Hair Growth-Promoting Effects of Astragalus sinicus Extracts in Human Follicle Dermal Papilla Cells
Abstract
1. Introduction
2. Materials and Methods
2.1. Preparation of ASEs
2.2. Cell Culture
2.3. Cell Viability Assay
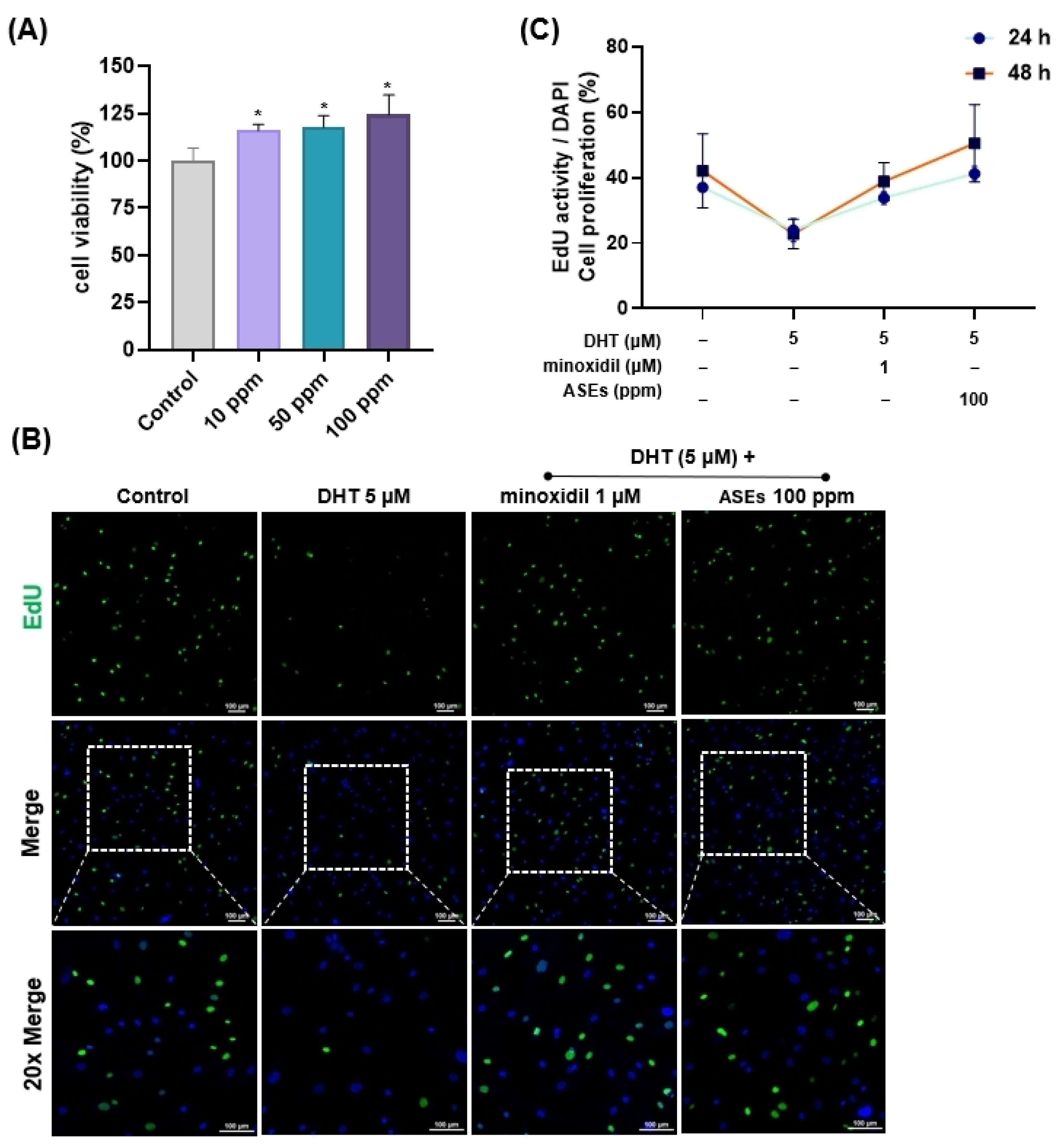
2.4. Cell Proliferation Assay
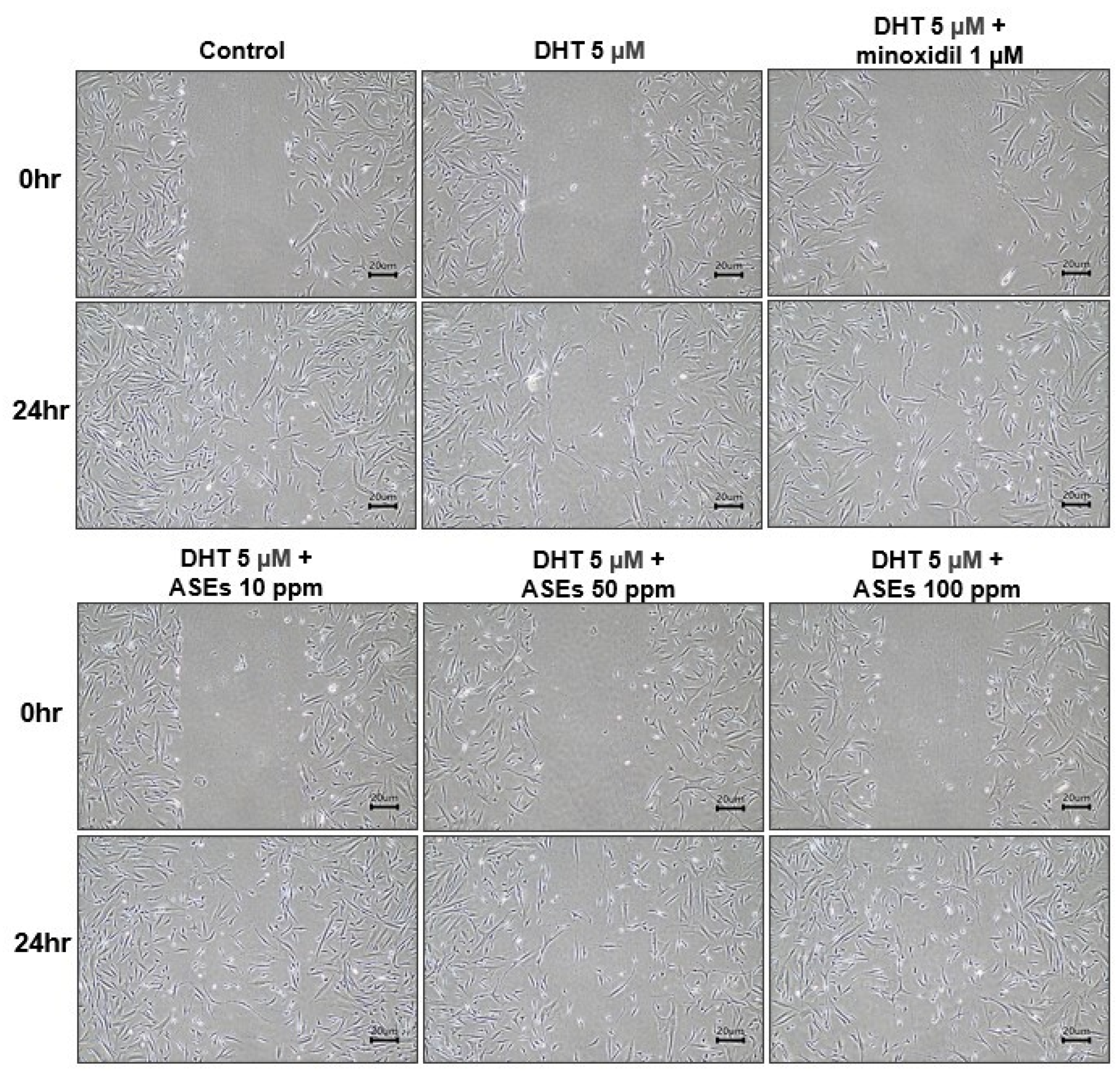
2.5. Wound Healing Assay
2.6. Alkaline Phosphatase Staining (ALP) Assay
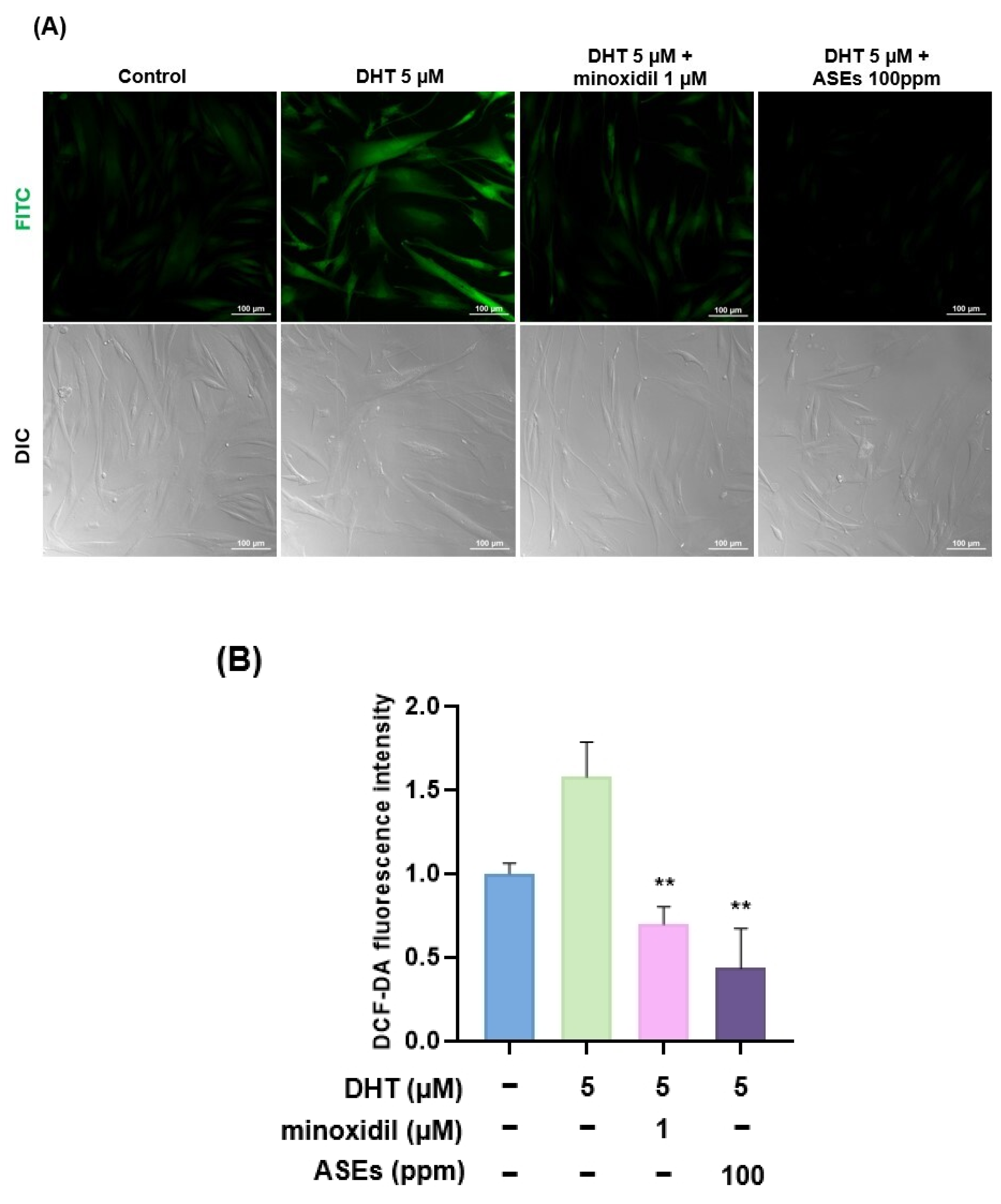
2.7. Measurement of Intracellular ROS
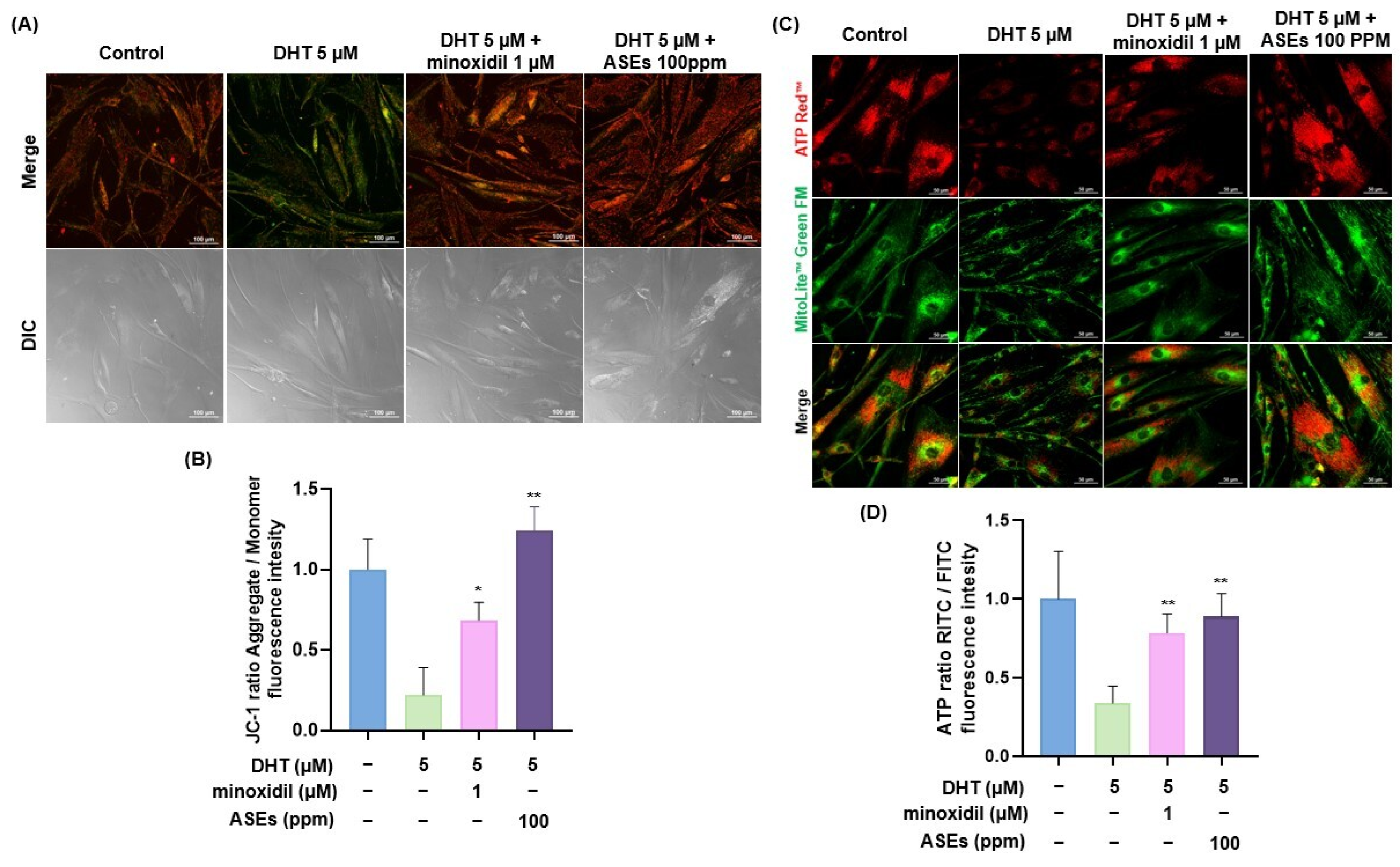
2.8. Measurement of Membrane Potential in Mitochondria
2.9. Live Cell ATP Assay
2.10. Western Blot Analysis
2.11. Statistical Analysis
3. Results
3.1. The Effects of ASEs on Cell Viability and Proliferation in HFDPCs
3.2. ASEs Improved Cellular Migration of HFDPCs Damaged by DHT
3.3. ASEs Improved the ALP Activity of HFDPCs Damaged by DHT
3.4. ASEs Reduced DHT-Induced ROS Levels in HFDPCs
3.5. ASEs Restored Mitochondrial Potential and ATP Levels in DHT-Damaged HFDPCs
3.6. ASEs Were Involved in the ERK/AKT Signaling Pathway and Wnt/β-Catenin Pathway in DHT-Damaged HFDPCs
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Shimizu, Y.; Ntege, E.H.; Sunami, H.; Inoue, Y. Regenerative medicine strategies for hair growth and regeneration: A narrative review of literature. Regen. Ther. 2022, 21, 527–539. [Google Scholar] [CrossRef]
- Choi, B.Y. Targeting Wnt/β-Catenin Pathway for Developing Therapies for Hair Loss. Int. J. Mol. Sci. 2020, 21, 4915. [Google Scholar] [CrossRef]
- Fu, D.; Huang, J.; Li, K.; Chen, Y.; He, Y.; Sun, Y.; Guo, Y.; Du, L.; Qu, Q.; Miao, Y.; et al. Dihydrotestosterone-induced hair regrowth inhibition by activating androgen receptor in C57BL6 mice simulates androgenetic alopecia. Biomed. Pharmacother. 2021, 137, 111247. [Google Scholar] [CrossRef]
- Kaliyadan, F.; Nambiar, A.; Vijayaraghavan, S. Androgenetic alopecia: An update. Indian J. Dermatol. Venereol. Leprol. 2013, 79, 613–625. [Google Scholar] [CrossRef] [PubMed]
- Nestor, M.S.; Ablon, G.; Gade, A.; Han, H.; Fischer, D.L. Treatment options for androgenetic alopecia: Efficacy, side effects, compliance, financial considerations, and ethics. J. Cosmet. Dermatol. 2021, 20, 3759–3781. [Google Scholar] [CrossRef]
- Upton, J.H.; Hannen, R.F.; Bahta, A.W.; Farjo, N.; Farjo, B.; Philpott, M.P. Oxidative stress-associated senescence in dermal papilla cells of men with androgenetic alopecia. J. Investig. Dermatol. 2015, 135, 1244–1252. [Google Scholar] [CrossRef]
- Trueb, R.M. Oxidative stress and its impact on skin, scalp and hair. Int. J. Cosmet. Sci. 2021, 43 (Suppl. S1), S9–S13. [Google Scholar] [CrossRef] [PubMed]
