Berry Fruit Extracts as Topical Cosmeceuticals for Skin Health Applications: A Systematic Review
Abstract
:1. Introduction
2. Materials and Methods
2.1. Focus Question and Registration on the Prospero Platform
2.2. Eligibility Criteria
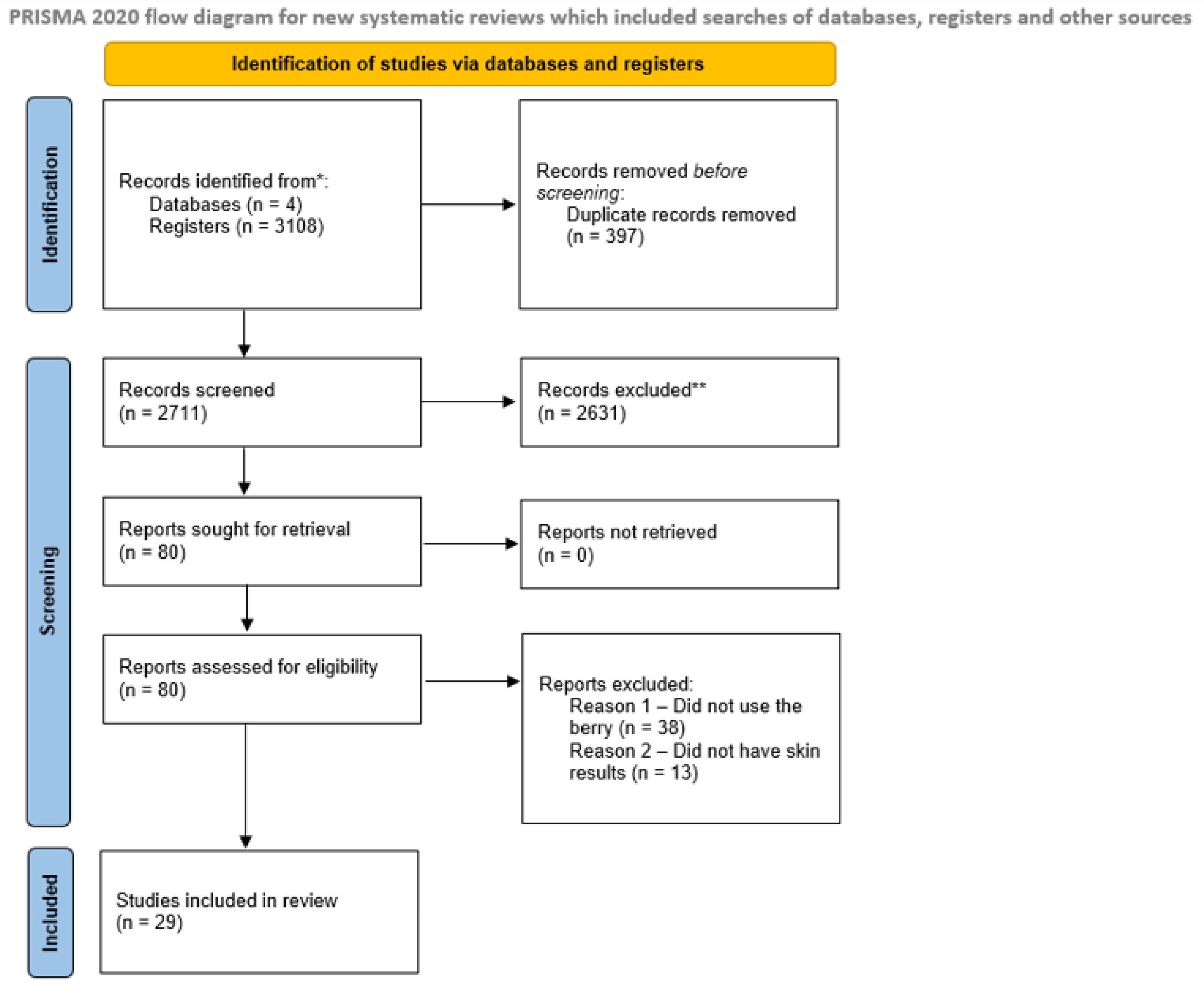
2.3. Literature Search Strategy
2.4. Data Extraction and Synthesis
2.5. Risk of Bias
3. Results
3.1. Characteristics of the Publications
3.2. Characteristics of Animal Models
3.3. Characteristics of the Berries
3.4. Characteristics of the Intervention
3.5. Main Outcomes
Oxidative Stress and Inflammation Markers
3.6. Secondary Outcomes
