Kv1.3 Ion Channels Mediate Electrical Stimulation-Induced Collagen Expression in Human Dermal Fibroblasts
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials and Reagents
2.2. Cell Culture
2.3. Electrical Stimulation of Cells
2.4. Quantification of Collagen Concentration
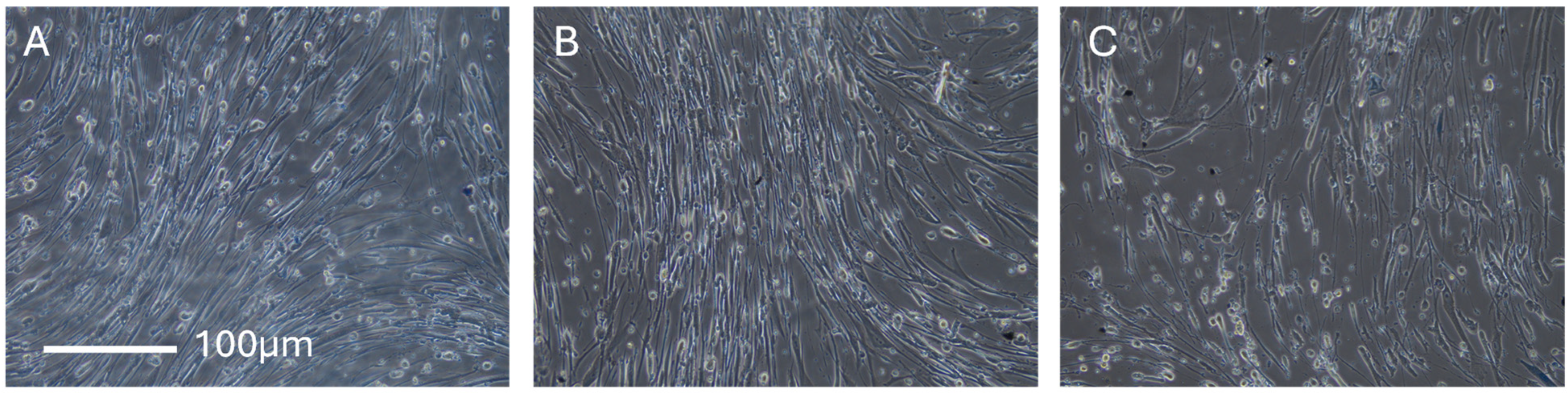
2.5. Microscopy
2.6. Proliferation Assay
2.7. Flow Cytometry
2.8. Data Analysis
3. Results
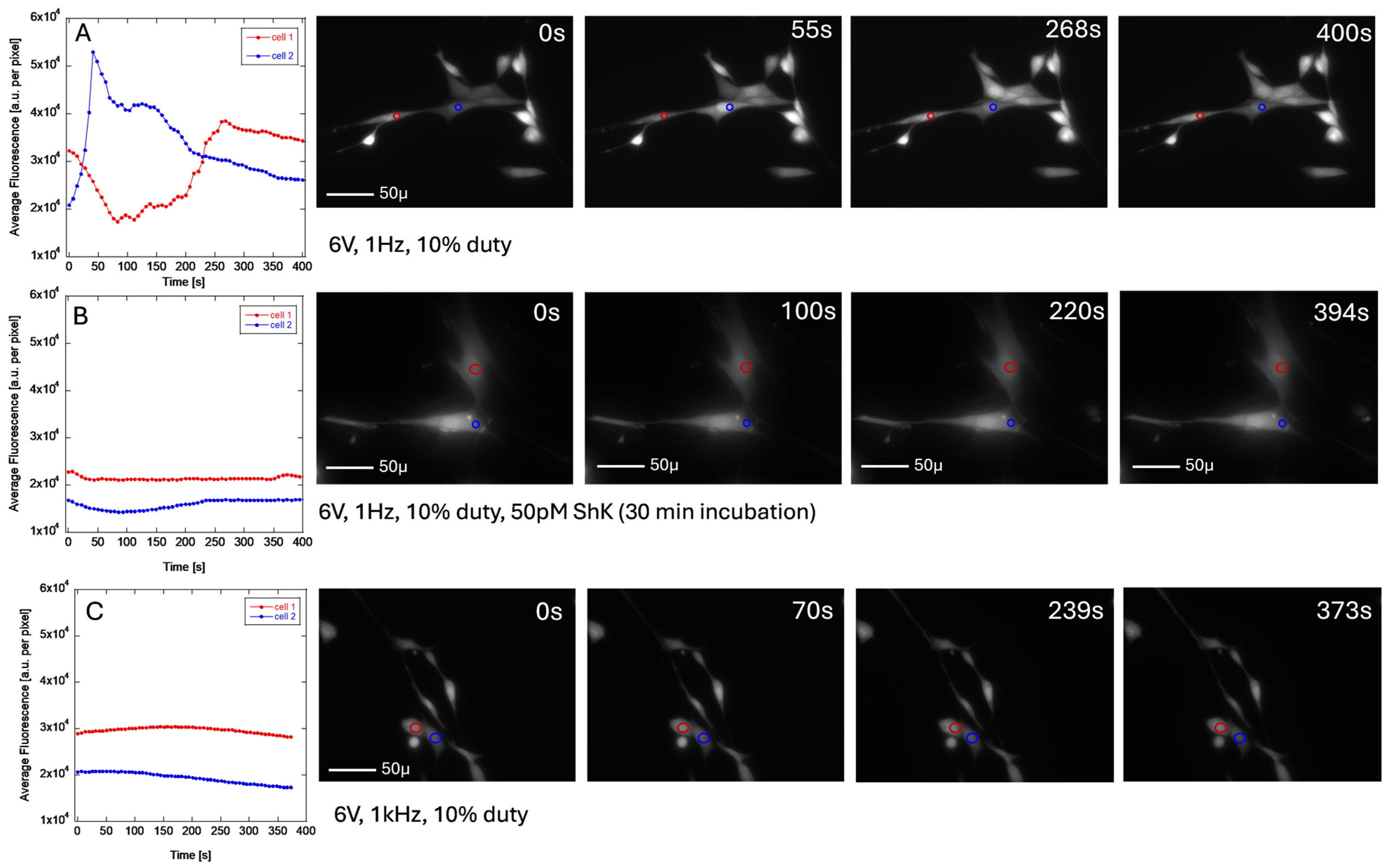
3.1. ES Measurements
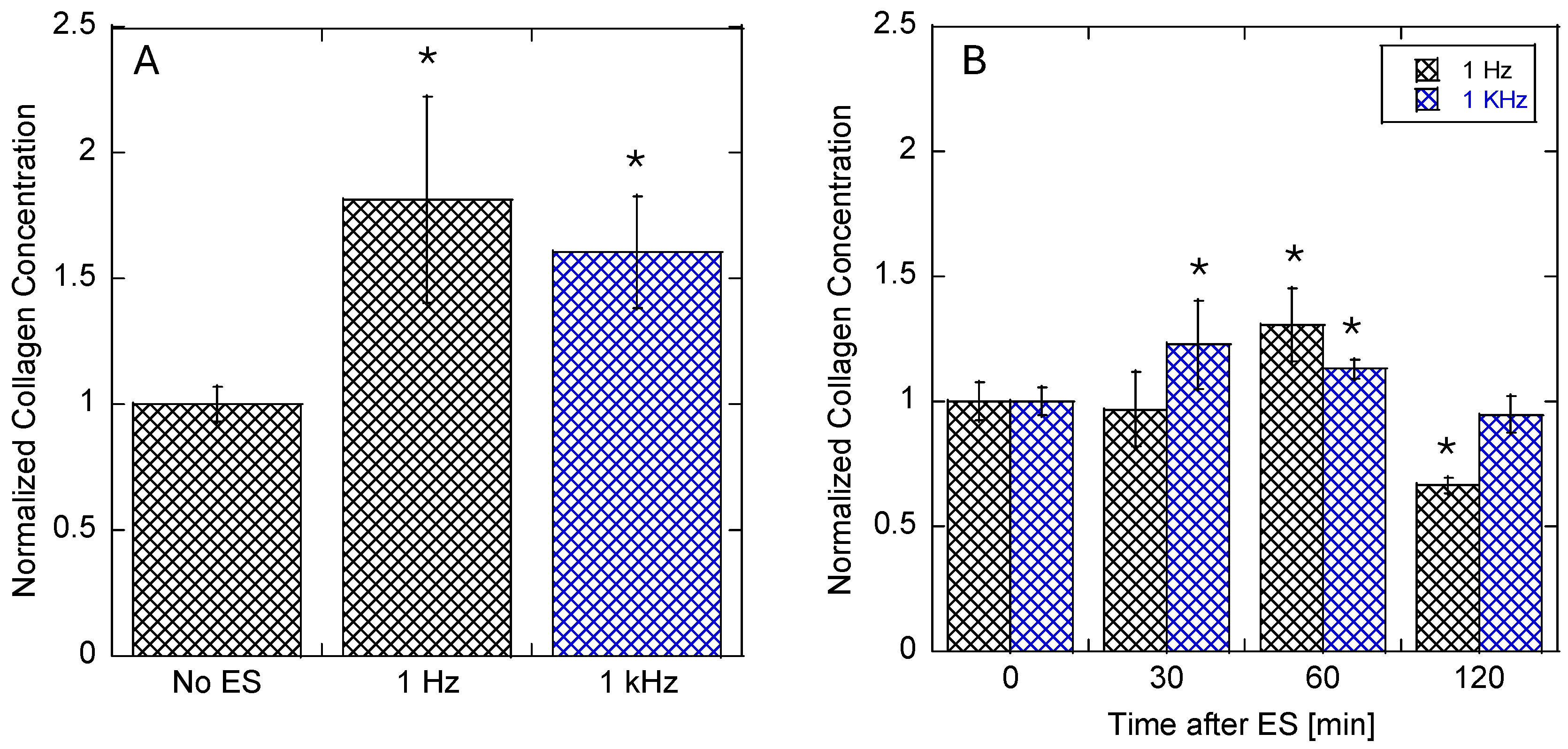
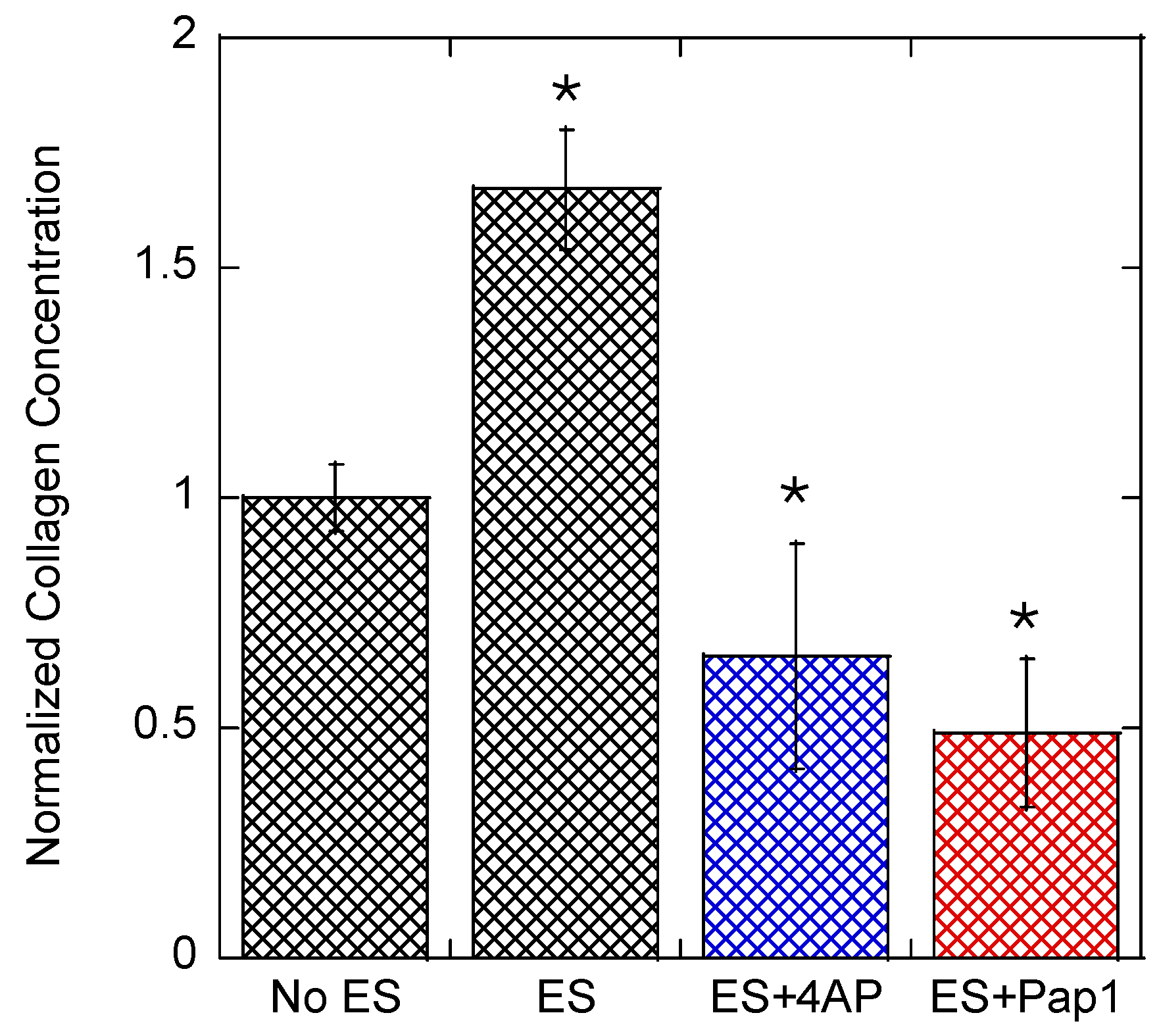
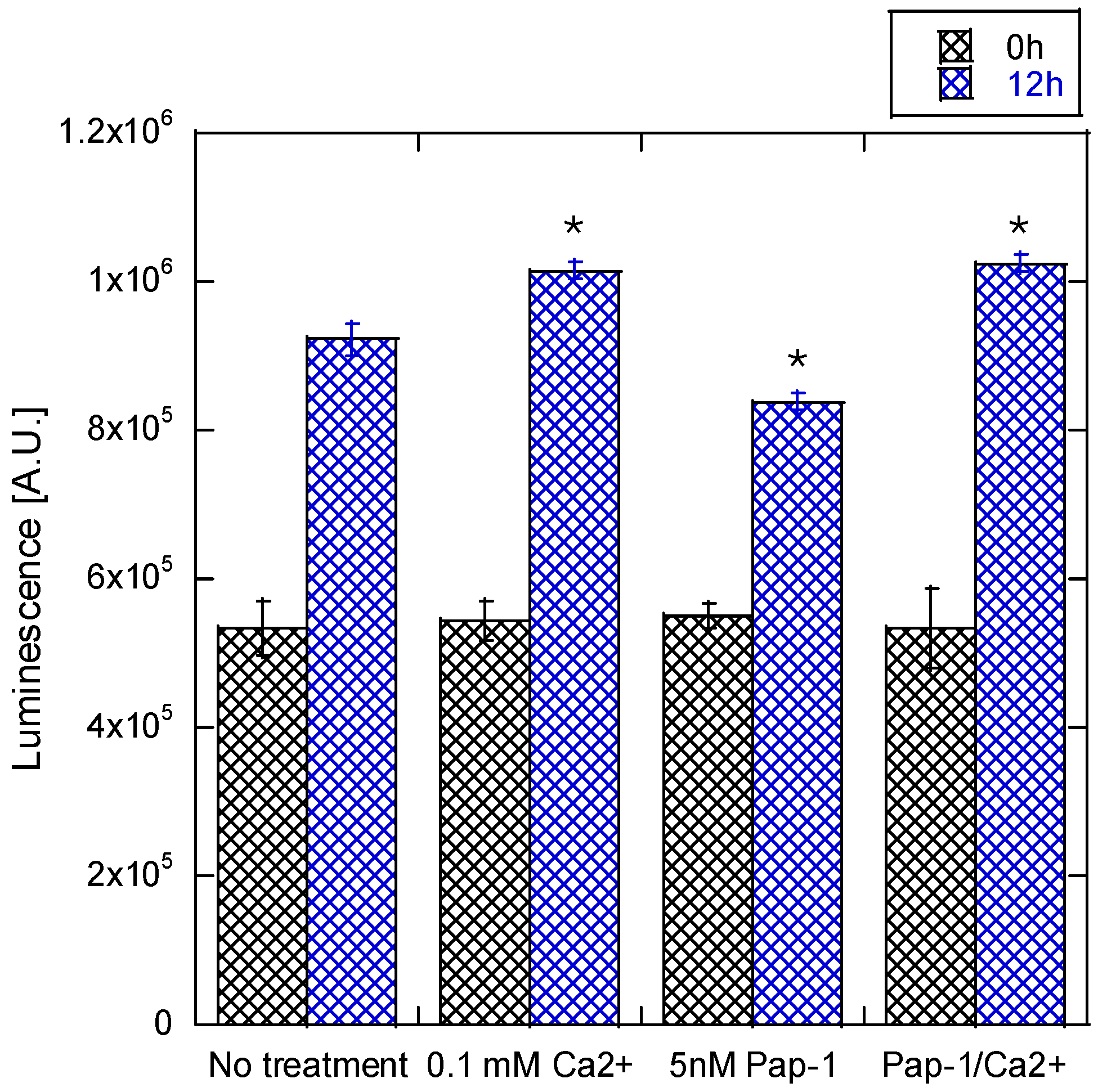
3.2. Collagen Expression
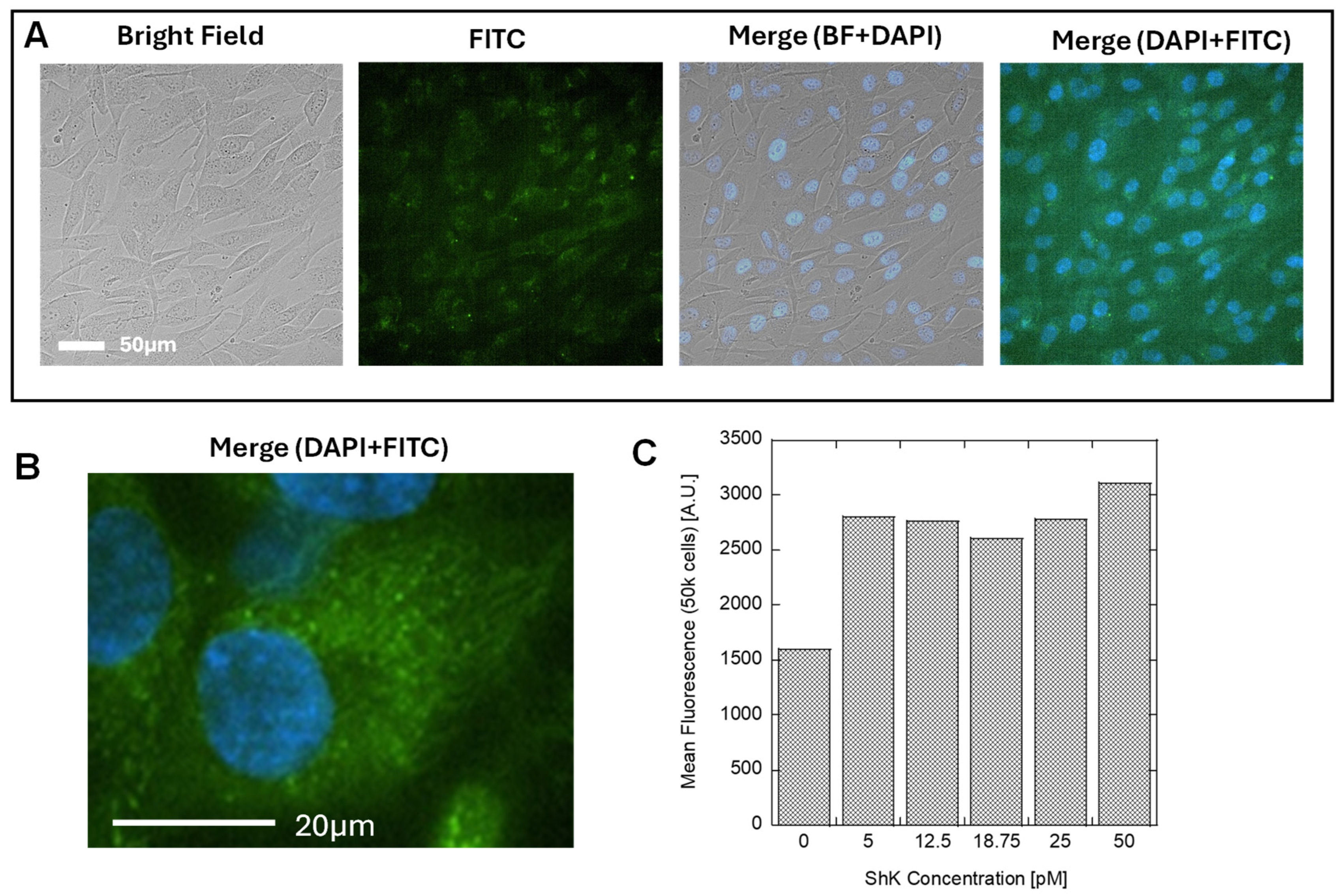
3.3. Identification of Kv1.3 Channel on Human Dermal Fibroblast Cell Surface
3.4. Cell Proliferation
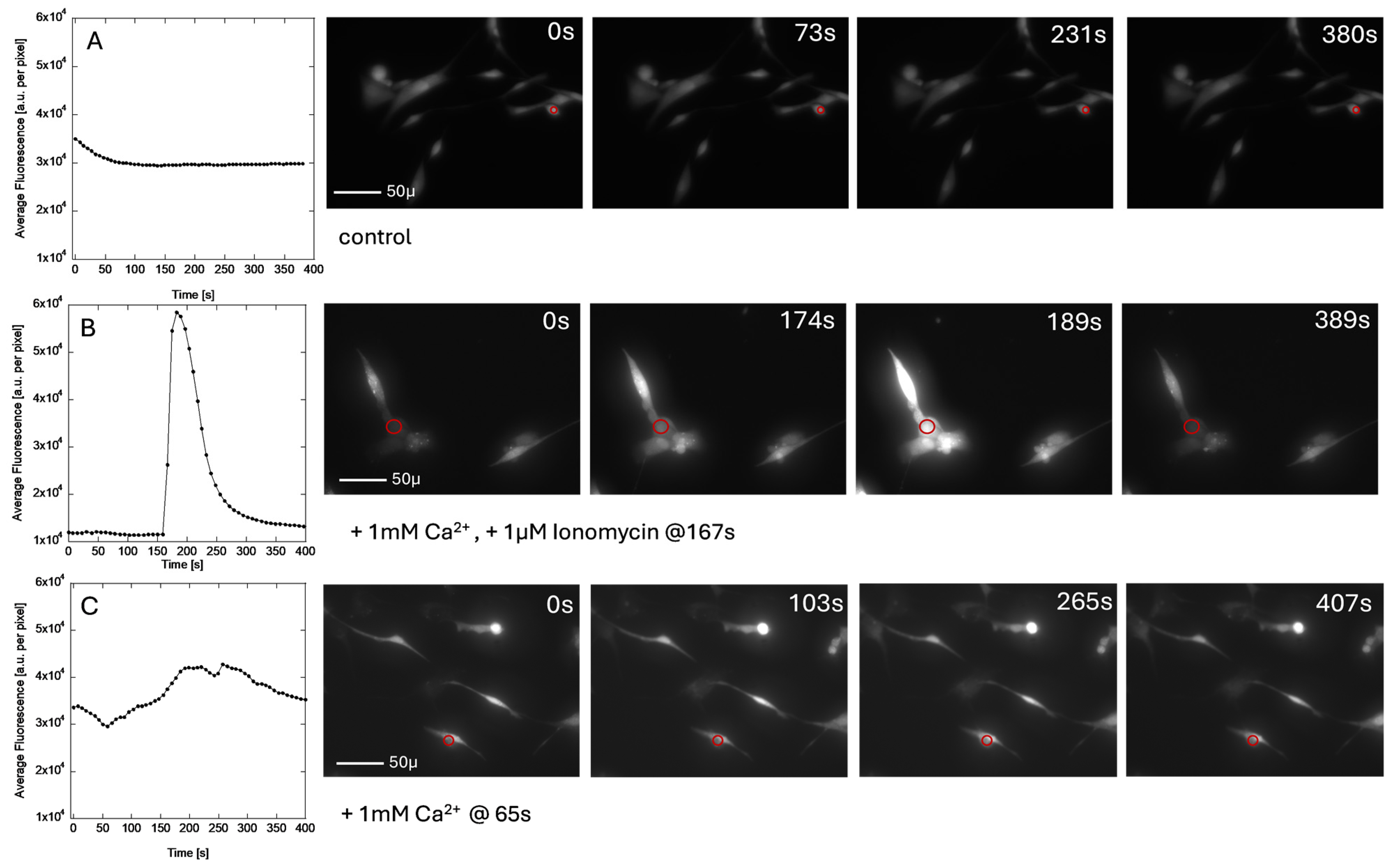
3.5. Calcium Imaging
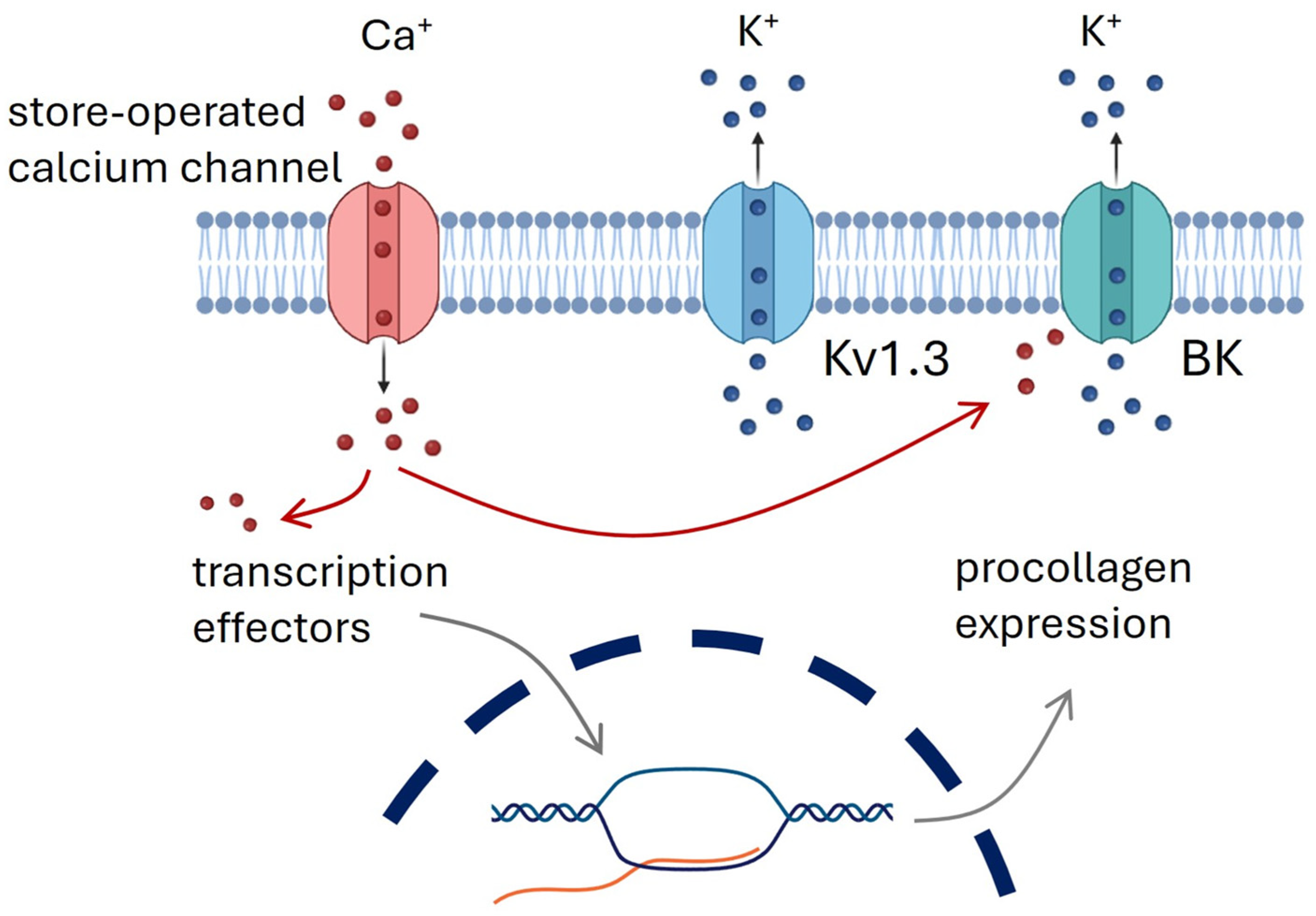
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| Kv | Voltage-Gated Potassium Channels |
| ES | Electrical Stimulation |
| IC50 | Half Maximal Inhibitory Concentration |
| ShK | Sea Anemone Toxin (Kv1.3 Channel Blocker) |
| 5FAM | 5-Carboxy Fluorescein |
| Kv1.3 | Voltage-Gated Potassium Channel 1.3 |
| Fluo-4 | Fluorescent Dye For Calcium Imaging |
| ORAI1 | Calcium Release-Activated Calcium Channel Protein 1 |
References
- Rajendran, S.B.; Challen, K.; Wright, K.L.; Hardy, J.G. Electrical Stimulation to Enhance Wound Healing. J. Funct. Biomater. 2021, 12, 40. [Google Scholar] [CrossRef] [PubMed]
- Preetam, S.; Ghosh, A.; Mishra, R.; Pandey, A.; Roy, D.S.; Rustagi, S.; Malik, S. Electrical Stimulation: A Novel Therapeutic Strategy to Heal Biological Wounds. RSC Adv. 2024, 14, 32142–32173. [Google Scholar] [CrossRef] [PubMed]