- Martinez-Jacobo, L.; Villarreal-Villarreal, C.D.; Ortiz-Lopez, R.; Ocampo-Candiani, J.; Rojas-Martinez, A. Genetic and molecular aspects of androgenetic alopecia. Indian J. Dermatol. Venereol. Leprol. 2018, 84, 263–268. [Google Scholar] [CrossRef] [PubMed]
- Taghiabadi, E.; Nilforoushzadeh, M.A.; Aghdami, N. Maintaining Hair Inductivity in Human Dermal Papilla Cells: A Review of Effective Methods. Ski. Pharmacol. Physiol. 2020, 33, 280–292. [Google Scholar] [CrossRef]
- Abreu, C.M.; Cerqueira, M.T.; Pirraco, R.P.; Gasperini, L.; Reis, R.L.; Marques, A.P. Rescuing key native traits in cultured dermal papilla cells for human hair regeneration. J. Adv. Res. 2020, 30, 103–112. [Google Scholar] [CrossRef] [PubMed]
- Gao, R.; Yu, Z.; Lv, C.; Geng, X.; Ren, Y.; Ren, J.; Wang, H.; Ai, F.; Zhang, B.; Yue, B.; et al. Medicinal and edible plant Allium macrostemon Bunge for the treatment of testosterone-induced androgenetic alopecia in mice. J. Ethnopharmacol. 2023, 315, 116657. [Google Scholar] [CrossRef]
- Kim, S.M.; Kang, J.; Yoon, H.; Choi, Y.K.; Go, J.S.; Oh, S.K.; Ahn, M.; Kim, J.; Koh, Y.S.; Hyun, J.W.; et al. HNG, A Humanin Analogue, Promotes Hair Growth by Inhibiting Anagen-to-Catagen Transition. Int. J. Mol. Sci. 2020, 21, 4553. [Google Scholar] [CrossRef] [PubMed]
- Tang, X.; Cao, C.; Liang, Y.; Han, L.; Tu, B.; Yu, M.; Wan, M. Adipose-Derived Stem Cell Exosomes Antagonize the Inhibitory Effect of Dihydrotestosterone on Hair Follicle Growth by Activating Wnt/β-Catenin Pathway. Stem Cells Int. 2023, 2023, 5548112. [Google Scholar] [CrossRef]
- Li, Y.; Zhang, K.; Ye, J.; Lian, X.; Yang, T. Wnt10b promotes growth of hair follicles via a canonical Wnt signalling pathway. Clin. Exp. Dermatol. 2011, 36, 534–540. [Google Scholar] [CrossRef]
- Enshell-Seijffers, D.; Lindon, C.; Kashiwagi, M.; Morgan, B.A. Β-Catenin Activity in the Dermal Papilla Regulates Morphogenesis and Regeneration of Hair. Dev. Cell. 2010, 18, 633–642. [Google Scholar] [CrossRef]
- Ryu, Y.C.; Kim, Y.; Park, J.; Choi, S.; Kim, G.; Kim, E.; Hwang, Y.; Kim, H.; Bak, S.S.; Lee, J.E.; et al. Wnt/β-catenin signaling activator restores hair regeneration suppressed by diabetes mellitus. BMB Rep. 2022, 55, 559–564. [Google Scholar] [CrossRef]
- Kesika, P.; Sivamaruthi, B.S.; Thangaleela, S.; Bharathi, M.; Chaiyasut, C. Role and Mechanisms of Phytochemicals in Hair Growth and Health. Pharmaceuticals 2023, 16, 206. [Google Scholar] [CrossRef]
- Sun, M.; Deng, Y.; Cao, X.; Xiao, L.; Ding, Q.; Luo, F.; Huang, P.; Gao, Y.; Liu, M.; Zhao, H. Effects of Natural Polyphenols on Skin and Hair Health: A Review. Molecules 2022, 27, 7832. [Google Scholar] [CrossRef] [PubMed]