Histological Markers and Associated Disorders
3.7. Reporting the Bias of Studies
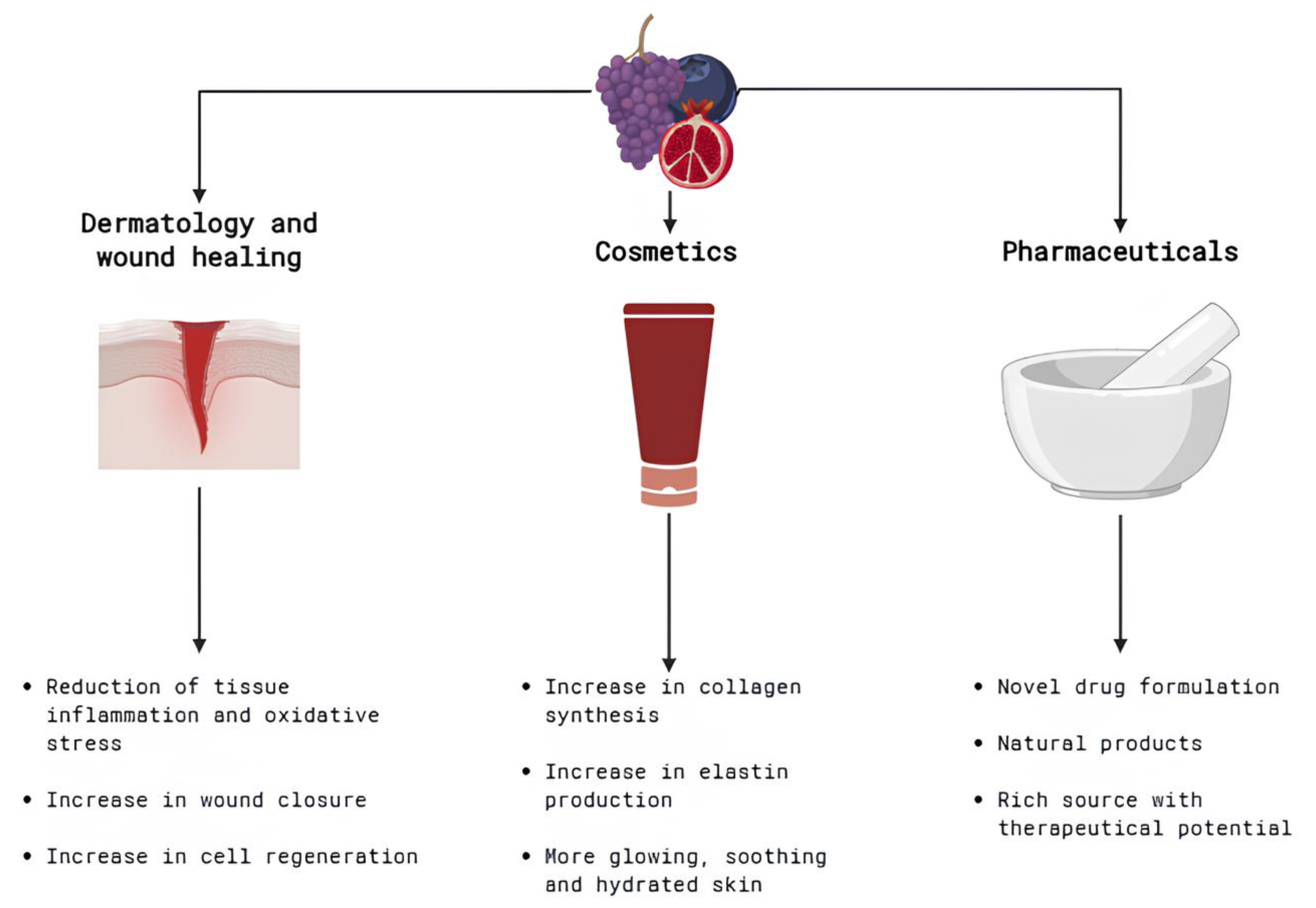
4. Discussion
4.1. General Information
4.2. Berries and Bioactive Compounds
4.3. Influence of Bioactive Compounds on Tissue Regeneration
4.4. Risk of Bias and Limitations
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Grand View Research. Natural Skin Care Products Market Size, Share & Trends Analysis Report. Retrieved 7 April 2025. Available online: https://www.grandviewresearch.com/industry-analysis/natural-skin-care-products-market (accessed on 16 November 2024).
