Investigation of the Anti-Aging Effects of Composite Nanocarriers Based on Autophagy Regulation and Oxidative Stress Inhibition
Abstract
:1. Introduction
2. Materials and Methods
2.1. Main Materials, Reagents, and Instruments
2.2. Experimental Methods
2.2.1. Preparation Method of RCDP NCs
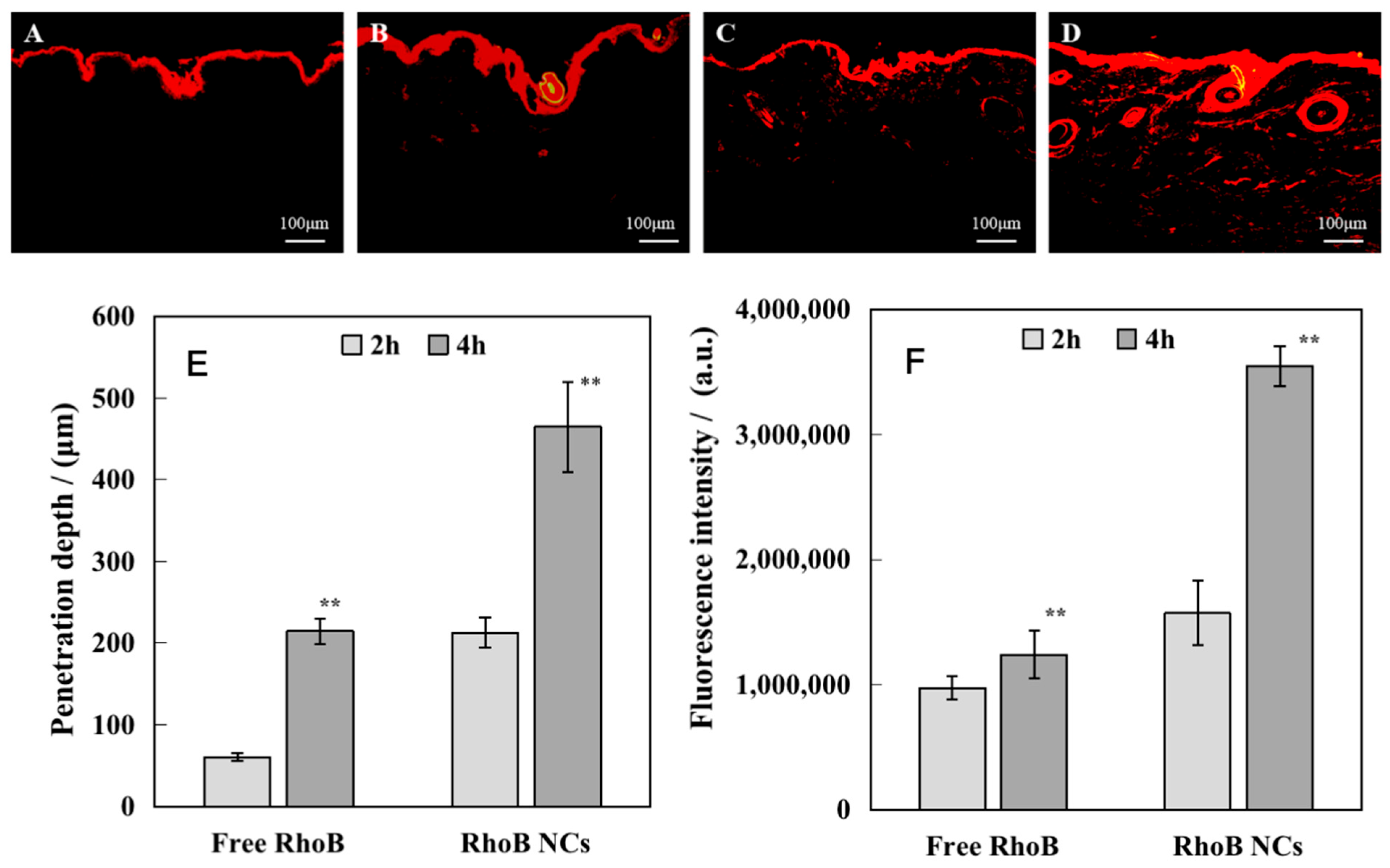
2.2.2. Percutaneous Penetration
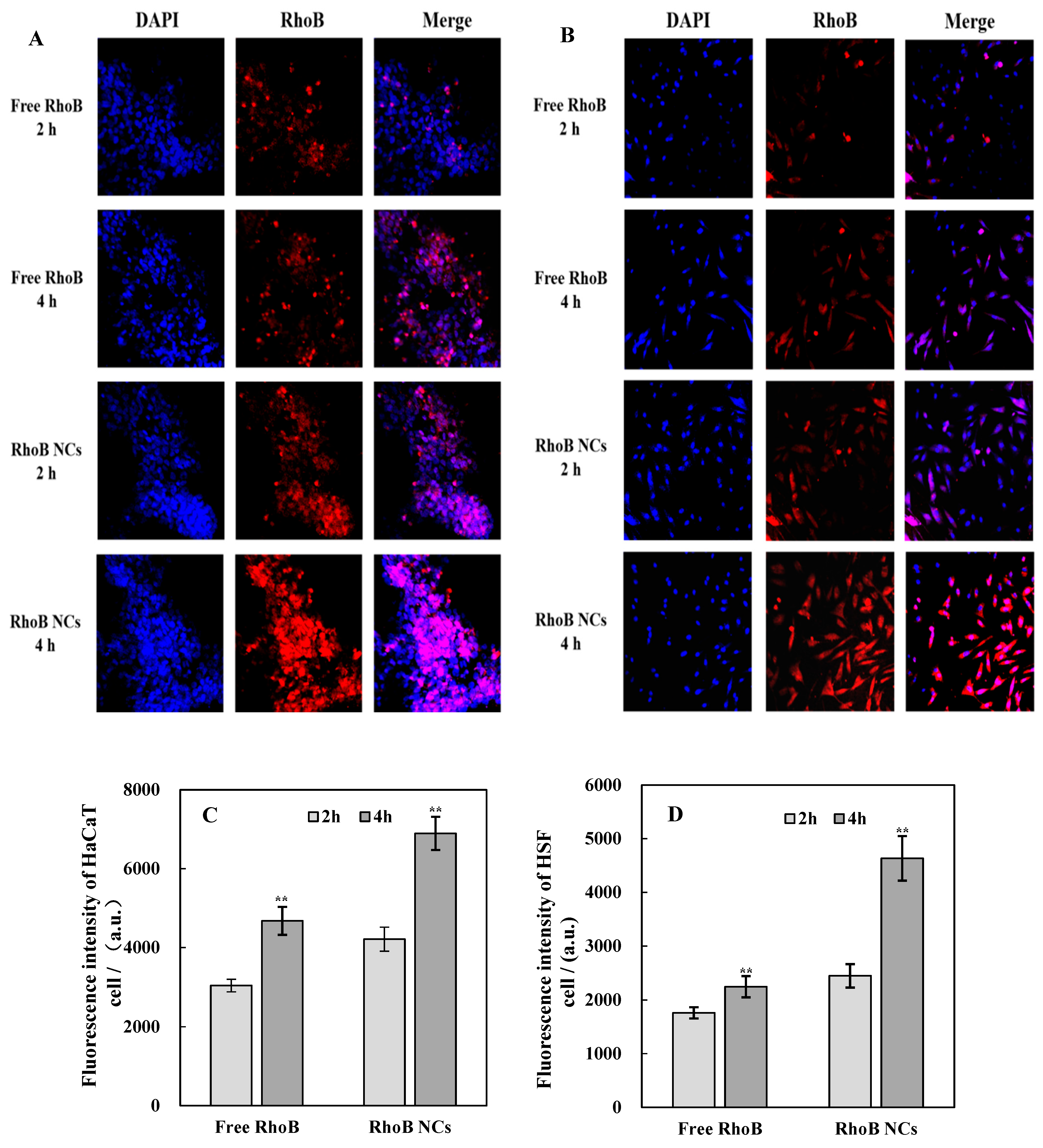
2.2.3. Quantification of Cellular Uptake
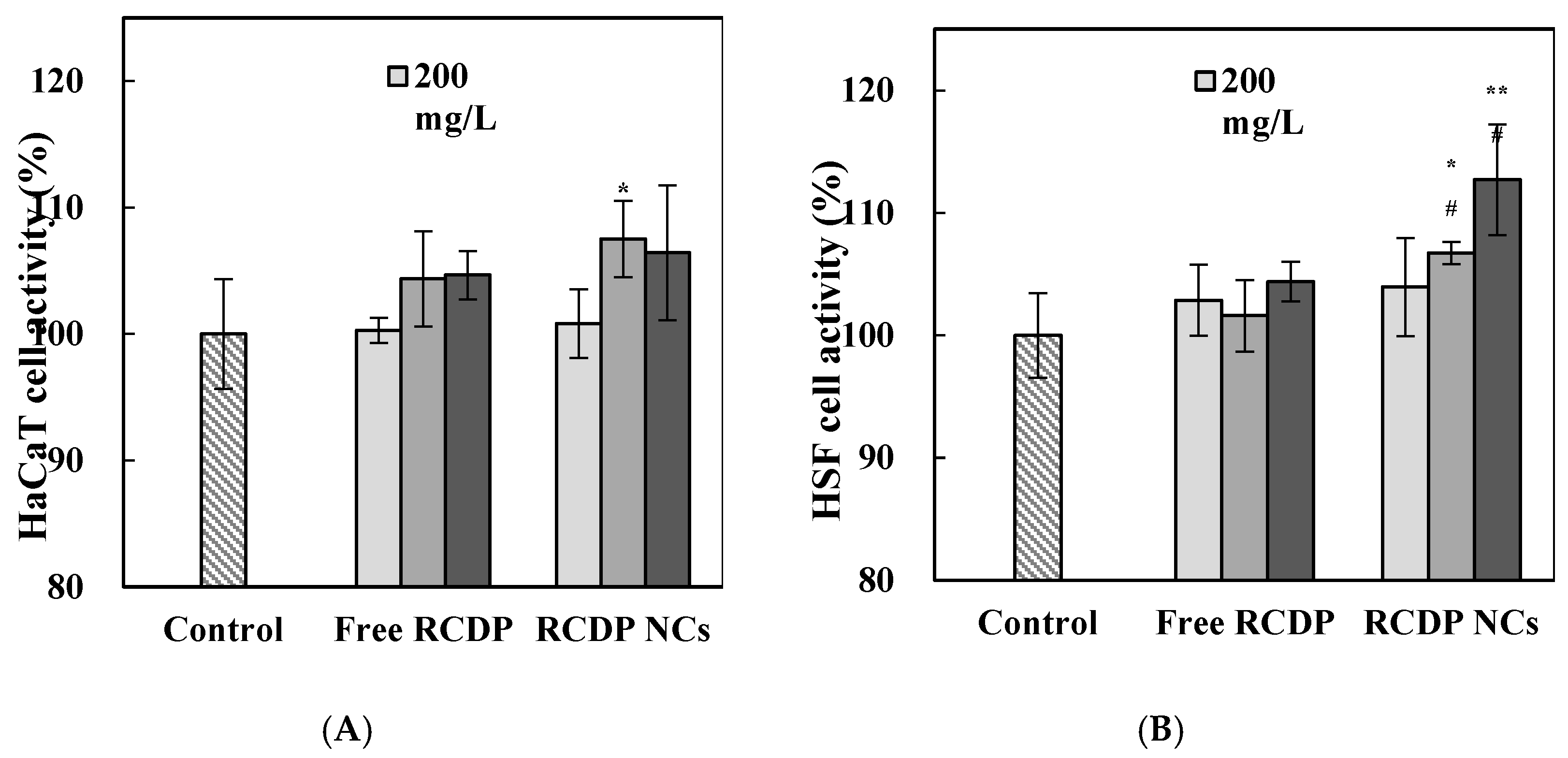
2.2.4. Cell Proliferation Activity
2.2.5. β-Galactosidase Staining Observation
2.2.6. Inhibition of Oxidative Stress
2.2.7. Autophagy-Related Protein Expression in Cells
2.2.8. Autophagosomes
2.2.9. Data Processing
3. Results
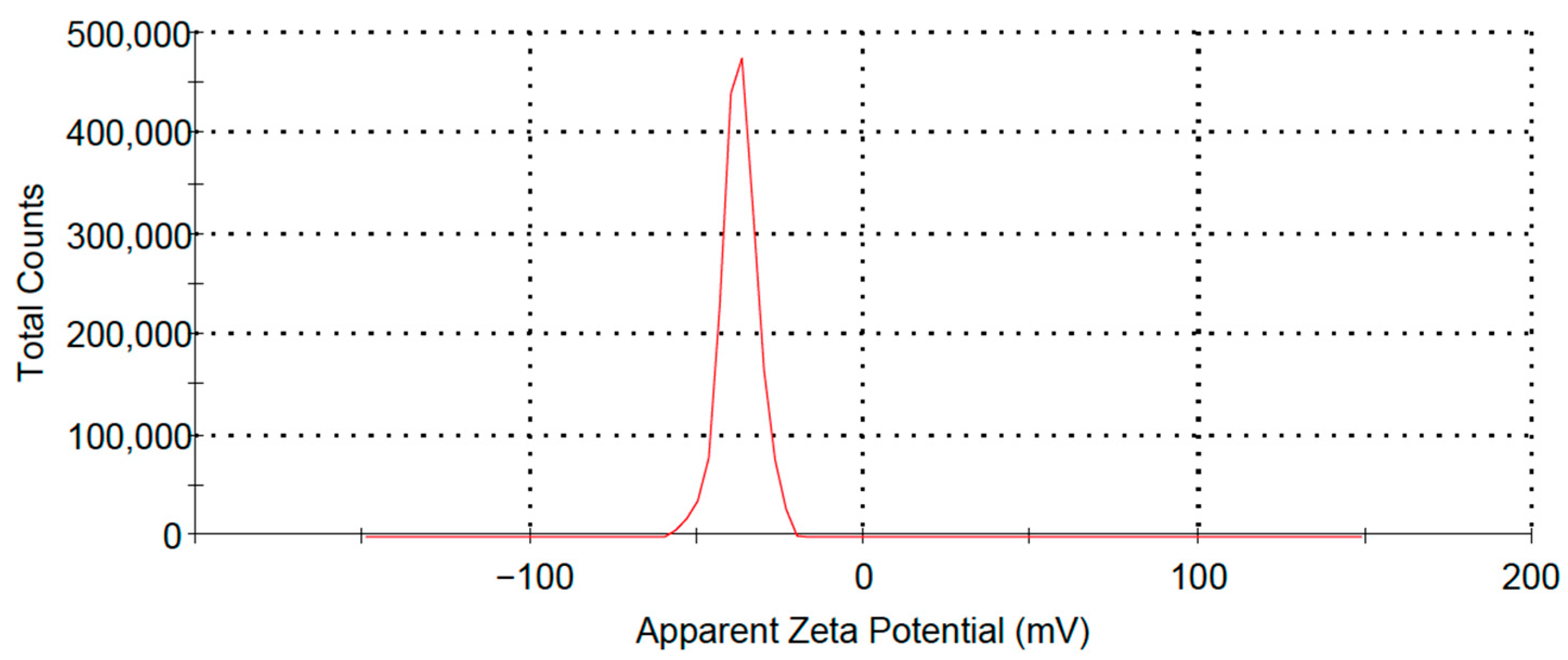
3.1. Particle Size, PDI, and Zeta Potential
3.2. In Vitro Skin Penetration
3.3. Cell Proliferation
3.4. The Uptake of RCDP NCs by Cells
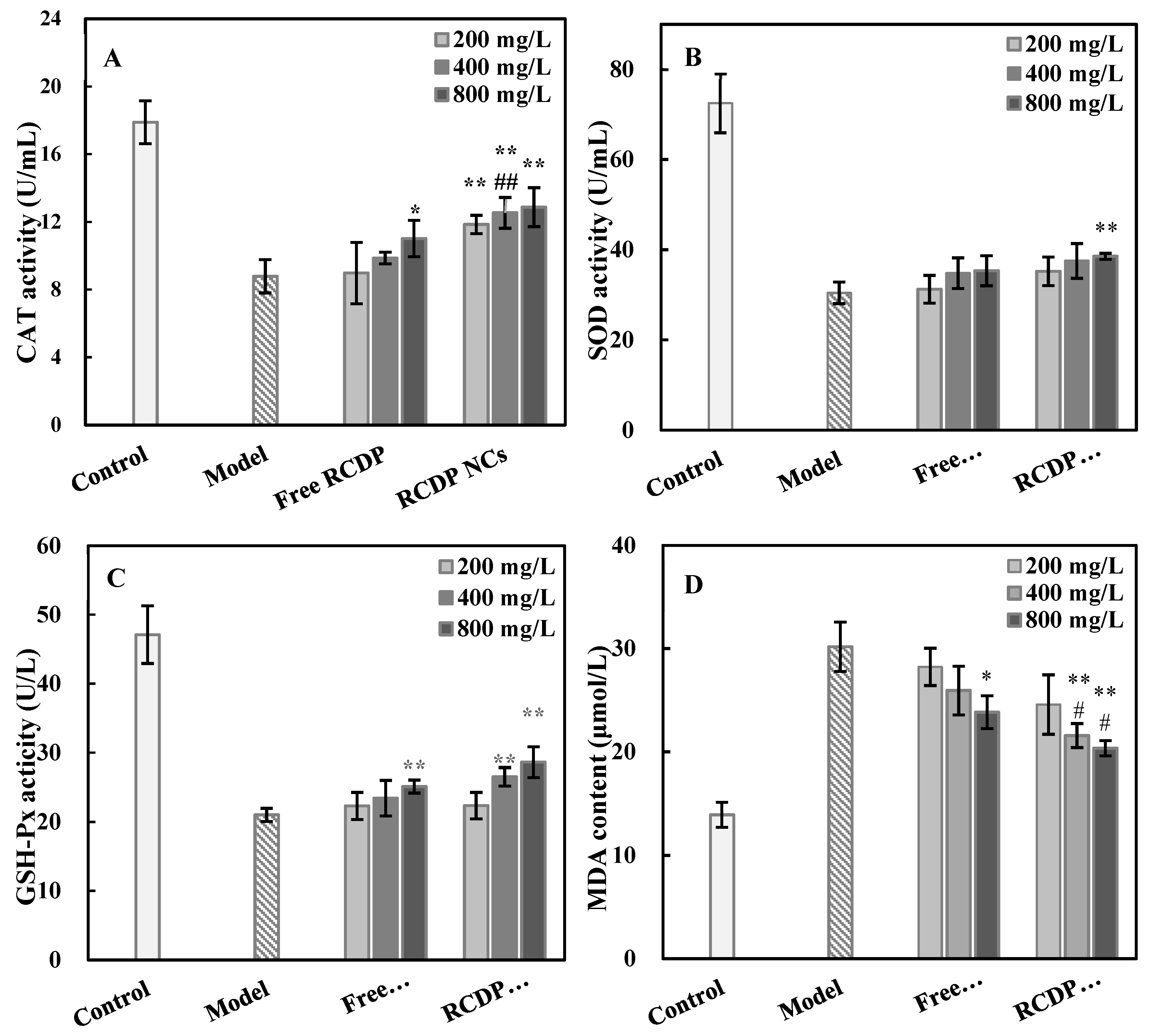
3.5. Effect of RCDP NCs on Cellular Oxidative Stress
3.6. β-Galactosidase
3.7. Expression of Autophagy-Related Proteins in Cells
3.8. Observation of Autophagosomes Using Transmission Electron Microscopy