- Kavanagh, S.; Newell, J.; Hennessy, M.; Sadick, N. Use of a Neuromuscular Electrical Stimulation Device for Facial Muscle Toning: A Randomized, Controlled Trial. J. Cosmet. Dermatol. 2012, 11, 261–266. [Google Scholar] [CrossRef]
- Rouabhia, M.; Park, H.; Meng, S.; Derbali, H.; Zhang, Z. Electrical Stimulation Promotes Wound Healing by Enhancing Dermal Fibroblast Activity and Promoting Myofibroblast Transdifferentiation. PLoS ONE 2013, 8, e71660. [Google Scholar] [CrossRef]
- Farber, P.L.; Hochman, B.; Furtado, F.; Ferreira, L.M. Electricity and Colloidal Stability: How Charge Distribution in the Tissue Can Affects Wound Healing. Med. Hypotheses 2014, 82, 199–204. [Google Scholar] [CrossRef]
- Rabbani, M.; Rahman, E.; Powner, M.B.; Triantis, I.F. Making Sense of Electrical Stimulation: A Meta-Analysis for Wound Healing. Ann. Biomed. Eng. 2024, 52, 153–177. [Google Scholar] [CrossRef]
- Cheng, K.; Goldman, R.J. Electric Fields and Proliferation in a Dermal Wound Model: Cell Cycle Kinetics. Bioelectromagnetics 1998, 19, 68–74. [Google Scholar] [CrossRef]
- Xu, X.; Zhang, H.; Yan, Y.; Wang, J.; Guo, L. Effects of Electrical Stimulation on Skin Surface. Acta Mech. Sin. 2021, 37, 1843–1871. [Google Scholar] [CrossRef]
- Golberg, A.; Khan, S.; Belov, V.; Quinn, K.P.; Albadawi, H.; Felix Broelsch, G.; Watkins, M.T.; Georgakoudi, I.; Papisov, M.; Mihm, M.C.; et al. Skin Rejuvenation with Non-Invasive Pulsed Electric Fields. Sci. Rep. 2015, 5, 10187. [Google Scholar] [CrossRef]