- Trakoolthong, P.; Ditthawuttikul, N.; Sivamaruthi, B.S.; Sirilun, S.; Rungseevijitprapa, W.; Peerajan, S.; Chaiyasut, C. Antioxidant and 5α-Reductase Inhibitory Activity of Momordica charantia Extract, and Development and Characterization of Microemulsion. Appl. Sci. 2022, 12, 4410. [Google Scholar] [CrossRef]
- Zhang, X.; Xu, J.; Si, L.; Cao, K.; Wang, Y.; Li, H.; Wang, J. Cloning, Identification, and Functional Analysis of the Chalcone Isomerase Gene from Astragalus sinicus. Genes 2023, 14, 1400. [Google Scholar] [CrossRef] [PubMed]
- Lv, M.; Wang, Y.; Chen, X.; Qin, W.; Shi, W.; Song, W.; Chen, J.; Xu, C. The moderate substitution of Astragalus sinicus returning for chemical fertilizer improves the N cycle function of key ecological bacterial clusters in soil. Front. Microbiol. 2023, 13, 1067939. [Google Scholar] [CrossRef] [PubMed]
- Lim, D.; Choi, D.; Choi, O.; Cho, K.; Kim, R.; Choi, H.; Cho, H. Effect of Astragalus sinicus L. seed extract on antioxidant activity. J. Ind. Eng. Chem. 2011, 17, 510–516. [Google Scholar] [CrossRef]
- D’Avino, D.; Cerqua, I.; Ullah, H.; Spinelli, M.; Di Matteo, R.; Granato, E.; Capasso, R.; Maruccio, L.; Ialenti, A.; Daglia, M.; et al. Beneficial Effects of Astragalus membranaceus (Fisch.) Bunge Extract in Controlling Inflammatory Response and Preventing Asthma Features. Int. J. Mol. Sci. 2023, 24, 10954. [Google Scholar] [CrossRef] [PubMed]
- Kim, B.; Oh, I.; Kim, J.; Jeon, J.; Jeon, B.; Shin, J.; Kim, T. Anti-inflammatory activity of compounds isolated from Astragalus sinicus L. in cytokine-induced keratinocytes and skin. Exp. Mol. Med. 2014, 46, e87. [Google Scholar] [CrossRef] [PubMed]
- Liu, S.; Wang, W.; Chen, J.; Ma, Z.; Xiao, Y.; Chen, Z.; Zhang, Y.; Du, X.; Mu, Y. Weed suppression and antioxidant activity of Astragalus sinicus L. decomposition leachates. Front. Plant. Sci. 2022, 13, 1013443. [Google Scholar] [CrossRef] [PubMed]
- Bassino, E.; Gasparri, F.; Munaron, L. Protective Role of Nutritional Plants Containing Flavonoids in Hair Follicle Disruption: A Review. Int. J. Mol. Sci. 2020, 21, 523. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.; Yoon, J.; Shin, S.H.; Zahoor, M.; Kim, H.J.; Park, P.J.; Park, W.; Min, D.S.; Kim, H.; Choi, K. Valproic acid induces hair regeneration in murine model and activates alkaline phosphatase activity in human dermal papilla cells. PLoS ONE 2012, 7, e34152. [Google Scholar] [CrossRef]
- Ohyama, M.; Zheng, Y.; Paus, R.; Stenn, K.S. The mesenchymal component of hair follicle neogenesis: Background, methods and molecular characterization. Exp. Dermatol. 2010, 19, 89–99. [Google Scholar] [CrossRef] [PubMed]
- Li, F.; Liu, H.; Wu, X.; Song, Z.; Tang, H.; Gong, M.; Liu, L.; Li, F. Tetrathiomolybdate Decreases the Expression of Alkaline Phosphatase in Dermal Papilla Cells by Increasing Mitochondrial ROS Production. Int. J. Mol. Sci. 2023, 24, 3123. [Google Scholar] [CrossRef]
