Recombinant Type XVII Collagen Inhibits EGFR/MAPK/AP-1 and Activates TGF-β/Smad Signaling to Enhance Collagen Secretion and Reduce Photoaging
Abstract
:1. Introduction
2. Materials and Methods
2.1. Experimental Raw Materials
2.2. Cell Lines and Materials
2.3. Selection of an Optimal UVA Dose for Photoaging
2.4. Establishment of a UVA Irradiation-Induced Model of Cell Damage
2.5. RT-qPCR
2.6. Western Blot Analysis
2.7. Immunofluorescent Staining
2.8. Statistical Analysis
3. Results
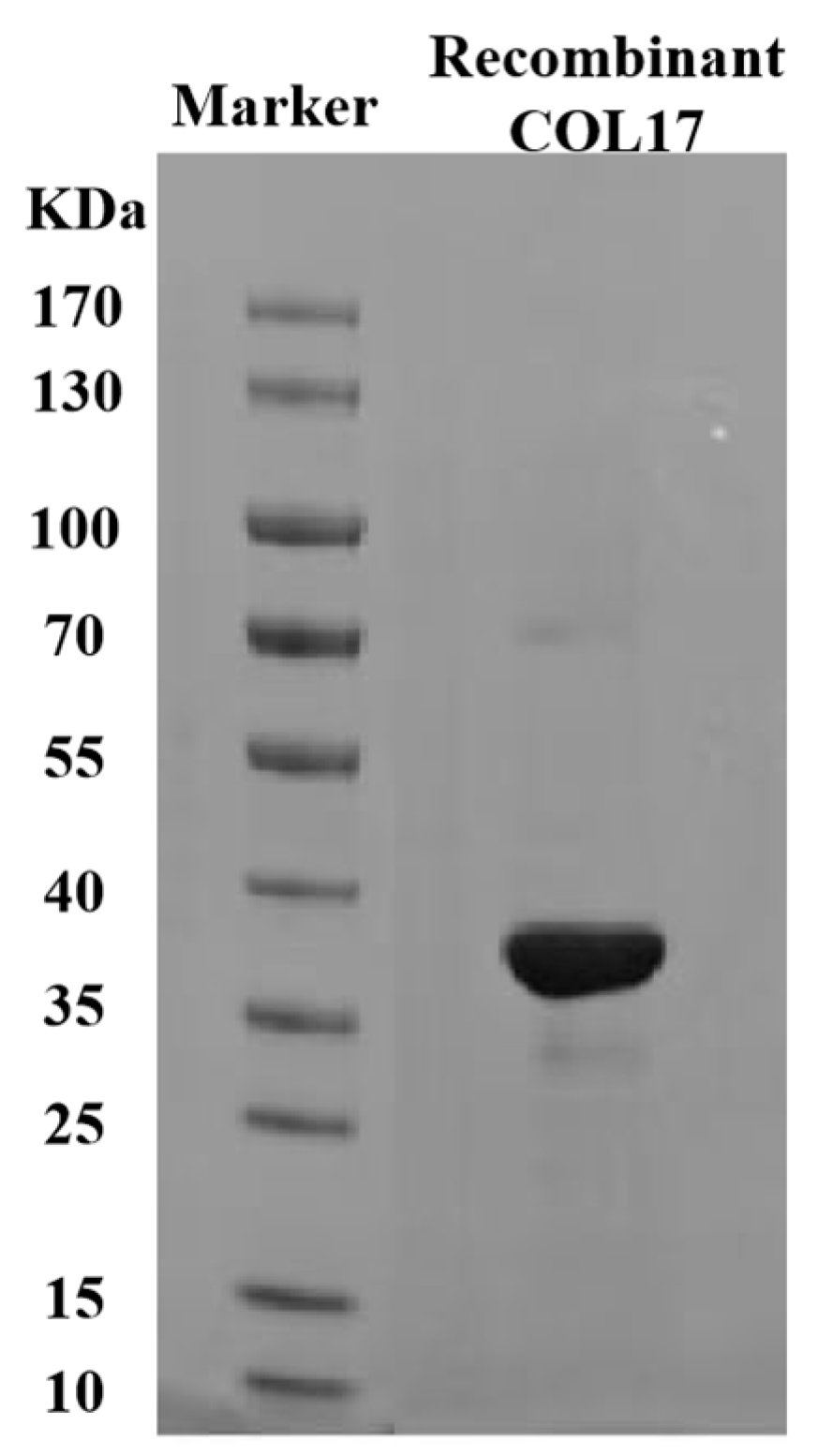
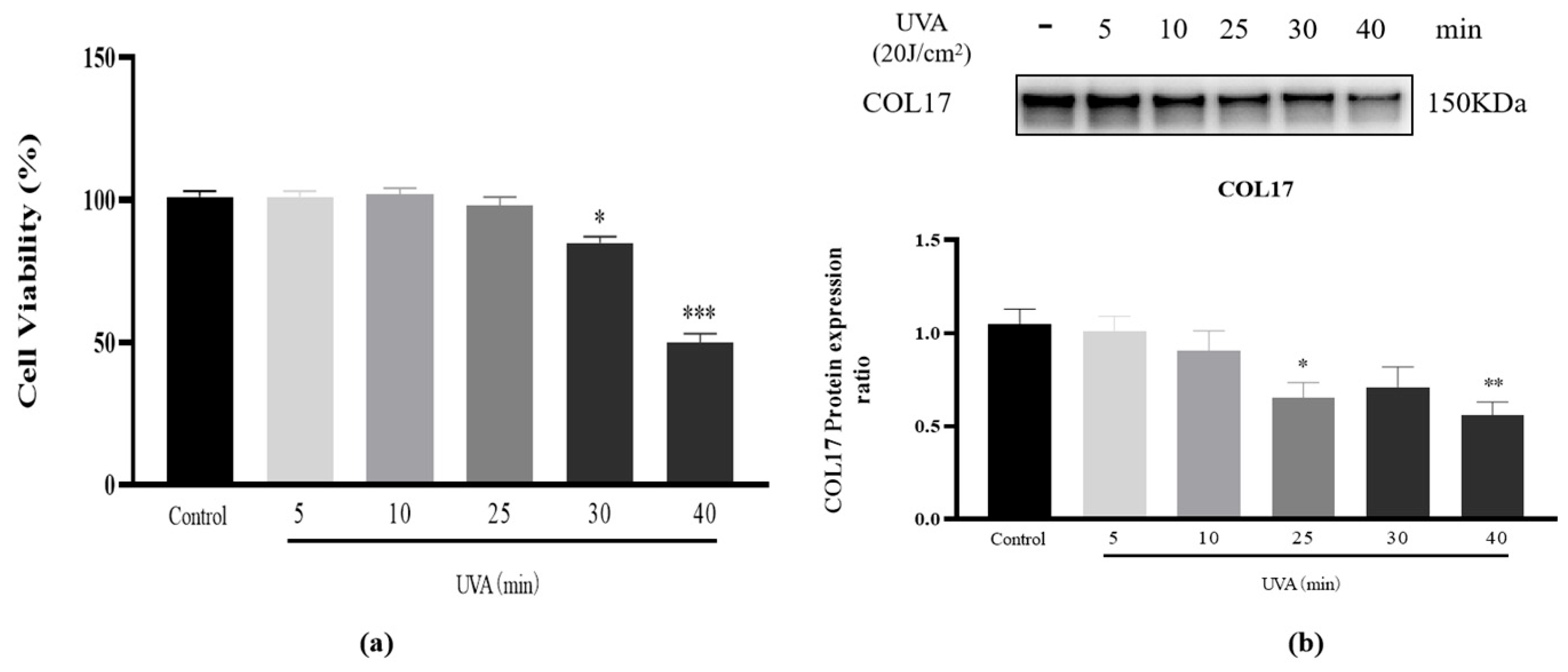
3.1. Cell Viability and COL17 Expression After UVA (20 J/cm2) Irradiation
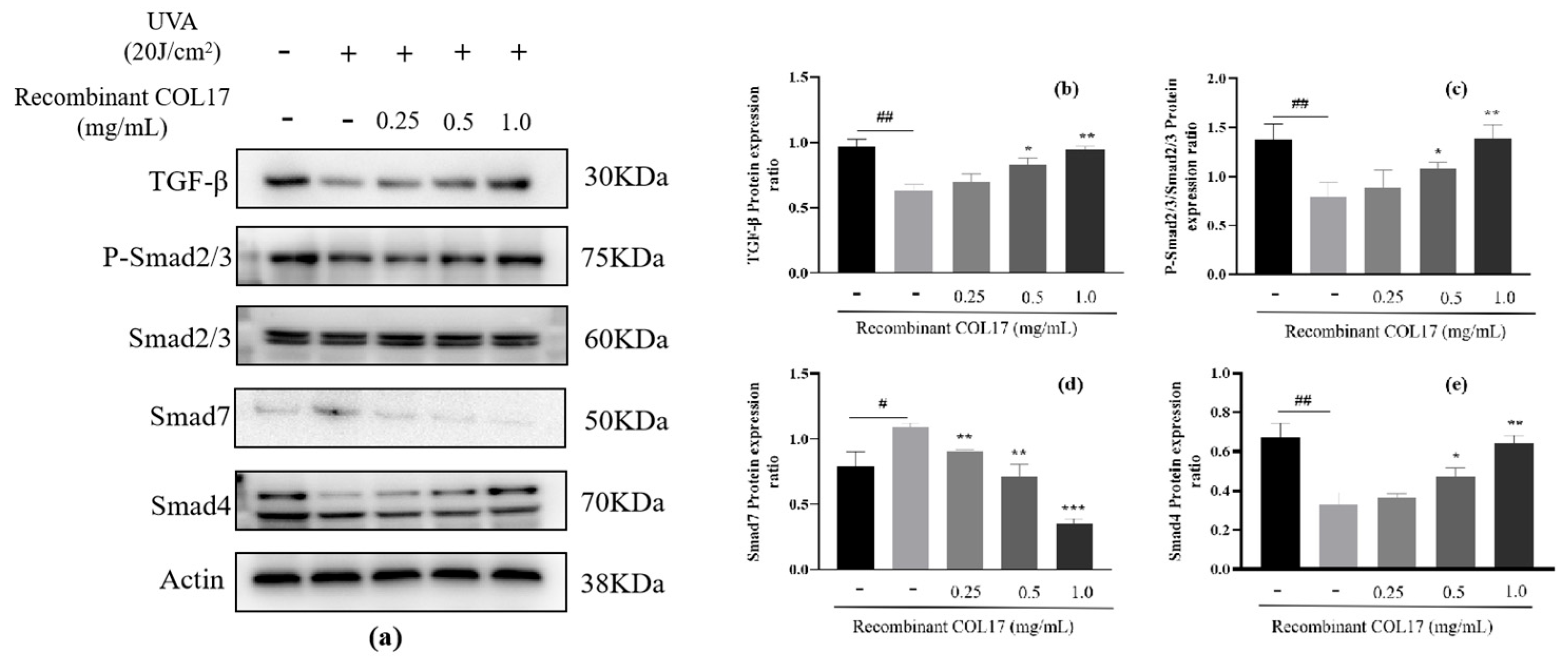
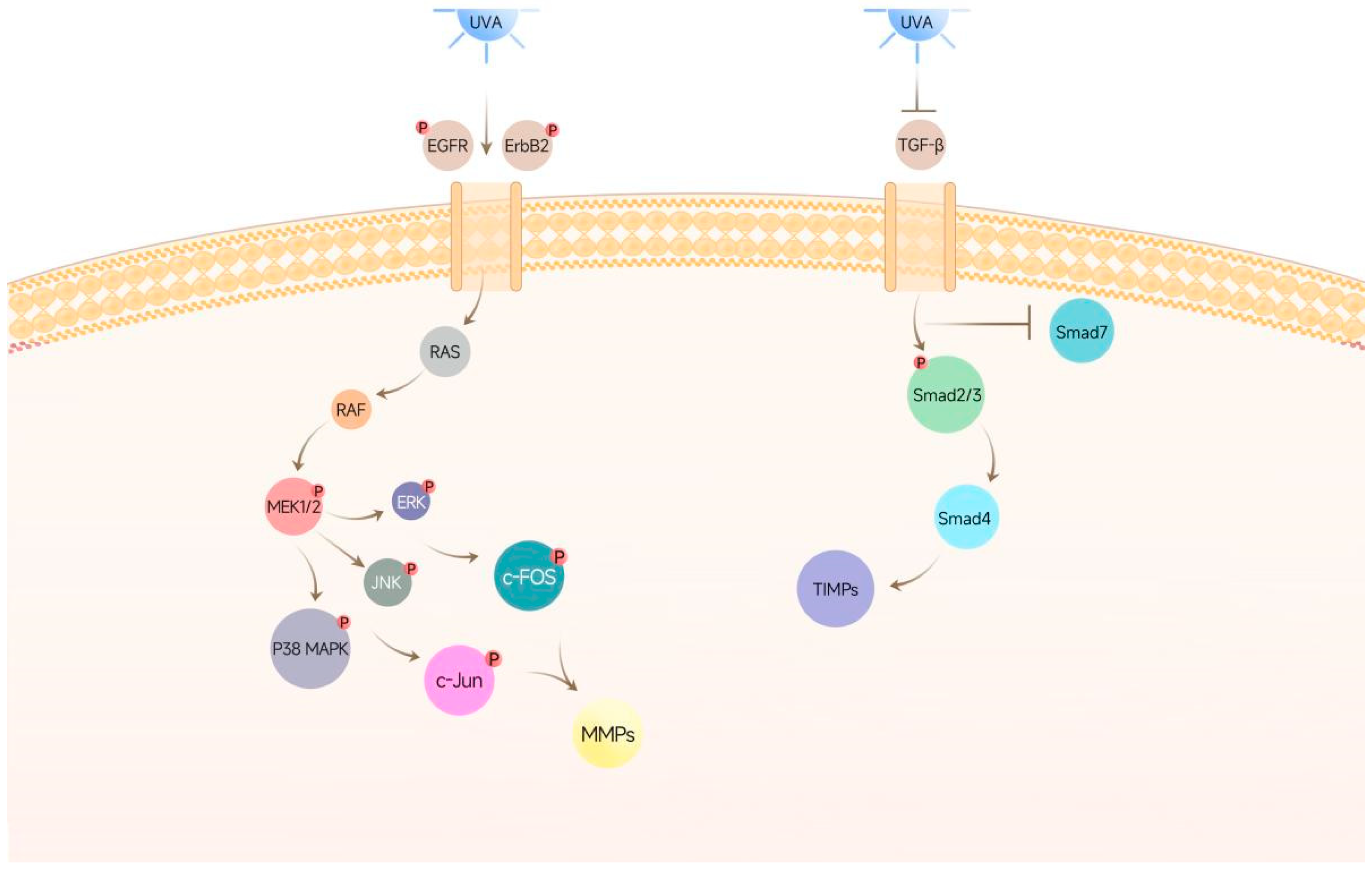
3.2. Effects of Recombinant COL17 on the TGF-β/Smad Pathway
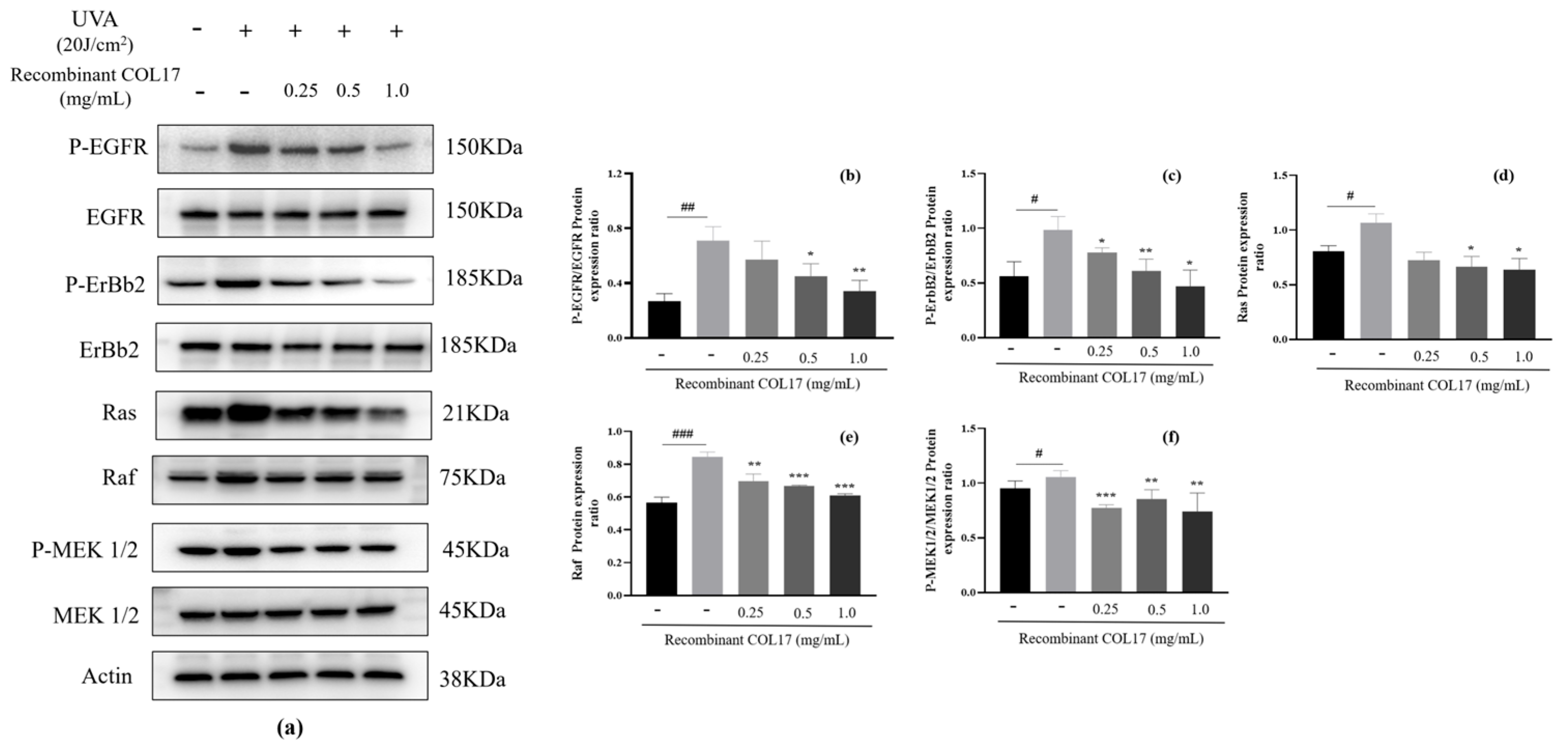
3.3. Effects of Recombinant COL17 on the EGFR/MAPK/AP-1 Pathway
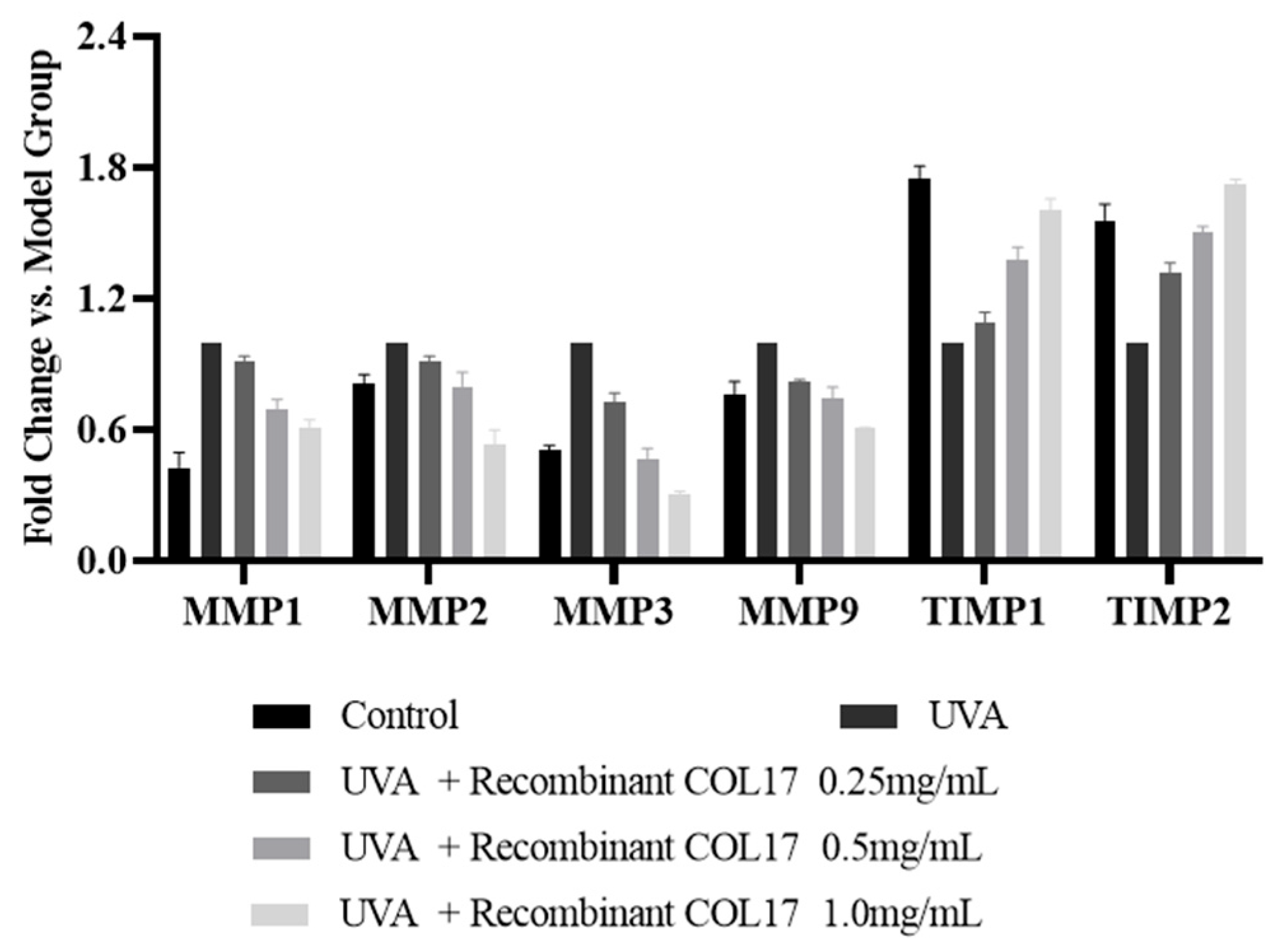
3.4. Effects of Recombinant COL17 on MMP and TIMP Expression in HaCaT Cells
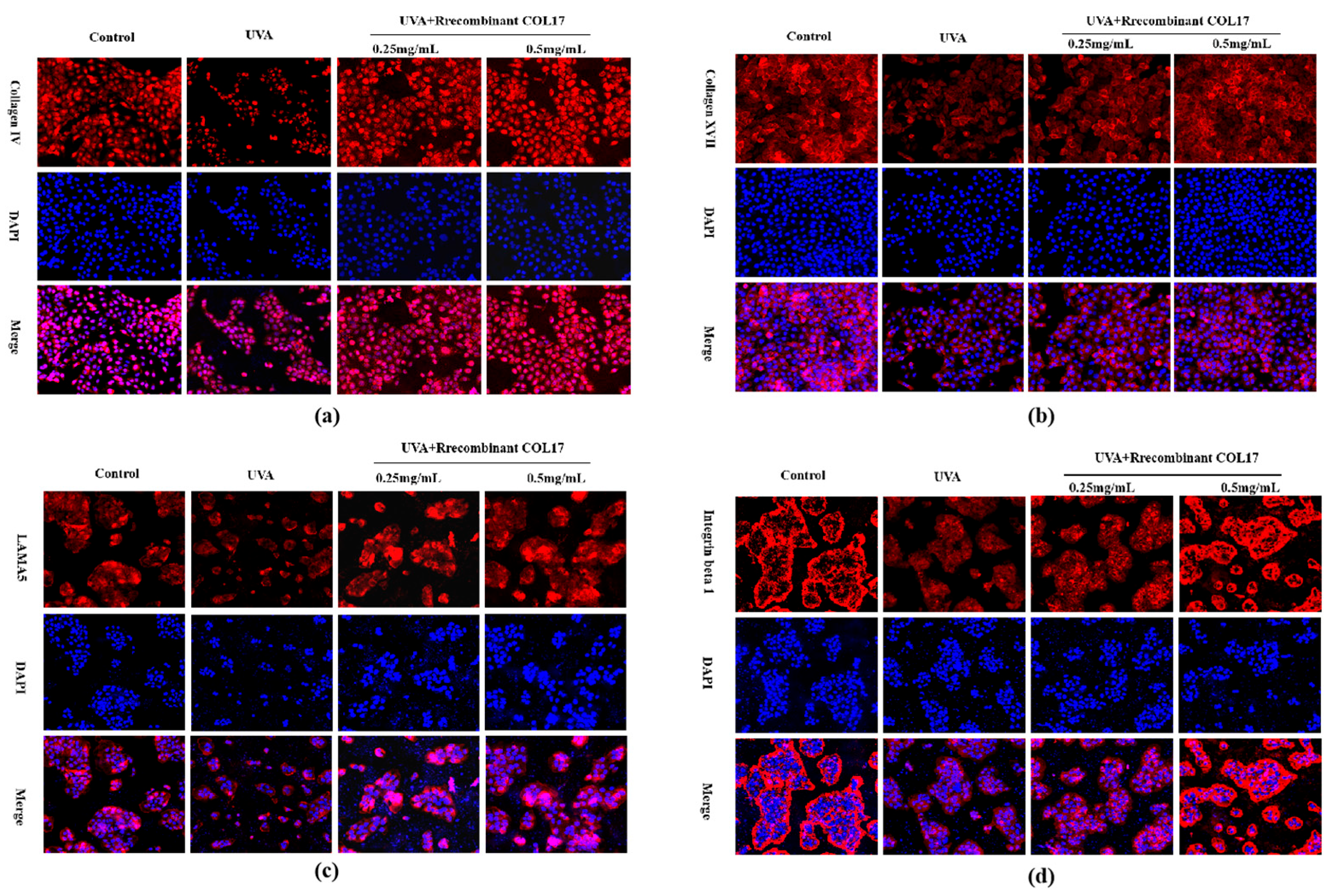
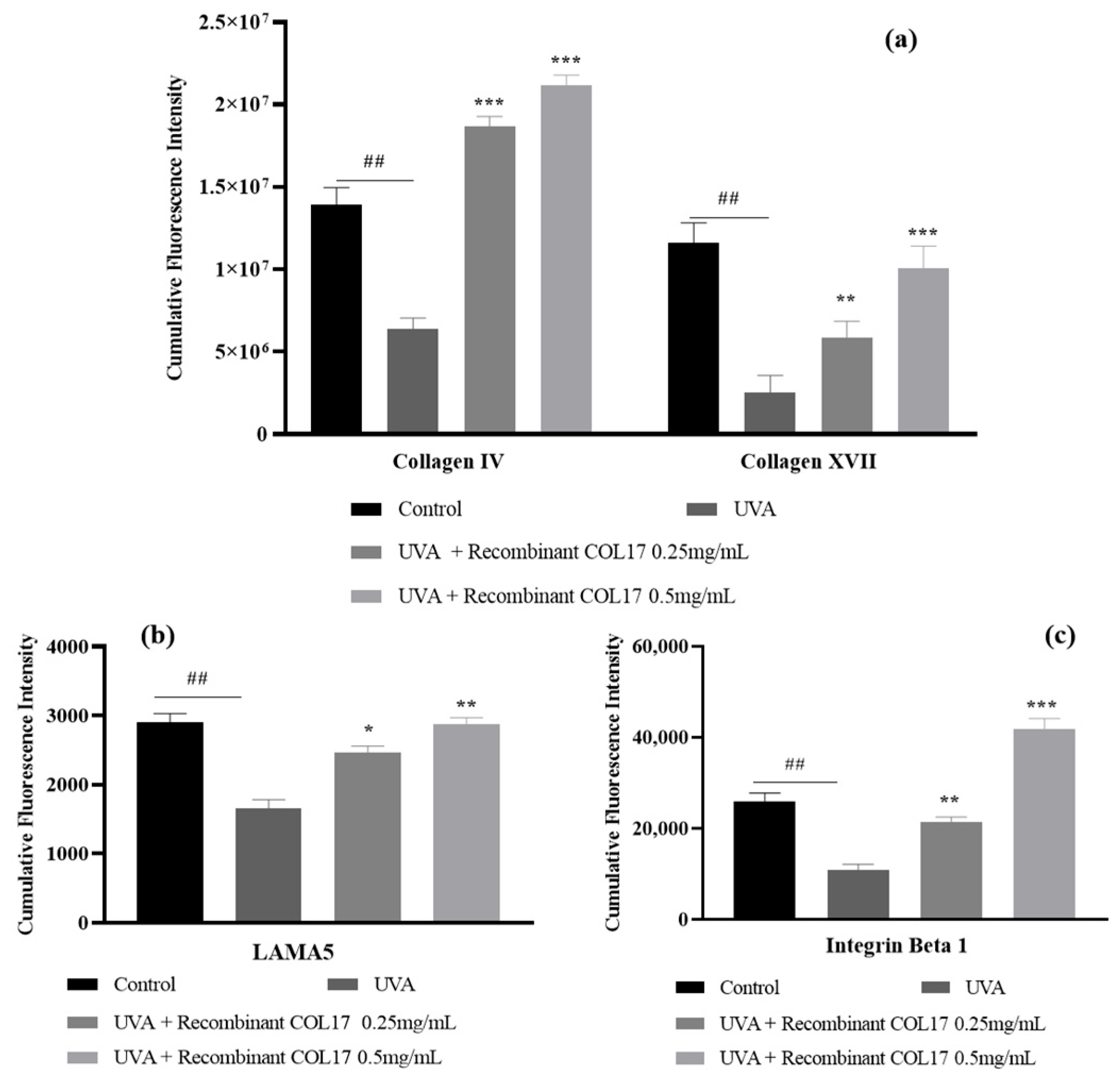
3.5. Effects of Recombinant COL17 on the UVA-Mediated Expression of Basement Membrane-Associated Proteins
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Gromkowska-Kępka, K.J.; Puścion-Jakubik, A.; Markiewicz-Żukowska, R.; Socha, K. The impact of ultraviolet radiation on skin photoaging—Review of in vitro studies. J. Cosmet. Dermatol. 2021, 20, 3427–3431. [Google Scholar] [CrossRef] [PubMed]
- Fitsiou, E.; Pulido, T.; Campisi, J.; Alimirah, F.; Demaria, M. Cellular Senescence and the Senescence-Associated Secretory Phenotype as Drivers of Skin Photoaging. J. Investig. Dermatol. 2021, 141, 1119–1126. [Google Scholar] [CrossRef] [PubMed]