- Lakshmikanthan, M.; Muthu, S.; Krishnan, K.; Altemimi, A.B.; Haider, N.N.; Govindan, L.; Francis, Y.M. A comprehensive review on anthocyanin-rich foods: Insights into extraction, medicinal potential, and sustainable applications. J. Agric. Food Res. 2024, 17, 101245. [Google Scholar] [CrossRef]
- Šimerdová, B.; Bobríková, M.; Lhotská, I.; Kaplan, J.; Křenová, A.; Šatínský, D. Evaluation of anthocyanin profiles in various blackcurrant cultivars over a three-year period using a fast HPLC-DAD method. Foods 2021, 10, 1745. [Google Scholar] [CrossRef]
- Liu, Y.; Zhang, M.; Zhang, H.; Qian, X.; Luo, L.; He, Z. Anthocyanins Inhibit Airway Inflammation by Downregulating the NF-κB Pathway via the miR-138-5p/SIRT1 Axis in Asthmatic Mice. Int. Arch. Allergy Immunol. 2022, 183, 539–551. [Google Scholar] [CrossRef] [PubMed]
- Bartel, S.; La Grutta, S.; Cilluffo, G.; Perconti, G.; Bongiovanni, A.; Giallongo, A.; Behrends, J.; Kruppa, J.; Hermann, S.; Chiang, D.; et al. Human airway epithelial extracellular vesicle miRNA signature is altered upon asthma development. Allergy 2020, 75, 346–356. [Google Scholar] [CrossRef] [PubMed]
- Andries, P.; Clarysse, B.; Costa, S. Technology ventures’ engagement of external actors in the search for viable market applications: On the relevance of technology broadcasting and systematic validation. J. Bus. Ventur. 2021, 36, 106–145. [Google Scholar]
- Ohlert, S.; Laibach, N.; Harms, R.; Bröring, S. Opportunity recognition in the tension field of knowledge and learning: The case of converging industries. J. Bus. Res. 2025, 186, 114993. [Google Scholar] [CrossRef]
- Chen, J. Essential role of medicine and food homology in health and wellness. Chin. Herb. Med. 2023, 15, 347–348. [Google Scholar] [CrossRef]
- Santos, J.D.; Mendonça, A.A.; Sousa, R.C.; Silva, T.G.; Bigonha, S.M.; Santos, E.C.; Novaes, R.D. Food-drug interaction: Anabolic steroids aggravate hepatic lipotoxicity and nonalcoholic fatty liver disease induced by trans fatty acids. Food Chem. Toxicol. 2018, 116, 360–368. [Google Scholar]
- Martins, T.E.; Sales de Oliveira Pinto, C.A.; Costa de Oliveira, A.; Robles Velasco, M.V.; Gorriti Guitiérrez, A.R.; Cosquillo Rafael, M.F.; Retuerto-Figueroa, M.G. Contribution of topical antioxidants to maintain healthy skin—A review. Sci. Pharm. 2020, 88, 27. [Google Scholar] [CrossRef]
- Michalak, M. Plant-Derived Antioxidants: Significance in Skin Health and the Ageing Process. Int. J. Mol. Sci. 2022, 23, 585. [Google Scholar] [CrossRef]
- Bilawal, A.; Ishfaq, M.; Gantumur, M.A.; Qayum, A.; Shi, R.; Fazilani, S.A.; Hou, J. A review of the bioactive ingredients of berries and their applications in curing diseases. Food Biosci. 2021, 44, 101407. [Google Scholar] [CrossRef]
- Qamar, M.; Akhtar, S.; Ismail, T.; Sestili, P.; Tawab, A.; Ahmed, N. Anticancer and anti-inflammatory perspectives of Pakistans indigenous berry Grewia asiatica Linn (Phalsa). J. Berry Res. 2020, 10, 115–131. [Google Scholar] [CrossRef]
- Tang, S.; Ruan, Z.; Ma, A.; Wang, D.; Kou, J. Effect of vitamin K on wound healing: A systematic review and meta-analysis based on preclinical studies. Front. Pharmacol. 2022, 13, 1063349. [Google Scholar] [CrossRef] [PubMed]
- Vendrame, S.; Alaba, T.; Marchi, N.; Tsakiroglou, P.; Klimis-Zacas, D. In vitro and in vivo evaluation of bioactive compounds from berries for wound healing. Curr. Dev. Nutr. 2024, 8, 102078. [Google Scholar] [CrossRef]
- Hoskin, R.T.; Xiong, J.; Esposito, D.A.; Lila, M.A. Blueberry polyphenol-protein food ingredients: The impact of spray drying on the in vitro antioxidant activity, anti-inflammatory markers, glucose metabolism and fibroblast migration. Food Chem. 2019, 280, 187–194. [Google Scholar] [CrossRef]
- Ravichandran, K.S.; Silva, E.S.; Moncada, M.; Perkins-Veazie, P.; Lila, M.A.; Greenlief, C.M.; Krishnaswamy, K. Spray drying to produce novel phytochemical-rich ingredients from juice and pomace of American elderberry. Food Biosci. 2023, 55, 102981. [Google Scholar] [CrossRef]