- Liu, K.; Hua, S.; Song, L. PM2.5 Exposure and Asthma Development: The Key Role of Oxidative Stress. Oxidative Med. Cell. Longev. 2022, 2022, 3618806. [Google Scholar] [CrossRef]
- Sahiner, U.M.; Birben, E.; Erzurum, S.; Sackesen, C.; Kalayci, O. Oxidative stress in asthma: Part of the puzzle. Pediatr. Allergy Immunol. 2018, 29, 789–800. [Google Scholar] [CrossRef]
- Nakamoto, K.; Watanabe, M.; Saito, M.; Kasuga, K.; Miyaoka, C.; Yoshida, Y.; Kobayashi, F.; Nunokawa, H.; Aso, J.; Nakamoto, Y.; et al. Serum Derivatives-Reactive Oxygen Metabolite Levels as a Marker of Clinical Conditions in Patients with Bronchial Asthma, COPD, or Asthma-COPD Overlap: A Prospective Study. J. Clin. Med. 2024, 13, 6022. [Google Scholar] [CrossRef] [PubMed]
- Tantawy, M.; Khabir, A.A.; Mahsoub, N.; Zohdy, M. Serum Paroxonase 1 level may be an Indicator and Predictor of the Severity of Androgenetic Alopecia. Int. J. Trichology 2021, 13, 26–31. [Google Scholar] [CrossRef] [PubMed]
- Shin, S.; Kim, K.; Lee, M.J.; Lee, J.; Choi, S.; Kim, K.; Ko, J.; Han, H.; Kim, S.Y.; Youn, H.J.; et al. Epigallocatechin Gallate-Mediated Alteration of the MicroRNA Expression Profile in 5alpha-Dihydrotestosterone-Treated Human Dermal Papilla Cells. Ann. Dermatol. 2016, 28, 327–334. [Google Scholar] [CrossRef] [PubMed]
- Natarelli, N.; Gahoonia, N.; Aflatooni, S.; Bhatia, S.; Sivamani, R.K. Dermatologic Manifestations of Mitochondrial Dysfunction: A Review of the Literature. Int. J. Mol. Sci. 2024, 25, 3303. [Google Scholar] [CrossRef]
- Stout, R.; Birch-Machin, M. Mitochondria’s Role in Skin Ageing. Biology 2019, 8, 29. [Google Scholar] [CrossRef]
- Wu, H.; Fan, X.; Hu, C.; Chao, Y.; Liu, C.; Chang, J.; Sen, Y. Comparison of mitochondrial transplantation by using a stamp-type multineedle injector and platelet-rich plasma therapy for hair aging in naturally aging mice. Biomed. Pharmacother. 2020, 130, 110520. [Google Scholar] [CrossRef] [PubMed]
- Kloepper, J.E.; Baris, O.R.; Reuter, K.; Kobayashi, K.; Weiland, D.; Vidali, S.; Tobin, D.J.; Niemann, C.; Wiesner, R.J.; Paus, R. Mitochondrial function in murine skin epithelium is crucial for hair follicle morphogenesis and epithelial-mesenchymal interactions. J. Investig. Dermatol. 2015, 135, 679–689. [Google Scholar] [CrossRef]
- Richani, D.; Dunning, K.R.; Thompson, J.G.; Gilchrist, R.B. Metabolic co-dependence of the oocyte and cumulus cells: Essential role in determining oocyte developmental competence. Hum. Reprod. Update 2021, 27, 27–47. [Google Scholar] [CrossRef] [PubMed]