- Omatsu, J.; Yamashita, T.; Mori, T.; Osuji, Y.; Kawanabe, R.; Kuzumi, A.; Yoshizaki, A.; Yokota, T.; Yamazaki, K.; Sato, S.; et al. Neuromuscular Electrical Stimulation for Facial Wrinkles and Sagging: The 8-week Prospective, Split-face, Controlled Trial in Asians. J. Cosmet. Dermatol. 2024, 23, 3222–3233. [Google Scholar] [CrossRef]
- Sadala, A.Y.; Rampazo, É.P.; Liebano, R.E. Analgesic Electrical Currents Associated with Carboxytherapy in Women with Cellulite: A Randomized Controlled Trial. J. Cosmet. Dermatol. 2023, 22, 2288–2296. [Google Scholar] [CrossRef] [PubMed]
- Liatsopoulou, A.; Varvaresou, A.; Mellou, F.; Protopapa, E. Iontophoresis in Dermal Delivery: A Review of Applications in Dermato-cosmetic and Aesthetic Sciences. Int. J. Cosmet. Sci. 2023, 45, 117–132. [Google Scholar] [CrossRef] [PubMed]
- Bai, Y.; Ni, W.; Zhang, Y.; Jiang, Z.; Zhou, S.; Yao, M. Radiofrequency Improves Facial Fine Lines by Thermal Effect: Damage or Just Stimulation? J. Cosmet. Dermatol. 2025, 24, e16600. [Google Scholar] [CrossRef] [PubMed]
- Snyder, S.; DeJulius, C.; Willits, R.K. Electrical Stimulation Increases Random Migration of Human Dermal Fibroblasts. Ann. Biomed. Eng. 2017, 45, 2049–2060. [Google Scholar] [CrossRef]
- Wang, Y.; Rouabhia, M.; Zhang, Z. Pulsed Electrical Stimulation Benefits Wound Healing by Activating Skin Fibroblasts through the TGFβ1/ERK/NF-ΚB Axis. Biochim. Biophys. Acta Gen. Subj. 2016, 1860, 1551–1559. [Google Scholar] [CrossRef]
- Urabe, H.; Akimoto, R.; Kamiya, S.; Hosoki, K.; Ichikawa, H.; Nishiyama, T. Pulsed Electrical Stimulation and Amino Acid Derivatives Promote Collagen Gene Expression in Human Dermal Fibroblasts. Cytotechnology 2024, 76, 139–151. [Google Scholar] [CrossRef]
