A Novel, Multifunctional, Natural-Based Emollient: An Exhaustive Characterization of Sharofeel DS
Abstract
:1. Introduction
2. Materials and Methods
2.1. Chemicals
2.2. Preparation of Emulsions
2.3. Physical Properties
2.3.1. IR and UV Analysis
2.3.2. Density
2.3.3. Dynamic Viscosity (Dyn. Visc.)
2.3.4. Refractive Index
2.3.5. Surface Tension
2.3.6. Contact Angle
2.3.7. Molar Polarizability
2.4. TGA
2.5. Hair Care
2.6. Viscoelastic Properties
2.7. Toxicity Tests
- The in vitro evaluation of skin irritation on reconstructed epidermal tissues was performed using the alternative method “In Vitro Skin Irritation: Reconstructed Human Epidermis Test Method (OECD TG 439)” with references to the protocol proposed by MatTek Corporation’s In Vitro EpidermTM Skin Irritation Test (EPI-200-SIT). The classification was performed according to the following criteria: mean tissue viability ≤ 50% = irritant and mean tissue viability > 50% = non-irritant. This study was carried out for regulatory purposes according to Regulation (EC) N.1272/2008—classification, labeling, and packaging of substances and mixtures (CLP legislation) and its annexes.
- The in vitro evaluation of ocular irritation potential on reconstructed corneal epithelium was performed using the SkinEthicTM HCE TTT model following the SkinEthicTM HCE TTL protocol. This method provides a categorization for chemicals, such as not requiring classification (No Cat., mean values > 50%), requiring classification for eye irritation (Cat. 2, any combination of values), and requiring classification for serious eye damage (Cat. 1, mean values < 50%), according to the UN GHS ocular hazard categories. This study was carried out for regulatory purposes according to Regulation (EC) N.1272/2008 —classification, labeling, and packaging of substances and mixtures (CLP legislation) and its annexes.
- Regarding the 48 h closed in vivo patch test, the product was applied as is by using the Finn Chamber, an 8 mm diameter aluminum disk containing a blotting paper disk soaked with the sample to be tested. The Finn Chamber was affixed with tape to the surfaces of the back of 25 volunteers. The product had already been tested and defined as ‘non-irritant’ during the in vitro skin irritation test before application to the volunteers. The amount applied was sufficient to saturate the pad without overflowing once applied to the skin. The product was left in contact with the skin surface for 48 h. The cutaneous reactions were analyzed at 15 min, 60 min, and 24 h after Finn Chamber removal. A Finn Chamber containing a blotting paper disk soaked with demineralized water was contextually applied, in the described conditions, to the same volunteers and used as a negative control. For each experimental time, Mean Irritation Index (MII) was calculated by adding erythema mean value and edema mean value (not irritating for mean value < 0.5, slightly irritating 0.5 ≤ MII < 2.0, moderately irritating 2.0 ≤ MII < 5.0, and highly irritating 5.0 ≤ MII ≤ 8.0).
2.8. Skin Care: In Vivo Tests
2.9. Subjects and Assessments
- All participants were healthy volunteers at least 18 years old.
- All participants were selected under the supervision of a dermatologist according to specific inclusion and exclusion criteria. The inclusion criterion was an age between 18 and 70 years. Exclusion criteria included being pregnant or breastfeeding, the presence of marks or dermatological conditions, the use of medication in the tested skin region, and undergoing pharmacological treatment.
- Volunteer participation in the study was entirely voluntary and free of any coercion, in accordance with the Helsinki Declaration.
- All the participants were informed of the aim and nature of the study and of the potential risks involved.
- All the participants signed the informed consent form prior to the beginning of the study.
- Before volunteers were exposed to the product to be tested, all relevant safety information about the product itself and each ingredient were collected and evaluated.
- All necessary precautions were taken to avoid adverse skin reactions.