- Page, M.J.; Moher, D.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; McKenzie, J.E. PRISMA 2020 explanation and elaboration: Updated guidance and exemplars for reporting systematic reviews. BMJ 2021, 372, n160. [Google Scholar] [CrossRef]
- Piazza, S.; Fumagalli, M.; Khalilpour, S.; Martinelli, G.; Magnavacca, A.; Dell’Agli, M.; Sangiovanni, E. A Review of the Potential Benefits of Plants Producing Berries in Skin Disorders. Antioxidants 2020, 9, 542. [Google Scholar] [CrossRef]
- Hooijmans, C.R.; Rovers, M.M.; De Vries, R.B.; Leenaars, M.; Ritskes-Hoitinga, M.; Langendam, M.W. SYRCLE’s risk of bias tool for animal studies. BMC Med. Res. Methodol. 2014, 14, 43. [Google Scholar] [CrossRef]
- Divya, S.P.; Wang, X.; Pratheeshkumar, P.; Son, Y.O.; Roy, R.V.; Kim, D.; Dai, J.; Hitron, J.A.; Wang, L.; Asha, P.; et al. Blackberry extract inhibits UVB-induced oxidative damage and inflammation through MAP kinases and NF-κB signaling pathways in SKH-1 mice skin. Toxicol. Appl. Pharmacol. 2015, 284, 92–99. [Google Scholar] [CrossRef]
- Nejati, H.; Farahpour, M.R. Effect of topical red grape seed hydroethanol extract on burn wound healing in rats. Indian J. Chem. Technol. 2014, 6, 2340–2346. [Google Scholar]
- Ayla, Ş.; Günal, M.Y.; SAYIN ŞAKUL, A.A.; Biçeroğlu, Ö.; Özdemir, E.M.; Okur, M.E.; Bilgiç, B.E. Effects of Prunus spinosa L. fruits on experimental wound healing. Medeni. Med. J. 2017, 32, 152–158. [Google Scholar] [CrossRef]
- Gouveia MC, P.; Minto, B.W.; Sargi, L.F.; Souza, R.L.; Pazzini, J.M.; Colodel, E.M.; Dias, L.G.G.G. Evaluation of the alcoholic extract of Dipteryx alata Vogel almonds and bark in skin wound healing in C57BL6 mice. Arq. Bras. De Med. Veterinária E Zootec. 2021, 73, 1315–1322. [Google Scholar] [CrossRef]
- Moalla Rekik, D.; Ben Khedir, S.; Ksouda Moalla, K.; Kammoun, N.G.; Rebai, T.; Sahnoun, Z. Evaluation of wound healing properties of grape seed, sesame, and fenugreek oils. Evid.-Based Complement. Altern. Med. 2016, 2016, 7965689. [Google Scholar] [CrossRef]
- Hanausek, M.; Spears, E.; Walaszek, Z.; Kowalczyk, M.C.; Kowalczyk, P.; Wendel, C.; Slaga, T.J. Inhibition of murine skin carcinogenesis by freeze-dried grape powder and other grape-derived major antioxidants. Nutr. Cancer 2011, 63, 28–38. [Google Scholar] [CrossRef]
- Filip, G.A.; Postescu, I.D.; Bolfa, P.; Catoi, C.; Muresan, A.; Clichici, S. Inhibition of UVB-induced skin phototoxicity by a grape seed extract as modulator of nitrosative stress, ERK/NF-κB signaling pathway and apoptosis, in SKH-1 mice. Food Chem. Toxicol. 2013, 57, 296–306. [Google Scholar] [CrossRef]
- Elshaer, E.E.; Elwakil, B.H.; Eskandrani, A.; Elshewemi, S.S.; Olama, Z.A. Novel Clotrimazole and Vitis vinifera loaded chitosan nanoparticles: Antifungal and wound healing efficiencies. Saudi J. Biol. Sci. 2022, 29, 1832–1841. [Google Scholar] [CrossRef] [PubMed]
- Filip, A.; Daicoviciu, D.; Clichici, S.; Mocan, T.; Muresan, A.; Postescu, I.D. Photoprotective effects of two natural products on ultraviolet B–induced oxidative stress and apoptosis in SKH-1 mouse skin. J. Med. Food 2011, 14, 761–766. [Google Scholar] [CrossRef]
- Wang, P.W.; Cheng, Y.C.; Hung, Y.C.; Lee, C.H.; Fang, J.Y.; Li, W.T.; Pan, T.L. Red raspberry extract protects the skin against UVB-induced damage with antioxidative and anti-inflammatory properties. Oxidative Med. Cell. Longev. 2019, 2019, 9529676. [Google Scholar] [CrossRef]
- Filip, A.; Daicoviciu, D.; Clichici, S.; Bolfa, P.; Catoi, C.; Baldea, I.; Postescu, I.D. The effects of grape seeds polyphenols on SKH-1 mice skin irradiated with multiple doses of UV-B. J. Photochem. Photobiol. B Biol. 2011, 105, 133–142. [Google Scholar] [CrossRef]
- Bralley, E.E.; Hargrove, J.L.; Greenspan, P.; Hartle, D.K. Topical anti-inflammatory activities of Vitis rotundifolia (muscadine grape) extracts in the tetradecanoylphorbol acetate model of ear inflammation. J. Med. Food 2007, 10, 636–642. [Google Scholar] [CrossRef]