- Battie, C.; Jitsukawa, S.; Bernerd, F.; Del Bino, S.; Marionnet, C.; Verschoore, M. New insights in photoaging, UVA induced damage and skin types. Exp. Dermatol. 2014, 23, 7–12. [Google Scholar] [CrossRef] [PubMed]
- Silvers, A.L.; Bachelor, M.A.; Bowden, G.T. The Role of JNK and p38 MAPK Activities in UVA-Induced Signaling Pathways Leading to AP-1 Activation and c-Fos Expression. Neoplasia 2003, 5, 319–329. [Google Scholar] [CrossRef]
- Zhang, M.; Zhang, T.; Tang, Y.; Ren, G.; Zhang, Y.; Ren, X. Concentrated growth factor inhibits UVA-induced photoaging in human dermal fibroblasts via the MAPK/AP-1 pathway. Biosci. Rep. 2020, 40, BSR20193566. [Google Scholar] [CrossRef]
- Oh, J.H.; Joo, Y.H.; Karadeniz, F.; Ko, J.; Kong, C.-S. Syringaresinol Inhibits UVA-Induced MMP-1 Expression by Suppression of MAPK/AP-1 Signaling in HaCaT Keratinocytes and Human Dermal Fibroblasts. Int. J. Mol. Sci. 2020, 21, 3981. [Google Scholar] [CrossRef]
- He, Y.-Y.; Council, S.E.; Feng, L.; Chignell, C.F. UVA-Induced Cell Cycle Progression Is Mediated by a Disintegrin and Metalloprotease/Epidermal Growth Factor Receptor/AKT/Cyclin D1 Pathways in Keratinocytes. Cancer Res. 2008, 68, 3752–3758. [Google Scholar] [CrossRef]
- Jagodzik, P.; Tajdel-Zielinska, M.; Ciesla, A.; Marczak, M.; Ludwikow, A. Mitogen-Activated Protein Kinase Cascades in Plant Hormone Signaling. Front. Plant Sci. 2018, 9, 1387. [Google Scholar] [CrossRef]
- Bahar, M.E.; Kim, H.J.; Kim, D.R. Targeting the RAS/RAF/MAPK pathway for cancer therapy: From mechanism to clinical studies. Signal. Transduct. Target. Ther. 2023, 8, 455. [Google Scholar] [CrossRef]
- Hong, J.-A.; Bae, D.; Oh, K.-N.; Oh, D.-R.; Kim, Y.; Kim, Y.; Jeong Im, S.; Choi, E.-J.; Lee, S.-G.; Kim, M.; et al. Protective effects of Quercus acuta Thunb. fruit extract against UVB-induced photoaging through ERK/AP-1 signaling modulation in human keratinocytes. BMC Complement. Med. Ther. 2022, 22, 6. [Google Scholar] [CrossRef]