2.10. DSC Analysis
3. Results
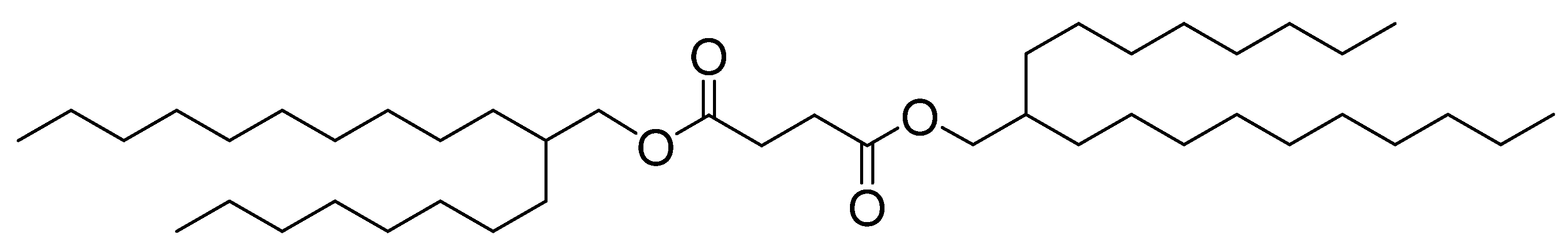
3.1. Characterization of DS
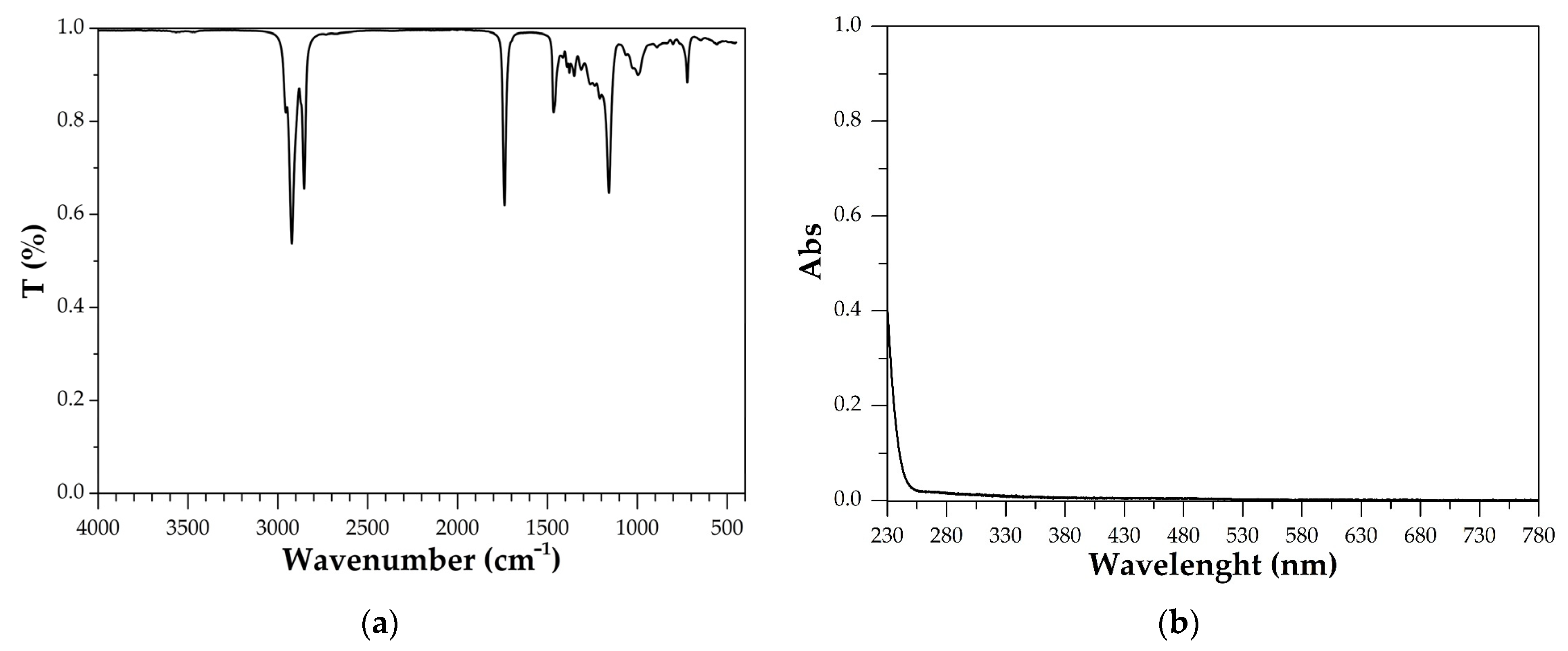
3.1.1. IR Spectroscopic Analysis