- Tang, Y.; Luo, B.; Deng, Z.; Wang, B.; Liu, F.; Li, J.; Shi, W.; Xie, H.; Hu, X.; Li, J. Mitochondrial aerobic respiration is activated during hair follicle stem cell differentiation, and its dysfunction retards hair regeneration. PeerJ 2016, 4, e1821. [Google Scholar] [CrossRef]
- Lan, S.; Liu, F.; Zhao, G.; Zhou, T.; Wu, C.; Kou, J.; Fan, R.; Qi, X.; Li, Y.; Jiang, Y.; et al. Cyclosporine A increases hair follicle growth by suppressing apoptosis-inducing factor nuclear translocation: A new mechanism. Fundam. Clin. Pharmacol. 2015, 29, 191–203. [Google Scholar] [CrossRef]
- Huang, H.; Lin, H.; Huang, M. Lactoferrin promotes hair growth in mice and increases dermal papilla cell proliferation through Erk/Akt and Wnt signaling pathways. Arch. Dermatol. Res. 2019, 311, 411–420. [Google Scholar] [CrossRef] [PubMed]
- Rastegar, H.; Ahmadi Ashtiani, H.; Aghaei, M.; Ehsani, A.; Barikbin, B. Combination of herbal extracts and platelet-rich plasma induced dermal papilla cell proliferation: Involvement of ERK and Akt pathways. J. Cosmet. Dermatol. 2013, 12, 116–122. [Google Scholar] [CrossRef] [PubMed]
- Li, W.; Man, X.; Li, C.; Chen, J.; Zhou, J.; Cai, S.; Lu, Z.; Zheng, M. VEGF induces proliferation of human hair follicle dermal papilla cells through VEGFR-2-mediated activation of ERK. Exp. Cell Res. 2012, 318, 1633–1640. [Google Scholar] [CrossRef] [PubMed]
- Li, W.; Lu, Z.; Man, X.; Li, C.; Zhou, J.; Chen, J.; Yang, X.; Wu, X.; Cai, S.; Zheng, M. VEGF upregulates VEGF receptor-2 on human outer root sheath cells and stimulates proliferation through ERK pathway. Mol. Biol. Rep. 2012, 39, 8687–8694. [Google Scholar] [CrossRef]
- Lee, Y.R.; Bae, S.; Kim, J.Y.; Lee, J.; Cho, D.; Kim, H.; An, I.; An, S. Monoterpenoid Loliolide Regulates Hair Follicle Inductivity of Human Dermal Papilla Cells by Activating the Akt/β-Catenin Signaling Pathway. J. Microbiol. Biotechnol. 2019, 29, 1830–1840. [Google Scholar] [CrossRef]
- Feutz, A.; Barrandon, Y.; Monard, D. Control of thrombin signaling through PI3K is a mechanism underlying plasticity between hair follicle dermal sheath and papilla cells. J. Cell Sci. 2008, 121, 1435–1443. [Google Scholar] [CrossRef]
- Lee, S.B.; Lee, J.W.; Lee, H.; Lee, K.; Hwang, H.W.; Shin, H.; Byun, J.W.; Shin, J.; Choi, G.S. Dickkopf-related Protein 2 Promotes Hair Growth by Upregulating the Wnt/β-catenin Signaling Pathway in Human Dermal Papilla Cells. Ann. Dermatol. 2024, 36, 292–299. [Google Scholar] [CrossRef] [PubMed]
- Andl, T.; Reddy, S.T.; Gaddapara, T.; Millar, S.E. WNT signals are required for the initiation of hair follicle development. Dev. Cell 2002, 2, 643–653. [Google Scholar] [CrossRef]
- Yang, Y.; Li, Y.; Wang, Y.; Wu, J.; Yang, G.; Yang, T.; Gao, Y.; Lu, Y. Versican gene: Regulation by the β-catenin signaling pathway plays a significant role in dermal papilla cell aggregative growth. J. Dermatol. Sci. 2012, 68, 157–163. [Google Scholar] [CrossRef]