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Warsito, M.F.; Kusumawati, I. The impact of herbal products in the prevention, regeneration and delay of skin aging. Adv. Exp. Med. Biol. 2019, 1178, 155–174. [Google Scholar] [CrossRef] [PubMed]
- Zhao, X.J.; Hong, Y.H.; Liu, W. Nanocarrier technology-from transdermal drug delivery to functional cosmetics. Deterg. Cosmet. 2016, 44, 12–16. [Google Scholar] [CrossRef]
- Martinez-Lopez, N.; Athonvarangku, D.; Singh, R. Autophagy and aging. Adv. Exp. Med. Biol. 2015, 847, 73–87. [Google Scholar] [CrossRef] [PubMed]
- Palmer, D.M.; Kitchin, J.S. Oxidative damage, skin aging, antioxidants and a novel antioxidant rating system. J. Drugs Dermatol. 2010, 9, 11–15. [Google Scholar] [PubMed]
- Gu, Y.; Han, J.; Jiang, C.; Zhang, Y. Biomarkers, oxidative stress and autophagy in skin aging. Ageing Res. Rev. 2020, 59, 101036. [Google Scholar] [CrossRef]
- Mariño, G.; Niso-Santano, M.; Baehrecke, E.H.; Kroemer, G. Self-consumption: The interplay of autophagy and apoptosis. Nat. Rev. Mol. Cell Biol. 2014, 15, 81–94. [Google Scholar] [CrossRef] [PubMed]
- Roupe, G. Skin of the aging human being. Lakartidningen 2001, 98, 1091–1095. [Google Scholar] [PubMed]
- Muggleton-Harris, A.L.; Defuria, R. Age-dependent metabolic changes in cultured human fibroblasts. In Vitro Cell Dev. Biol. 1985, 21, 271–276. [Google Scholar] [CrossRef]