- Qin, Z.; Balimunkwe, R.M.; Quan, T. Age-related reduction of dermal fibroblast size upregulates multiple matrix metalloproteinases as observed in aged human skin in vivo. Br. J. Dermatol. 2017, 177, 1337–1348. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Huang, H.; Tao, K.; Guo, L.; Hu, X.; Chang, H. Novel Thermus thermophilus and Bacillus subtilis mixed-culture ferment extract provides potent skin benefits in vitro and protects skin from aging. J. Cosmet. Dermatol. 2024, 23, 4334–4342. [Google Scholar] [CrossRef] [PubMed]
- Quan, T.; He, T.; Kang, S.; Voorhees, J.J.; Fisher, G.J. Solar Ultraviolet Irradiation Reduces Collagen in Photoaged Human Skin by Blocking Transforming Growth Factor-β Type II Receptor/Smad Signaling. Am. J. Pathol. 2004, 165, 741–751. [Google Scholar] [CrossRef]
- Chen, B.; Li, R.; Yan, N.; Chen, G.; Qian, W.; Jiang, H.L.; Ji, C.; Bi, Z.G. Astragaloside IV controls collagen reduction in photoaging skin by improving transforming growth factor-β/Smad signaling suppression and inhibiting matrix metalloproteinase-1. Mol. Med. Rep. 2015, 11, 3344–3348. [Google Scholar] [CrossRef]
- Jabłońska-Trypuć, A.; Matejczyk, M.; Rosochacki, S. Matrix metalloproteinases (MMPs), the main extracellular matrix (ECM) enzymes in collagen degradation, as a target for anticancer drugs. J. Enzym. Inhib. Med. Chem. 2016, 31, 177–183. [Google Scholar] [CrossRef]
- Pittayapruek, P.; Meephansan, J.; Prapapan, O.; Komine, M.; Ohtsuki, M. Role of Matrix Metalloproteinases in Photoaging and Photocarcinogenesis. Int. J. Mol. Sci. 2016, 17, 868. [Google Scholar] [CrossRef]
- Wang, Y.; Chen, H.; Wang, W.; Wang, R.; Liu, Z.-L.; Zhu, W.; Lian, S. N-terminal 5-mer peptide analog P165 of amyloid precursor protein inhibits UVA-induced MMP-1 expression by suppressing the MAPK pathway in human dermal fibroblasts. Eur. J. Pharmacol. 2014, 734, 1–8. [Google Scholar] [CrossRef]