- Xiong, Y.; Liu, Y.; Song, Z.; Hao, F.; Yang, X. Identification of Wnt/β-catenin signaling pathway in dermal papilla cells of human scalp hair follicles: TCF4 regulates the proliferation and secretory activity of dermal papilla cell. J. Dermatol. 2014, 41, 84–91. [Google Scholar] [CrossRef]
- Kim, Y.; Lee, J.O.; Lee, J.M.; Lee, M.; Kim, H.; Chung, H.; Kim, D.; Lee, J.; Kim, B.J. Low Molecular Weight Collagen Peptide (LMWCP) Promotes Hair Growth by Activating the Wnt/GSK-3β/β-Catenin Signaling Pathway. J. Microbiol. Biotechnol. 2024, 34, 17–28. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.O.; Kim, Y.; Lee, J.M.; Suk, J.M.; Jung, I.; Choi, S.Y.; Yoo, K.H.; Seok, J.; Kim, B.J. AP collagen peptides (APCPs) promote hair growth by activating the GSK-3β/β-catenin pathway and improve hair condition. Exp. Dermatol. 2024, 33, e15137. [Google Scholar] [CrossRef] [PubMed]
- Han, S.; Byun, J.; Lee, W.; Kang, H.; Kye, Y.; Kim, K.; Kim, D.; Kim, M.; Kim, S.; Kim, H.; et al. Quality of life assessment in male patients with androgenetic alopecia: Result of a prospective, multicenter study. Ann. Dermatol. 2012, 24, 311–318. [Google Scholar] [CrossRef] [PubMed]
- Gupta, S.; Goyal, I.; Mahendra, A. Quality of Life Assessment in Patients with Androgenetic Alopecia. Int. J. Trichology 2019, 11, 147–152. [Google Scholar] [CrossRef]
- English, R.S.J.; Ruiz, S.; DoAmaral, P. Microneedling and Its Use in Hair Loss Disorders: A Systematic Review. Dermatol. Ther. 2022, 12, 41–60. [Google Scholar] [CrossRef]
- Ocampo-Garza, S.S.; Fabbrocini, G.; Ocampo-Candiani, J.; Cinelli, E.; Villani, A. Micro needling: A novel therapeutic approach for androgenetic alopecia, A Review of Literature. Dermatol. Ther. 2020, 33, e14267. [Google Scholar] [CrossRef] [PubMed]
- Stevens, J.; Khetarpal, S. Platelet-rich plasma for androgenetic alopecia: A review of the literature and proposed treatment protocol. Int. J. Women’s Dermatol. 2018, 5, 46–51. [Google Scholar] [CrossRef]
- Gentile, P.; Garcovich, S. Systematic Review of Platelet-Rich Plasma Use in Androgenetic Alopecia Compared with Minoxidil®, Finasteride®, and Adult Stem Cell-Based Therapy. Int. J. Mol. Sci. 2020, 21, 2702. [Google Scholar] [CrossRef] [PubMed]
- Devjani, S.; Ezemma, O.; Kelley, K.J.; Stratton, E.; Senna, M. Androgenetic Alopecia: Therapy Update. Drugs 2023, 83, 701–715. [Google Scholar] [CrossRef]
- Adil, A.; Godwin, M. The effectiveness of treatments for androgenetic alopecia: A systematic review and meta-analysis. J. Am. Acad. Dermatol. 2017, 77, 136–141.e5. [Google Scholar] [CrossRef]