- Shecterle, L.M.; St Cyr, J.A. Dermal benefits of topical D-ribose. Clin. Cosmet. Investig. Dermatol. 2009, 2, 151–152. [Google Scholar] [CrossRef]
- Wang, J.; Hu, H.; Wang, J.; Qiu, H.; Gao, Y.; Xu, Y.; Liu, Z.; Tang, Y.; Song, L.; Ramshaw, J.; et al. Characterization of recombinant humanized collagen type Ⅲ and its influence on cell behavior and phenotype. Collagen Leather 2023, 5, 84–96. [Google Scholar] [CrossRef]
- Hua, C.; Zhu, Y.; Xu, W.; Ye, S.; Zhang, R.; Lu, L.; Jiang, S. Characterization by high-resolution crystal structure analysis of a triple-helix region of human collagen type III with potent cell adhesion activity. Biochem. Biophys. Res. Commun. 2019, 508, 1018–1023. [Google Scholar] [CrossRef] [PubMed]
- Baek, J.H.; Kong, U.S.; Lee, G.M. Cosmetic Composition for Preventing Skin Wrinkles, Containing Carcinine(Decarboxy Carnosine 2HCl) Having Collagen Synthetic and Antioxidant Effects. GB Patent Application No. KR20030010259, 11 September 2024. [Google Scholar]
- Yang, F.; Zhang, X.; Wang, H.; Guo, M.; Zhang, J.; Feng, X.; Yu, J.; Yang, J.; Zhu, J.; Wang, Y. Comprehensive evaluation of the efficacy and safety of a new multi-component anti-aging topical eye cream. Skin Res. Technol. 2024, 30, e13790. [Google Scholar] [CrossRef]