- Yu, S.-C.; Chiu, W.-C.; Loe, P.Y.; Chien, Y.-W. Effects of Hydroxysafflor Yellow A (HSYA) on UVA-Induced Damage in HaCaT Keratinocytes. Int. J. Mol. Sci. 2024, 25, 7573. [Google Scholar] [CrossRef]
- Oh, J.H.; Karadeniz, F.; Seo, Y.; Kong, C.-S. Isopimpinellin inhibits UVA-induced overproduction of MMPs via suppression of MAPK/AP-1 signaling in human dermal fibroblasts. Food Sci. Biotechnol. 2024, 33, 3579–3589. [Google Scholar] [CrossRef]
- Yao, C.; Lee, D.H.; Oh, J.-H.; Kim, M.-K.; Kim, K.H.; Park, C.-H.; Chung, J.H. Poly(I:C) induces expressions of MMP-1, -2, and -3 through various signaling pathways including IRF3 in human skin fibroblasts. J. Dermatol. Sci. 2015, 80, 54–60. [Google Scholar] [CrossRef]
- Chen, Y.-L.; Zhang, M.-H.; Su, L.-L.; Sun, L.-C.; Qiu, X.-J.; Lin, D.; Zhang, L.-J.; Jin, T.; Cao, M.-J. Relationships of Matrix Metalloproteinase 1 and a Tissue Inhibitor of Metalloproteinase to Collagen Metabolism in Haliotis discus hannai. J. Agric. Food Chem. 2022, 70, 14886–14897. [Google Scholar] [CrossRef]
- Laronha, H.; Caldeira, J. Structure and Function of Human Matrix Metalloproteinases. Cells 2020, 9, 1076. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Ho, C.; Wen, D.; Sun, J.; Huang, L.; Gao, Y.; Li, Q.; Zhang, Y. Targeting the stem cell niche: Role of collagen XVII in skin aging and wound repair. Theranostics 2022, 12, 6446–6454. [Google Scholar] [CrossRef] [PubMed]
- Liu, N.; Matsumura, H.; Kato, T.; Ichinose, S.; Takada, A.; Namiki, T.; Asakawa, K.; Morinaga, H.; Mohri, Y.; De Arcangelis, A.; et al. Stem cell competition orchestrates skin homeostasis and ageing. Nature 2019, 568, 344–350. [Google Scholar] [CrossRef] [PubMed]