- Gasmi, A.; Mujawdiya, P.K.; Beley, N.; Shanaida, M.; Lysiuk, R.; Lenchyk, L.; Noor, S.; Muhammad, A.; Strus, O.; Piscopo, S.; et al. Natural Compounds Used for Treating Hair Loss. Curr. Pharm. Des. 2023, 29, 1231–1244. [Google Scholar] [CrossRef] [PubMed]
- Gupta, A.K.; Talukder, M.; Williams, G. Comparison of oral minoxidil, finasteride, and dutasteride for treating androgenetic alopecia. J. Dermatol. Treat. 2022, 33, 2946–2962. [Google Scholar] [CrossRef] [PubMed]
- Suchonwanit, P.; Thammarucha, S.; Leerunyakul, K. Minoxidil and its use in hair disorders: A review. Drug Des. Dev. Ther. 2019, 13, 2777–2786. [Google Scholar] [CrossRef] [PubMed]
- Zheng, C.; Liu, C.; Ren, W.; Li, B.; Lu, Y.; Pan, Z.; Cao, W. Flower and pod development, grain-setting characteristics and grain yield in Chinese milk vetch (Astragalus sinicus L.) in response to pre-anthesis foliar application of paclobutrazol. PLoS ONE 2021, 16, e0245554. [Google Scholar] [CrossRef]
- Mukhtar, I.; Chen, R.; Cheng, Y.; Chen, J. First report of powdery mildew on Astragalus sinicus (Chinese milk vetch) caused by Erysiphe trifoliorum in China. Plant Dis. 2021, 106, 2535. [Google Scholar] [CrossRef]
- Tan, W.J.T.; Song, L. Role of mitochondrial dysfunction and oxidative stress in sensorineural hearing loss. Hear. Res. 2023, 434, 108783. [Google Scholar] [CrossRef] [PubMed]
- Baek, J.; Kim, Y.; Lee, K.; Kim, U. Mitochondrial redox system: A key target of antioxidant therapy to prevent acquired sensorineural hearing loss. Front. Pharmacol. 2023, 14, 1176881. [Google Scholar] [CrossRef]
- Huang, P.; Yan, R.; Zhang, X.; Wang, L.; Ke, X.; Qu, Y. Activating Wnt/β-catenin signaling pathway for disease therapy: Challenges and opportunities. Pharmacol. Ther. 2019, 196, 79–90. [Google Scholar] [CrossRef]
- Han, S.H.; Jo, K.W.; Kim, Y.; Kim, K. Piperonylic Acid Promotes Hair Growth by Activation of EGFR and Wnt/β-Catenin Pathway. Int. J. Mol. Sci. 2024, 25, 10774. [Google Scholar] [CrossRef] [PubMed]
- Wang, F.; He, G.; Liu, M.; Sun, Y.; Ma, S.; Sun, Z.; Wang, Y. Pilose antler extracts promotes hair growth in androgenetic alopecia mice by activating hair follicle stem cells via the AKT and Wnt pathways. Front. Pharmacol. 2024, 15, 1410810. [Google Scholar] [CrossRef]
- Kang, J.; Kim, M.; Lee, J.; Jeon, Y.; Hwang, E.; Koh, Y.; Hyun, J.; Kwon, S.; Yoo, E.; Kang, H. Undariopsis peterseniana Promotes Hair Growth by the Activation of Wnt/β-Catenin and ERK Pathways. Mar. Drugs 2017, 15, 130. [Google Scholar] [CrossRef]
- Kang, J.; Choi, Y.K.; Han, S.; Nam, H.; Lee, G.; Kang, J.; Koh, Y.S.; Hyun, J.W.; Yoo, E.; Kang, H. 5-Bromo-3,4-dihydroxybenzaldehyde Promotes Hair Growth through Activation of Wnt/β-Catenin and Autophagy Pathways and Inhibition of TGF-β Pathways in Dermal Papilla Cells. Molecules 2022, 27, 2176. [Google Scholar] [CrossRef]