- Zhang, S.; Zhou, H.; Chen, X.; Zhu, S.; Chen, D.; Luo, D.; Chen, S.; Liu, W. Microneedle Delivery Platform Integrated with Codelivery Nanoliposomes for Effective and Safe Androgenetic Alopecia Treatment. ACS Appl. Mater. Interfaces 2024, 16, 15701–15717. [Google Scholar] [CrossRef]
- Zhou, H.; Luo, D.; Chen, D.; Tan, X.; Bai, X.; Liu, Z.; Yang, X.; Liu, W. Current Advances of Nanocarrier Technology-Based Active Cosmetic Ingredients for Beauty Applications. Clin. Cosmet. Investig. Dermatol. 2021, 14, 867–887. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.P.; Shen, H.H.; Luo, D.; Chen, D.; Sheng, J.; Liu, W. Preparation and efficacy evaluation of nanoliposomes for co-delivery of anti-alopecia agents. Chin. Surf. Deter. Cos. 2020, 50, 396–401. [Google Scholar] [CrossRef]
- Kim, B.S.; Na, Y.G.; Choi, J.H.; Kim, I.; Lee, E.; Kim, S.-Y.; Lee, J.-Y.; Cho, C.-W. The Improvement of Skin Whitening of Phenylethyl Resorcinol by Nanostructured Lipid Carriers. Nanomaterials 2017, 7, 241. [Google Scholar] [CrossRef]
- Wang, M.; Charareh, P.; Lei, X.; Zhong, J.L. Autophagy: Multiple Mechanisms to Protect Skin from Ultraviolet Radiation-Driven Photoaging. Oxidative Med. Cell. Longev. 2019, 2019, 8135985. [Google Scholar] [CrossRef]
