacillus helveticus 34.9. World J. Microbiol. Biotechnol. 2022, 38, 220. [Google Scholar] [CrossRef] [PubMed]
- Ahmad, C.; Natascha, C.; Haiqin, C.; Jianxin, Z.; Jian, T.; Hao, Z.; Wei, C. Bifidin I—A new bacteriocin produced by Bifidobacterium infantis BCRC 14602: Purification and partial amino acid sequence—ScienceDirect. Food Control 2010, 21, 746–753. [Google Scholar]
- Nissen-Meyer, J.; Rogne, P.; Oppegard, C.; Haugen, H.; Kristiansen, P. Structure-Function Relationships of the Non-Lanthionine-Containing Peptide (class II) Bacteriocins Produced by Gram-Positive Bacteria. Curr. Pharm. Biotechnol. 2009, 10, 19–37. [Google Scholar] [CrossRef]
- Salomskiene, J.; Jonkuviene, D.; Macioniene, I.; Abraitiene, A.; Zeime, J.; Repeckiene, J.; Vaiciulyte-Funk, L. Differences in the occurence and efficiency of antimicrobial compounds produced by lactic acid bacteria. Eur. Food Res. Technol. 2019, 245, 569–579. [Google Scholar] [CrossRef]


| Name | Strain | Growth Inhibition of M. furfur | Growth Inhibition of C. acnes | ||||
|---|---|---|---|---|---|---|---|
| 48 h | 72 h | 96 h | 48 h | 72 h | 96 h | ||
| Bifidobacterium lactis | HN019 | ++ | ++ | ++ | ++ | ++ | ++ |
| Bifidobacterium lactis | Bl-04 | − | − | − | + | + | + |
| Bifidobacterium lactis | B420 | ++ | ++ | ++ | ++ | + | + |
| Bifidobacterium lactis | Bi-07 | ++ | ++ | ++ | ++ | + | + |
| Bifidobacterium animalis | Bb-12 | ++ | − | − | ++ | + | + |
| Lactobacillus brevis | Lbr-35 | − | − | − | + | + | + |
| Lactobacillus acidophilus | NCFM | + | − | − | + | + | + |
| Lactobacillus plantarum | Lp-115 | ++ | + | − | ++ | ++ | ++ |
| Lactobacillus paracasei | Lpc-37 | + | − | − | + | + | − |
| Lactobacillus rhamnosus | HN001 | ++ | + | − | ++ | + | + |
| Lactobacillus acidophilus | La-14 | − | − | − | ++ | − | − |
| Lactobacillus casei | Lc-11 | + | − | − | ++ | + | − |
| Lactobacillus rhamnosus | GG | ++ | − | − | ++ | + | + |
| Lactobacillus salivarius | Ls-33 | + | − | − | ++ | + | + |
| Lactobacillus rhamnosus | Lr-32 | ++ | − | − | ++ | + | + |
| Lactobacillus reuteri | 1E1 | + | − | − | + | − | − |
| Lactobacillus fermentum | SBS-1 | − | − | − | + | + | + |
| Lactobacillus bulgaricus | Lb-87 | − | − | − | ++ | + | − |
| No. | Strain | Lactic Acid (mg/L) | Acetic Acid (mg/L) | Propionic Acid (mg/L) | Butyric Acid (mg/L) |
|---|---|---|---|---|---|
| 1 | B420 | 5.64 | 9.26 | 1.82 | 0.2 |
| 2 | Bi-07 | 17.39 | 6.25 | 1.65 | 0.17 |
| 3 | HN019 | 10.82 | 8.85 | 1.06 | 0.05 |
| 4 | Lp-115 | 19.88 | 4.04 | 1.80 | 0.06 |
| No. | Strain | Growth Inhibition of M. furfur | ||
|---|---|---|---|---|
| Crude Protein Extract | Crude Supernatant Extracts | Organic Acid Mixture | ||
| 1 | B420 | - | 55% | 72% |
| 2 | Bi-07 | 31% | 50% | 92% |
| 3 | HN019 | 30% | 79% | 71% |
| 4 | Lp-115 | - | 88% | 54% |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Meng, Q.; Xiao, C.; Wang, Z.; Shang, Y. Inhibitory Effect of Probiotic Metabolites on Seborrheic Dermatitis and Acne-Related Pathogenic Bacteria. Cosmetics 2025, 12, 3. https://doi.org/10.3390/cosmetics12010003
Meng Q, Xiao C, Wang Z, Shang Y. Inhibitory Effect of Probiotic Metabolites on Seborrheic Dermatitis and Acne-Related Pathogenic Bacteria. Cosmetics. 2025; 12(1):3. https://doi.org/10.3390/cosmetics12010003
Chicago/Turabian StyleMeng, Qingpeng, Ciying Xiao, Zejian Wang, and Yazhuo Shang. 2025. "Inhibitory Effect of Probiotic Metabolites on Seborrheic Dermatitis and Acne-Related Pathogenic Bacteria" Cosmetics 12, no. 1: 3. https://doi.org/10.3390/cosmetics12010003
APA StyleMeng, Q., Xiao, C., Wang, Z., & Shang, Y. (2025). Inhibitory Effect of Probiotic Metabolites on Seborrheic Dermatitis and Acne-Related Pathogenic Bacteria. Cosmetics, 12(1), 3. https://doi.org/10.3390/cosmetics12010003