- Kroeger, J.; Hoppe, E.; Galiger, C.; Has, C.; Franzke, C.-W. Amino acid substitution in the C-terminal domain of collagen XVII reduces laminin-332 interaction causing mild skin fragility with atrophic scarring. Matrix Biol. 2019, 80, 72–84. [Google Scholar] [CrossRef]
- Jeong, S.; Yoon, S.; Kim, S.; Jung, J.; Kor, M.; Shin, K.; Lim, C.; Han, H.S.; Lee, H.; Park, K.-Y.; et al. Anti-Wrinkle Benefits of Peptides Complex Stimulating Skin Basement Membrane Proteins Expression. Int. J. Mol. Sci. 2020, 21, 73. [Google Scholar]
- Jacków, J.; Löffek, S.; Nyström, A.; Bruckner-Tuderman, L.; Franzke, C.-W. Collagen XVII Shedding Suppresses Re-Epithelialization by Directing Keratinocyte Migration and Dampening mTOR Signaling. J. Investig. Dermatol. 2016, 136, 1031–1041. [Google Scholar] [CrossRef]
- Xiang, Y.; Liu, Y.; Yang, Y.; Yan, Y.; Kim, A.J.; Guo, C.; Fisher, G.J.; Quan, T. Reduced expression of Collagen 17A1 in naturally aged, photoaged, and UV-irradiated human skin in vivo: Potential links to epidermal aging. J. Cell Commun. Signal. 2022, 16, 421–432. [Google Scholar] [CrossRef]
- Has, C.; Kern, J.S. Collagen XVII. Dermatol. Clin. 2010, 28, 61–66. [Google Scholar] [CrossRef]
- Wang, Y.; Kitahata, H.; Kosumi, H.; Watanabe, M.; Fujimura, Y.; Takashima, S.; Osada, S.-I.; Hirose, T.; Nishie, W.; Nagayama, M.; et al. Collagen XVII deficiency alters epidermal patterning. Lab. Investig. 2022, 102, 581–588. [Google Scholar] [CrossRef]
- Wang, Y.; Hess, M.E.; Tan, Y.; Esser, P.R.; Nyström, A.; Boerries, M.; Sayar, S.B.; Has, C. Alterations in the microenvironment of junctional epidermolysis bullosa keratinocytes: A gene expression study. Matrix Biol. 2025, 135, 12–23. [Google Scholar] [CrossRef] [PubMed]