- Tlili, C.; Reybier, K.; Géloën, A.; Ponsonnet, L.; Martelet, C.; Ouada, H.B.; Lagarde, M.; Jaffrezic-Renault, N. Fibroblast Cells: A Sensing Bioelement for Glucose Detection by Impedance Spectroscopy. Anal. Chem. 2003, 75, 3340–3344. [Google Scholar] [CrossRef]
- Hagedorn, R.; Fuhr, G.; Lichtwardt-Zinke, K.; Richter, E.; Hornung, J.; Voigt, A. Characterisation of Cell Movement by Impedance Measurement on Fibroblasts Grown on Perforated Si-Membranes. Biochim. Biophys. Acta 1995, 1269, 221–232. [Google Scholar] [CrossRef]
- Mellström, B.; Savignac, M.; Gomez-Villafuertes, R.; Naranjo, J.R. Ca2+-Operated Transcriptional Networks: Molecular Mechanisms and In Vivo Models. Physiol. Rev. 2008, 88, 421–449. [Google Scholar] [CrossRef]
- Naranjo, J.R.; Mellström, B. Ca2+-Dependent Transcriptional Control of Ca2+ Homeostasis. J. Biol. Chem. 2012, 287, 31674–31680. [Google Scholar] [CrossRef]
- Plikus, M.V.; Wang, X.; Sinha, S.; Forte, E.; Thompson, S.M.; Herzog, E.L.; Driskell, R.R.; Rosenthal, N.; Biernaskie, J.; Horsley, V. Fibroblasts: Origins, Definitions, and Functions in Health and Disease. Cell 2021, 184, 3852–3872. [Google Scholar] [CrossRef] [PubMed]
- Worthen, C.A.; Cui, Y.; Orringer, J.S.; Johnson, T.M.; Voorhees, J.J.; Fisher, G.J. CD26 Identifies a Subpopulation of Fibroblasts That Produce the Majority of Collagen during Wound Healing in Human Skin. J. Investig. Dermatol. 2020, 140, 2515–2524.e3. [Google Scholar] [CrossRef] [PubMed]