- Ghabaee, D.N.Z.; Ebrahimzadeh, M.A.; Akbari, J.; Amiri, F.T. Wound healing activity of Sambucus ebulus. Int. J. Pharm. Sci. Res. 2017, 8, 132. [Google Scholar]
- Nayak, B.S.; Ramdath, D.D.; Marshall, J.R.; Isitor, G.N.; Eversley, M.; Xue, S.; Shi, J. Wound-healing activity of the skin of the common grape (Vitis Vinifera) variant, cabernet sauvignon. Phytother. Res. 2010, 24, 1151–1157. [Google Scholar] [CrossRef]
- Bomser, J.; Singletary, K.; Meline, B. Inhibition of 12-O-tetradecanoylphorbol-13-acetate (TPA)-induced mouse skin ornithine decarboxylase and protein kinase C by polyphenolics from grapes. Chem. -Biol. Interact. 2000, 127, 45–59. [Google Scholar] [CrossRef] [PubMed]
- Bolfa, P.; Sarac, F.; Filip, A.; Gal, A.; Taulescu, M.; Cuc, C.; Catoi, C. Preclinical evaluation of red grapes seeds extract from vitis vinifera, burgund mare, recas, romania as skin photochemoprotective agent. Planta Medica 2011, 77, PK4. [Google Scholar] [CrossRef]
- Fukuda, T.; Ito, H.; Mukainaka, T.; Tokuda, H.; Nishino, H.; Yoshida, T. Anti-tumor promoting effect of glycosides from Prunus persica seeds. Biol. Pharm. Bull. 2003, 26, 271–273. [Google Scholar] [CrossRef] [PubMed]
- Odah, S.M.; Salama, M.M.; Aziz, W.M.; El-Alfy, T.S.; Ezzat, S.M. Anti-wrinkle activity and UPLC-MS/MS metabolic profiling of pomegranate and grape seeds extracts. IJSPR 2020, 11, 3679–3689. [Google Scholar]
- Son, H.U.; Choi, H.J.; Alam, M.B.; Jeong, C.G.; Lee, H.I.; Kim, S.L.; Lee, S.H. Prunus mume Seed Exhibits Inhibitory Effect on Skin Senescence via SIRT1 and MMP-1 Regulation. Oxidative Med. Cell. Longev. 2021, 2021, 5528795. [Google Scholar] [CrossRef]
- Kamiya, T.; Tanimoto, Y.; Fujii, N.; Negishi, T.; Suzuki, T.; Hatano, T.; Arimoto-Kobayashi, S. 2,6-Dimethoxy-1,4-benzoquinone, isolation and identification of anti-carcinogenic, anti-mutagenic and anti-inflammatory component from the juice of Vitis coignetiae. Food Chem. Toxicol. 2018, 122, 172–180. [Google Scholar] [CrossRef]
- Kamel, R.; Abbas, H.; Fayez, A. Diosmin/essential oil combination for dermal photo-protection using a lipoid colloidal carrier. J. Photochem. Photobiol. B Biol. 2017, 170, 49–57. [Google Scholar] [CrossRef]
- Lujan de Pinedo, M.E.; Ayala-Jara, C.I.; Castillo Saavedra, E.F.; Pinedo-Torres, C.A.; González-Puetate, I.; Durand-Luján, C.S. Dressing based on Vaccinium corymbosum L. and chitosan with high skin regenerative capacity. CABI Databases 2018, 9, 223–229. [Google Scholar] [CrossRef]
- Singh, D.; Gohil, K.J.; Sharma, V.; Shukla, S.S. In vivo wound Healing Activity of Ointment based Formulation for Prunus amygdalus (Batsch.) on Rats. Indian J. Pharm. Educ. Res. 2022, 56, 184–190. [Google Scholar] [CrossRef]
- Alsadi, N.; Yasavoli-Sharahi, H.; Mueller, R.; Cuenin, C.; Chung, F.; Herceg, Z.; Matar, C. Protective Mechanisms of Polyphenol-Enriched Blueberry Preparation in Preventing Inflammation in the Skin against UVB-Induced Damage in an Animal Model. Antioxidants 2023, 13, 25. [Google Scholar] [CrossRef]
- Jabri, M.A.; Hajaji, S.; Omrani, A.; Ben Youssef, M.; Sebai, H. Myrtle berries seeds prevent dyslipidemia, inflammation, and excessive cardiac reactive oxygen species production in response to high-fat diet–induced obesity. J. Med. Food 2023, 26, 631–640. [Google Scholar] [CrossRef] [PubMed]
- Zhou, F.; Guo, J.; Han, X.; Gao, Y.; Chen, Q.; Huang, W.; Zhan, J.; Huang, D.; You, Y. Cranberry Polyphenolic Extract Exhibits an Antiobesity Effect on High-Fat Diet-Fed Mice through Increased Thermogenesis. J. Nutr. 2020, 150, 2131–2138. [Google Scholar] [CrossRef] [PubMed]
- Bai, B.; Chen, H. Metformin: A Novel Weapon Against Inflammation. Front. Pharmacol. 2021, 12, 622262. [Google Scholar] [CrossRef]
- Chavda, V.P.; Feehan, J.; Apostolopoulos, V. Inflammation: The cause of all diseases. Cells 2024, 13, 1906. [Google Scholar] [CrossRef]