- Pigors, M.; Patzelt, S.; Reichhelm, N.; Dworschak, J.; Khil’chenko, S.; Emtenani, S.; Bieber, K.; Hofrichter, M.; Kamaguchi, M.; Goletz, S.; et al. Bullous pemphigoid induced by IgG targeting type XVII collagen non-NC16A/NC15A extracellular domains is driven by Fc gamma receptor- and complement-mediated effector mechanisms and is ameliorated by neonatal Fc receptor blockade. J. Pathol. 2024, 262, 161–174. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Ho, C.; Wen, D.; Sun, J.; Liu, Y.; Li, Q.; Zhang, Y.; Gao, Y. PKM2-mediated collagen XVII expression is critical for wound repair. JCI Insight 2025, 10, e184457. [Google Scholar] [CrossRef] [PubMed]
- Hwang, Y.P.; Oh, K.N.; Yun, H.J.; Jeong, H.G. The flavonoids apigenin and luteolin suppress ultraviolet A-induced matrix metalloproteinase-1 expression via MAPKs and AP-1-dependent signaling in HaCaT cells. J. Dermatol. Sci. 2011, 61, 23–31. [Google Scholar] [CrossRef]
- Iriyama, S.; Yasuda, M.; Nishikawa, S.; Takai, E.; Hosoi, J.; Amano, S. Decrease of laminin-511 in the basement membrane due to photoaging reduces epidermal stem/progenitor cells. Sci. Rep. 2020, 10, 12592. [Google Scholar] [CrossRef]
- Ge, Y.; Li, M.; Bai, S.; Chen, C.; Zhang, S.; Cheng, J.; Wang, X. Doxercalciferol alleviates UVB-induced HaCaT cell senescence and skin photoaging. Int. Immunopharmacol. 2024, 127, 111357. [Google Scholar] [CrossRef]
- Oh, J.H.; Kim, J.; Karadeniz, F.; Kim, H.R.; Park, S.Y.; Seo, Y.; Kong, C.-S. Santamarine Shows Anti-Photoaging Properties via Inhibition of MAPK/AP-1 and Stimulation of TGF-β/Smad Signaling in UVA-Irradiated HDFs. Molecules 2021, 26, 3585. [Google Scholar] [CrossRef]
- Cui, B.; Wang, Y.; Jin, J.; Yang, Z.; Guo, R.; Li, X.; Yang, L.; Li, Z. Resveratrol Treats UVB-Induced Photoaging by Anti-MMP Expression, through Anti-Inflammatory, Antioxidant, and Antiapoptotic Properties, and Treats Photoaging by Upregulating VEGF-B Expression. Oxid. Med. Cell Longev. 2022, 2022, 6037303. [Google Scholar] [CrossRef]