- Ascensión, A.M.; Fuertes-Álvarez, S.; Ibañez-Solé, O.; Izeta, A.; Araúzo-Bravo, M.J. Human Dermal Fibroblast Subpopulations Are Conserved across Single-Cell RNA Sequencing Studies. J. Investig. Dermatol. 2021, 141, 1735–1744.e35. [Google Scholar] [CrossRef] [PubMed]
- Chang, H.Y.; Chi, J.-T.; Dudoit, S.; Bondre, C.; Van De Rijn, M.; Botstein, D.; Brown, P.O. Diversity, Topographic Differentiation, and Positional Memory in Human Fibroblasts. Proc. Natl. Acad. Sci. USA 2002, 99, 12877–12882. [Google Scholar] [CrossRef]
- Estacion, M. Characterization of ion channels seen in subconfluent human dermal fibroblasts. J. Physiol. 1991, 436, 579–601. [Google Scholar] [CrossRef]
- Shao, P.P.; Liu, C.J.; Xu, Q.; Zhang, B.; Li, S.H.; Wu, Y.; Sun, Z.; Cheng, L.F. Eplerenone Reverses Cardiac Fibrosis via the Suppression of Tregs by Inhibition of Kv1.3 Channel. Front. Physiol. 2018, 9, 899. [Google Scholar] [CrossRef]
- Teisseyre, A.; Palko-Labuz, A.; Sroda-Pomianek, K.; Michalak, K. Voltage-Gated Potassium Channel Kv1.3 as a Target in Therapy of Cancer. Front. Oncol. 2019, 9, 933. [Google Scholar] [CrossRef]
- Hernandez-Resendiz, I.; Hartung, F.; Pardo, L.A. Antibodies Targeting K V Potassium Channels: A Promising Treatment for Cancer. Bioelectricity 2019, 1, 180–187. [Google Scholar] [CrossRef]
- Shah, V.N.; Chagot, B.; Chazin, W.J. Calcium-Dependent Regulation of Ion Channels. Calcium Bind. Proteins 2006, 1, 203. [Google Scholar]
- Zajac, M.; Modi, S.; Krishnan, Y. The Evolution of Organellar Calcium Mapping Technologies. Cell Calcium 2022, 108, 102658. [Google Scholar] [CrossRef]
- Bonsignore, G.; Martinotti, S.; Ranzato, E. Wound Repair and Ca2+ Signalling Interplay: The Role of Ca2+ Channels in Skin. Cells 2024, 13, 491. [Google Scholar] [CrossRef] [PubMed]