3.1.2. UV Spectrophotometry Analysis
3.1.3. Physical Properties
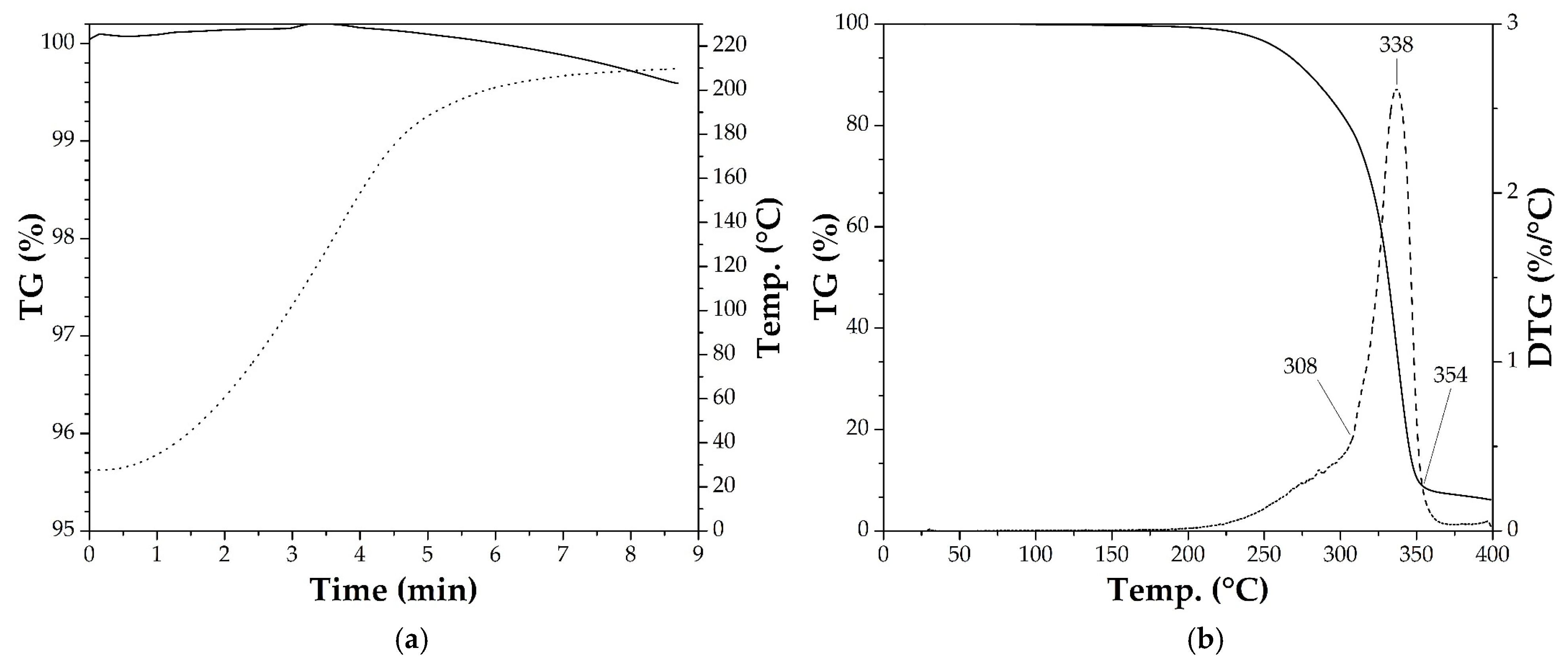
3.2. Thermogravimetric Analysis (TGA)
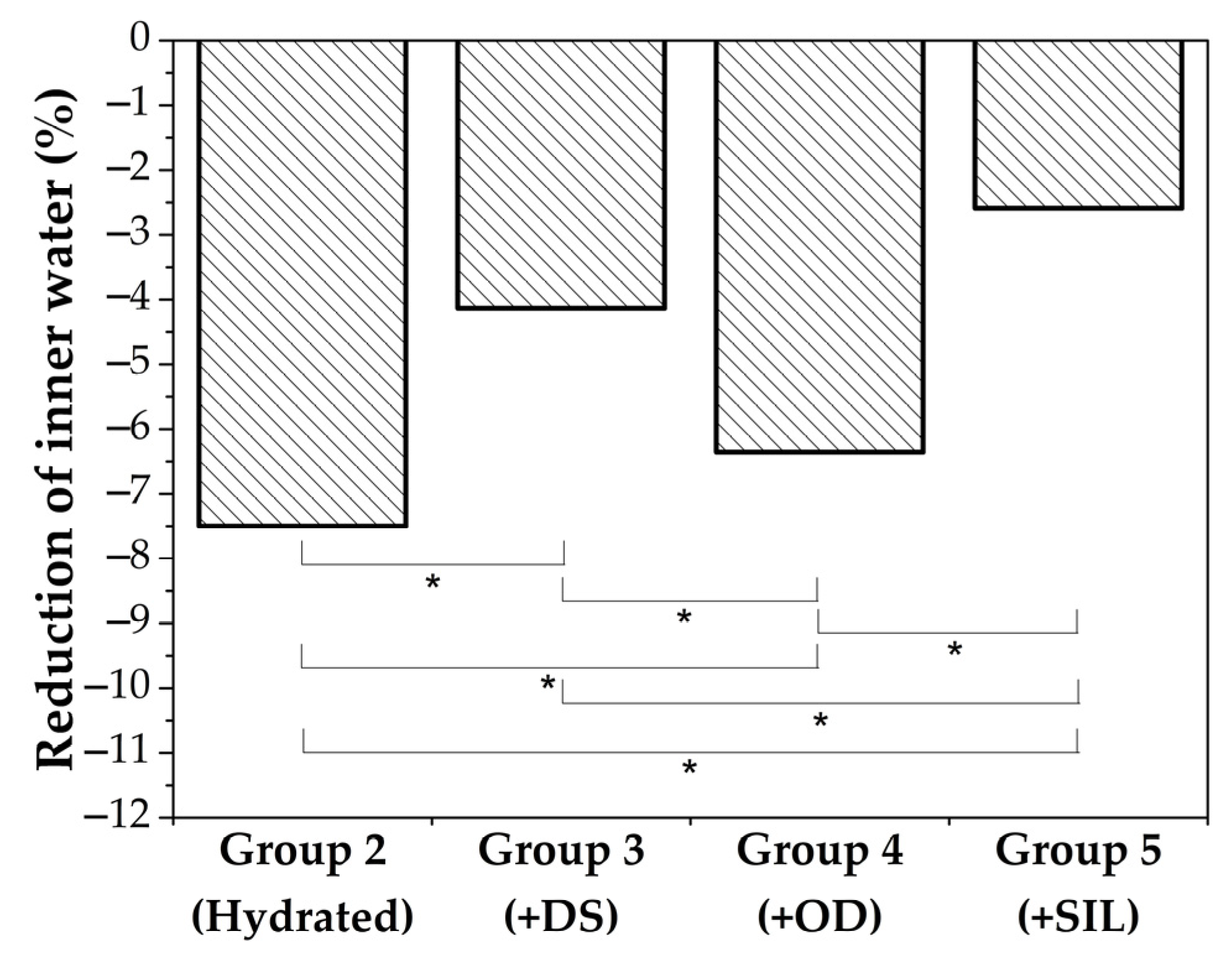