- Berneburg, M.; Plettenberg, H.; Krutmann, J. Photoaging of human skin. Photodermatol. Photoimmunol. Photomed. 2000, 16, 239–244. [Google Scholar] [CrossRef]
- Martínez-Carpio, P.A.; Trelles, M.A. Cutaneous epidermal growth factor receptor system following ultraviolet irradiation: Exploring the role of molecular mechanisms. Photodermatol. Photoimmunol. Photomed. 2010, 26, 250–256. [Google Scholar] [CrossRef]
- Jeon, H.; Tkacik, E.; Eck, M.J. Signaling from RAS to RAF: The Molecules and Their Mechanisms. Annu. Rev. Biochem. 2024, 93, 289–316. [Google Scholar] [CrossRef] [PubMed]
- Surve, S.; Watkins, S.C.; Sorkin, A. EGFR-RAS-MAPK signaling is confined to the plasma membrane and associated endorecycling protrusions. J. Cell Biol. 2021, 220, e202107103. [Google Scholar] [CrossRef] [PubMed]
- Han, S.H.; Ballinger, E.; Choung, S.-Y.; Kwon, J.Y. Anti-Photoaging Effect of Hydrolysates from Pacific Whiting Skin via MAPK/AP-1, NF-κB, TGF-β/Smad, and Nrf-2/HO-1 Signaling Pathway in UVB-Induced Human Dermal Fibroblasts. Mar. Drugs 2022, 20, 308. [Google Scholar] [CrossRef] [PubMed]
- Nanba, D.; Toki, F.; Asakawa, K.; Matsumura, H.; Shiraishi, K.; Sayama, K.; Matsuzaki, K.; Toki, H.; Nishimura, E.K. EGFR-mediated epidermal stem cell motility drives skin regeneration through COL17A1 proteolysis. J. Cell Biol. 2021, 220, e202012073. [Google Scholar] [CrossRef]
- Honda, A.; Abe, R.; Makino, T.; Norisugi, O.; Fujita, Y.; Watanabe, H.; Nishihira, J.; Iwakura, Y.; Yamagishi, S.-i.; Shimizu, H.; et al. Interleukin-1β and macrophage migration inhibitory factor (MIF) in dermal fibroblasts mediate UVA-induced matrix metalloproteinase-1 expression. J. Dermatol. Sci. 2008, 49, 63–72. [Google Scholar] [CrossRef]
- Liu, Z.; Li, Y.; Song, H.; He, J.; Li, G.; Zheng, Y.; Li, B. Collagen peptides promote photoaging skin cell repair by activating the TGF-β/Smad pathway and depressing collagen degradation. Food Funct. 2019, 10, 6121–6134. [Google Scholar] [CrossRef]