- Schleinhege, R.; Neumann, I.; Oeckinghaus, A.; Schwab, A.; Pethő, Z. A CNA-35-Based High-Throughput Fibrosis Assay Reveals ORAI1 as a Regulator of Collagen Release from Pancreatic Stellate Cells. Matrix Biol. 2025, 135, 70–86. [Google Scholar] [CrossRef] [PubMed]
- Tandon, N.; Cannizzaro, C.; Chao, P.H.G.; Maidhof, R.; Marsano, A.; Au, H.T.H.; Radisic, M.; Vunjak-Novakovic, G. Electrical Stimulation Systems for Cardiac Tissue Engineering. Nat. Protoc. 2009, 4, 155–173. [Google Scholar] [CrossRef]
- Nguyen, E.B.; Wishner, J.; Slowinska, K. The Effect of Pulsed Electric Field on Expression of ECM Proteins: Collagen, Elastin, and MMP1 in Human Dermal Fibroblasts. J. Electroanal. Chem. 2018, 812, 265–272. [Google Scholar] [CrossRef]
- Kim, D.M.; Nimigean, C.M. Voltage-Gated Potassium Channels: A Structural Examination of Selectivity and Gating. Cold Spring Harb. Perspect. Biol. 2016, 8, a029231. [Google Scholar] [CrossRef]
- Rodríguez-Rangel, S.; Bravin, A.D.; Ramos-Torres, K.M.; Brugarolas, P.; Sánchez-Rodríguez, J.E. Structure-Activity Relationship Studies of Four Novel 4-Aminopyridine K+ Channel Blockers. Sci. Rep. 2020, 10, 52. [Google Scholar] [CrossRef]
- Tian, C.; Zhu, R.; Zhu, L.; Qiu, T.; Cao, Z.; Kang, T. Potassium Channels: Structures, Diseases, and Modulators. Chem. Biol. Drug Des. 2014, 83, 1–26. [Google Scholar] [CrossRef]
- Pennington, M.W.; Chang, S.C.; Chauhan, S.; Huq, R.; Tajhya, R.B.; Chhabra, S.; Norton, R.S.; Beeton, C. Development of Highly Selective Kv1.3-Blocking Peptides Based on the Sea Anemone Peptide ShK. Mar. Drugs 2015, 13, 529–542. [Google Scholar] [CrossRef]