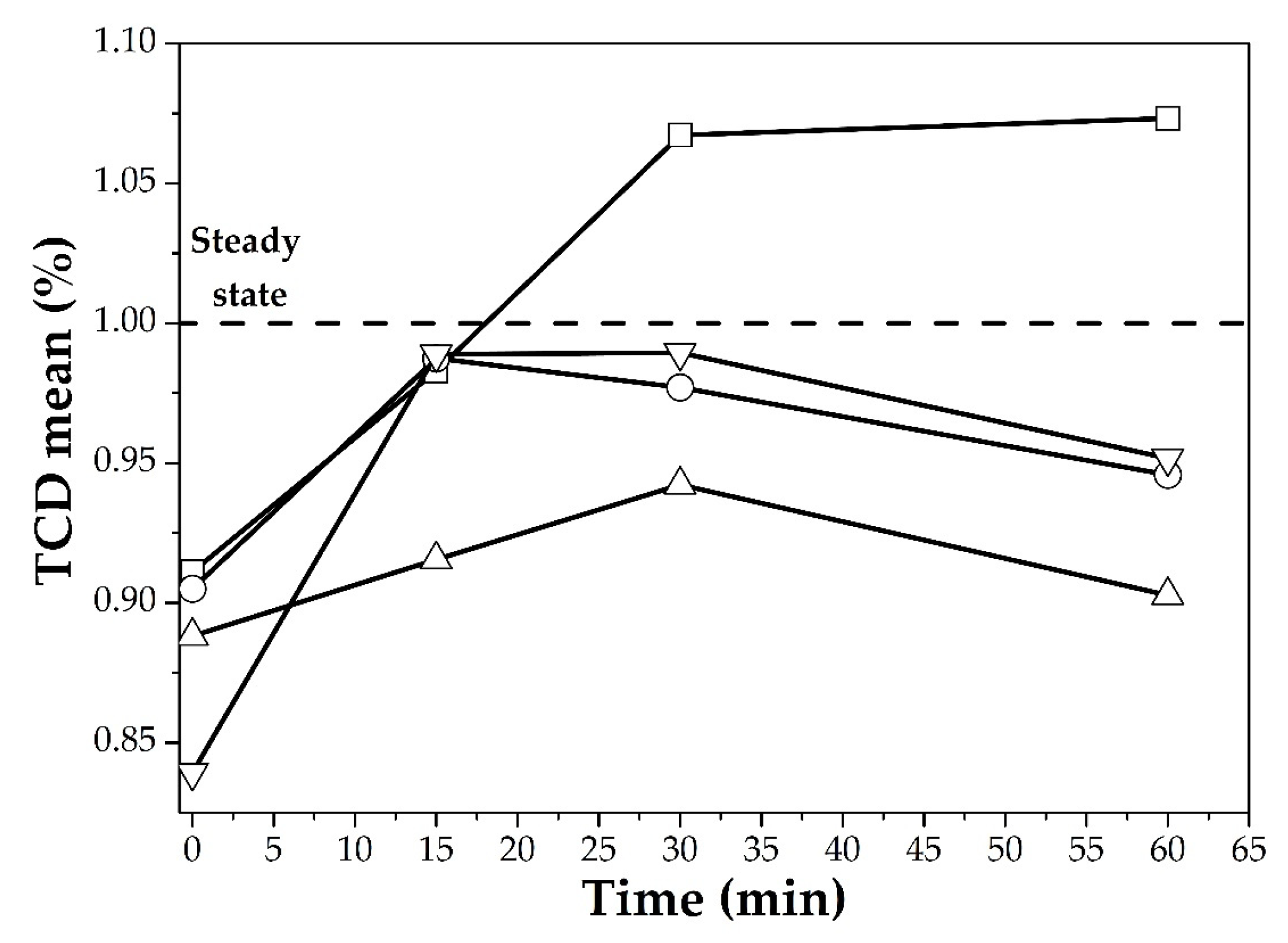
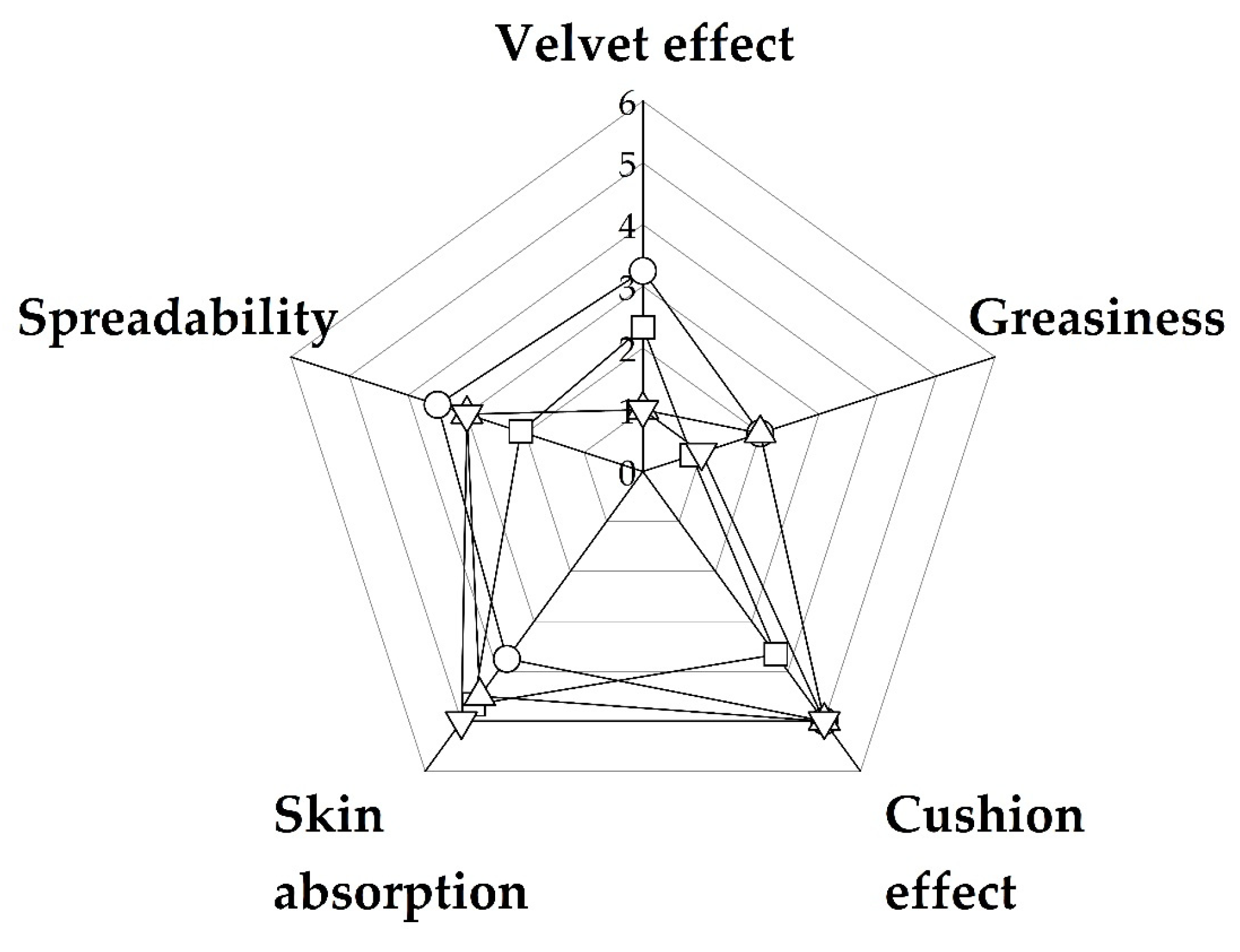
3.3. Hair Care
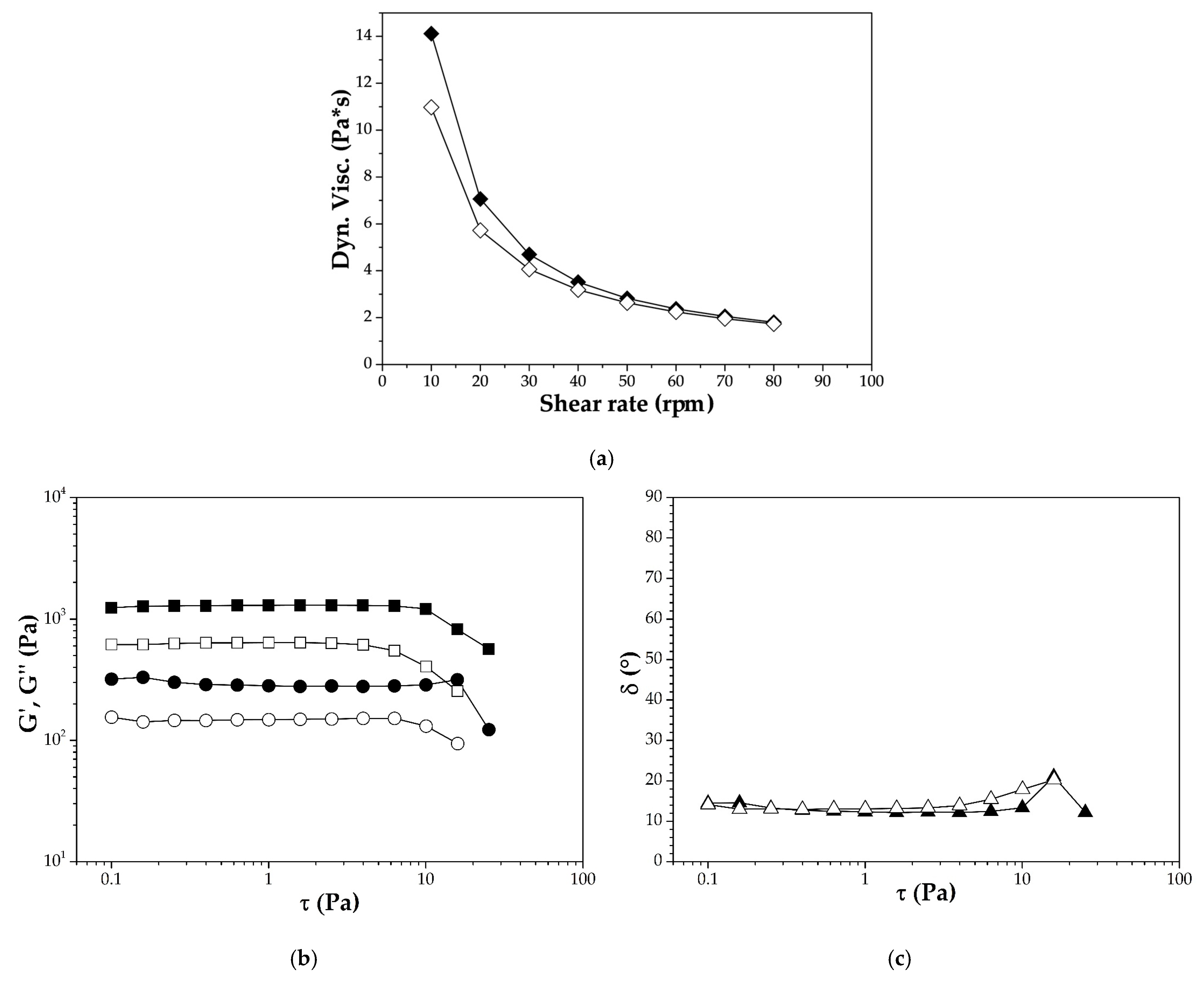
3.4. Viscoelastic Properties
3.5. Toxicity Test
- The in vitro evaluation of skin irritation potential on reconstructed epidermis tissues assessed DS as non-irritant (Mean Value ± SD 98.8% ± 2.1%).