- Wang, J.; Lin, S.; Wei, Y.; Ye, Z. Recombinant human collagen XVII protects skin basement membrane integrity by inhibiting the MAPK and Wnt signaling pathways. Mol. Med. Rep. 2025, 31, 100. [Google Scholar] [CrossRef]
- Vázquez, F.; Palacios, S.; Alemañ, N.; Guerrero, F. Changes of the basement membrane and type IV collagen in human skin during aging. Maturitas 1996, 25, 209–215. [Google Scholar] [CrossRef]
- Amano, S. Possible Involvement of Basement Membrane Damage in Skin Photoaging. J. Investig. Dermatol. Symp. Proc. 2009, 14, 2–7. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
He, Y.; Yin, S.; Xu, R.; Zhao, Y.; Du, Y.; Duan, Z.; Fan, D. Recombinant Type XVII Collagen Inhibits EGFR/MAPK/AP-1 and Activates TGF-β/Smad Signaling to Enhance Collagen Secretion and Reduce Photoaging. Cosmetics 2025, 12, 59. https://doi.org/10.3390/cosmetics12020059
He Y, Yin S, Xu R, Zhao Y, Du Y, Duan Z, Fan D. Recombinant Type XVII Collagen Inhibits EGFR/MAPK/AP-1 and Activates TGF-β/Smad Signaling to Enhance Collagen Secretion and Reduce Photoaging. Cosmetics. 2025; 12(2):59. https://doi.org/10.3390/cosmetics12020059
Chicago/Turabian StyleHe, Ying, Shiyu Yin, Ru Xu, Yan Zhao, Yuhang Du, Zhiguang Duan, and Daidi Fan. 2025. "Recombinant Type XVII Collagen Inhibits EGFR/MAPK/AP-1 and Activates TGF-β/Smad Signaling to Enhance Collagen Secretion and Reduce Photoaging" Cosmetics 12, no. 2: 59. https://doi.org/10.3390/cosmetics12020059
APA StyleHe, Y., Yin, S., Xu, R., Zhao, Y., Du, Y., Duan, Z., & Fan, D. (2025). Recombinant Type XVII Collagen Inhibits EGFR/MAPK/AP-1 and Activates TGF-β/Smad Signaling to Enhance Collagen Secretion and Reduce Photoaging. Cosmetics, 12(2), 59. https://doi.org/10.3390/cosmetics12020059