- Han, S.; Li, H.; Luo, F.; Chen, X.; Cen, Y.; Liu, P.; Chen, Z.; Lan, T.; Lin, J. Inhibitory Effect of Seawater Pearl Hydrolysate on UVA-Induced Photoaging of Human Skin Fibroblasts. Evid. Based. Complement. Alternat. Med. 2022, 2022, 1558288. [Google Scholar] [CrossRef]
- Misovic, M.; Milenkovic, D.; Martinovic, T.; Ciric, D.; Bumbasirevic, V.; Kravic-Stevovic, T. Short-term exposure to UV-A, UV-B, and UV-C irradiation induces alteration in cytoskeleton and autophagy in human keratinocytes. Ultrastruct. Pathol. 2013, 37, 241–248. [Google Scholar] [CrossRef]
- Jung, C.H.; Ro, S.H.; Cao, J.; Otto, N.M.; Kim, D.-H. mTOR regulation of autophagy. FEBS Lett. 2010, 584, 1287–1295. [Google Scholar] [CrossRef]
- Klionsky, D.J.; Emr, S.D. Autophagy as a regulated pathway of cellular degradation. Science 2000, 290, 1717–1721. [Google Scholar] [CrossRef]
- Gilchrest, B.A. A review of skin aging and its medical therapy. Br. J. Dermatol. 1996, 135, 867–875. [Google Scholar] [CrossRef] [PubMed]
- Gilchrest, B.A.; Garmys, M.; Yaar, M.A. Aging and photoaging effectgene expression in cultured human Keratinocyte. Arch. Dermatol. 1994, 130, 82–86. [Google Scholar] [CrossRef]