- Iwakawa, N.; Baxter, N.J.; Wai, D.C.C.; Fowler, N.J.; Morales, R.A.V.; Sugase, K.; Norton, R.S.; Williamson, M.P. Conformational Exchange in the Potassium Channel Blocker ShK. Sci. Rep. 2019, 9, 19307. [Google Scholar] [CrossRef]
- Bhavsar, M.B.; Leppik, L.; Costa Oliveira, K.M.; Barker, J.H. Role of Bioelectricity During Cell Proliferation in Different Cell Types. Front. Bioeng. Biotechnol. 2020, 8, 603. [Google Scholar] [CrossRef]
- Gee, K.R.; Brown, K.A.; Chen, W.N.U.; Bishop-Stewart, J.; Gray, D.; Johnson, I. Chemical and Physiological Characterization of Fluo-4 Ca2+-Indicator Dyes. Cell Calcium 2000, 27, 97–106. [Google Scholar] [CrossRef] [PubMed]
- Nakamura, S.; Nakanishi, A.; Takazawa, M.; Okihiro, S.; Urano, S.; Fukui, K. Ionomycin-Induced Calcium Influx Induces Neurite Degeneration in Mouse Neuroblastoma Cells: Analysis of a Time-Lapse Live Cell Imaging System. Free Radic. Res. 2016, 50, 1214–1225. [Google Scholar] [CrossRef] [PubMed]
- Kolimechkov, S.; Seijo, M.; Swaine, I.; Thirkell, J.; Colado, J.C.; Naclerio, F. Physiological Effects of Microcurrent and Its Application for Maximising Acute Responses and Chronic Adaptations to Exercise. Eur. J. Appl. Physiol. 2022, 123, 451–465. [Google Scholar] [CrossRef] [PubMed]
- Prakriya, M. The Molecular Physiology of CRAC Channels. Immunol. Rev. 2009, 231, 88–98. [Google Scholar] [CrossRef]
- Schneggenburger, R.; Neher, E. Intracellular Calcium Dependence of Transmitter Release Rates at a Fast Central Synapse. Nature 2000, 406, 889–893. [Google Scholar] [CrossRef]