- The in vitro evaluation of ocular irritation potential on reconstructed corneal epithelium assessed DS as no category (Mean Value ± SD 96.38% ± 9.68%), resulting in not requiring classification (the product is not an ocular irritant).
- On the basis of data obtained from the 48 h closed in vivo patch test, the product was assessed as non-irritant and safe for dermatological use (mean irritation index < 0.5).
3.6. Skin Care: In Vivo Tests
3.6.1. Test 1
3.6.2. Test 2
3.7. DSC Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Gore, E.; Picard, C.; Savary, G. Spreading Behavior of Cosmetic Emulsions: Impact of the Oil Phase. Biotribology 2018, 16, 17–24. [Google Scholar] [CrossRef]
- Alander, J.T. Chemical and Physical Properties of Emollients. In Treatment of Dry Skin Syndrome; Springer: Berlin/Heidelberg, Germany, 2012; Volume 9783642276057, pp. 399–417. ISBN 9783642276064. [Google Scholar]
- Wenninger, J.A.M.G.N. International Cosmetic Ingredient Dictionary; Cosmetic, Toiletry and Fragrance Association: Washington, DC, USA, 1993. [Google Scholar]
- Terescenco, D.; Savary, G.; Picard, C.; Clemenceau, F.; Merat, E.; Grisel, M. Influence of the Emollient on Emulsions Containing Lamellar Liquid Crystals: From Molecular Organization towards Applicative Properties. Int. J. Cosmet. Sci. 2018, 40, 565–574. [Google Scholar] [CrossRef] [PubMed]
- Araviiskaia, E.; Pincelli, C.; Sparavigna, A.; Luger, T. The Role of a Novel Generation of Emollients, “Emollients Plus”, in Atopic Dermatitis. Clin. Cosmet. Investig. Dermatol. 2022, 15, 2705–2719. [Google Scholar] [CrossRef] [PubMed]
- Chao, C.; Génot, C.; Rodriguez, C.; Magniez, H.; Lacourt, S.; Fievez, A.; Len, C.; Pezron, I.; Luart, D.; van Hecke, E. Emollients for Cosmetic Formulations: Towards Relationships between Physico-Chemical Properties and Sensory Perceptions. Colloids Surfaces A Physicochem. Eng. Asp. 2018, 536, 156–164. [Google Scholar] [CrossRef]
- Fabbron-Appas, C.T.; Pandey, P.; Parekh, H.S.; Sales, C.C.; Duque, M.D.; Andréo-Filho, N.; Lopes, P.S.; de Noronha, R.L.F.; Leite-Silva, V.R. Impact of Different Emollient Esters on Body Emulsions: Sensory, Physicochemical, and Biometrological Characterization. J. Sens. Stud. 2021, 36, e12660. [Google Scholar] [CrossRef]
- Mayrovitz, H.N.; Shams, E.; Astudillo, A.; Jain, A. Tissue Dielectric Constant and Skin Stiffness Relationships in Lower Extremity Lymphedema. Lymphat. Res. Biol. 2023, 21, 439–446. [Google Scholar] [CrossRef] [PubMed]
- De Clermont-Gallerande, H. Functional Roles of Lipids in Make-up Products. OCL Oilseeds Fats Crop. Lipids 2020, 27, 33. [Google Scholar] [CrossRef]
- Liu, Z.; Graf, K.; Hub, J.; Kellermeier, M. Effects of Cosmetic Emulsions on the Surface Properties of Mongolian Hair. ACS Omega 2022, 7, 10910–10920. [Google Scholar] [CrossRef] [PubMed]
- Calvo, F.; Gómez, J.M.; Alvarez, O.; Ricardez-Sandoval, L. Effect of Emulsification Parameters on the Rheology, Texture, and Physical Stability of Cosmetic Emulsions: A Multiscale Approach. Chem. Eng. Res. Des. 2022, 186, 407–415. [Google Scholar] [CrossRef]
- Ogorzałek, M.; Klimaszewska, E.; Mirowski, M.; Kulawik-Pióro, A.; Tomasiuk, R. Natural or Synthetic Emollients? Physicochemical Properties of Body Oils in Relation to Selected Parameters of Epidermal Barrier Function. Appl. Sci. 2024, 14, 2783. [Google Scholar] [CrossRef]
- Savary, G.; Grisel, M.; Picard, C. Impact of emollients on the spreading properties of cosmetic products: A combined sensory and instrumental characterization. Colloids Surf. B Biointerfaces 2013, 102, 371–378. [Google Scholar] [CrossRef] [PubMed]
- August, S.; Granier, S.; Tighe, M.P.; Tbaily, L.W.; Ahlbom, H. A Clinical Investigation of the Performance and Safety of Epaderm®, an Emollient Cream. Clin. Cosmet. Investig. Dermatol. 2021, 14, 909–920. [Google Scholar] [CrossRef] [PubMed]
- ASTM E1958-22; Standard Guide for Sensory Claim Substantiation. ASTM International: West Conshohocken, PA, USA, 2022.