- Altoé, L.S.; Alves, R.S.; Sarandy, M.M.; Morais-Santos, M.; Novaes, R.D.; Gonçalves, R.V. Does antibiotic use accelerate or retard cutaneous repair? A systematic review in animal models. PLoS ONE 2019, 14, e0223511. [Google Scholar] [CrossRef] [PubMed]
- Panigrahy, D.; Gilligan, M.M.; Serhan, C.N.; Kashfi, K. Resolution of inflammation: An organizing principle in biology and medicine. Pharmacol. Ther. 2021, 227, 107879. [Google Scholar] [CrossRef]
- Liu, M.; Yun, P.; Hu, Y.; Yang, J.; Khadka, R.B.; Peng, X. Effects of Grape Seed Proanthocyanidin Extract on Obesity. Obes. Facts 2020, 13, 279–291. [Google Scholar] [CrossRef]
- Rosa, D.F.; Sarandy, M.M.; Novaes, R.D.; Freitas, M.B.; do Carmo Gouveia Pelúzio, M.; Gonçalves, R.V. High-fat diet and alcohol intake promotes inflammation and impairs skin wound healing in Wistar rats. Mediat. Inflamm. 2018, 2018, 4658583. [Google Scholar] [CrossRef] [PubMed]
- Sarandy, M.M.; Novaes, R.D.; Xavier, A.A.; Vital, C.E.; Leite, J.P.; Melo, F.C.; Gonçalves, R.V. Hydroethanolic extract of Strychnos pseudoquina accelerates skin wound healing by modulating the oxidative status and microstructural reorganization of scar tissue in experimental type I diabetes. BioMed Res. Int. 2017, 2017, 9538351. [Google Scholar] [CrossRef]
- Keewan, E.; Naser, S.A. The Role of Notch Signaling in Macrophages during Inflammation and Infection: Implication in Rheumatoid Arthritis? Cells 2020, 9, 111. [Google Scholar] [CrossRef]
- Li, M.; Hou, Q.; Zhong, L.; Zhao, Y.; Fu, X. Macrophage related chronic inflammation in non-healing wounds. Front. Immunol. 2021, 16, 681710. [Google Scholar] [CrossRef]
- Valacchi, G.; Virgili, F.; Cervellati, C.; Pecorelli, A. OxInflammation: From subclinical condition to pathological biomarker. Front. Physiol. 2018, 9, 858. [Google Scholar] [CrossRef] [PubMed]
- Kalt, W.; Cassidy, A.; Howard, L.R.; Krikorian, R.; Stull, A.J.; Tremblay, F.; Zamora-Ros, R. Recent Research on the Health Benefits of Blueberries and Their Anthocyanins. Adv. Nutr. 2020, 11, 224–236. [Google Scholar] [CrossRef] [PubMed]
- Lee, H.S.; Choi, C.I. Black Goji Berry (Lycium ruthenicum Murray): A Review of Its Pharmacological Activity. Nutrients 2023, 15, 4181. [Google Scholar] [CrossRef] [PubMed]
- Nogueira, B.C.F.; Campos, A.K.; Alves, R.S.; Sarandy, M.M.; Novaes, R.D.; Esposito, D.; Gonçalves, R.V. What Is the Impact of Depletion of Immunoregulatory Genes on Wound Healing? Systematic Review of Preclinical Evidence. Oxidative Med. Cell Longev. 2020, 2020, 8862953. [Google Scholar] [CrossRef]
- Steen, E.H.; Wang, X.; Balaji, S.; Butte, M.J.; Bollyky, P.L.; Keswani, S.G. The Role of the Anti-Inflammatory Cytokine Interleukin-10 in Tissue Fibrosis. Adv. Wound Care 2020, 9, 184–198. [Google Scholar] [CrossRef]
- Khan, A.Q.; Travers, J.B.; Kemp, M.G. Roles of UVA radiation and DNA damage responses in melanoma pathogenesis. Environ. Mol. Mutagen. 2018, 59, 438–460. [Google Scholar] [CrossRef]
- Alemu, B.K.; Getahun, K.B.; Kahaliw, W. In vitro Antioxidant and in vivo Wound Healing Activities of the 80% Methanol Extract and Solvent Fractions of Seeds of Brassica carinata A. Braun (Brassicaceae) in Mice. J. Exp. Pharmacol. 2020, 12, 463–474. [Google Scholar] [CrossRef]
- Mattosinhos, P.D.S.; Sarandy, M.M.; Novaes, R.D.; Esposito, D.; Goncalves, R.V. Anti-inflammatory, antioxidant, and skin regenerative potential of secondary metabolites from plants of the Brassicaceae family: A systematic review of in vitro and in vivo preclinical evidence (biological activities Brassicaceae skin diseases). Antioxidants 2022, 11, 1346. [Google Scholar] [CrossRef] [PubMed]
- Nikolic, M.; Andjic, M.; Bradic, J.; Kocovic, A.; Tomovic, M.; Samanovic, A.M.; Jakovljevic, V.; Veselinovic, M.; Capo, I.; Krstonosic, V.; et al. Topical Application of Siberian Pine Essential Oil Formulations Enhance Diabetic Wound Healing. Pharmaceutics 2023, 15, 2437. [Google Scholar] [CrossRef]