- Sun, G.X.; Wang, L.J.; Xiang, C.; Qin, K.R. A Dynamic Model for Intracellular Calcium Response in Fibroblasts Induced by Electrical Stimulation. Math. Biosci. 2013, 244, 47–57. [Google Scholar] [CrossRef] [PubMed]
- Cho, M.R. Control of Calcium Entry in Human Fibroblasts by Frequency-Dependent Electrical Stimulation. Front. Biosci. 2002, 7, A733. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Obiajulu, C.; Nguyen, D.; Bui, K.H.N.; Tran, T.; Vu, A.; Ngo, C.; Slowinski, I.A.; Miyazawa, K.; Slowinska, K. Kv1.3 Ion Channels Mediate Electrical Stimulation-Induced Collagen Expression in Human Dermal Fibroblasts. Cosmetics 2025, 12, 86. https://doi.org/10.3390/cosmetics12030086
Obiajulu C, Nguyen D, Bui KHN, Tran T, Vu A, Ngo C, Slowinski IA, Miyazawa K, Slowinska K. Kv1.3 Ion Channels Mediate Electrical Stimulation-Induced Collagen Expression in Human Dermal Fibroblasts. Cosmetics. 2025; 12(3):86. https://doi.org/10.3390/cosmetics12030086
Chicago/Turabian StyleObiajulu, Catherine, Diem Nguyen, Kim Hoang Ngan Bui, Timothy Tran, Annamarie Vu, Cortney Ngo, Ian A. Slowinski, Kazuyuki Miyazawa, and Katarzyna Slowinska. 2025. "Kv1.3 Ion Channels Mediate Electrical Stimulation-Induced Collagen Expression in Human Dermal Fibroblasts" Cosmetics 12, no. 3: 86. https://doi.org/10.3390/cosmetics12030086
APA StyleObiajulu, C., Nguyen, D., Bui, K. H. N., Tran, T., Vu, A., Ngo, C., Slowinski, I. A., Miyazawa, K., & Slowinska, K. (2025). Kv1.3 Ion Channels Mediate Electrical Stimulation-Induced Collagen Expression in Human Dermal Fibroblasts. Cosmetics, 12(3), 86. https://doi.org/10.3390/cosmetics12030086