- Nam, G.; Jo, K.; Park, Y.; Kawk, H.W.; Yoo, J.; Jang, J.D.; Kang, S.M.; Kim, S.; Kim, Y. Bacillus/Trapa japonica Fruit Extract Ferment Filtrate enhances human hair follicle dermal papilla cell proliferation via the Akt/ERK/GSK-3β signaling pathway. BMC Complement. Altern. Med. 2019, 19, 104–108. [Google Scholar] [CrossRef]
- Fu, H.; Li, W.; Weng, Z.; Huang, Z.; Liu, J.; Mao, Q.; Ding, B. Water extract of cacumen platycladi promotes hair growth through the Akt/GSK3β/β-catenin signaling pathway. Front. Pharmacol. 2023, 14, 1038039. [Google Scholar] [CrossRef]
- Nam, G.H.; Jo, K.; Park, Y.; Kawk, H.W.; Yoo, J.; Jang, J.D.; Kang, S.M.; Kim, S.; Kim, Y. The peptide AC 2 isolated from Bacillus-treated Trapa japonica fruit extract rescues DHT (dihydrotestosterone)-treated human dermal papilla cells and mediates mTORC1 signaling for autophagy and apoptosis suppression. Sci. Rep. 2019, 9, 16903. [Google Scholar] [CrossRef]
- Sharma, A.; Mohapatra, H.; Arora, K.; Babbar, R.; Arora, R.; Arora, P.; Kumar, P.; Algin Yapar, E.; Rani, K.; Meenu, M.; et al. Bioactive Compound-Loaded Nanocarriers for Hair Growth Promotion: Current Status and Future Perspectives. Plants 2023, 12, 3739. [Google Scholar] [CrossRef] [PubMed]
- Popescu, M.N.; Berteanu, M.; Beiu, C.; Popa, L.G.; Mihai, M.M.; Iliescu, M.G.; Stanescu, A.M.A.; Ionescu, A.M. Complementary Strategies to Promote Hair Regrowth in Post-COVID-19 Telogen Effluvium. Clin. Cosmet. Investig. Dermatol. 2022, 15, 735–743. [Google Scholar] [CrossRef] [PubMed]



Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jeon, C.Y.; Go, M.Y.; Kim, I.-K.; Park, M.-R.; Lee, H.W.; Kim, Y.-K.; Shin, D.W. Hair Growth-Promoting Effects of Astragalus sinicus Extracts in Human Follicle Dermal Papilla Cells. Cosmetics 2025, 12, 6. https://doi.org/10.3390/cosmetics12010006
Jeon CY, Go MY, Kim I-K, Park M-R, Lee HW, Kim Y-K, Shin DW. Hair Growth-Promoting Effects of Astragalus sinicus Extracts in Human Follicle Dermal Papilla Cells. Cosmetics. 2025; 12(1):6. https://doi.org/10.3390/cosmetics12010006
Chicago/Turabian StyleJeon, Chae Young, Min Young Go, In-Kyung Kim, Myung-Rye Park, Hyean Woo Lee, Youn-Kyu Kim, and Dong Wook Shin. 2025. "Hair Growth-Promoting Effects of Astragalus sinicus Extracts in Human Follicle Dermal Papilla Cells" Cosmetics 12, no. 1: 6. https://doi.org/10.3390/cosmetics12010006
APA StyleJeon, C. Y., Go, M. Y., Kim, I.-K., Park, M.-R., Lee, H. W., Kim, Y.-K., & Shin, D. W. (2025). Hair Growth-Promoting Effects of Astragalus sinicus Extracts in Human Follicle Dermal Papilla Cells. Cosmetics, 12(1), 6. https://doi.org/10.3390/cosmetics12010006