- Boismal, F.; Serror, K.; Dobos, G.; Zuelgaray, E.; Bensussan, A.; Michel, L. Skin aging: Pathophysiology and innovative therapies. Med. Sci. 2020, 36, 1163–1172. [Google Scholar] [CrossRef]
- Csekes, E.; Račková, L. Skin Aging, Cellular senescence and natural polyphenols. Int. J. Mol. Sci. 2021, 22, 12641. [Google Scholar] [CrossRef] [PubMed]
- Fisher, G.J.; Kang, S.; Varani, J.; Bata-Csorgo, Z.; Wan, Y.; Datta, S.; Voorhees, J.J. Mechanisms of photoaging and chronological skin aging. Arch. Dermatol. 2002, 138, 1462–1470. [Google Scholar] [CrossRef] [PubMed]
- Pozos-Nonato, S.; Domínguez-Delgado, C.L.; Campos-Santander, K.A.; Benavides, A.A.; Pacheco-Ortin, S.M.; Higuera-Piedrahita, R.I.; Resendiz-González, G.; Molina-Trinidad, E.M. Novel nanotechnological strategies for skin anti-aging. Curr. Pharm. Biotechnol. 2023, 24, 1397–1419. [Google Scholar] [CrossRef]
- He, X.; Gao, X.; Guo, Y.; Xie, W. Research progress on bioactive factors against skin aging. Int. J. Mol. Sci. 2024, 25, 3797. [Google Scholar] [CrossRef]
- He, X.; Wan, F.; Su, W.; Xie, W. Research progress on skin aging and active Ingredients. Molecules 2023, 28, 5556. [Google Scholar] [CrossRef]