- Sitohang, N.A.; Putra, E.D.L.; Kamil, H.; Musman, M. Acceleration of wound healing by topical application of gel formulation of Barringtonia racemosa (L.) Spreng kernel extract. F1000Res 2022, 11, 191. [Google Scholar] [CrossRef]
- Farooq, N.; Anwar, F.; Saleem, U.; Ashfaq, M.; Shafi, A.; Ismail, T. The wound healing potential of Hedychium spicatum Sm. and Zinnia peruviana (L.) ethanolic extracts against excision wound model in rats. J. Ethnopharmacol. 2023, 311, 116404. [Google Scholar] [CrossRef] [PubMed]
- Coutinho-Wolino, K.S.; Almeida, P.P.; Mafra, D.; Stockler-Pinto, M.B. Bioactive compounds modulating Toll-like 4 receptor (TLR4)-mediated inflammation: Pathways involved and future perspectives. Nutr. Res. 2022, 107, 96–116. [Google Scholar] [CrossRef] [PubMed]
- Joseph, S.V.; Edirisinghe, I.; Burton-Freeman, B.M. Berries: Anti-inflammatory effects in humans. J. Agric. Food Chem. 2014, 62, 3886–3903. [Google Scholar] [CrossRef]
- Calvo-Castro, L.; Syed, D.N.; Chamcheu, J.C.; Vilela, F.M.P.; Perez, A.M.; Vaillant, F.; Rojas, M.; Mukhtar, H. Protective effect of tropical highland blackberry juice (rubus adenotrichos schltdl.) against UVB-mediated damage in human epidermal keratinocytes and in a reconstituted skin equivalent model. Photochem. Photobiol. 2013, 89, 1199–1207. [Google Scholar] [CrossRef]
- de Araújo, F.F.; de Paulo Farias, D.; Neri-Numa, I.A.; Pastore, G.M. Polyphenols and their applications: An approach in food chemistry and innovation potential. Food Chem. 2021, 338, 127535. [Google Scholar] [CrossRef]
- Guo, Q.; Li, F.; Duan, Y.; Wen, C.; Wang, W.; Zhang, L.; Huang, R.; Yin, Y. Oxidative stress, nutritional antioxidants and beyond. Sci. China Life Sci. 2020, 63, 866–874. [Google Scholar] [CrossRef]
- Jin, Q.; Liu, T.; Qiao, Y.; Liu, D.; Yang, L.; Mao, H.; Ma, F.; Wang, Y.; Peng, L.; Zhan, Y. Oxidative stress and inflammation in diabetic nephropathy: Role of polyphenols. Front. Immunol. 2023, 14, 1185317. [Google Scholar] [CrossRef]
- Sahoo, D.K.; Heilmann, R.M.; Paital, B.; Patel, A.; Yadav, V.K.; Wong, D.; Jergens, A.E. Oxidative stress, hormones, and effects of natural antioxidants on intestinal inflammation in inflammatory bowel disease. Front. Endocrinol. 2023, 14, 1217165. [Google Scholar] [CrossRef]
- Fernandes, A.; Rodrigues, P.M.; Pintado, M.; Tavaria, F.K. A systematic review of natural products for skin applications: Targeting inflammation, wound healing, and photo-aging. Phytomedicine 2023, 115, 154824. [Google Scholar] [CrossRef]
- Lee, Y.M.; Yoon, Y.; Yoon, H.; Park, H.M.; Song, S.; Yeum, K.J. Dietary Anthocyanins against Obesity and Inflammation. Nutrients 2017, 9, 1089. [Google Scholar] [CrossRef]
- Zhang, J.; Ye, Y.; Chang, D.W.; Lin, S.H.; Huang, M.; Tannir, N.M.; Matin, S.; Karam, J.A.; Wood, C.G.; Chen, Z.N.; et al. Global and Targeted miRNA Expression Profiling in Clear Cell Renal Cell Carcinoma Tissues Potentially Links miR-155-5p and miR-210-3p to both Tumorigenesis and Recurrence. Am. J. Pathol. 2018, 188, 2487–2496. [Google Scholar] [CrossRef]
- Sajadimajd, S.; Bahramsoltani, R.; Iranpanah, A.; Kumar Patra, J.; Das, G.; Gouda, S.; Rahimi, R.; Rezaeiamiri, E.; Cao, H.; Giampieri, F.; et al. Advances on Natural Polyphenols as Anticancer Agents for Skin Cancer. Pharmacol. Res. 2020, 151, 104584. [Google Scholar] [CrossRef]
- Cremonini, E.; Iglesias, D.E.; Matsukuma, K.E.; Hester, S.N.; Wood, S.M.; Bartlett, M.; Fraga, C.G.; Oteiza, P.I. Supplementation with cyanidin and delphinidin mitigates high fat diet-induced endotoxemia and associated liver inflammation in mice. Food Funct. 2022, 13, 781–794. [Google Scholar] [CrossRef]
- Gado, F.; Ferrario, G.; Della Vedova, L.; Zoanni, B.; Altomare, A.; Carini, M.; Aldini, G.; D’Amato, A.; Baron, G. Targeting Nrf2 and NF-κB Signaling Pathways in Cancer Prevention: The Role of Apple Phytochemicals. Molecules 2023, 28, 1356. [Google Scholar] [CrossRef]
- Dai, K.; Agarwal, N.; Rodriguez-Palacios, A.; Basson, A.R. Regulation of Intestinal Inflammation by Walnut-Derived Bioactive Compounds. Nutrients 2024, 16, 2643. [Google Scholar] [CrossRef]