- Goussard, V.; Aubry, J.-M.; Nardello-Rataj, V. Bio-Based Alternatives to Volatile Silicones: Relationships between Chemical Structure, Physicochemical Properties and Functional Performances. Adv. Colloid Interface Sci. 2022, 304, 102679. [Google Scholar] [CrossRef] [PubMed]
- Ravotti, R.; Fellmann, O.; Lardon, N.; Fischer, L.J.; Stamatiou, A.; Worlitschek, J. Analysis of Bio-Based Fatty Esters PCM’s Thermal Properties and Investigation of Trends in Relation to Chemical Structures. Appl. Sci. 2019, 9, 225. [Google Scholar] [CrossRef]
- Ravotti, R.; Fellmann, O.; Lardon, N.; Fischer, L.J.; Stamatiou, A.; Worlitschek, J. Synthesis and Investigation of Thermal Properties of Highly Pure Carboxylic Fatty Esters to Be Used as PCM. Appl. Sci. 2018, 8, 1069. [Google Scholar] [CrossRef]
- Ravotti, R.; Fellmann, O.; Fischer, L.J.; Worlitschek, J.; Stamatiou, A. Investigation of the Thermal Properties of Diesters from Methanol, 1-Pentanol, and 1-Decanol as Sustainable Phase Change Materials. Materials 2020, 13, 810. [Google Scholar] [CrossRef] [PubMed]
- Tinto, W.F.; Elufioye, T.O.; Roach, J. Waxes. In Pharmacognosy; Elsevier: Amsterdam, The Netherlands, 2017; pp. 443–455. ISBN 9780128020999. [Google Scholar]
- Bucio, A.; Moreno-Tovar, R.; Bucio, L.; Espinosa-Dávila, J.; Anguebes-franceschi, F. Characterization of Beeswax, Candelilla Wax and Paraffin Wax for Coating Cheeses. Coatings 2021, 11, 261. [Google Scholar] [CrossRef]



| Ingredient Name | (a) Anionic | (b) Cationic | (c) Non-Ionic | |
|---|---|---|---|---|
| Water Phase | Water | 79.25 | 79.25 | 79.25 |
| Glycerin | 3.00 | 3.00 | 3.00 | |
| EDTA | 0.10 | 0.10 | 0.10 | |
| Oil Phase | Cetearyl Alcohol | 2.50 | 2.50 | 2.50 |
| Emollient | 10.00 | 10.00 | 10.00 | |
| Glyceryl Stearate Citrate | 5.00 | - | - | |
| Behentrimonium Chloride | - | 5.00 | - | |
| PEG-100 Stearate; Glyceryl Stearate | - | - | 5.00 | |
| Preser-vative | CMIT-MIT | 0.15 | 0.15 | 0.15 |
| Step N° | Start Temp. | Limit Temp. | Rate | Holding Time |
|---|---|---|---|---|
| (°C) | (°C) | (°C/min) | (min) | |
| 1 | 20 | 100 | 10 | 10 |
| 2 | 100 | −20 | 40 | 10 |
| 3 | −20 | 100 | 10 | 10 |
| 4 | 100 | −20 | 40 | 10 |
| 5 | −20 | 20 | 10 | - |
| Emollients | Density | Dyn. Viscosity | RI | Molar Polarizability | Surface Tension | Contact Angle (°) | |
|---|---|---|---|---|---|---|---|
| (g/mL) | (mPa·s) | (mL/mol) | (mN/m) | t = 0 | Equilibrium | ||
| DS | 0.881 | 86 | 1.457 | 267.8 | 22.53 ± 0.11 | 37.43 | 18.12 |
| SIL | 0.948 | 121 | 1.402 | 2119.4 | 19.44 ± 0.43 | 26.33 | 14.5 |
| OD | 0.839 | 81 | 1.453 | 123.3 | 25.57 ± 0.35 | 17.52 | 14.14 |
| SAO | 0.916 | 68 | 1.469 | 370.8 | 29.38 ± 0.41 | 32.82 | 8.97 |
| INI | 0.845 | 14 | 1.437 | 115.4 | 24.49 ± 0.38 | 39.5 | 8.11 |
| TGCC | 0.954 | 32 | 1.447 | 185.5 | 26.68 ± 0.33 | 53.58 | 15.38 |
| DISM | 0.907 | 2800 | 1.458 | 245.0 | 22.05 ± 0.06 | 59.06 | 47.67 |
| Group 1 (Dry) | Group 2 (Hydrated) | Group 3 (+DS) | Group 4 (+OD) | Group 5 (+SIL) | |
|---|---|---|---|---|---|
| Initial mass | 1.0387 ± 0.0458 | 1.0452 ± 0.0622 | 1.0518 ± 0.0416 | 1.0512 ± 0.0406 | 1.0658 ± 0.0475 |