- Pintea, A.; Manea, A.; Pintea, C.; Vlad, R.-A.; Bîrsan, M.; Antonoaea, P.; Rédai, E.M.; Ciurba, A. Peptides: Emerging candidates for the prevention and treatment of skin senescence: A review. Biomolecules 2025, 15, 88. [Google Scholar] [CrossRef]
- Alotaibi, M.R.; As, S.H.; Alaqil, F.A.; Almutairi, M.; Alhazzani, K.; Sulaiman, A.A.; Isab, A.A.; Alotaibi, N.H. A newly synthesizedplatinum-based compound (PBC-Ⅱ) increases chemosensitivity of HeLa ovarian cancer cells via inhibition of autophagy. Saudi Pharm. J. 2019, 27, 1203–1209. [Google Scholar] [CrossRef] [PubMed]
- Chen, S.M.; Gong, Y.C.; Sui, L.; Chu, M.; Dong, Y. Role of tangeretin on autophagy in human gastric cancer AGS cells and its mechanism. Chin. Pharmacol. Bull. 2019, 35, 1671–1676. [Google Scholar] [CrossRef]
- Qi, M.; Tan, B.E. Molecular mechanism of autophagy regulating oxidative stress in animals. Chin. J. Anim. Nut. 2020, 32, 10. [Google Scholar]
- Sun, D.; Wu, R.; Zheng, J.; Li, P.; Yu, L. Polyubiquitin chain-induced p62 phase separation drives autophagic cargo segregation. Cell Res. 2018, 28, 405–415. [Google Scholar] [CrossRef]
- Ferreira, M.S.; Magalhães, M.C.; Oliveira, R.; Sousa-Lobo, J.M.; Almeida, I.F. Trends in the Use of Botanicals in Anti-Aging Cosmetics. Molecules 2021, 26, 3584. [Google Scholar] [CrossRef]