- Petagna, L.; Antonelli, A.; Ganini, C.; Bellato, V.; Campanelli, M.; Divizia, A.; Sica, G.S. Pathophysiology of Crohn’s disease inflammation and recurrence. Biol. Direct 2020, 15, 23. [Google Scholar] [CrossRef]
- Niu, Y.; Zhang, J.; Shi, D.; Zang, W.; Niu, J. Glycosides as Potential Medicinal Components for Ulcerative Colitis: A Review. Molecules 2023, 28, 5210. [Google Scholar] [CrossRef]
- Wang, X.; Xu, X.; Wu, P.; Wu, M.; Gan, L.; Jin, J.; Wu, R.; Liu, W.; Zhang, K.; Li, D.; et al. Complanatuside alleviates inflammatory cell damage induced by pro-inflammatory cytokines in skin keratinocytes. Front. Chem. 2022, 10, 909651. [Google Scholar] [CrossRef]
- Merecz-Sadowska, A.; Sitarek, P.; Kowalczyk, T.; Zajdel, K.; Jęcek, M.; Nowak, P.; Zajdel, R. Food Anthocyanins: Malvidin and Its Glycosides as Promising Antioxidant and Anti-Inflammatory Agents with Potential Health Benefits. Nutrients 2023, 15, 3016. [Google Scholar] [CrossRef]
- Wen, L.; Wu, D.; Tan, X.; Zhong, M.; Xing, J.; Li, W.; Li, D.; Cao, F. The Role of Catechins in Regulating Diabetes: An Update Review. Nutrients 2022, 14, 4681. [Google Scholar] [CrossRef]
- Kim, J.M.; Heo, H.J. The roles of catechins in regulation of systemic inflammation. Food Sci. Biotechnol. 2022, 31, 957–970. [Google Scholar] [CrossRef]
- Shen, M.; You, Y.; Xu, C.; Chen, Z. Epigallocatechin-3-Gallate attenuates lipopolysacharide-induced pneumonia via modification of inflammation, oxidative stress, apoptosis, and autophagy. BMC Complement. Med. Ther. 2024, 24, 147. [Google Scholar] [CrossRef]
- Aljuffali, I.A.; Lin, C.H.; Yang, S.C.; Alalaiwe, A.; Fang, J.Y. Nanoencapsulation of Tea Catechins for Enhancing Skin Absorption and Therapeutic Efficacy. AAPS PharmSciTech 2022, 23, 187. [Google Scholar] [CrossRef]
- Moretti, L.; Stalfort, J.; Barker, T.H.; Abebayehu, D. The interplay of fibroblasts, the extracellular matrix, and inflammation in scar formation. J. Biol. Chem. 2022, 298, 101530. [Google Scholar] [CrossRef]
- Valacchi, G.; Sticozzi, C.; Pecorelli, A.; Cervellati, F.; Cervellati, C.; Maioli, E. Cutaneous responses to environmental stressors. Ann. N. Y. Acad. Sci. 2012, 1271, 75–81. [Google Scholar] [CrossRef]
- El-Benna, J.; Hurtado-Nedelec, M.; Gougerot-Pocidalo, M.A.; Dang, P.M. Effects of venoms on neutrophil respiratory burst: A major inflammatory function. J. Venom. Anim. Toxins Incl. Trop. Dis. 2021, 27, e20200179. [Google Scholar] [CrossRef]
- Gardeazabal, L.; Izeta, A. Elastin and collagen fibres in cutaneous wound healing. Exp. Dermatol. 2024, 33, e15052. [Google Scholar] [CrossRef] [PubMed]



Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Azevedo, F.S.; Pires, A.R.; Lila, M.A.; Valacchi, G.; Hoskin, R.T.; Sarandy, M.M.; Novaes, R.D.; Goncalves, R.V. Berry Fruit Extracts as Topical Cosmeceuticals for Skin Health Applications: A Systematic Review. Cosmetics 2025, 12, 87. https://doi.org/10.3390/cosmetics12030087
Azevedo FS, Pires AR, Lila MA, Valacchi G, Hoskin RT, Sarandy MM, Novaes RD, Goncalves RV. Berry Fruit Extracts as Topical Cosmeceuticals for Skin Health Applications: A Systematic Review. Cosmetics. 2025; 12(3):87. https://doi.org/10.3390/cosmetics12030087
Chicago/Turabian StyleAzevedo, Filipe Silveira, Allan Rodrigues Pires, Mary Ann Lila, Giuseppe Valacchi, Roberta Targino Hoskin, Mariaurea Matias Sarandy, Rômulo Dias Novaes, and Reggiani Vilela Goncalves. 2025. "Berry Fruit Extracts as Topical Cosmeceuticals for Skin Health Applications: A Systematic Review" Cosmetics 12, no. 3: 87. https://doi.org/10.3390/cosmetics12030087
APA StyleAzevedo, F. S., Pires, A. R., Lila, M. A., Valacchi, G., Hoskin, R. T., Sarandy, M. M., Novaes, R. D., & Goncalves, R. V. (2025). Berry Fruit Extracts as Topical Cosmeceuticals for Skin Health Applications: A Systematic Review. Cosmetics, 12(3), 87. https://doi.org/10.3390/cosmetics12030087