| After hydration | - | 1.3554 ± 0.0826 | 1.3659 ± 0.0639 | 1.3685 ± 0.0429 | 1.3855 ± 0.0678 |
| After emollient application | - | - | 1.5026 ± 0.075 | 1.4944 ± 0.0482 | 1.5399 ± 0.0906 |
| After heating treatment | 0.9364 ± 0.039 | 0.9668 ± 0.0408 | 1.145 ± 0.0566 | 1.1102 ± 0.051 | 1.1927 ± 0.0637 |
| DS | OD | |
|---|---|---|
| Anionic | ||
| LVE region | 25.11 | 10.01 |
| G′ (Pa) | 1980 | 819 |
| Phase angle (°) | 12.6 | 10.9 |
| Cationic | ||
| LVE region | 10.06 | 4.00 |
| G′ (Pa) | 248 | 270 |
| Phase angle (°) | 20.1 | 16.2 |
| Non-ionic | ||
| LVE region | 10.00 | 3.98 |
| G′ (Pa) | 1284 | 634 |
| Phase angle (°) | 11.8 | 13.5 |
| Texture Parameters | TGCC | DS | OD | INI |
|---|---|---|---|---|
| Velvet effect | 1.00 | 2.33 | 3.25 | 1.00 |
| Greasiness | 1.00 | 0.83 | 2.00 | 2.00 |
| Cushion effect | 5.00 | 3.67 | 5.00 | 5.00 |
| Skin absorption | 5.00 | 4.67 | 3.75 | 4.50 |
| Spreadability | 3.00 | 2.08 | 3.75 | 3.00 |
| Step1: 1° Heat | Step 2: 1° Cool | Step 3: 2° Heat | Step 4: 2° Cool | Step 5: 3° Heat | |
|---|---|---|---|---|---|
| Carnauba wax | |||||
| DS | 55.59 °C/76.80 °C | 44.30 °C/65.70 °C | 49.53 °C/76.10 °C | 44.15 °C/65.25 °C | 49.52 °C/75.30 °C |
| OD | 48.78 °C/71.51 °C | 44.30 °C/71.40 °C | 44.25 °C/72.28 °C | 41.16 °C/68.40 °C | 44.21 °C/73.00 °C |
| PURE | 62.50 °C/85.80 °C | 65.58 °C/71.49 °C | 57.80 °C/78.90 °C/85.50 °C | 65.25 °C/71.30 °C | 58.30 °C/85.70 °C |
| Candelilla wax | |||||
| DS | 52.57 °C | 44.10 °C/59.30 °C | 51.81 °C | 44.30 °C/59.47 °C | 51.80 °C |
| OD | 51.06 °C | 44.10 °C/59.30 °C | 50.30 °C | 44.30 °C/59.70 °C | 50.20 °C |
| PURE | 63.60 °C/69.10 °C | 56.41 °C | 61.30 °C/68.90 °C | 56.58 °C | 61.50 °C/68.44 °C |
| Polyethylene | |||||
| DS | 68.48 °C | 62.55 °C | 63.52 °C | 62.67 °C | 64.65 °C |
| OD | 68.48 °C | 59.48 °C | 63.52 °C | 62.67 °C | 64.65 °C |
| PURE | 74.58 °C | 62.55 °C | 55.83 °C/73.78 °C | 59.61 °C | 57.83 °C/74.40 °C |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Puzzo, F.; Di Liberto, M.; Valente, G.; Donadelli, R.M.; Beretta, G.; Gelmini, F. A Novel, Multifunctional, Natural-Based Emollient: An Exhaustive Characterization of Sharofeel DS. Cosmetics 2025, 12, 58. https://doi.org/10.3390/cosmetics12020058
Puzzo F, Di Liberto M, Valente G, Donadelli RM, Beretta G, Gelmini F. A Novel, Multifunctional, Natural-Based Emollient: An Exhaustive Characterization of Sharofeel DS. Cosmetics. 2025; 12(2):58. https://doi.org/10.3390/cosmetics12020058
Chicago/Turabian StylePuzzo, Francesco, Melania Di Liberto, Gabriele Valente, Roberta Maria Donadelli, Giangiacomo Beretta, and Fabrizio Gelmini. 2025. "A Novel, Multifunctional, Natural-Based Emollient: An Exhaustive Characterization of Sharofeel DS" Cosmetics 12, no. 2: 58. https://doi.org/10.3390/cosmetics12020058
APA StylePuzzo, F., Di Liberto, M., Valente, G., Donadelli, R. M., Beretta, G., & Gelmini, F. (2025). A Novel, Multifunctional, Natural-Based Emollient: An Exhaustive Characterization of Sharofeel DS. Cosmetics, 12(2), 58. https://doi.org/10.3390/cosmetics12020058