- Cronin, H.; Draelos, Z.D. Top 10 botanical ingredients in 2010 anti-aging creams. J. Cosmet. Dermatol. 2010, 9, 218–225. [Google Scholar] [CrossRef]




Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, M.; Ye, L.; Jiang, L.; Wang, X.; Sun, C.; Zheng, J.; Liu, W. Investigation of the Anti-Aging Effects of Composite Nanocarriers Based on Autophagy Regulation and Oxidative Stress Inhibition. Cosmetics 2025, 12, 83. https://doi.org/10.3390/cosmetics12020083
Liu M, Ye L, Jiang L, Wang X, Sun C, Zheng J, Liu W. Investigation of the Anti-Aging Effects of Composite Nanocarriers Based on Autophagy Regulation and Oxidative Stress Inhibition. Cosmetics. 2025; 12(2):83. https://doi.org/10.3390/cosmetics12020083
Chicago/Turabian StyleLiu, Min, Lei Ye, Lingling Jiang, Xi Wang, Cui Sun, Jiuyan Zheng, and Wei Liu. 2025. "Investigation of the Anti-Aging Effects of Composite Nanocarriers Based on Autophagy Regulation and Oxidative Stress Inhibition" Cosmetics 12, no. 2: 83. https://doi.org/10.3390/cosmetics12020083
APA StyleLiu, M., Ye, L., Jiang, L., Wang, X., Sun, C., Zheng, J., & Liu, W. (2025). Investigation of the Anti-Aging Effects of Composite Nanocarriers Based on Autophagy Regulation and Oxidative Stress Inhibition. Cosmetics, 12(2), 83. https://doi.org/10.3390/cosmetics12020083





